Enhanced Corrosion Resistance of OL 37 Steel in Hydrochloric Acid Using a Novel Composite Polymer Film
Abstract
1. Introduction
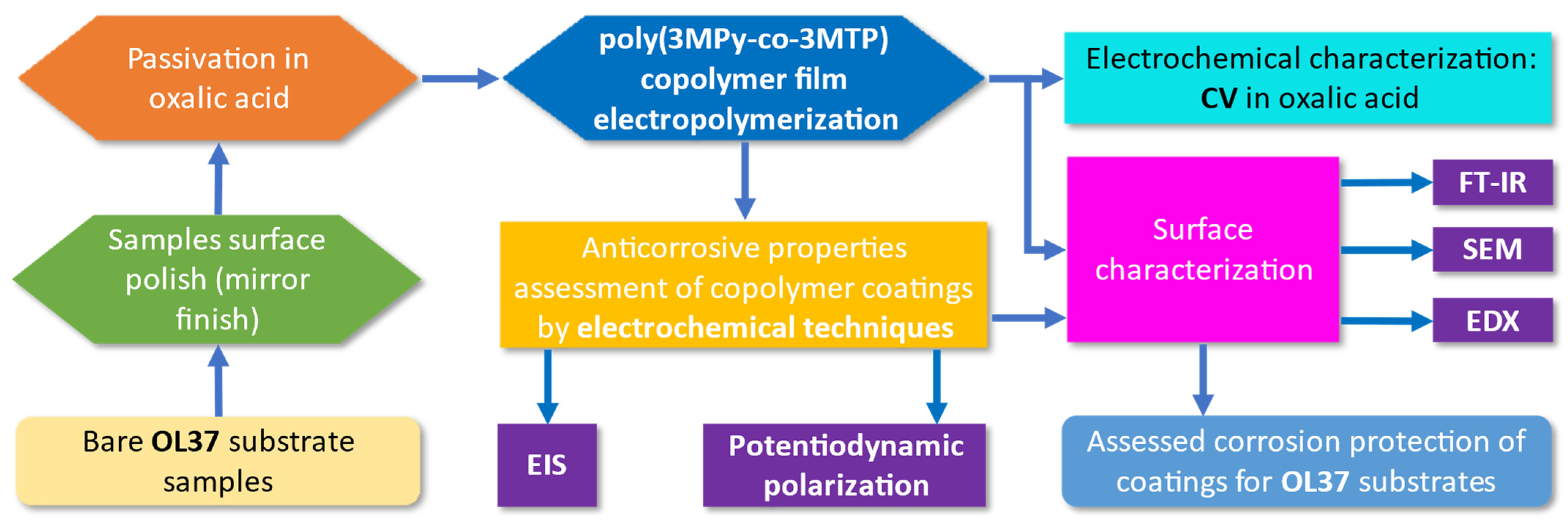
2. Experimental
2.1. Materials and Methods
2.2. Instruments
3. Results and Discussion
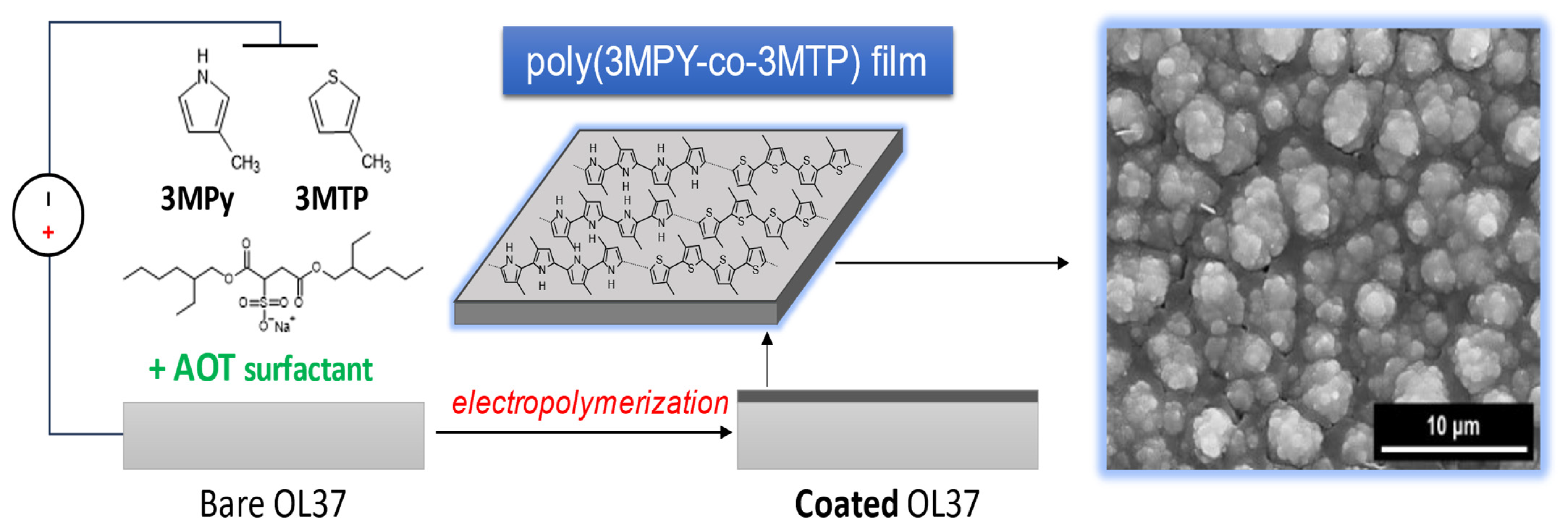
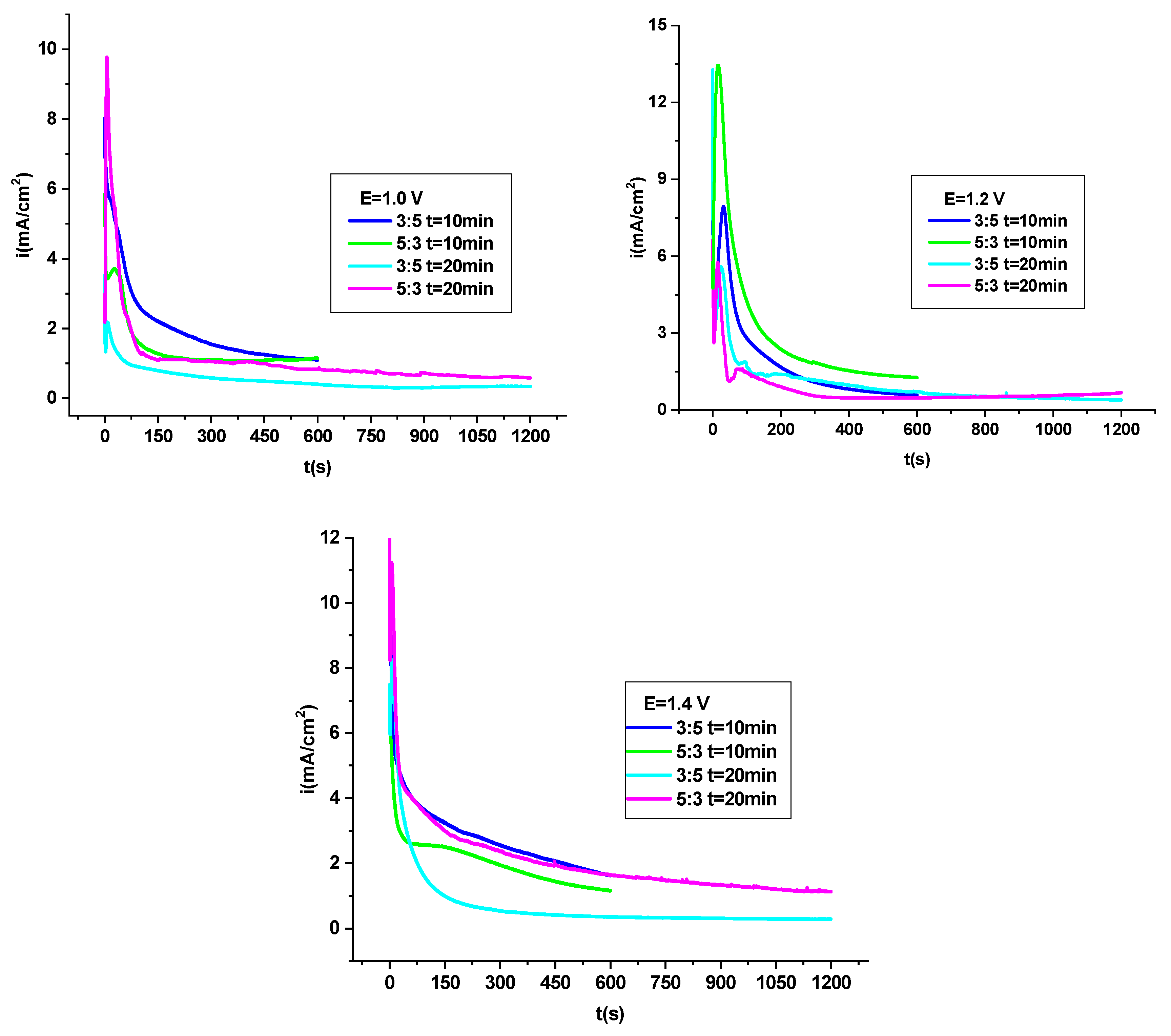
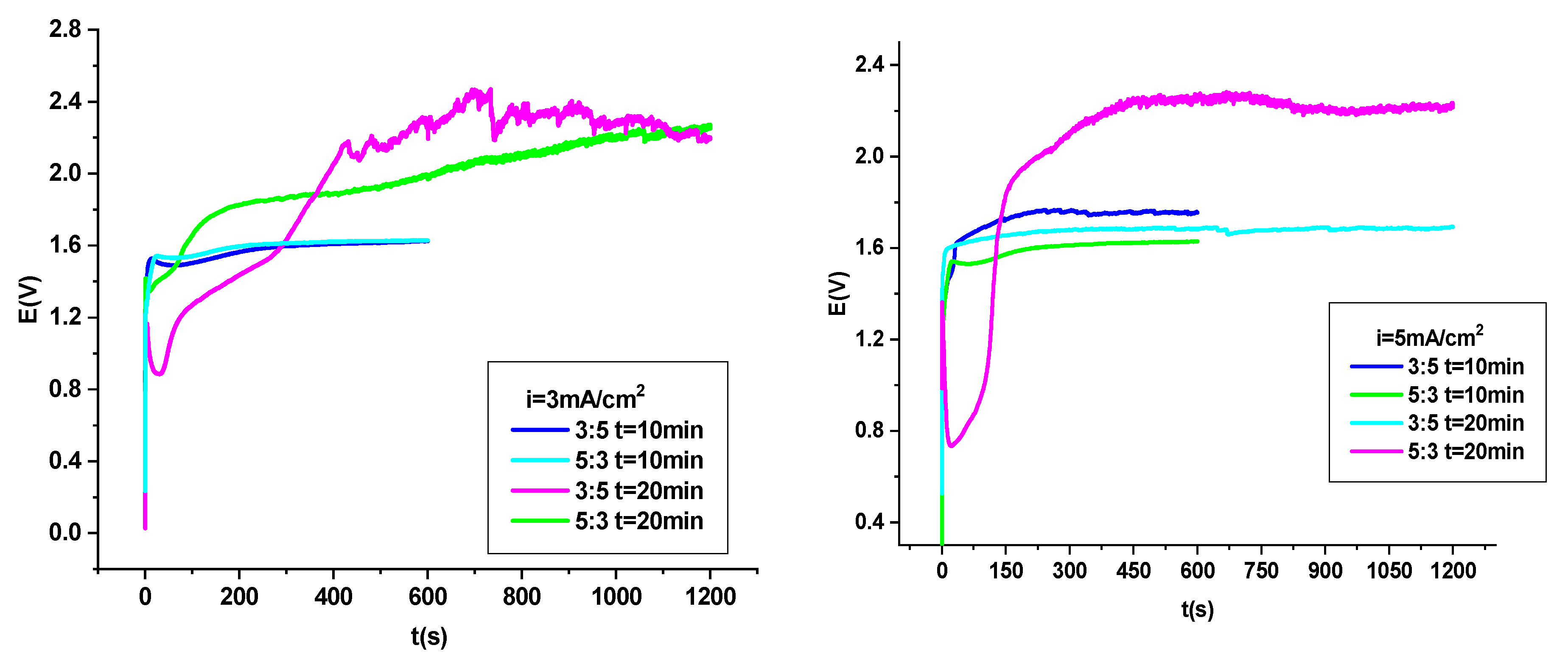
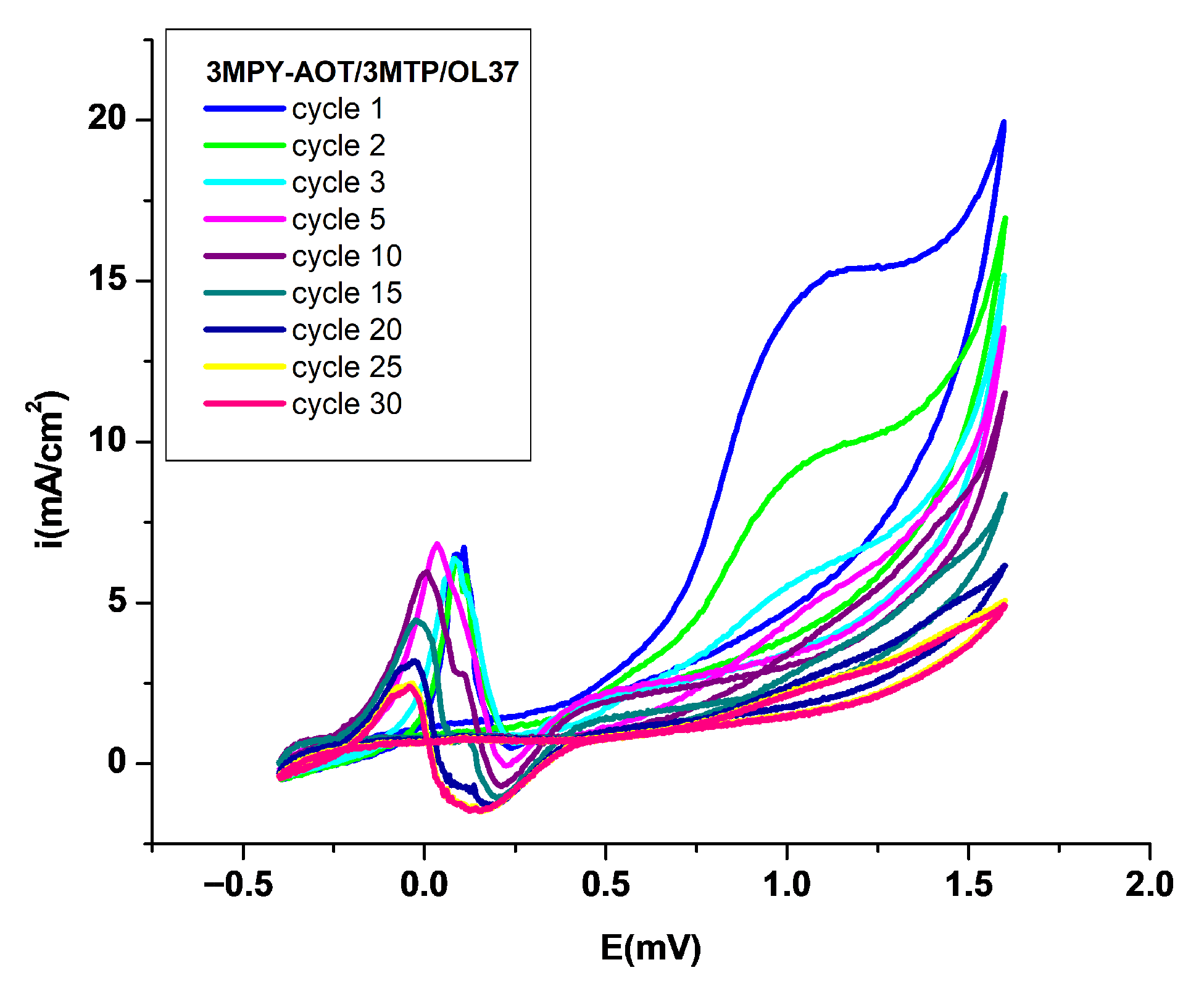
3.1. Electrodeposition of P3MPY–AOT/P3MTP/OL 37
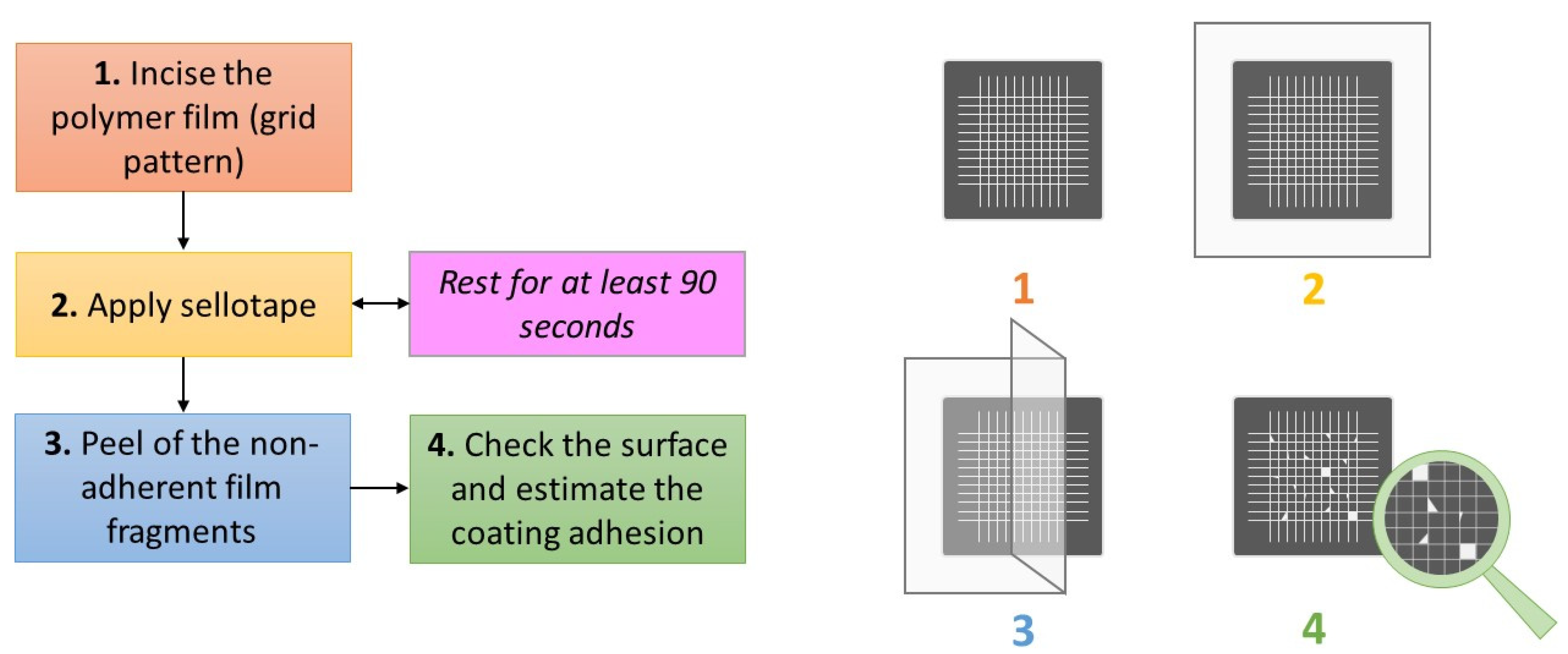
3.2. Electrochemical Analysis of the P3MPY–AOT/P3MTP/OL 37 Composite Coating
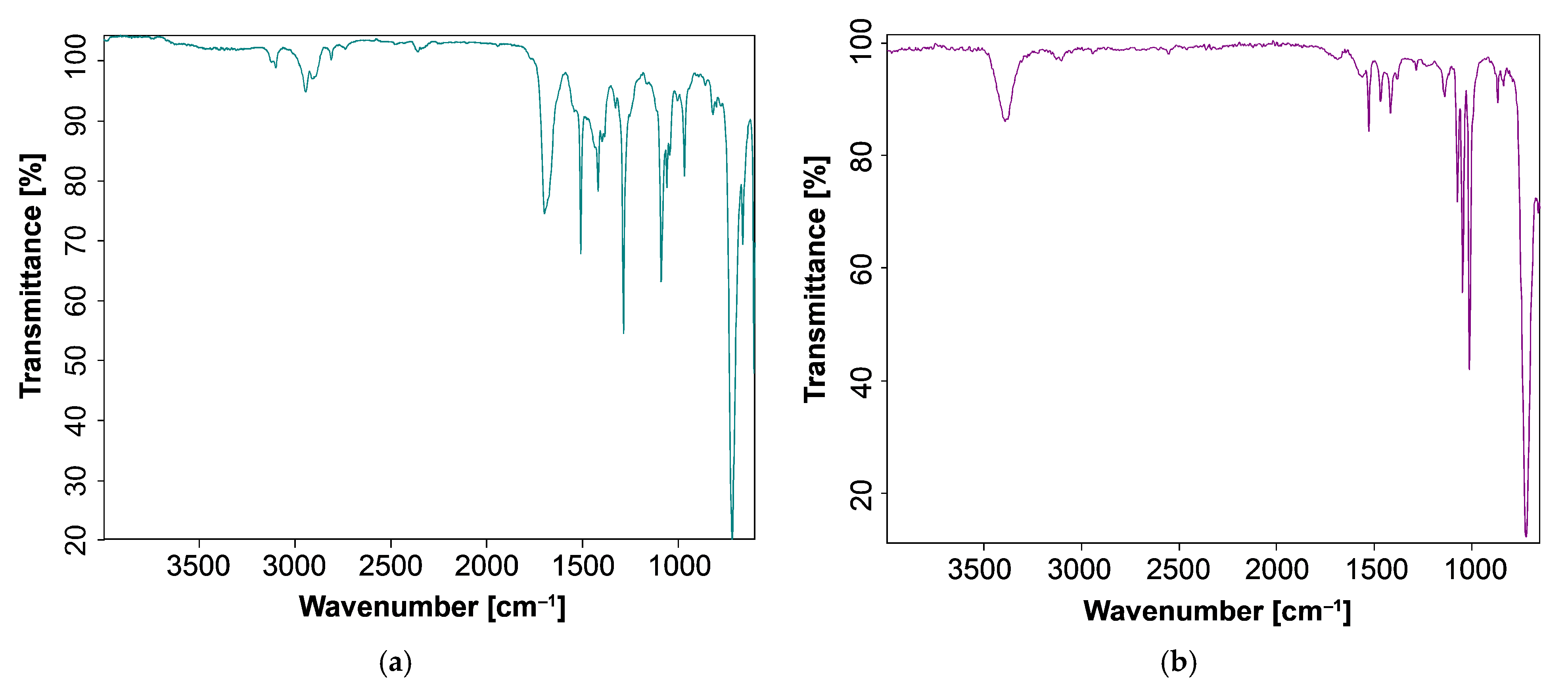
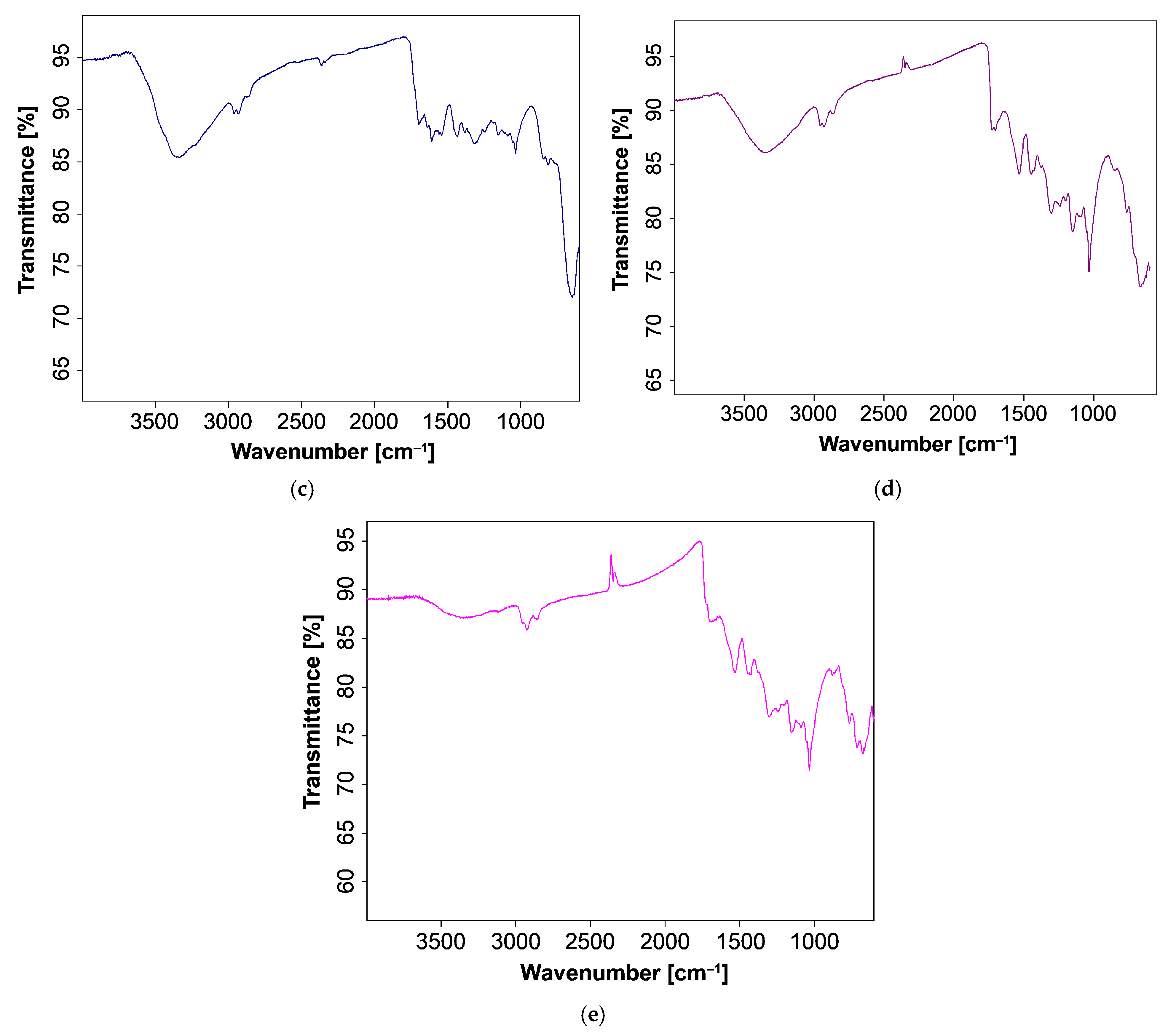
3.3. FT-IR Spectroscopic Study
3.4. Electrochemical Study of P3MPY–AOT/P3MTP/OL 37 Composite
3.4.1. Potentiodynamic Polarization Analysis
3.4.2. Electrochemical Impedance Spectroscopy (EIS) Investigation
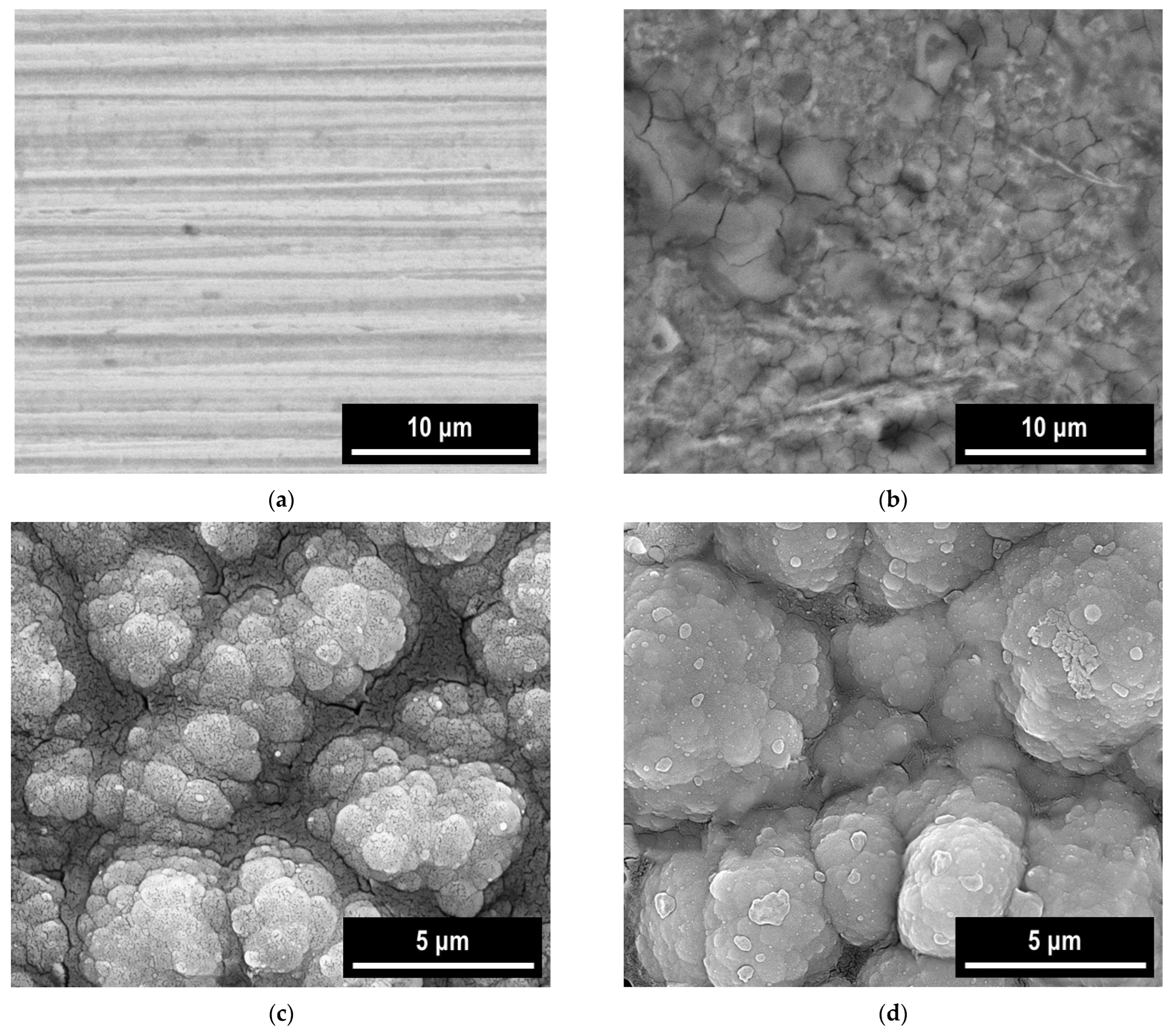
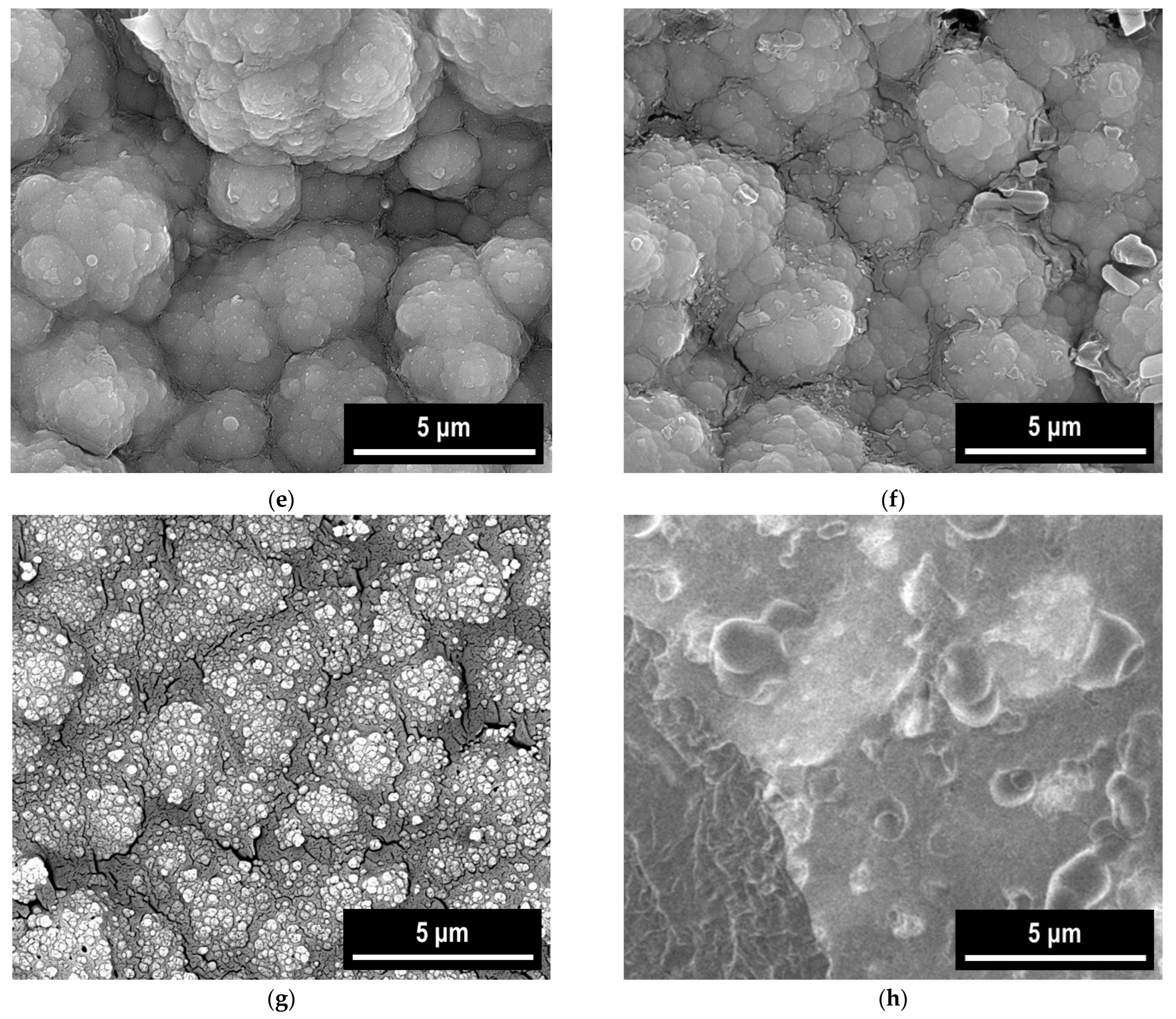
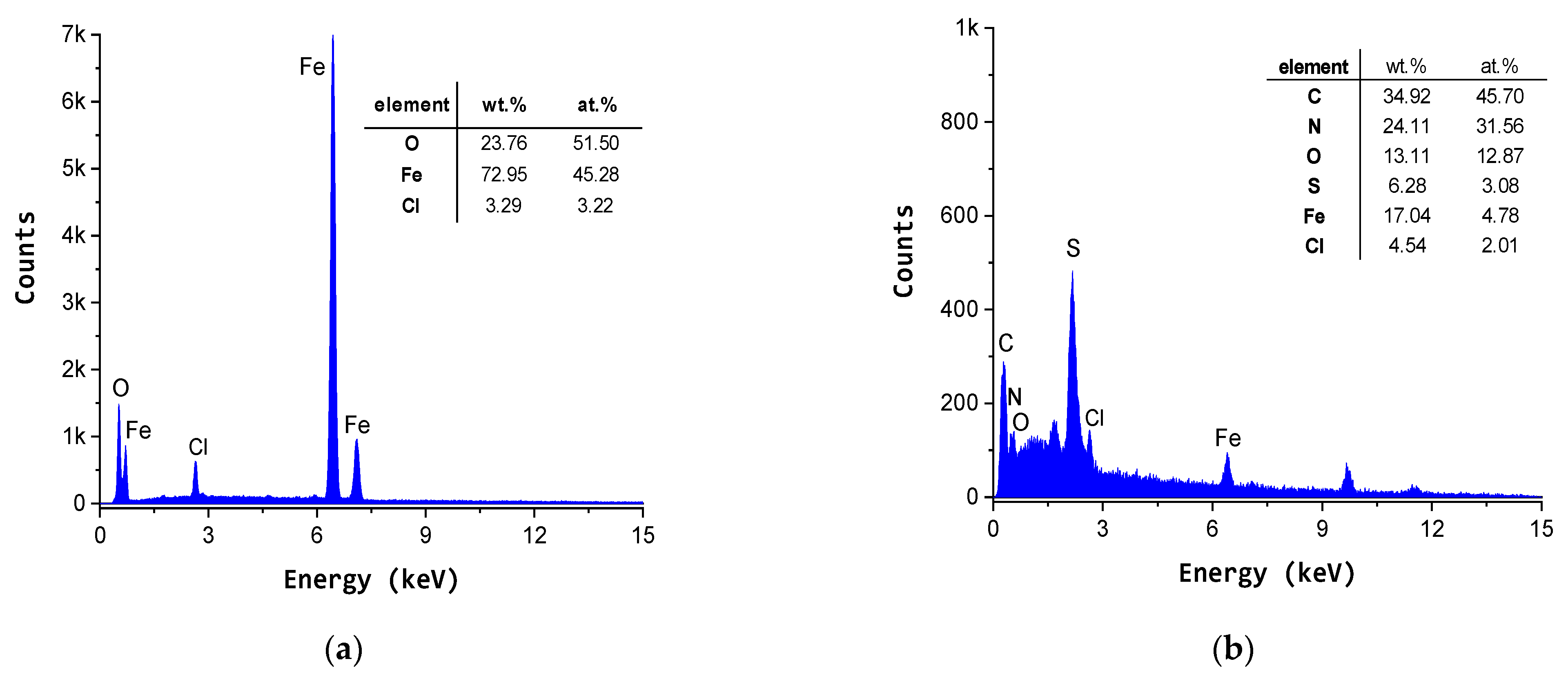
3.5. SEM-EDX Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouabdallaoui, M.; Aouzal, Z.; El Guerraf, A.; Ben Jadi, S.; Bazzaoui, M.; Wang, R.; Bazzaoui, E.A. Influence of Polythiophene Overoxidation on Its Physicochemical Properties and Corrosion Protection Performances. Mater. Today Proc. 2020, 31, S69–S74. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-Situ Electrodeposition of Conductive Polypyrrole-Graphene Oxide Composite Coating for Corrosion Protection of 304SS Bipolar Plates. J. Alloys Compd. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-C.; Gao, C.-J. On the mechanism of conductivity enhancement in PEDOT/PSS film doped with multi-walled carbon nanotubes. J. Polym. Res. 2010, 17, 713–718. [Google Scholar] [CrossRef]
- Branzoi, F.; Băran, A.; Mihai, M.A.; Zaki, M.Y. The Inhibition Action of Some Brij-Type Nonionic Surfactants on the Corrosion of OLC 45 in Various Aggressive Environments. Materials 2024, 17, 1378. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y.; Ma, J.; Yang, J.; Chen, D.; Gong, H.; Li, G.L. Intrinsic Self-Healing Polymer Coatings with Autonomous Corrosion Protection Based on Synergistic Healing Mechanisms. Appl. Surf. Sci. 2025, 709, 163641. [Google Scholar] [CrossRef]
- Nimet Ceren Güven, Hatice Ozkazanc, Corrosion protection behavior of poly (Nmethylpyrrole)/boron nitride composite film on aluminum-1050. Prog. Org. Coat. 2022, 164, 106696. [CrossRef]
- Sarıarslan, H.; Karaca, E.; Şahin, M.; Pekmez, N.Ö. Electrochemical Synthesis and Corrosion Protection of Poly(3-Aminophenylboronic Acid-Co-Pyrrole) on Mild Steel. RSC Adv. 2020, 10, 38548–38560. [Google Scholar] [CrossRef]
- Branzoi, F.; Mihai, A.M.; Zaki, M.Y. Anticorrosion Protection of New Composite Coating for Cobalt-Based Alloy in Hydrochloric Acid Solution Obtained by Electrodeposition Methods. Coatings 2024, 14, 106. [Google Scholar] [CrossRef]
- Pekmez, N.Ö.; Cınkıllı, K.; Zeybek, B. The electrochemical copolymerization of pyrrole and bithiophene on stainless steel in the presence of SDS in aqueous medium and its anticorrosive performance. Prog. Org. Coat. 2014, 77, 1277–1287. [Google Scholar] [CrossRef]
- Gopi, D.; Saraswathy, R.; Kavitha, L.; Kim, D.-K. Electrochemical synthesis of poly(indole-co-thiophene) on low-nickel stainless steel and its anticorrosive performance in 0.5 mol L−1 H2SO4. Polym. Int. 2014, 63, 280–289. [Google Scholar] [CrossRef]
- Kanwal, S.; Akhter, Z.; Ali, N.Z.; Hussain, R.; Qamar, S. Corrosion Protection of Aluminum Alloy (AA2219-T6) Using Sulfonic Acid-Doped Conducting Polymer Coatings. New J. Chem. 2022, 46, 14557–14564. [Google Scholar] [CrossRef]
- Ramírez, A.M.R.; Mieres, F.; Pineda, F.; Grez, P.; Heyser, C. Electrosynthesis of polyindole-carboxylic acids on stainless steel and their corrosion protection at different temperatures in acidic solution. Prog. Org. Coat. 2022, 177, 107075. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Eisa, M.H.; Maaza, M. Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance—A Review. Corros. Mater. Degrad. 2023, 4, 33–53. [Google Scholar] [CrossRef]
- Saikia, M.; Dutta, T.; Jadhav, N.; Kalita, D.J. Insights into the Development of Corrosion Protection Coatings—A Review. Polymers 2025, 17, 1548. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, C.; Cui, J.; Shen, S.; Qiu, H.; Li, J. Electrodeposition of polypyrrole coatings doped by benzenesulfonic acid-modified graphene oxide on metallic bipolar plates. Prog. Org. Coat. 2022, 170, 106995. [Google Scholar] [CrossRef]
- Cao, G.; Teng, Z.; Wu, A.; Zhang, C.; Wang, Z.; Wang, L.; Han, E. Enhanced Corrosion Protection of Waterborne Polyurethane Coating Using Highly Conductive and Dispersed Polythiophene Nanoparticles. Polym. Degrad. Stab. 2025, 241, 111520. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.; Yıldız, A. Poly(N-methylaniline) coatings on stainless steel by electropolymerization. Corros. Sci. 2007, 49, 2905–2919. [Google Scholar] [CrossRef]
- Yılmaz, S.M.; Atun, G. Corrosion protection efficiency of the electrochemically synthesized polypyrrole-azo dye composite coating on stainless steel. Prog. Org. Coat. 2022, 169, 106942. [Google Scholar] [CrossRef]
- Qiao, M.; Ji, G.; Lu, Y.; Zhang, B. Sustainable Corrosion-Resistant Superhydrophobic Composite Coating with Strengthened Robustness. J. Ind. Eng. Chem. 2023, 121, 215–227. [Google Scholar] [CrossRef]
- Song, L.; Gao, Z.; Sun, Q.; Chu, G.; Shi, H.; Xu, N.; Li, Z.; Hao, N.; Zhang, X.; Ma, F.; et al. Corrosion protection performance of a coating with 2-aminino-5-mercato-1,3,4-thiadizole-loaded hollow mesoporous silica on copper. Prog. Org. Coat. 2023, 175, 107331. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.; Luo, Z.; Chen, L.; Han, G. Enhanced adhesive and anti-corrosive performances of polymer composite coating for rusted metallic substrates by capillary filling. Prog. Org. Coat. 2023, 178, 107467. [Google Scholar] [CrossRef]
- Raheem, H.M.; Salman, T.A.; Jabor, A.A. Anti-Corrosion Behavior of Copolymer (Thiophene-Co-Pyrrole) Coating for Carbon Steel Alloy at Different Deposition Times in 3.5% NaCl Solution. Int. J. Corros. Scale Inhib. 2025, 14, 876–893. [Google Scholar]
- Pahuja, P.; Malik, R.; Saini, N.; Kumar, S.; Lata, S. PPy–TiO2–Phenylalanine Composites as Anticorrosive Coatings Electrodeposited over Fe–C Steel Surface in the Marine Environment. J. Adhes. Sci. Technol. 2020, 34, 1823–1839. [Google Scholar] [CrossRef]
- Ali, M.R.R.; Honeyman, J.S.; Domalanta, M.R.B.; Caldona, E.B. Superhydrophobic, Highly Adhesive, and Corrosion-Protective Fluoropolymer-Modified Polythiophene Coatings. Ind. Eng. Chem. Res. 2024, 63, 10243–10255. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; León-Silva, U.; Nicho, M.E. Corrosion protection of steel by poly(3-hexylthiophene) polymer blends. Anti-Corros. Methods Mater. 2015, 62, 229–240. [Google Scholar] [CrossRef]
- Branzoi, F.; Băran, A.; Mihai, M.A.; Paraschiv, A. Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures. Coatings 2025, 15, 359–361. [Google Scholar] [CrossRef]
- Huang, H.; Hou, L.; Du, H.; Wei, H.; Liu, X.; Wang, Q.; Wei, Y. In Situ Electrochemical Deposition Enables Dense Polypyrrole Grown on AZ31 Surface for Improving Corrosion Resistance. Mater. Lett. 2024, 362, 136214. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Kazempour, A. Incorporation of Organic/Inorganic Materials into Polypyrrole Matrix to Reinforce Its Anticorrosive Properties for the Protection of Steel Alloys: A Review. J. Mol. Liq. 2020, 309, 113085. [Google Scholar] [CrossRef]
- García-Cabezón, C.; Godinho, V.; Pérez-González, C.; Torres, Y.; Martín-Pedrosa, F. Electropolymerized Polypyrrole Silver Nanocomposite Coatings on Porous Ti Substrates with Enhanced Corrosion and Antibacterial Behavior for Biomedical Applications. Mater. Today Chem. 2023, 29, 101433. [Google Scholar] [CrossRef]
- Xue, S.; Ma, Y.; Miao, Y.; Li, W. Anti-Corrosion Performance of Conductive Copolymers of Polyaniline/Polythiophene on a Stainless-Steel Surface in Acidic Media. Int. J. Nanosci. 2020, 19, 1950023. [Google Scholar] [CrossRef]
- Mirzaee, M.; Kianpour, E.; Rashidi, A.; Rahimi, A.; Pourhashem, S.; Duan, J.; Iravani, D.; Sirati Gohari, M. Construction of a High-Performance Anti-Corrosion Epoxy Coating in the Presence of Poly (Aniline-Co-Pyrrole) Nanospheres. React. Funct. Polym. 2024, 194, 105794. [Google Scholar] [CrossRef]
- Awoyemi, R.F.; Almtiri, M.; Giri, H.; Scott, C.N.; Wipf, D.O. Corrosion Protective Coatings from Poly (Heterocyclic Diphenylamine): Polyaniline Analogues. ACS Appl. Polym. Mater. 2024, 6, 3060–3072. [Google Scholar] [CrossRef]
- González-Tejera, M.; García, M.; de la Blanca, E.S.; Redondo, M.; Raso, M.; Carrillo, I. Electrochemical synthesis of N-methyl and 3-methyl pyrrole perchlorate doped copolymer films. Thin Solid Film. 2007, 515, 6805–6811. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, K.; Zhang, K.; Wang, Z.; Liu, Z.; Hu, S.; Fang, Y.; He, C. Studies on Corrosion Behaviors of Q235 Steel Coated by the Polypyrrole Films Doped with different dopants. Int. J. Electrochem. Sci. 2020, 15, 2594–2603. [Google Scholar] [CrossRef]
- Yadav, P.K.; Prakash, O.; Ray, B.; Maiti, P. Functionalized Polythiophene for Corrosion Inhibition and Photovoltaic Application. J. Appl. Polym. Sci. 2021, 138, 51306. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G. Cyclic Voltammetric Synthesis of Poly(N-methyl pyrrole) on Copper and Effects of Polymerization Parameters on Corrosion Performance. Ind. Eng. Chem. Res. 2012, 51, 5246–5255. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, M.; Cui, Y.; Bao, D.; Peng, J.; Gao, Y.; Lin, D.; Geng, H.; Zhu, Y.; Wang, H. A Novel Polymer Composite Coating with High Thermal Conductivity and Unique Anti-Corrosion Performance. Chem. Eng. J. 2022, 439, 135660. [Google Scholar] [CrossRef]
- Xie, Z.-H.; Teng, Y.; Huang, Z.; Feng, P.; Yong, Q.; Jiang, X.; Wu, L. Corrosion-Resistant and Superhydrophobic Nickel-Based Composite Coating on Magnesium Alloy. Surf. Coat. Technol. 2025, 496, 131638. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, X.; Fang, B. Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis: A Review. Appl. Sci. 2023, 13, 1244. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Ravi, R.P.; Udoh, I.I.; Mathew, G.M.; Rajan, T.P.D. Polymer-Based Coating for Steel Protection, Highlighting Metal–Organic Framework as Functional Actives: A Review. Corros. Mater. Degrad. 2023, 4, 284–316. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Hu, H.; Bi, C.; Zhang, H.; Ye, Y.; Yan, C. Research Progress of Polymer-Based Coatings for Anti-Corrosion Application: A Mini Review. J. Polym. Sci. 2025, 1–14. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y. A Review on Conducting Polymers and Nanopolymer Composite Coatings for Steel Corrosion Protection. Coatings 2019, 9, 807. [Google Scholar] [CrossRef]
- Yan, L.; Deng, W.; Wang, N.; Xue, X.; Hua, J.; Chen, Z. Anti-Corrosion Reinforcements Using Coating Technologies—A Review. Polymers 2022, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
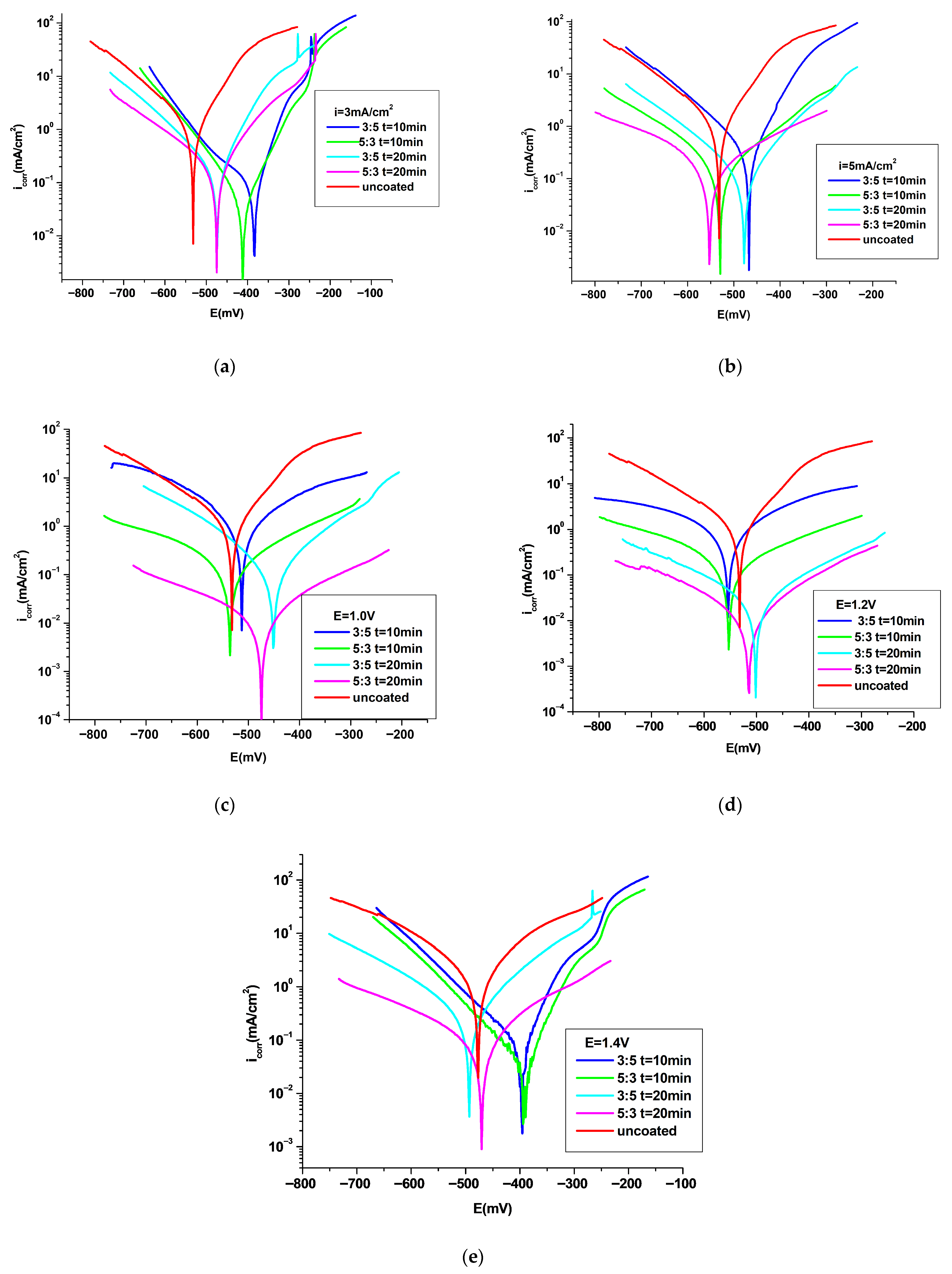
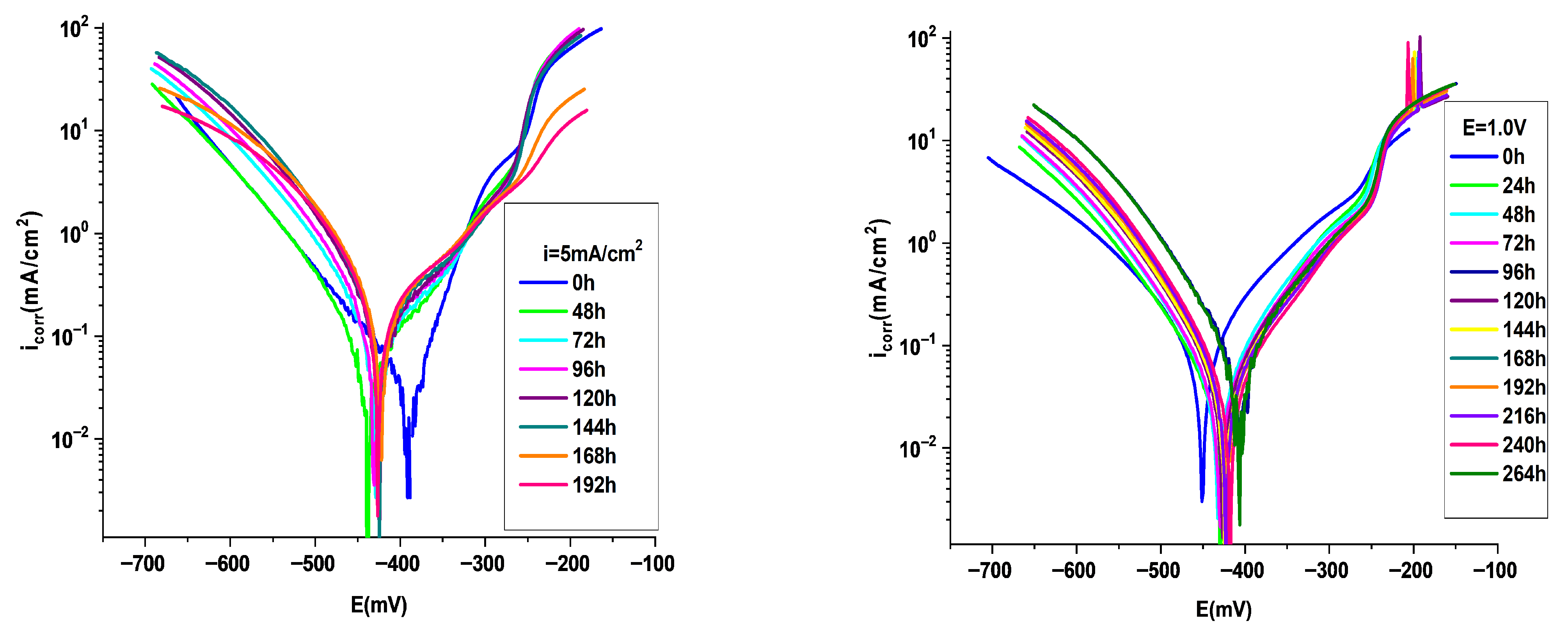
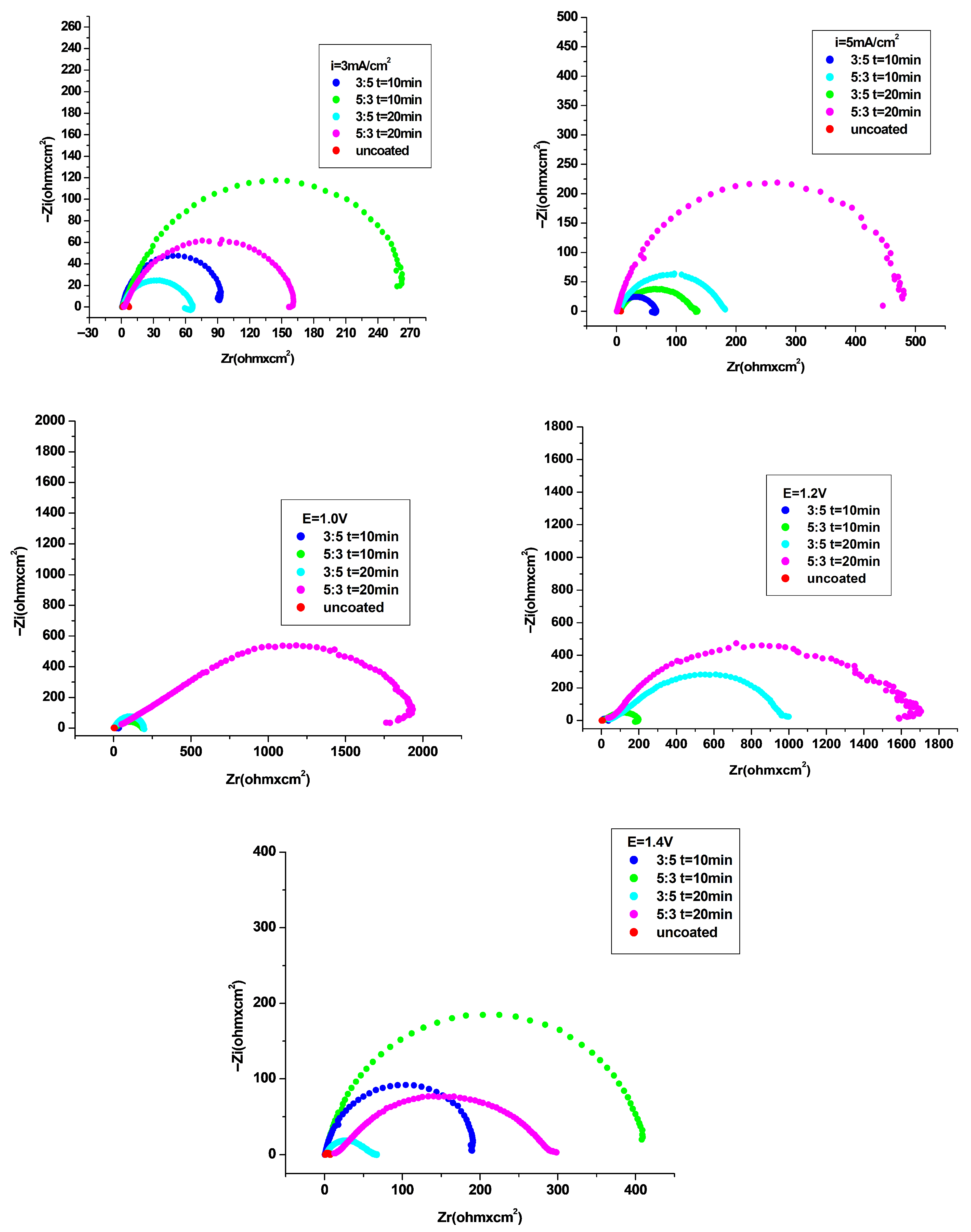
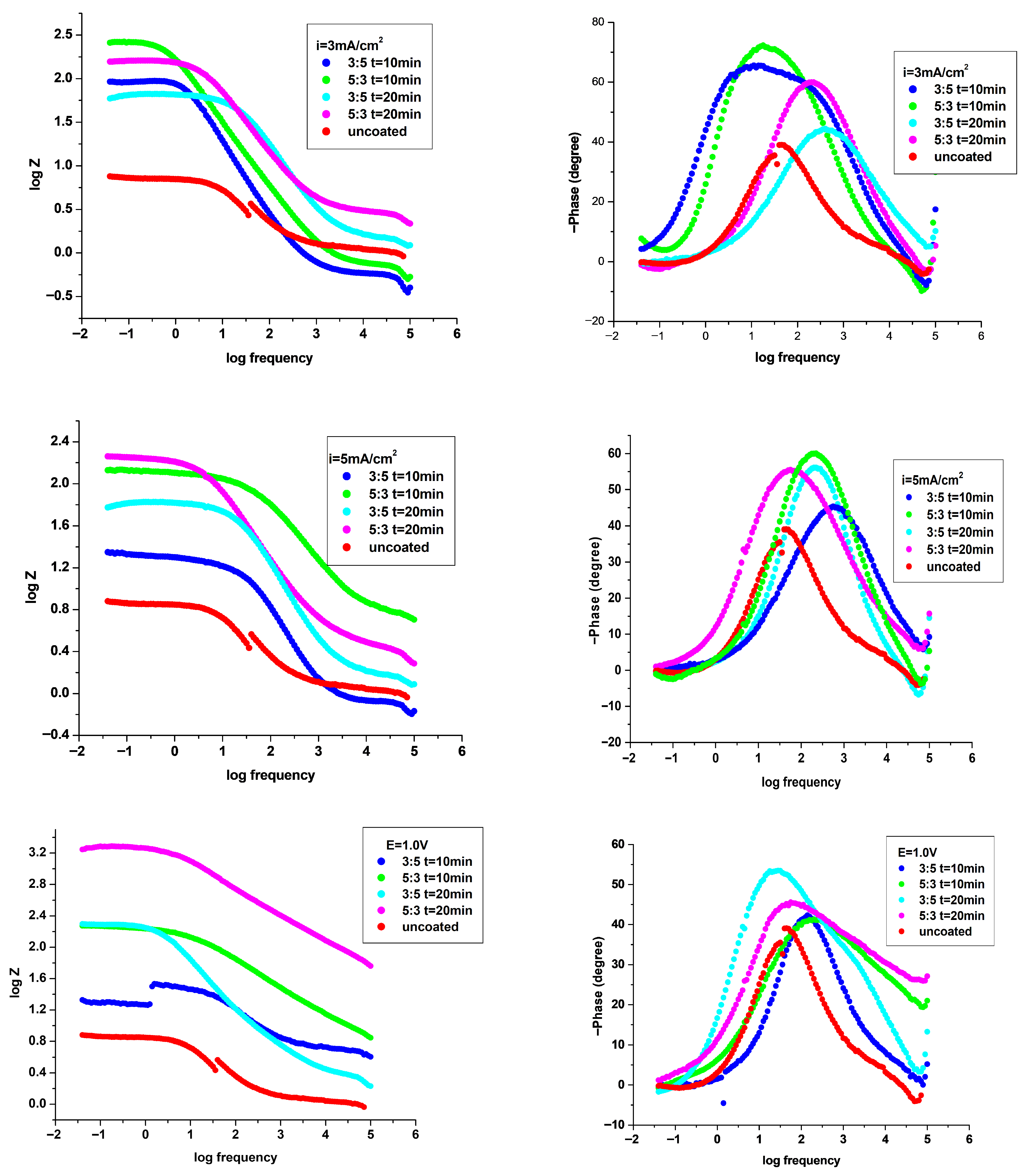
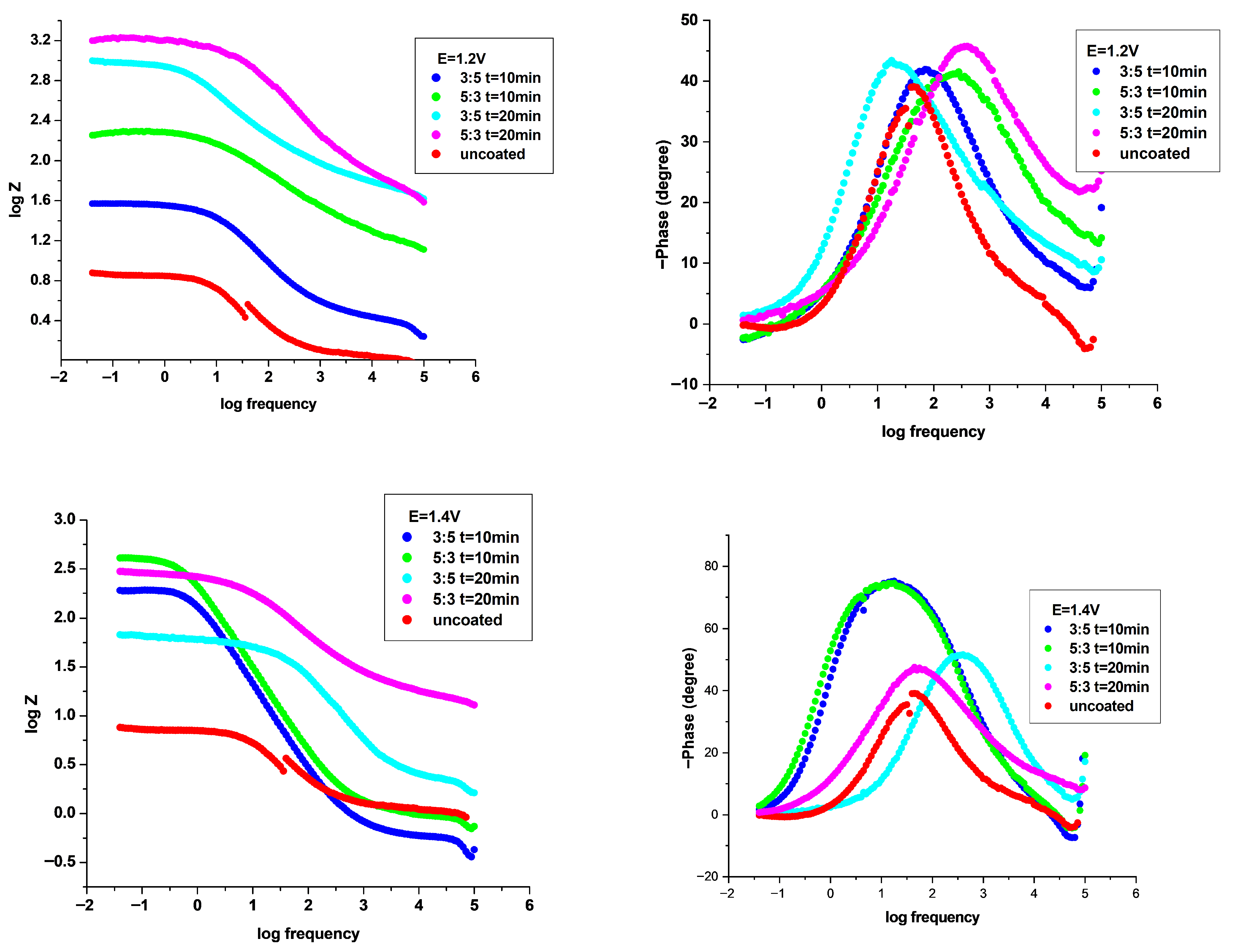
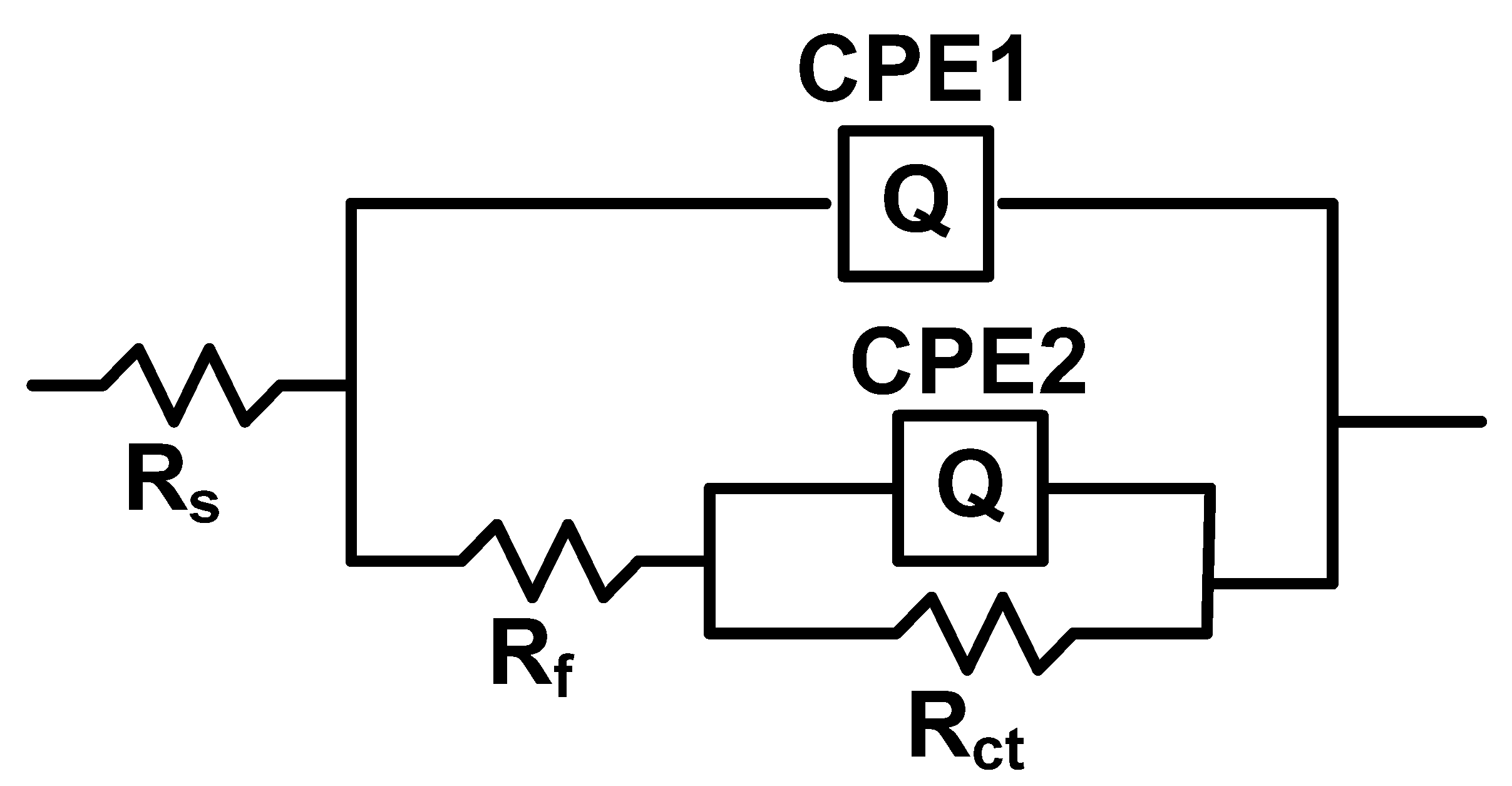
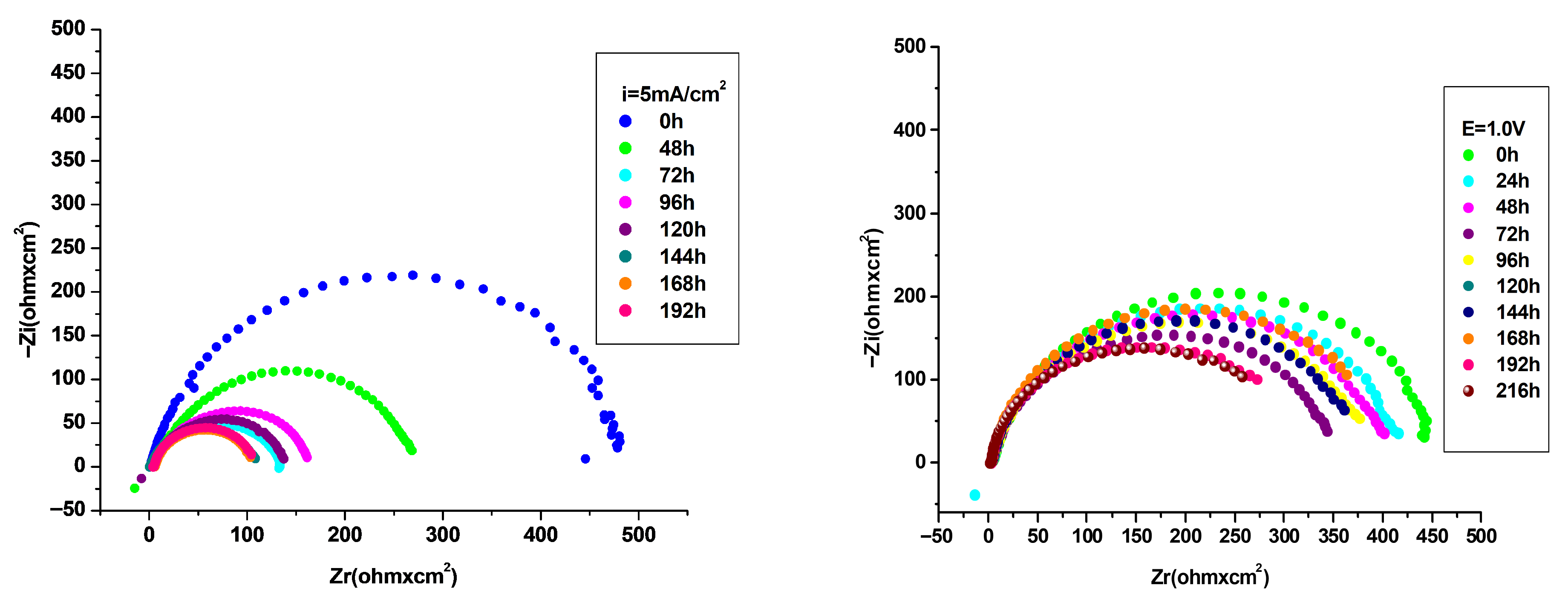
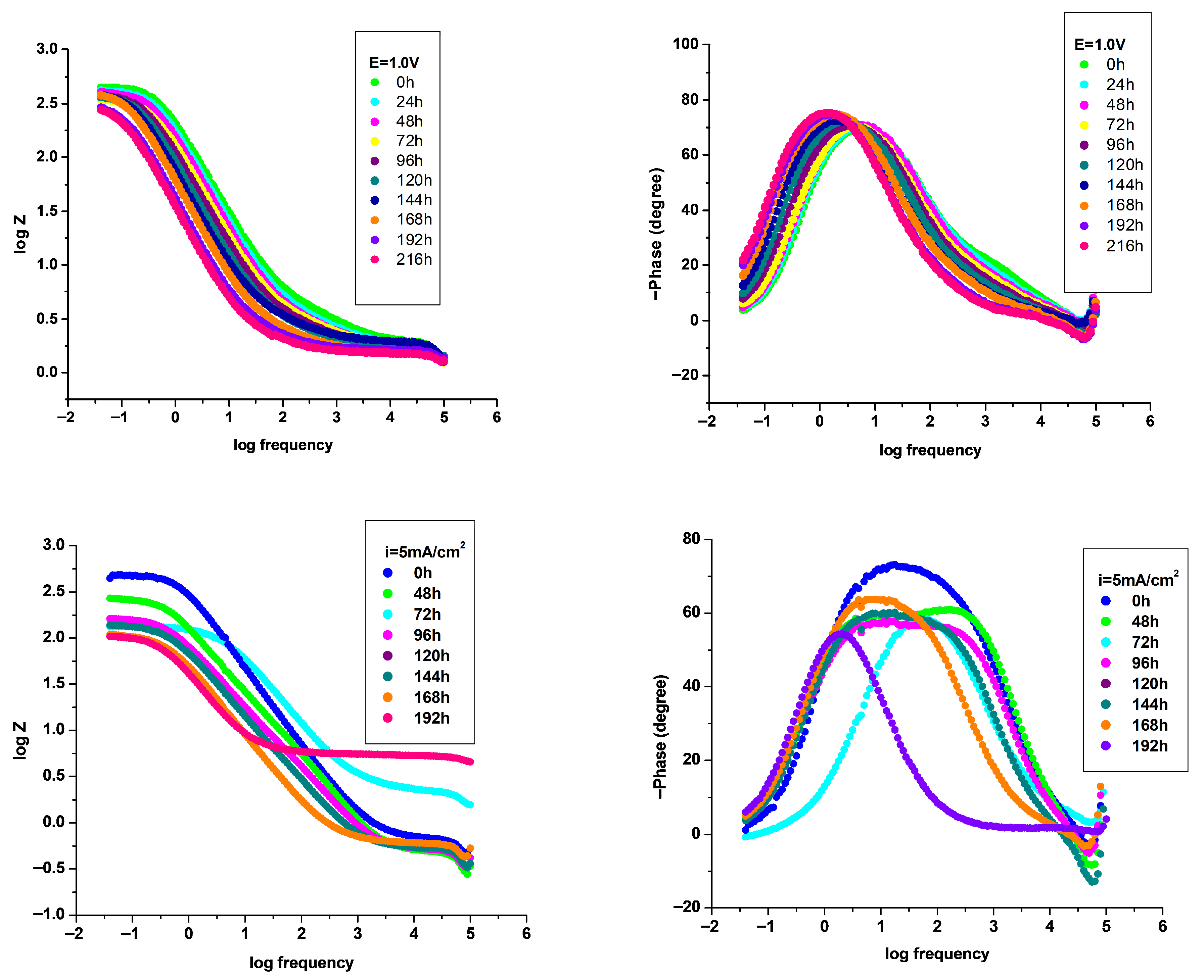

| Coating System | −Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) |
|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 1 M HCl | 529 | 0.873 | 26 | 409 | 10.39 | 9.29 | 88 | 115 | - |
| P3MPY–AOT/P3MTP 3 mA/cm2 3:5 molar ratio, t = 10 min | 401 | 0.057 | 179 | 26.6 | 0.675 | 0.60 | 49 | 102 | 93 |
| P3MPY–AOT/P3MTP 3 mA/cm2 5:3 molar ratio, t = 10 min | 415 | 0.030 | 350 | 14 | 0.355 | 0.32 | 60 | 71 | 97 |
| P3MPY–AOT/P3MTP 3 mA/cm2 3:5 molar ratio, t = 20 min | 480 | 0.115 | 116 | 53.66 | 1.36 | 1.22 | 61 | 99 | 87 |
| P3MPY–AOT/P3MTP 3 mA/cm2 5:3 molar ratio, t = 20 min | 485 | 0.098 | 139 | 45.98 | 1.16 | 1.06 | 73 | 106 | 90 |
| P3MPY–AOT/P3MTP 5 mA/cm2 3:5 molar ratio, t = 10 min | 470 | 0.19 | 64 | 89.15 | 2.26 | 2.02 | 100 | 94 | 78 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 10 min | 526 | 0.081 | 211 | 38 | 0.964 | 0.86 | 105 | 100 | 91 |
| P3MPY–AOT/P3MTP 5 mA/cm2 3:5 molar ratio, t = 20 min | 480 | 0.091 | 182 | 42.7 | 1.083 | 0.97 | 100 | 94 | 90 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min | 532 | 0.064 | 339 | 30.03 | 0.762 | 0.68 | 75 | 90 | 93 |
| Coating System | −Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) |
|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 1 M HCl | 529 | 0.873 | 26 | 409 | 10.39 | 9.29 | 88 | 115 | - |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 10 min | 513 | 0.34 | 61 | 159.3 | 4.06 | 3.62 | 92 | 82 | 61 |
| P3MPY–AOT/P3MTP 1.0 V 5:3 molar ratio, t = 10 min | 521 | 0.075 | 225 | 35.19 | 0.893 | 0.799 | 94 | 106 | 91 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min | 450 | 0.063 | 220 | 29.26 | 0.74 | 0.60 | 78 | 86 | 93 |
| P3MPY–AOT/P3MTP 1.0 V 5:3 molar ratio, t = 20 min | 490 | 0.006 | 2580 | 3.28 | 0.083 | 0.07 | 85 | 112 | 99 |
| P3MPY–AOT/P3MTP 1.2 V 3:5 molar ratio, t = 10 min | 515 | 0.25 | 65 | 117.3 | 2.977 | 2.66 | 90 | 102 | 71 |
| P3MPY–AOT/P3MTP 1.2 V 5:3 molar ratio, t = 10 min | 523 | 0.069 | 216 | 32.37 | 0.822 | 0.73 | 102 | 93 | 92 |
| P3MPY–AOT/P3MTP 1.2 V 3:5 molar ratio, t = 20 min | 516 | 0.014 | 1120 | 6.57 | 0.166 | 0.15 | 93 | 101 | 98 |
| P3MPY–AOT/P3MTP 1.2 V 5:3 molar ratio, t = 20 min | 512 | 0.006 | 2690 | 2.81 | 0.071 | 0.06 | 84 | 98 | 99 |
| P3MPY–AOT/P3MTP 1.4 V 3:5 molar ratio, t = 10 min | 400 | 0.075 | 163 | 35.19 | 0.893 | 0.79 | 50 | 99 | 91 |
| P3MPY–AOT/P3MTP 1.4 V 5:3 molar ratio, t = 10 min | 410 | 0.031 | 360 | 14.54 | 0.369 | 0.33 | 49 | 91 | 97 |
| P3MPY–AOT/P3MTP 1.4 V 3:5 molar ratio, t = 20 min | 499 | 0.151 | 98 | 70.85 | 1.798 | 1.61 | 75 | 93 | 83 |
| P3MPY–AOT/P3MTP 1.4 V 5:3 molar ratio, t = 20 min | 520 | 0.076 | 198 | 35.66 | 0.905 | 0.81 | 97 | 98 | 90 |
| Coating System | −Ecorr (mV) | icorr (mA/cm2) | Rp (Ωcm) | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ Decade) | −bc (mV/ Decade) | E (%) |
|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 1 M HCl | 529 | 0.873 | 26 | 409 | 10.39 | 9.29 | 88 | 115 | - |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 0 h | 432 | 0.026 | 495 | 12.13 | 0.333 | 0.275 | 66 | 72 | 97 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 24 h | 434 | 0.034 | 387 | 15.86 | 0.402 | 0.36 | 69 | 72 | 96 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 48 h | 433 | 0.033 | 404 | 15.4 | 0.391 | 0.35 | 71 | 71 | 96 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 72 h | 410 | 0.064 | 217 | 29.86 | 0.758 | 0.6378 | 81 | 74 | 93 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 96 h | 430 | 0.036 | 361 | 16.8 | 0.426 | 0.381 | 74 | 69 | 96 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 120 h | 427 | 0.037 | 373 | 17.26 | 0.438 | 0.392 | 75 | 70 | 95 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 144 h | 425 | 0.038 | 363 | 17.73 | 0.451 | 0.403 | 78 | 68 | 95 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 168 h | 426 | 0.0385 | 353 | 17.96 | 0.456 | 0.41 | 80 | 67 | 95 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 192 h | 421 | 0.0375 | 375 | 16.70 | 0.426 | 0.380 | 80 | 66 | 95 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 216 h | 432 | 0.026 | 495 | 12.13 | 0.333 | 0.275 | 66 | 72 | 97 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min 240 h | 410 | 0.0576 | 234 | 26.88 | 0.682 | 0.61 | 77 | 70 | 93 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 0 h | 480 | 0.064 | 215 | 30.03 | 0.762 | 0.68 | 75 | 90 | 93 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 48 h | 440 | 0.066 | 287 | 26.77 | 0.66 | 0.61 | 106 | 71 | 93 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 72 h | 431 | 0.087 | 177 | 40.6 | 1.03 | 0.922 | 114 | 70 | 90 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 96 h | 432 | 0.111 | 148 | 51.8 | 1.314 | 1.17 | 119 | 70 | 87 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 120 h | 427 | 0.130 | 129 | 60.66 | 1.54 | 1.38 | 118 | 68 | 87 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 144 h | 427 | 0.14 | 108 | 65.33 | 1.658 | 1.484 | 120 | 66 | 84 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 168 h | 425 | 0.16 | 100 | 74.66 | 1.89 | 1.69 | 114 | 71 | 82 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min 196 h | 428 | 0.17 | 100 | 79.33 | 2.01 | 1.80 | 121 | 74 | 81 |
| Coating System | Rs ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rf ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rct ohm·cm2 | χ |
|---|---|---|---|---|---|---|---|---|
| OL 37 + 1 M HCl | 0.77 | 0.0026 | 0.68 | 1.74 | 0.00518 | 0.72 | 9.34 | 4.645 × 10−3 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 10 min | 5.70 | 0.000133 | 0.70 | 29 | 0.000236 | 0.72 | 128 | 9.645 × 10−5 |
| P3MPY–AOT/P3MTP 1.0 V 5:3molar ratio, t = 10 min | 8.4 | 0.000101 | 072 | 36 | 0.000189 | 0.76 | 162 | 4.33 × 10−4 |
| P3MPY–AOT/P3MTP 1.0 V 3:5 molar ratio, t = 20 min | 2.29 | 0.000206 | 0.78 | 14 | 0.000253 | 0.82 | 191 | 1.089 × 10−3 |
| P3MPY–AOT/P3MTP 1.0 V 5:3 molar ratio, t = 20 min | 41 | 0.000022 | 0.71 | 460 | 0.000019 | 0.78 | 1608 | 4.269 × 10−4 |
| P3MPY–AOT/P3MTP 1.2 V 3:5 molar ratio, t = 10 min | 2.28 | 0.000266 | 0.76 | 12 | 0.000674 | 0.77 | 38 | 4.024 × 10−4 |
| P3MPY–AOT/P3MTP 1.2 V 5:3molar ratio, t = 10 min | 12 | 0.000093 | 0.73 | 38 | 0.000177 | 0.76 | 166 | 7.882 × 10−4 |
| P3MPY–AOT/P3MTP 1.2 V 3:5 molar ratio, t = 20 min | 45 | 0.000045 | 0.75 | 118 | 0.000058 | 0.78 | 841 | 7.494 × 10−4 |
| P3MPY–AOT/P3MTP 1.2 V 5:3 molar ratio, t = 20 min | 32 | 0.000021 | 0.73 | 201 | 0.000011 | 0.91 | 1478 | 3.280 × 10−4 |
| P3MPY–AOT/P3MTP 1.4 V 3:5molar ratio, t = 10 min | 0.63 | 0.000547 | 0.94 | 18 | 0.000448 | 0.92 | 198 | 8.414 × 10−4 |
| P3MPY–AOT/P3MTP 1.4 V 5:3 molar ratio, t = 10 min | 0.95 | 0.000338 | 0.93 | 22 | 0.000396 | 0.90 | 420 | 7.318 × 10−4 |
| P3MPY–AOT/P3MTP 1.4 V 3:5 molar ratio, t = 20 min | 2.17 | 0.000183 | 0.81 | 16 | 0.000814 | 0.78 | 61 | 1.313 × 10−3 |
| P3MPY–AOT/P3MTP 1.4 V 5:3 molar ratio, t = 20 min | 12 | 0.000252 | 0.71 | 39 | 0.000732 | 0.77 | 265 | 1.379 × 10−4 |
| Coating System | Rs ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rf ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rct ohm·cm2 | χ |
|---|---|---|---|---|---|---|---|---|
| OL 37 + 1 M HCl | 0.77 | 0.0026 | 0.68 | 1.74 | 0.00518 | 0.72 | 9.34 | 4.645 × 10−3 |
| P3MPY–AOT/P3MTP 3 mA/cm2 3:5 molar ratio, t = 10 min | 0.59 | 0.000589 | 0.95 | 12 | 0.000398 | 0.94 | 94 | 1.752 × 10−3 |
| P3MPY–AOT/P3MTP 3 mA/cm2 5:3molar ratio, t = 10 min | 0.73 | 0.000697 | 0.88 | 28 | 0.000236 | 0.92 | 260 | 1.490 × 10−3 |
| P3MPY–AOT/P3MTP 3 mA/cm2 3:5 molar ratio, t = 20 min | 1.513 | 0.000036 | 0.93 | 18 | 0.000214 | 0.78 | 66 | 1.032 × 10−3 |
| P3MPY–AOT/P3MTP 3 mA/cm2 5:3 molar ratio, t = 20 min | 2.886 | 0.000234 | 0.85 | 21 | 0.000142 | 0.83 | 153 | 6.020 × 10−4 |
| P3MPY–AOT/P3MTP 5 mA/cm2 3:5 molar ratio, t = 10 min | 0.84 | 0.000433 | 0.89 | 12 | 0.000486 | 0.76 | 68 | 1.657 × 10−3 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 10 min | 2.5 | 0.000112 | 0.82 | 14 | 0.000116 | 0.81 | 178 | 2.406 × 10−4 |
| P3MPY–AOT/P3MTP 5 mA/cm2 3:5 molar ratio, t = 20 min | 5.5 | 0.000103 | 0.73 | 23 | 0.000331 | 0.69 | 121 | 1.682 × 10−4 |
| P3MPY–AOT/P3MTP 5 mA/cm2 5:3 molar ratio, t = 20 min | 0.681 | 0.000379 | 0.89 | 16 | 0.000125 | 0.89 | 487 | 1.228 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branzoi, F.; Neacsu, E.I.; Mihai, M.A.; Paraschiv, A. Enhanced Corrosion Resistance of OL 37 Steel in Hydrochloric Acid Using a Novel Composite Polymer Film. Materials 2025, 18, 5351. https://doi.org/10.3390/ma18235351
Branzoi F, Neacsu EI, Mihai MA, Paraschiv A. Enhanced Corrosion Resistance of OL 37 Steel in Hydrochloric Acid Using a Novel Composite Polymer Film. Materials. 2025; 18(23):5351. https://doi.org/10.3390/ma18235351
Chicago/Turabian StyleBranzoi, Florina, Elena Ionela Neacsu, Marius Alexandru Mihai, and Alexandru Paraschiv. 2025. "Enhanced Corrosion Resistance of OL 37 Steel in Hydrochloric Acid Using a Novel Composite Polymer Film" Materials 18, no. 23: 5351. https://doi.org/10.3390/ma18235351
APA StyleBranzoi, F., Neacsu, E. I., Mihai, M. A., & Paraschiv, A. (2025). Enhanced Corrosion Resistance of OL 37 Steel in Hydrochloric Acid Using a Novel Composite Polymer Film. Materials, 18(23), 5351. https://doi.org/10.3390/ma18235351






