Halide-Assisted Synthesis of V-WSe2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
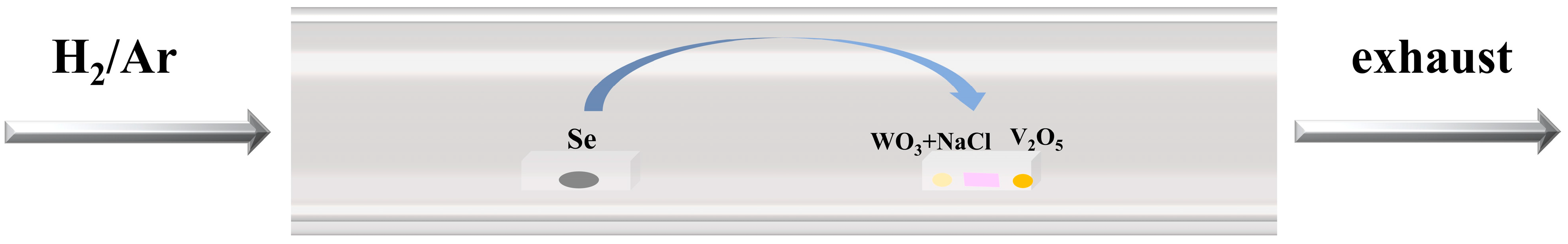
2.2. Growth Preparation and Processes
2.3. Characterization
2.4. Methods
2.4.1. Preparation of Si/SiO2 Substrate
2.4.2. TEM Sample Preparation
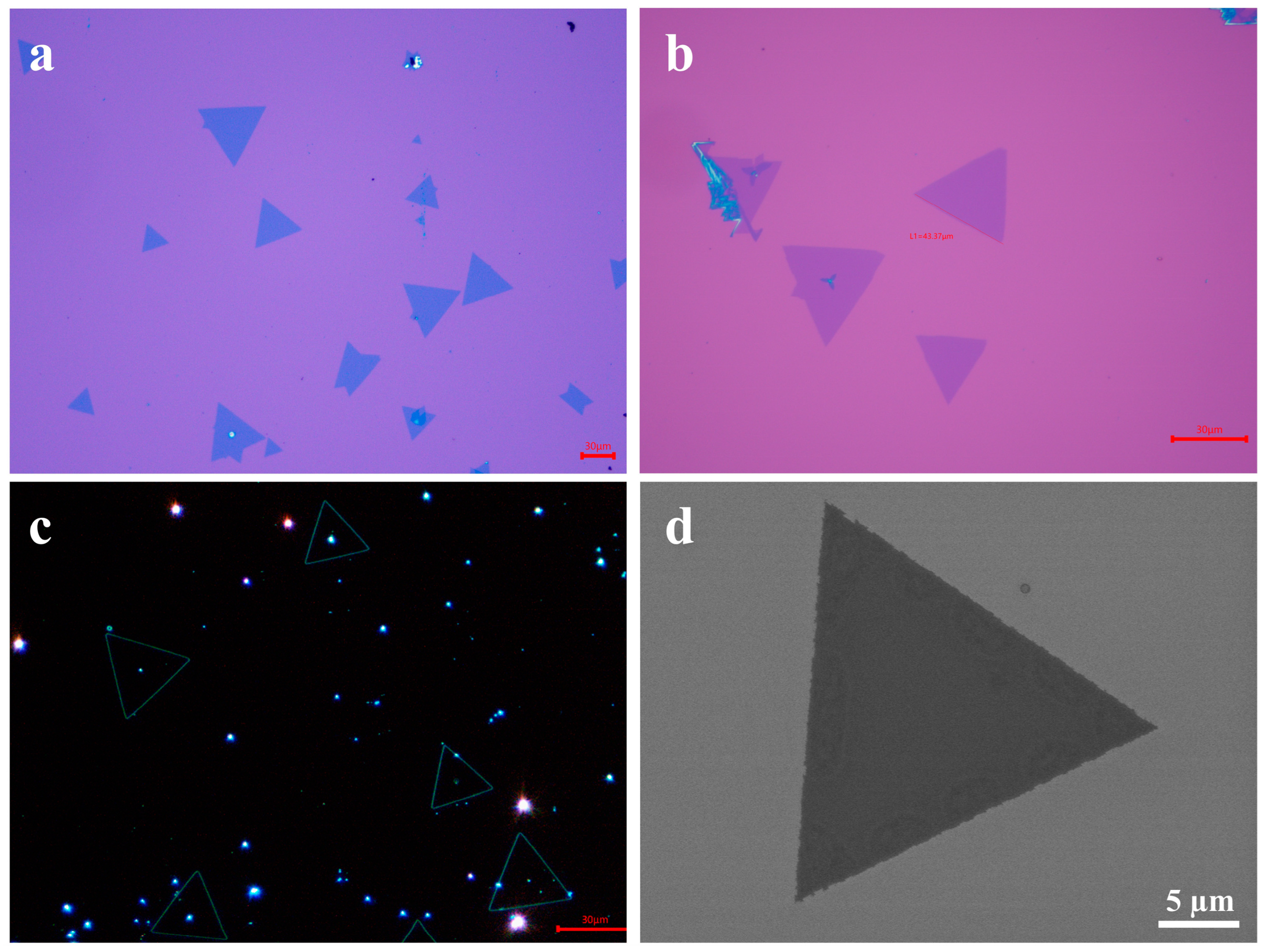
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Grigorenko, A.N.; Polini, M.; Novoselov, K.S. Graphene plasmonics. Nat. Photonics 2012, 6, 749–758. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Yin, Y.; Kang, X.; Han, B. Two-dimensional materials: Synthesis and applications in the electro-reduction of carbon dioxide. Chem. Synth. 2022, 2, 109313. [Google Scholar] [CrossRef]
- Sakurai, M. On-surface synthesized magnetic nanoclusters of ferrocene derivatives. Chem. Synth. 2014, 4, 42. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, D.; Wang, X.; Jiao, Y.; Liu, M.; Liu, C.; Zhang, Q.; Ren, S.; Liu, Y. One-Pot Synthesis of Pd@Pt Core-Shell Icosahedron for Efficient Oxygen Reduction. Materials 2025, 18, 1279. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Zhou, J.D.; Zhu, C.; Zhou, Y.; Dong, J.C.; Li, P.L.; Zhang, Z.W.; Wang, Z.; Lin, Y.C.; Shi, J.; Zhang, R.W.; et al. Composition and phase engineering of metal chalcogenides and phosphorous chalcogenides. Nat. Mater. 2023, 22, 450–458. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Lembke, D.; Bertolazzi, S.; Kis, A. Single-Layer MoS2 Electronics. Acc. Chem. Res. 2015, 48, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, S.; Hu, L.; Hoang, A.T.; Choi, J.Y.; Ahn, J.H. Wafer-scale monolithic integration of full-colour micro-LED display using MoS2 transistor. Nat. Nanotechnol. 2022, 17, 500–506. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Wu, S.F.; Buckley, S.; Schaibley, J.R.; Feng, L.F.; Yan, J.Q.; Mandrus, D.G.; Hatami, F.; Yao, W.; Vuckovic, J.; Majumdar, A.; et al. Monolayer semiconductor nanocavity lasers with ultralow thresholds. Nature 2015, 520, 69–72. [Google Scholar] [CrossRef]
- Choi, M.; Bae, S.R.; Hu, L.; Hoang, A.T.; Kim, S.Y.; Ahn, J.H. Full-color active-matrix organic light-emitting diode display on human skin based on a large-area MoS2 backplane. Sci. Adv. 2020, 6, eabb5898. [Google Scholar] [CrossRef]
- Hoang, A.T.; Hu, L.H.; Kim, B.J.; Van, T.T.N.; Park, K.D.; Jeong, Y.; Lee, K.; Ji, S.; Hong, J.; Katiyar, A.K.; et al. Low-temperature growth of MoS2 on polymer and thin glass substrates for flexible electronics. Nat. Nanotechnol. 2023, 18, 1439–1447. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, X.X.; Xu, M.S.; Liu, L.W.; Yang, D.R.; Zhou, P. Two-dimensional devices and integration towards the silicon lines. Nat. Mater. 2022, 21, 1225–1239. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, X.X.; Zhou, P. The Road for 2D Semiconductors in the Silicon Age. Adv. Mater. 2022, 34, 2106886. [Google Scholar] [CrossRef]
- Ahn, E.C. 2D materials for spintronic devices. npj 2D Mater. Appl. 2020, 4, 17. [Google Scholar] [CrossRef]
- Ross, J.S.; Rivera, P.; Schaibley, J.; Lee-Wong, E.; Yu, H.Y.; Taniguchi, T.; Watanabe, K.; Yan, J.Q.; Mandrus, D.; Cobden, D.; et al. Interlayer Exciton Optoelectronics in a 2D Heterostructure p-n Junction. Nano Lett. 2017, 17, 638–643. [Google Scholar] [CrossRef]
- Liu, L.T.; Kumar, S.B.; Ouyang, Y.; Guo, J. Performance Limits of Monolayer Transition Metal Dichalcogenide Transistors. IEEE Trans. Electron Devices 2011, 58, 3042–3047. [Google Scholar] [CrossRef]
- Feng, L.P.; Jiang, W.Z.; Su, J.; Zhou, L.Q.; Liu, Z.T. Performance of field-effect transistors based on NbxW1-xS2 monolayers. Nanoscale 2016, 8, 6507–6513. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Balendhran, S.; Wang, Y.C.; Ab Kadir, R.; Zoolfakar, A.S.; Atkin, P.; Ou, J.Z.; Sriram, S.; Kalantar-zadeh, K.; Bhaskaran, M. Characterization of metal contacts for two-dimensional MoS2 nanoflakes. Appl. Phys. Lett. 2013, 103, 232105. [Google Scholar] [CrossRef]
- Das, S.; Chen, H.Y.; Penumatcha, A.V.; Appenzeller, J. High Performance Multilayer MoS2 Transistors with Scandium Contacts. Nano Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Kozhakhmetov, A.; Stolz, S.; Tan, A.M.Z.; Pendurthi, R.; Bachu, S.; Turker, F.; Alem, N.; Kachian, J.; Das, S.; Hennig, R.G.; et al. Controllable p-Type Doping of 2D WSe2 via Vanadium Substitution. Adv. Funct. Mater. 2021, 31, 2105252. [Google Scholar] [CrossRef]
- Xu, H.L.; Fathipour, S.; Kinder, E.W.; Seabaugh, A.C.; Fullerton-Shirey, S.K. Reconfigurable Ion Gating of 2H-MoTe2 Field-Effect Transistors Using Poly(ethylene oxide)-CsClO4 Solid Polymer Electrolyte. ACS Nano 2015, 9, 4900–4910. [Google Scholar] [CrossRef]
- Zhang, K.H.; Feng, S.M.; Wang, J.J.; Azcatl, A.; Lu, N.; Addou, R.; Wang, N.; Zhou, C.J.; Lerach, J.; Bojan, V.; et al. Manganese Doping of Monolayer MoS2: The Substrate Is Critical. Nano Lett. 2016, 16, 2125. [Google Scholar] [CrossRef]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable Photoluminescence of Monolayer MoS2 via Chemical Doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tosun, M.; Seol, G.; Chang, T.C.; Takei, K.; Guo, J.; Javey, A. Degenerate n-Doping of Few-Layer Transition Metal Dichalcogenides by Potassium. Nano Lett. 2013, 13, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.Y.; Jang, H.K.; Lee, K.J.; Piao, M.X.; Ko, S.P.; Shin, M.; Huh, J.; Kim, G.T. Triethanolamine doped multilayer MoS2 field effect transistors. Phys. Chem. Chem. Phys. 2017, 19, 13133–13139. [Google Scholar] [CrossRef]
- Afaneh, T.; Sahoo, P.K.; Nobrega, I.A.P.; Xin, Y.; Gutiérrez, H.R. Laser-Assisted Chemical Modification of Monolayer Transition Metal Dichalcogenides. Adv. Funct. Mater. 2018, 28, 1802949. [Google Scholar] [CrossRef]
- Luo, P.; Zhuge, F.W.; Zhang, Q.F.; Chen, Y.Q.; Lv, L.; Huang, Y.; Li, H.Q.; Zhai, T.Y. Doping engineering and functionalization of two-dimensional metal chalcogenides. Nanoscale Horiz. 2019, 4, 26–51. [Google Scholar] [CrossRef]
- Fan, M.; Guo, J.; Fang, G.; Tian, H.; You, Y.; Huang, Z.; Huang, J. Microwave-pulse assisted synthesis of tunable ternary-doped 2D molybdenum carbide for efficient hydrogen evolution. Chem. Synth. 2024, 4, 36. [Google Scholar] [CrossRef]
- Zhao, P.D.; Kiriya, D.; Azcatl, A.; Zhang, C.X.; Tosun, M.; Liu, Y.S.; Hettick, M.; Kang, J.S.; McDonnell, S.; Santosh, K.C.; et al. Air Stable p-Doping of WSe2 by Covalent Functionalization. ACS Nano 2014, 8, 10808–10814. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhao, W.J.; Giustiniano, F.; Eda, G. Effect of oxygen and ozone on p-type doping of ultra-thin WSe2 and MoSe2 field effect transistors. Phys. Chem. Chem. Phys. 2016, 18, 4304–4309. [Google Scholar] [CrossRef]
- Das, S.; Demarteau, M.; Roelofs, A. Nb-doped single crystalline MoS2 field effect transistor. Appl. Phys. Lett. 2015, 106, 173506. [Google Scholar] [CrossRef]
- Kozhakhmetov, A.; Schuler, B.; Tan, A.M.Z.; Cochrane, K.A.; Nasr, J.R.; El-Sherif, H.; Bansal, A.; Vera, A.; Bojan, V.; Redwing, J.M.; et al. Scalable Substitutional Re-Doping and its Impact on the Optical and Electronic Properties of Tungsten Diselenide. Adv. Mater. 2020, 32, 2005159. [Google Scholar] [CrossRef]
- Sun, L.Z.; Yuan, G.W.; Gao, L.B.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z.F. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Hou, T.Y.; Li, D.; Qu, Y.; Hao, Y.F.; Lai, Y. The Role of Carbon in Metal-Organic Chemical Vapor Deposition-Grown MoS2 Films. Materials 2023, 16, 7030. [Google Scholar] [CrossRef]
- Luo, X.M.; Jiao, Y.H.; Li, H.; Liu, Q.; Liu, J.F.; Wang, M.W.; Liu, Y. Impact of Carrier Gas Flow Rate on the Synthesis of Monolayer WSe2 via Hydrogen-Assisted Chemical Vapor Deposition. Materials 2024, 17, 2190. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Gleason, K.K. Device Fabrication Based on Oxidative Chemical Vapor Deposition (oCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Interfaces 2019, 6, 1801564. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M. Fabrication of Conjugated Conducting Polymers by Chemical Vapor Deposition (CVD) Method. Nanomaterials 2025, 15, 452. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Tang, D.M.; Zhao, W.; Xu, H.; Chu, L.; Eda, G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 2015, 1, 60–66. [Google Scholar] [CrossRef]
- Wang, W.D.; Clark, N.; Hamer, M.; Carl, A.; Tovari, E.; Sullivan-Allsop, S.; Tillotson, E.; Gao, Y.Z.; de Latour, H.; Selles, F.; et al. Clean assembly of van der Waals heterostructures using silicon nitride membranes. Nat. Electron. 2023, 6, 981–990. [Google Scholar] [CrossRef]
- Pham, P.V.; Mai, T.H.; Dash, S.P.; Biju, V.; Chueh, Y.L.; Jariwala, D.; Tung, V. Transfer of 2D Films: From Imperfection to Perfection. ACS Nano 2024, 18, 14841–14876. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.F.; Zhou, S.R.; Chen, H.H.; Gong, Y.M.; Kong, L.K.; Yin, Y.; Lan, C.Y.; Li, C.; Liu, Y. Contamination-free assembly of two-dimensional van der Waals heterostructures toward high-performance electronics and optoelectronics. Appl. Mater. Today 2025, 43, 102657. [Google Scholar] [CrossRef]
- Younas, R.; Zhou, G.Y.; Hinkle, C.L. Scalable and Contamination-Free Selenium-Assisted Exfoliation of Transition Metal Dichalcogenides WSe2 and MoSe2. Processes 2025, 13, 791. [Google Scholar] [CrossRef]
- Liu, B.L.; Fathi, M.; Chen, L.; Abbas, A.; Ma, Y.Q.; Zhou, C.W. Chemical Vapor Deposition Growth of Monolayer WSe2 with Tunable Device Characteristics and Growth Mechanism Study. ACS Nano 2015, 9, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Liu, B.; Liu, Y.P.; Tang, W.; Nai, C.T.; Li, L.J.; Zheng, J.; Gao, L.B.; Zheng, Y.; Shin, H.S.; et al. Chemical Vapor Deposition of Large-Sized Hexagonal WSe2 Crystals on Dielectric Substrates. Adv. Mater. 2015, 27, 6722–6727. [Google Scholar] [CrossRef]
- Wang, X.L.; Gong, Y.J.; Shi, G.; Chow, W.L.; Keyshar, K.; Ye, G.L.; Vajtai, R.; Lou, J.; Liu, Z.; Ringe, E.; et al. Chemical Vapor Deposition Growth of Crystalline Mono layer MoSe2. ACS Nano 2014, 8, 5125–5131. [Google Scholar] [CrossRef]
- Susarla, S.; Kutana, A.; Hachtel, J.A.; Kochat, V.; Apte, A.; Vajtai, R.; Idrobo, J.C.; Yakobson, B.I.; Tiwary, C.S.; Ajayan, P.M. Quaternary 2D Transition Metal Dichalcogenides (TMDs) with Tunable Bandgap. Adv. Mater. 2017, 29, 1702457. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.G.; Solís-Fernández, P.; Yoshimura, D.; Maruyama, M.; Endo, T.; Miyata, Y.; Okada, S.; Ago, H. Chemically Tuned p- and n-Type WSe2 Monolayers with High Carrier Mobility for Advanced Electronics. Adv. Mater. 2019, 31, 1903613. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Duong, D.L.; Ha, D.M.; Singh, K.; Phan, T.L.; Choi, W.; Kim, Y.M.; Lee, Y.H. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. 2020, 7, 1903076. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef]
- Kang, D.H.; Shim, J.; Jang, S.K.; Jeon, J.; Jeon, M.H.; Yeom, G.Y.; Jung, W.S.; Jang, Y.H.; Lee, S.; Park, J.H. Controllable Nondegenerate p-Type Doping of Tungsten Diselenide by Octadecyltrichlorosilane. ACS Nano 2015, 9, 1099–1107. [Google Scholar] [CrossRef]
- Wu, K.; Wang, H.; Yang, M.; Liu, L.; Sun, Z.Y.; Hu, G.J.; Song, Y.P.; Han, X.; Guo, J.A.; Wu, K.H.; et al. Gold-Template-Assisted Mechanical Exfoliation of Large-Area 2D Layers Enables Efficient and Precise Construction of Moiré Superlattices. Adv. Mater. 2024, 36, 2313511. [Google Scholar] [CrossRef]
- Faggio, G.; Politano, G.G.; Lisi, N.; Capasso, A.; Messina, G. The structure of chemical vapor deposited graphene substrates for graphene-enhanced Raman spectroscopy. J. Phys. Condens. Matter 2024, 36, 195303. [Google Scholar] [CrossRef]
- Duan, X.D.; Wang, C.; Shaw, J.C.; Cheng, R.; Chen, Y.; Li, H.L.; Wu, X.P.; Tang, Y.; Zhang, Q.L.; Pan, A.L.; et al. Lateral epitaxial growth of two-dimensional layered semiconductor heterojunctions. Nat. Nanotechnol. 2014, 9, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yun, S.J.; Park, J.C.; Park, M.H.; Park, J.H.; Kim, K.K.; Lee, Y.H. Seed Growth of Tungsten Diselenide Nanotubes from Tungsten Oxides. Small 2015, 11, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Wang, Y.H.; Xiong, Z.Z.; Zhang, H.J.; Liang, F. Preparation and characterization of WSe2 nano-films by magnetron sputtering and vacuum selenization. Nanotechnology 2018, 29, 275201. [Google Scholar] [CrossRef] [PubMed]
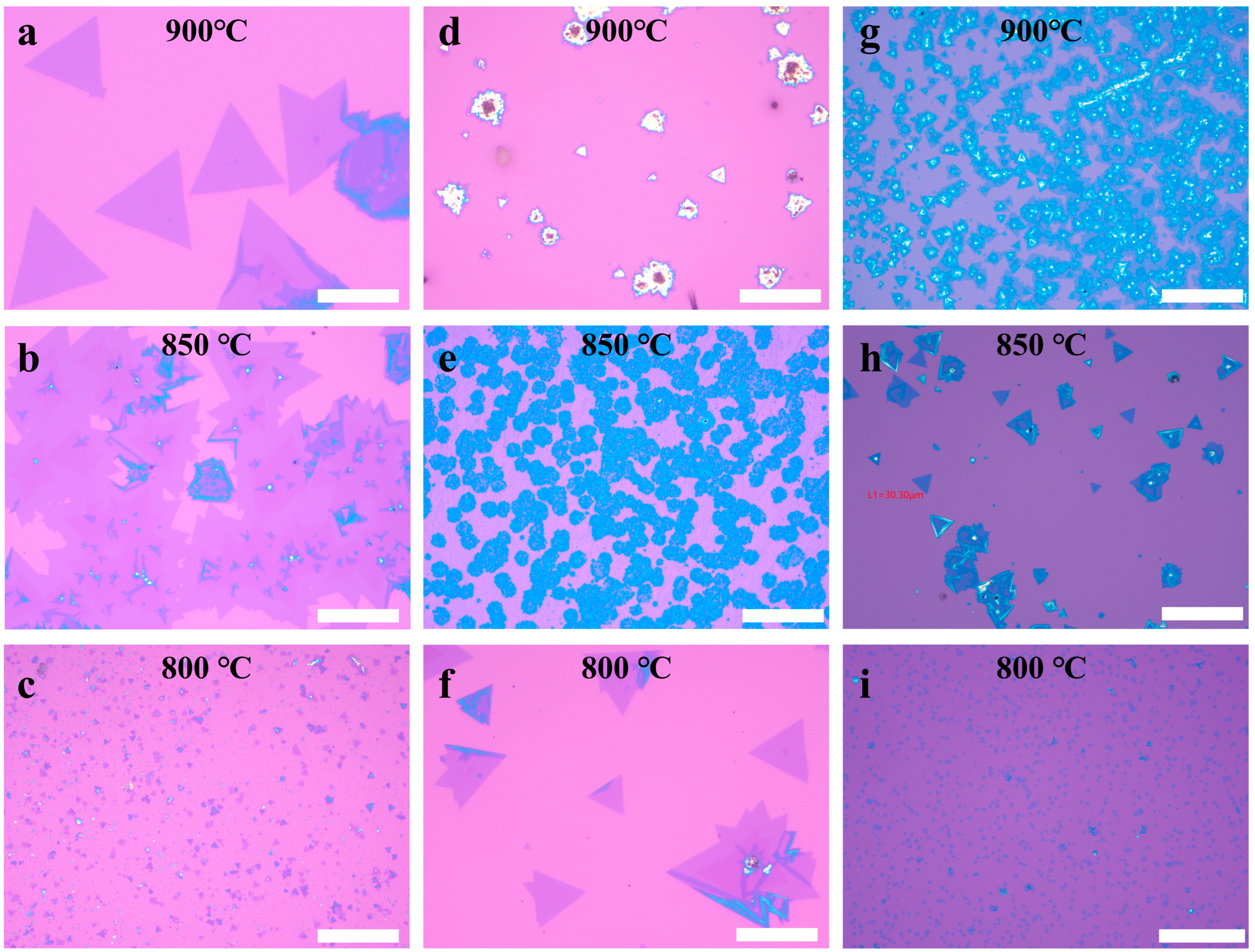


| Halide | KCl | KI | NaCl |
|---|---|---|---|
| Temperature (°C) | 900 | 800 | 850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Wang, X.; Tang, Z.; Liu, M.; Liu, C.; Zhang, Q.; Liu, Y. Halide-Assisted Synthesis of V-WSe2. Materials 2025, 18, 5360. https://doi.org/10.3390/ma18235360
Jiao Y, Wang X, Tang Z, Liu M, Liu C, Zhang Q, Liu Y. Halide-Assisted Synthesis of V-WSe2. Materials. 2025; 18(23):5360. https://doi.org/10.3390/ma18235360
Chicago/Turabian StyleJiao, Yanhui, Xiaoqian Wang, Zisheng Tang, Manrui Liu, Chengqi Liu, Qi Zhang, and Yong Liu. 2025. "Halide-Assisted Synthesis of V-WSe2" Materials 18, no. 23: 5360. https://doi.org/10.3390/ma18235360
APA StyleJiao, Y., Wang, X., Tang, Z., Liu, M., Liu, C., Zhang, Q., & Liu, Y. (2025). Halide-Assisted Synthesis of V-WSe2. Materials, 18(23), 5360. https://doi.org/10.3390/ma18235360







