Characterization of Eco-Friendly Fabricated and Induction-Sintered Hydroxyapatite-Based Hybrid Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Green Process-Based Hydroxyapatite Powders
2.3. Fabrication and Characterization of Composites
2.4. Bioactivity Testing
2.5. Cell Culture Studies—Isolation and Culture of Mesenchymal Stem Cells
2.6. MTT Assay Studies
2.7. Osteogenic Differentiation Studies
2.8. Antimicrobial Activity Studies
2.9. Statistical Analysis
3. Results and Discussions
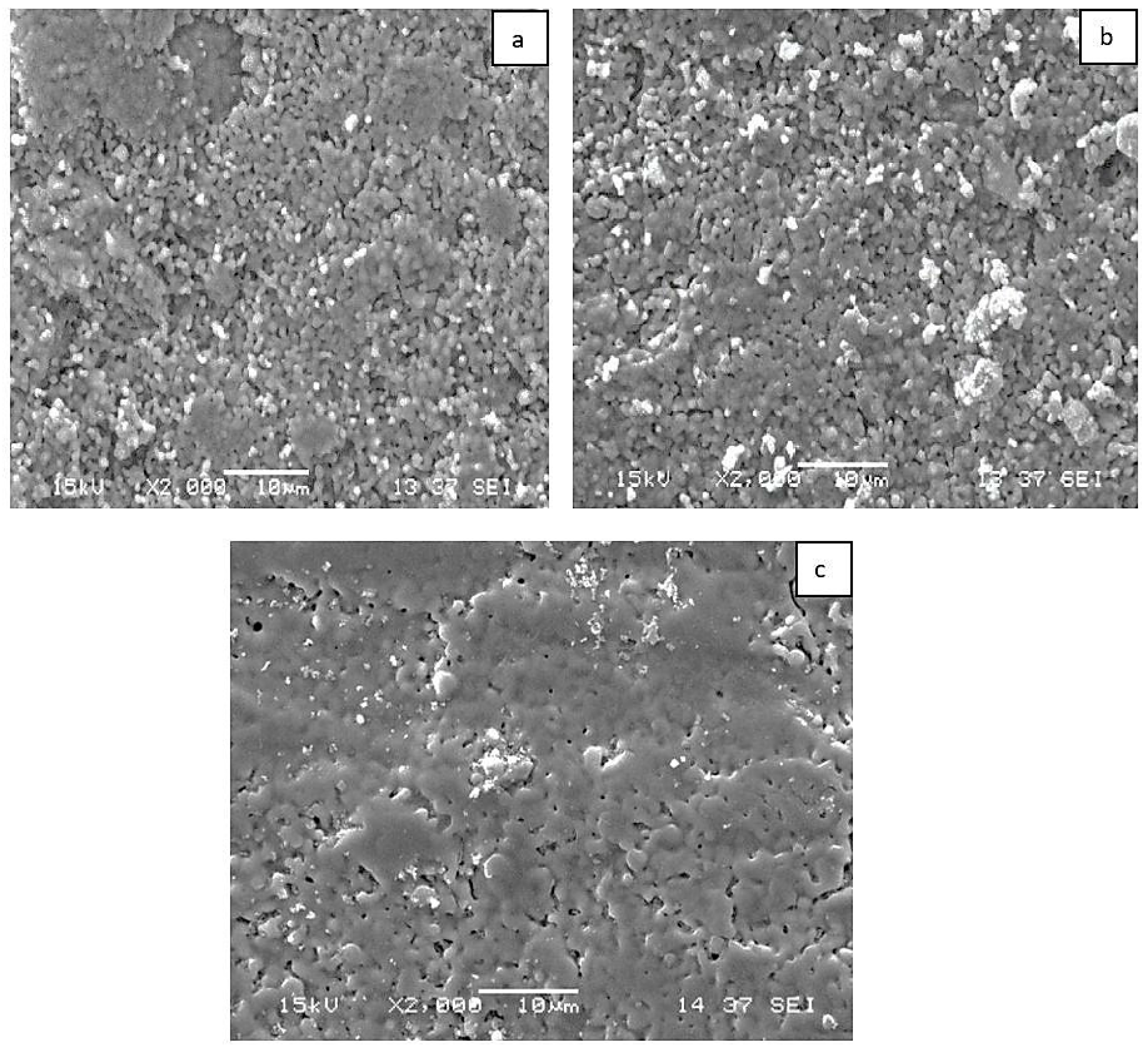
3.1. Composite Characterization
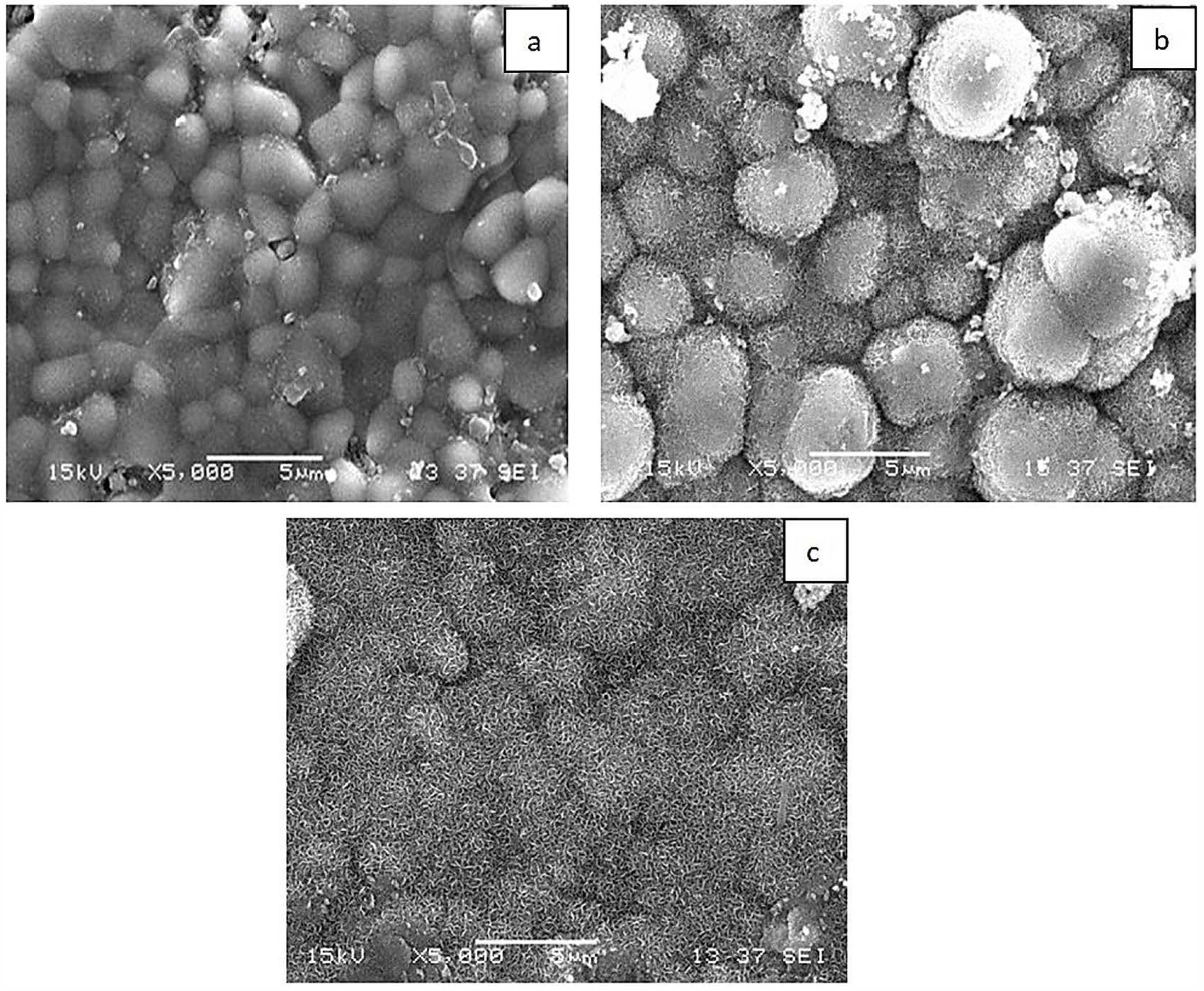
3.2. Bioactivity Results
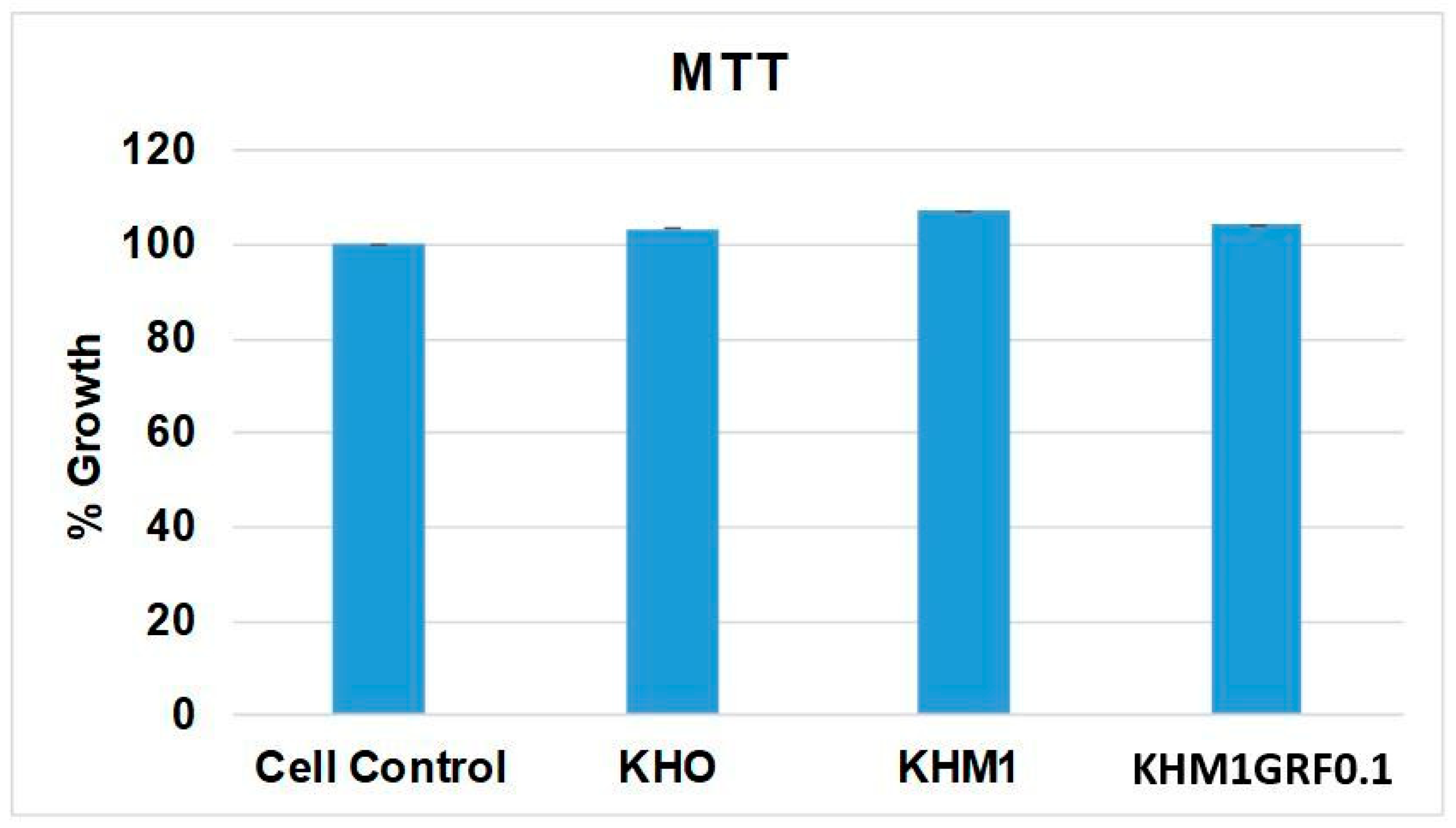
3.3. MTT Assay
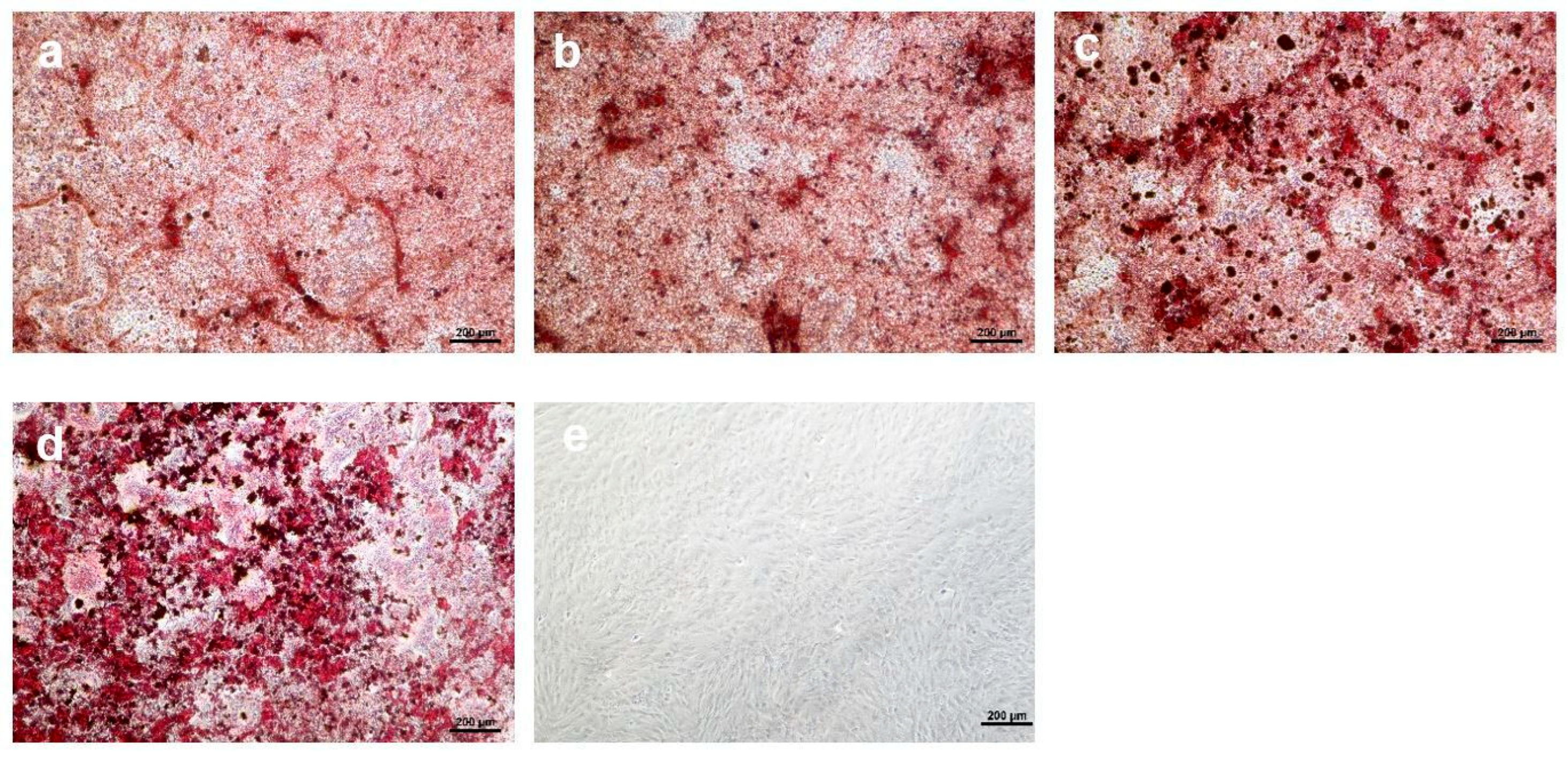
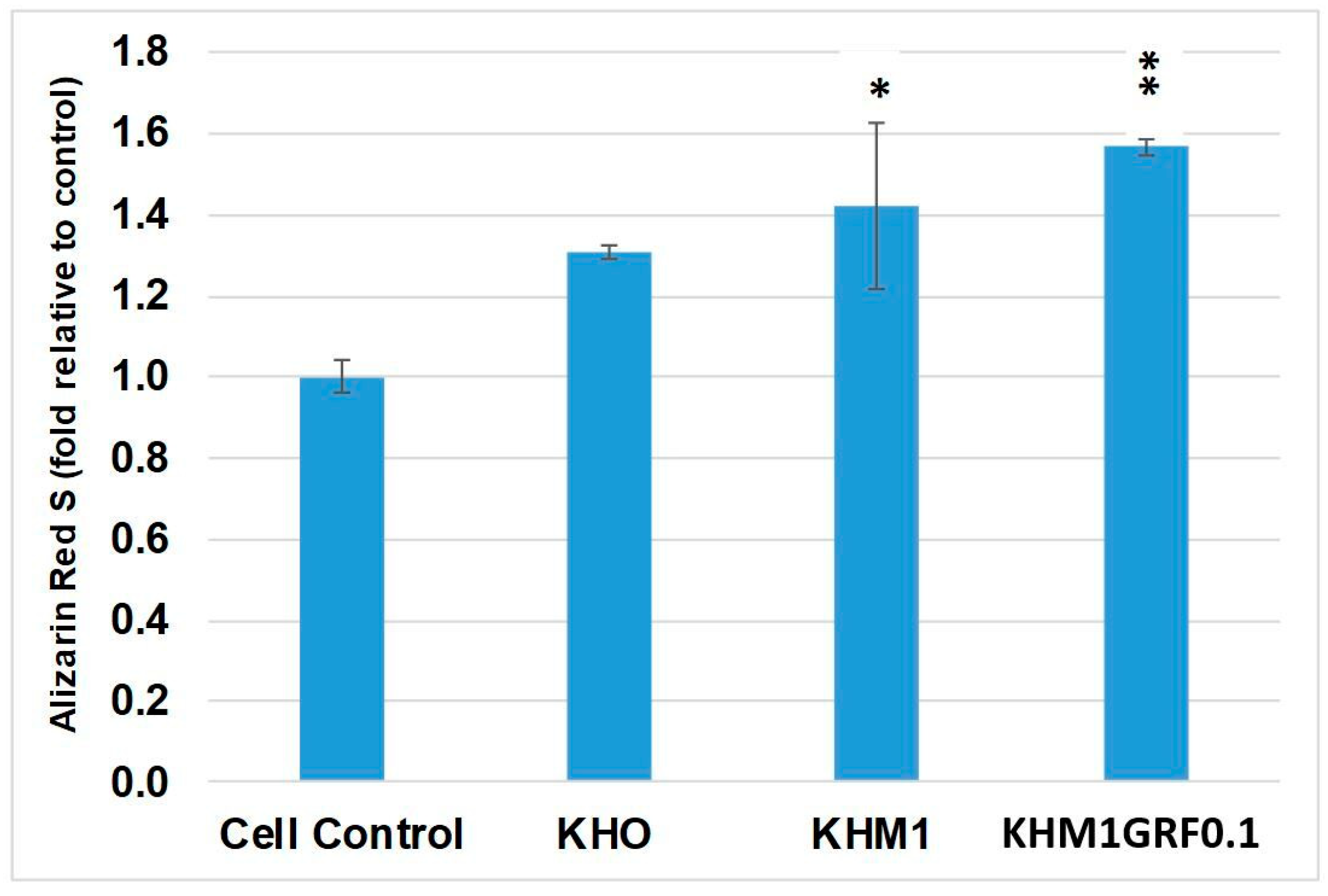
3.4. Osteogenic Differentiation
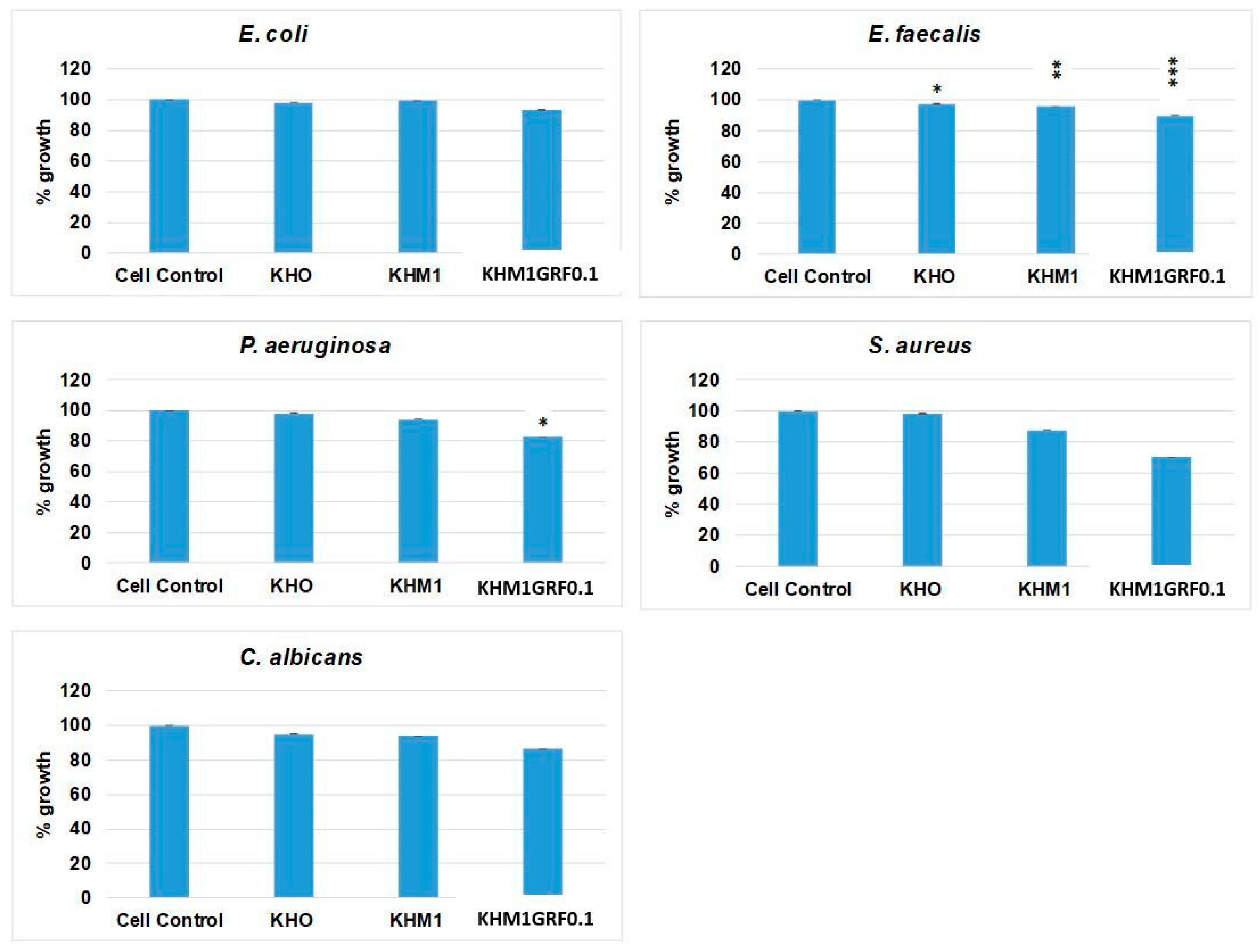
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, 561–570. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noël, L.; Strong, D.M. Adverse reactions and events related to musculoskeletal allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Orthop. 2012, 36, 633–641. [Google Scholar] [CrossRef]
- Ni, X.; Feng, J.; Liang, M.; Zhou, F.; Xia, Y.; Dong, Z.; Xue, Q.; Li, Z.; Pu, F.; Xia, P. Enhancing Bone Repair with β-TCP-Based Composite Scaffolds: A Review of Design Strategies and Biological Mechanisms. Orthop. Res. Rev. 2025, 14, 313–340. [Google Scholar] [CrossRef]
- Redhwi, I.; Fallatah, A.; Alshabona, F. Hydroxyapatite: A Comprehensive Review of Its Properties, Applications, and Future Trends. Int. J. Biomed. Mater. Res. 2024, 12, 1–6. [Google Scholar] [CrossRef]
- Ilelo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Etinosa, P.O.; Osuchukwu, O.A.; Anisiji, E.O.; Lawal, M.Y.; Mohammed, S.A.; Ibitoye, O.I.; Oni, P.G.; Aderibigbe, V.D.; Aina, T.; Oyebode, D.; et al. In-depth review of synthesis of hydroxyapatite biomaterials from natural resources and chemical regents for biomedical applications. Arab. J. Chem. 2024, 17, 106010. [Google Scholar] [CrossRef]
- Kamal, M.; Mahmud, S.; Plabon, I.A.; Kader, A.; Islam, N. Effects of sintering temperature on the physical, structural, mechanical and antimicrobial properties of extracted hydroxyapatite ceramics from Anabas testudineus bone and head scull for biomedical applications. Res. Mater. 2024, 22, 100590. [Google Scholar] [CrossRef]
- Wang, P.E.; Chaki, T.K. Sintering behaviour and mechanical properties of hydroxyapatite and dicalcium phosphate. J. Mater. Sci. Mater. Med. 1993, 4, 150–158. [Google Scholar] [CrossRef]
- Tan, C.Y.; Aw, K.L.; Yeo, W.H.; Ramesh, S.; Hamdi, M.; Sopyan, I. Influence of Magnesium Doping in Hydroxyapatite Ceramics. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering 2008, Kuala Lumpur, Malaysia, 25–28 June 2008; IFMBE Proceedings. Volume 21, pp. 326–329. [Google Scholar]
- Kanasan, N.; Adzila, S.; Koh, C.T.; Rahman, H.A.; Panerselvan, G. Effects of magnesium doping on the properties of hydroxyapatite/sodium alginate biocomposite. Adv. Appl. Ceram Struc. Func. Biocera. 2019, 118, 381–386. [Google Scholar] [CrossRef]
- Szilvia, K.; Csaba, B.; Katalin, B.; Eszter, B.; Péter, F.; Anna, M.K.; János, S.; Zoltán, K. Spark plasma sintering of graphene reinforced hydroxyapatite composites. Ceram. Int. 2015, 41, 3647–3652. [Google Scholar] [CrossRef]
- Indu, B.; Duk-Yeon, K.; Young-Hwan, H.; Byung-Koog, J.; Sukyoung, K. Directional property evaluation of spark plasma sintered GNPs-reinforced hydroxyapatite composites. Mater. Lett. 2015, 158, 62–65. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, K.K.; Rahman, O.A.; Haldar, S.; Lahiri, D.; Keshri, A.K. Investigation of crystallinity, mechanical properties, fracture toughness and cell proliferation in plasma sprayed graphene nano platelets reinforced hydroxyapatite coating. Mater. Res. Express 2020, 7, 015415. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Dohrn, M.; Neuroth, M.; Geisler, T. High-temperature phase transformations of hydroxylapatite and the formation of silicocarnotite in the hydroxylapatite–quartz–lime system studied in situ and in operando by Raman spectroscopy. J. Mater. Sci. 2022, 57, 15239–15266. [Google Scholar] [CrossRef]
- Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. [Google Scholar] [CrossRef]
- Gu, Y.W.; Loh, N.H.; Khor, K.A.; Tor, S.B.; Cheang, P. Spark plasma sintering of hydroxyapatite powders. Biomaterials 2002, 23, 37–43. [Google Scholar] [CrossRef]
- Kwon, S.M.; Lee, S.J.; Shon, I.J. Enhanced properties of nanostructured ZrO2–graphene composites rapidly sintered via high-frequency induction heating. Ceram. Int. 2015, 41, 835–842. [Google Scholar] [CrossRef]
- Shon, I.J. High-frequency induction sintering of B4C ceramics and its mechanical properties. Ceram. Int. 2016, 42, 19406–19412. [Google Scholar] [CrossRef]
- Khalil, A.K.; Sug, W.K.; Hak, Y.K. Consolidation and mechanical properties of nanostructured hydroxyapatite–(ZrO2+3mol% Y2O3) bioceramics by high-frequency induction heat sintering. Mater. Sci. Eng. A 2007, 456, 368–372. [Google Scholar] [CrossRef]
- Oh, S.J.; Byung-Su Kim, B.S.; Shon, I.J. Mechanical properties and rapid consolidation of nanostructured WC and WC–Al2O3 composites by high-frequency induction-heated sintering. Int. J. Refract. Met. Hard Mater. 2016, 58, 189–195. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, B.; Yoon, J.; Hong, K.; Shon, I. The effect of graphene on the properties of 8YSZ ceramics rapidly sintered by high-frequency induction heating. J. Cera. Process. Res. 2015, 16, 472–478. [Google Scholar]
- Khalil, K.A.; Almajid, A.A. Effect of high-frequency induction heat sintering conditions on the microstructure and mechanical properties of nanostructured magnesium/hydroxyapatite nanocomposites. Mater. Des. 2012, 36, 58–68. [Google Scholar] [CrossRef]
- Szewczyk, A.İ.; Skwira-Rucińska, A.; Osińska, M.; Prokopowicz, M. The apatite-forming ability of bioactive glasses—A comparative study in human serum and Kokubo’s simulated body fluid. Ceram. Int. 2024, 50, 51030–51042. [Google Scholar] [CrossRef]
- Prokopiev, O.; Sevostianov, I. Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. Mater. Sci. Eng. A 2006, 431, 218–227. [Google Scholar] [CrossRef]
- Obada, D.O.; Dauda, E.T.; Abifarin, J.K.; Dodoo-Arhin, D.; Bansod, N.D. Mechanical properties of natural hydroxyapatite using low cold compaction pressure: Effect of sintering temperature. Mater. Chem. Phys. 2020, 239, 122099. [Google Scholar] [CrossRef]
- Abifarin, F.B.; Musa, Z.; Abifarin, J.K. Mechanical processing of hydroxyapatite through sintering and multi-objective optimization technique for biomedical application. MRS Adv. 2023, 8, 532–537. [Google Scholar] [CrossRef]
- Gautam, C.R.; Kumar, S.; Biradar, S.; Josec, S.; Mishrad, V.K. Synthesis and enhanced mechanical properties of MgO substituted hydroxyapatite: A bone substitute material. RSC Adv. 2016, 6, 67565. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Ya, H.B.; Azeem, M.; Mustapha, M.; Yusuf, M.; Masood, F.; Marode, R.V.; Sapuan, S.M.; Ansari, A.H. Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review. Nanotechnol. Rev. 2023, 12, 20230111. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Gao, H.; Shi, X.; Zhao, N.; Wang, Y. Hydrothermal growth of whitlockite coating on β-tricalcium phosphate surfaces for enhancing bone repair potential. J. Mater. Sci. Technol. 2018, 34, 1054–1059. [Google Scholar] [CrossRef]
- Maroua, T.; Ibrahim, A.; Algarni, H.; Ayed, F.B.; Yousef, E.S. Mechanical and tribological properties of the tricalcium phosphate-magnesium oxide composites. Mater. Sci. Eng. C 2019, 96, 716–729. [Google Scholar]
- Bellucci, D.; Sola, A.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. Role of magnesium oxide and strontium oxide as modifiers in silicate-based bioactive glasses: Effects on thermal behaviour, mechanical properties and in-vitro bioactivity. Mater. Sci. Eng. C 2017, 72, 566–575. [Google Scholar] [CrossRef]
- Liga, S.; Kristine, S.A.; Dmitrijs, J.; Natalija, B.; Liga, B.C. The Study of Magnesium Substitution Effect on Physicochemical Properties of Hydroxyapatite. Mater. Sci. App. Chem. 2013, 28, 51–57. [Google Scholar]
- Tan, C.Y.; Yaghoubi, A.; Ramesh, S.; Adzila, S.; Purbolaksono, J.; Hassan, M.A.; Kutty, M.G. Sintering and mechanical properties of MgO-doped nanocrystalline hydroxyapatite. Ceram. Int. 2013, 39, 8979–8983. [Google Scholar] [CrossRef]
- Yu, L.; Sun, F.; Wang, Y.; Li, W.; Zheng, Y.; Shen, G.; Wang, Y.; Chen, M. Effects of MgO nanoparticle addition on the mechanical properties, degradation properties, antibacterial properties and in vitro and in vivo biological properties of 3D-printed Zn scaffolds. Bioac. Mater. 2024, 37, 72–85. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Padrão, T.; Costa, L.; Marta, T.P.; Paulo, C.C.; Valentina, F.D.; Paulo, A.Q.; Fernando, J.M.; Susana, R.S. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci. Rep. 2020, 10, 19098. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Joshi, A.; Agrawal, A.; Nain, A.; Bagde, A.; Patel, A.; Syed, Z.Q.; Asthana, S.; Chatterjee, K. NIR-Responsive Deployable and Self-Fitting 4D-Printed Bone Tissue Scaffold. ACS Appl. Mater. Interfaces 2024, 16, 49135–49147. [Google Scholar] [CrossRef] [PubMed]


| Component | Amount |
|---|---|
| NaCI | 11.994 g |
| NaHCO3 | 0.525 g |
| KCI | 0.336 g |
| K2HPO4·3H2O | 0.342 g |
| MgCl26H20 | 0.458 g |
| 1 M-HCI | 60 mL |
| CaCI2 | 0.417 g |
| Na2SO4 | 0.107 g |
| NH2C(CH2OH)3 | 9.086 g |
| Sample Code | Density (g/cm3) | Porosity (%) | Compressive Strength (MPa) |
|---|---|---|---|
| KHO | 2.36 ± 0.26 | 26.18 ± 0.26 | 7.50 ± 2.14 |
| KHM1 | 2.77 ± 0.18 | 12.30 ± 0.18 | 28.42 ± 4.22 |
| KHM5 | 2.46 ± 0.22 | 22.12 ± 0.22 | 18.89 ± 2.83 |
| KHM10 | 2.54 ± 0.20 | 19.31 ± 0.20 | 12.05 ± 3.46 |
| KHM1GRF0.1 | 2.63 ± 0.16 | 16.48 ± 0.16 | 26.25 ± 4.21 |
| KHM1GRF0.5 | 2.55 ± 0.24 | 19.30 ± 0.24 | 19.03 ± 3.71 |
| KHM1GRF1 | 2.46 ± 0.19 | 22.15 ± 0.19 | 18.68 ± 3.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usta, E.N.; Demirkol, N.; Turan, B.C.; Gürbüz, M.; Göller, G.; Barbaro, K.; Sagrafoli, D.; Fosca, M.; Rau, J.V. Characterization of Eco-Friendly Fabricated and Induction-Sintered Hydroxyapatite-Based Hybrid Composites. Materials 2025, 18, 5359. https://doi.org/10.3390/ma18235359
Usta EN, Demirkol N, Turan BC, Gürbüz M, Göller G, Barbaro K, Sagrafoli D, Fosca M, Rau JV. Characterization of Eco-Friendly Fabricated and Induction-Sintered Hydroxyapatite-Based Hybrid Composites. Materials. 2025; 18(23):5359. https://doi.org/10.3390/ma18235359
Chicago/Turabian StyleUsta, Esra Nur, Nermin Demirkol, Bilgehan Cem Turan, Mevlüt Gürbüz, Gültekin Göller, Katia Barbaro, Daniele Sagrafoli, Marco Fosca, and Julietta V. Rau. 2025. "Characterization of Eco-Friendly Fabricated and Induction-Sintered Hydroxyapatite-Based Hybrid Composites" Materials 18, no. 23: 5359. https://doi.org/10.3390/ma18235359
APA StyleUsta, E. N., Demirkol, N., Turan, B. C., Gürbüz, M., Göller, G., Barbaro, K., Sagrafoli, D., Fosca, M., & Rau, J. V. (2025). Characterization of Eco-Friendly Fabricated and Induction-Sintered Hydroxyapatite-Based Hybrid Composites. Materials, 18(23), 5359. https://doi.org/10.3390/ma18235359







