Performance Evolution and Degradation Mechanism of Chemically Bonded Phosphate Ceramic Cement Under Freeze–Thaw Cycles
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Test
2.3.1. Mass Loss Rate
- Δm is the mass loss rate;
- m0 is the initial mass;
- m is the mass after the nth cycle.
2.3.2. Compressive Strength
2.3.3. pH
2.3.4. TG/DTG
2.3.5. XRD
2.3.6. FTIR
2.3.7. SEM
3. Results and Discussion
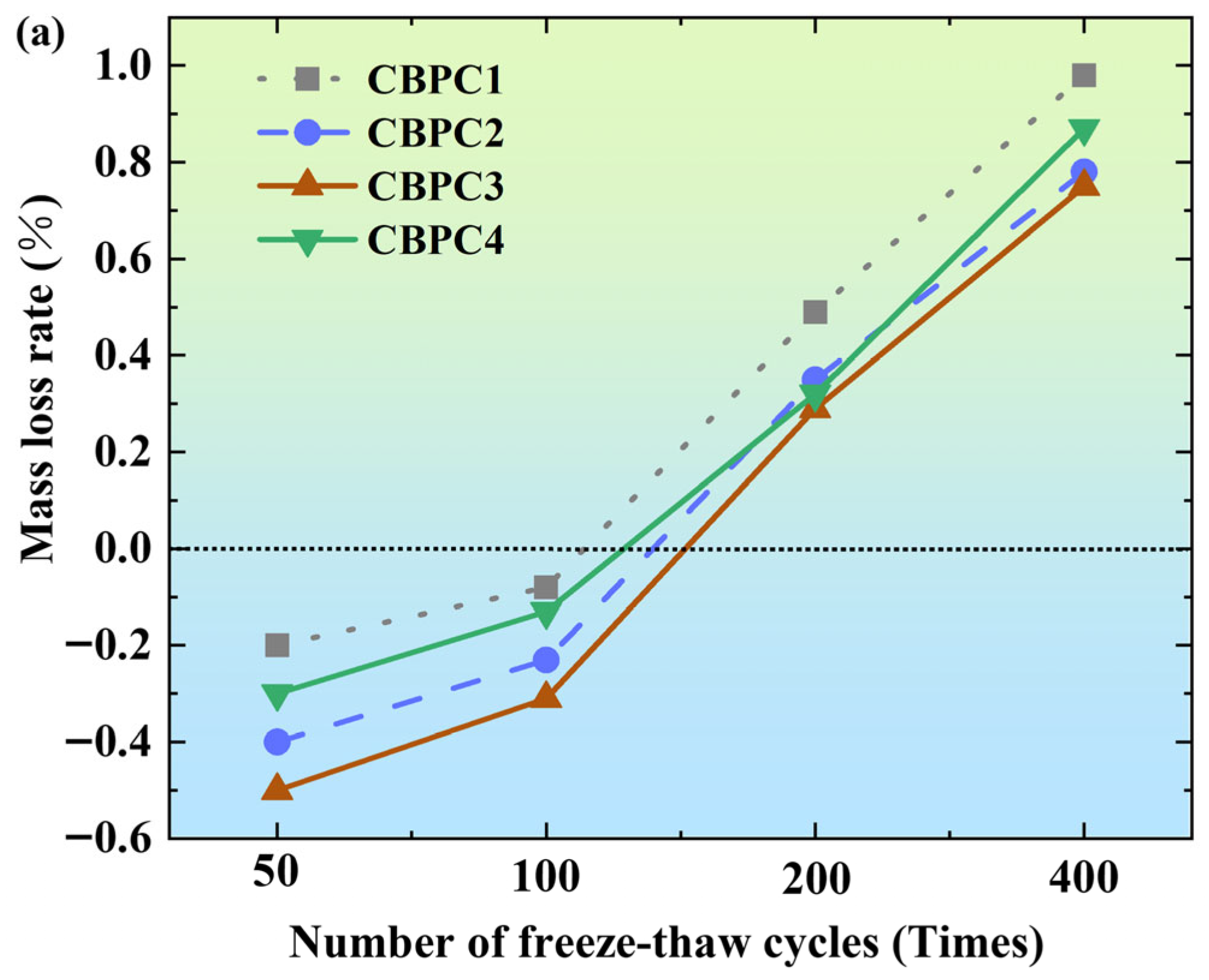
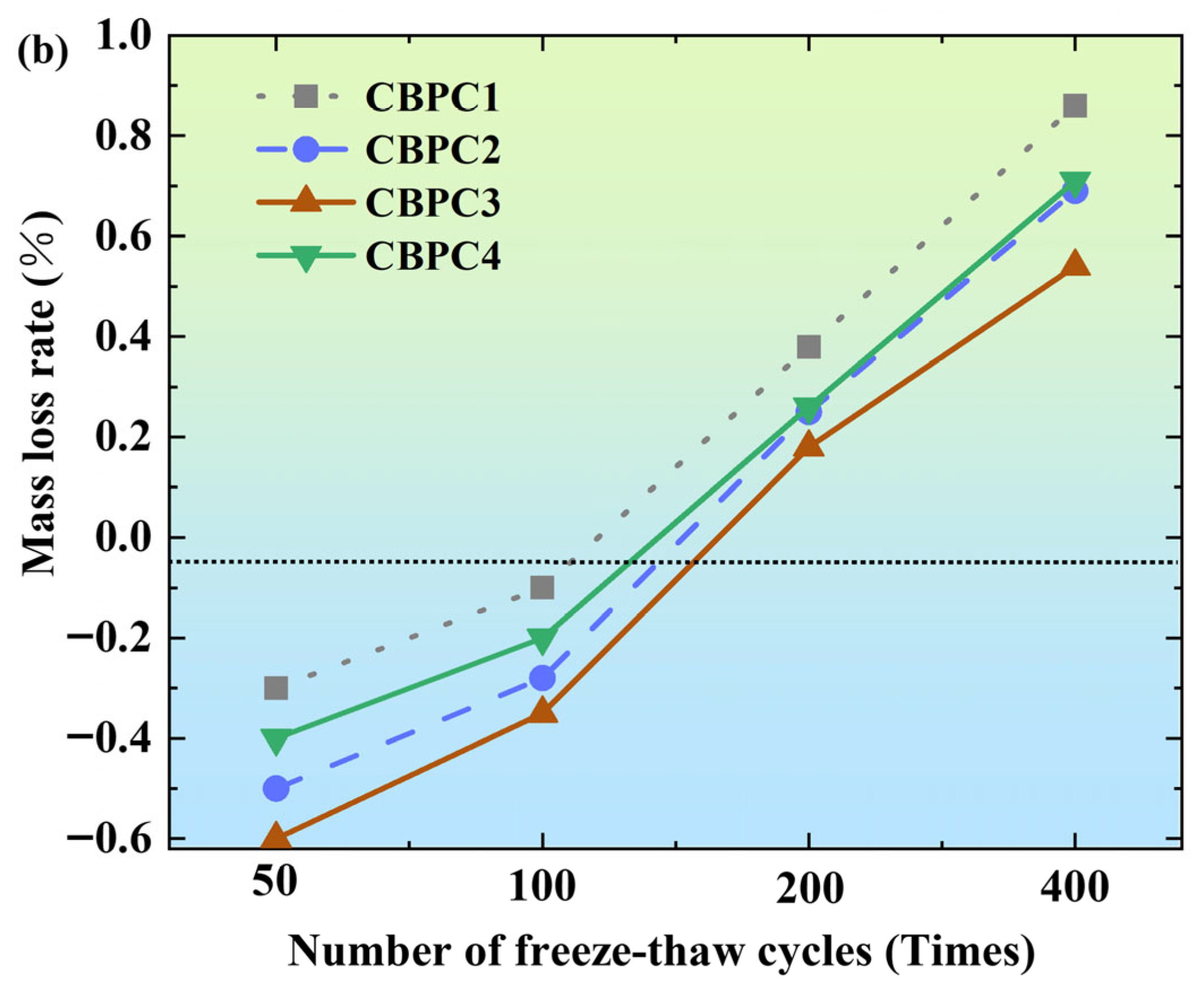
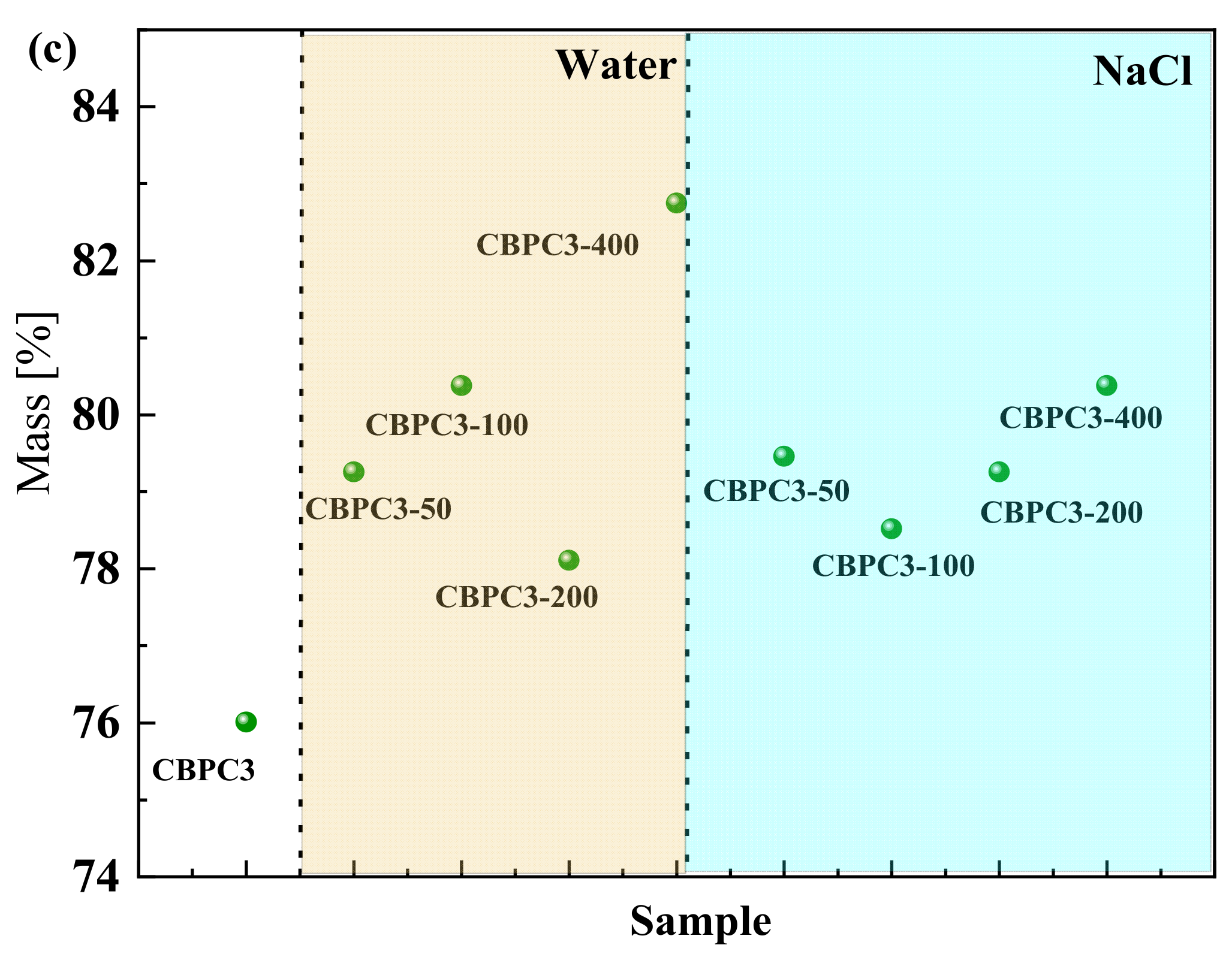
3.1. Mass Loss Rate
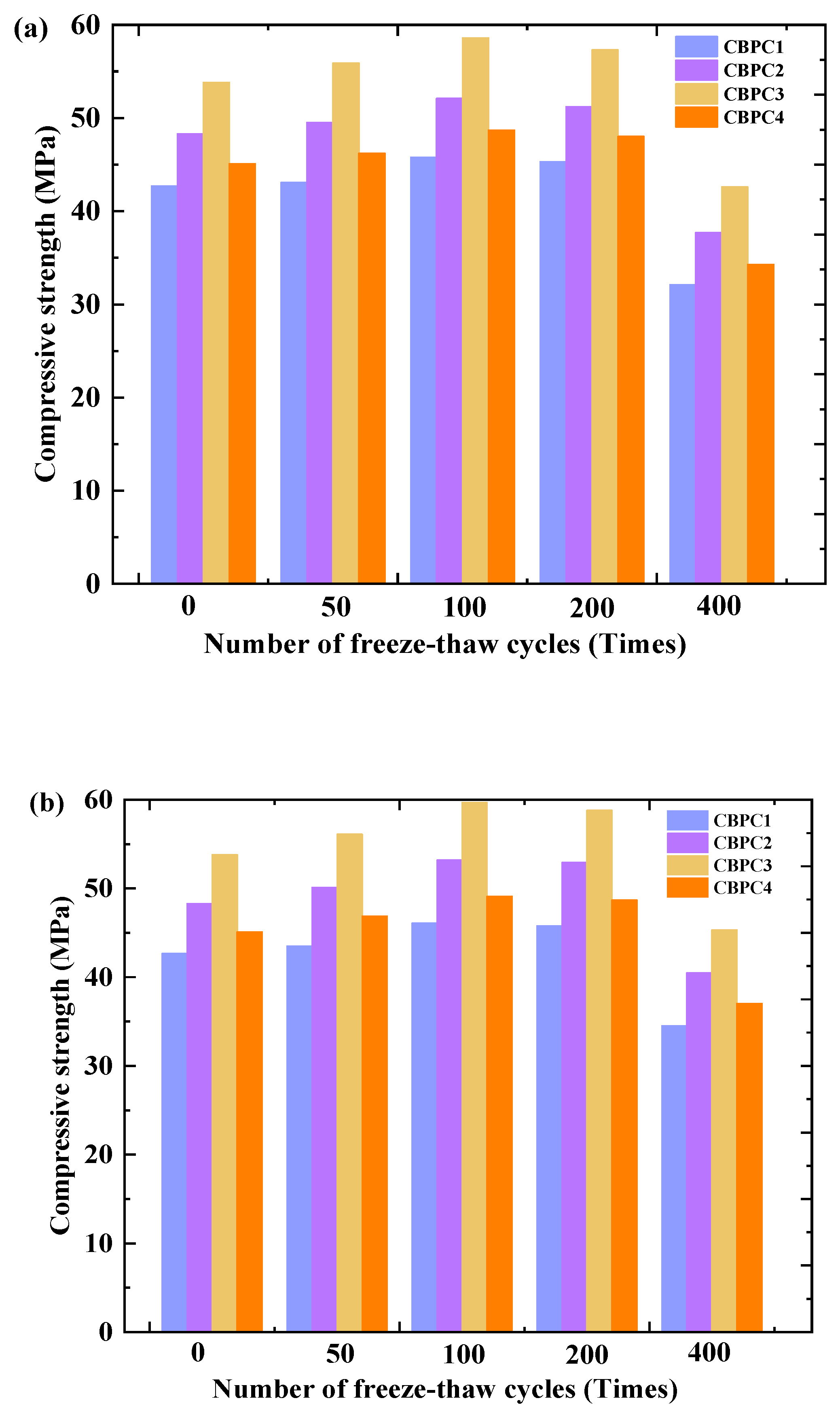
3.2. Compressive Strength
3.3. pH and Ion Concentration Analysis
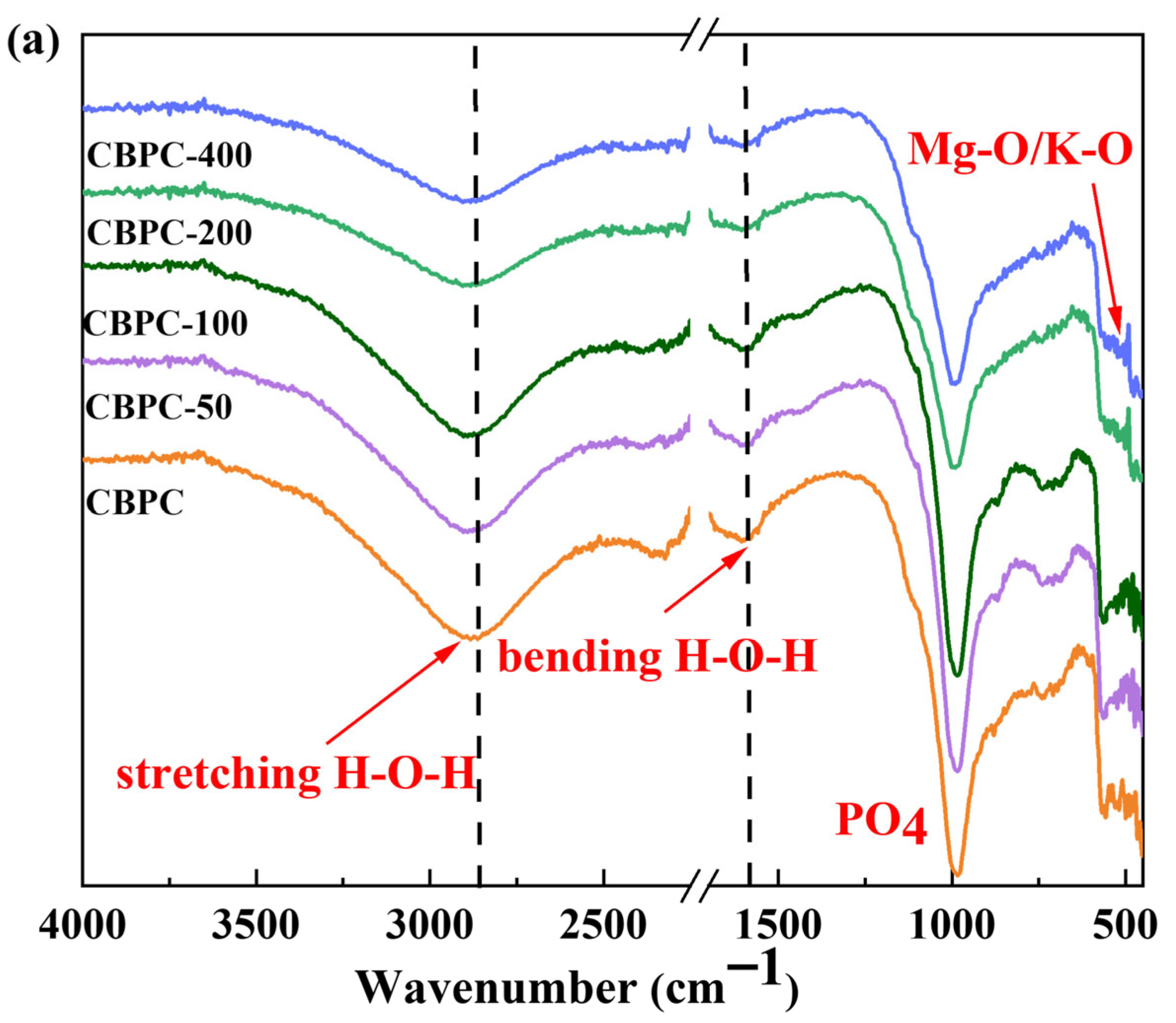
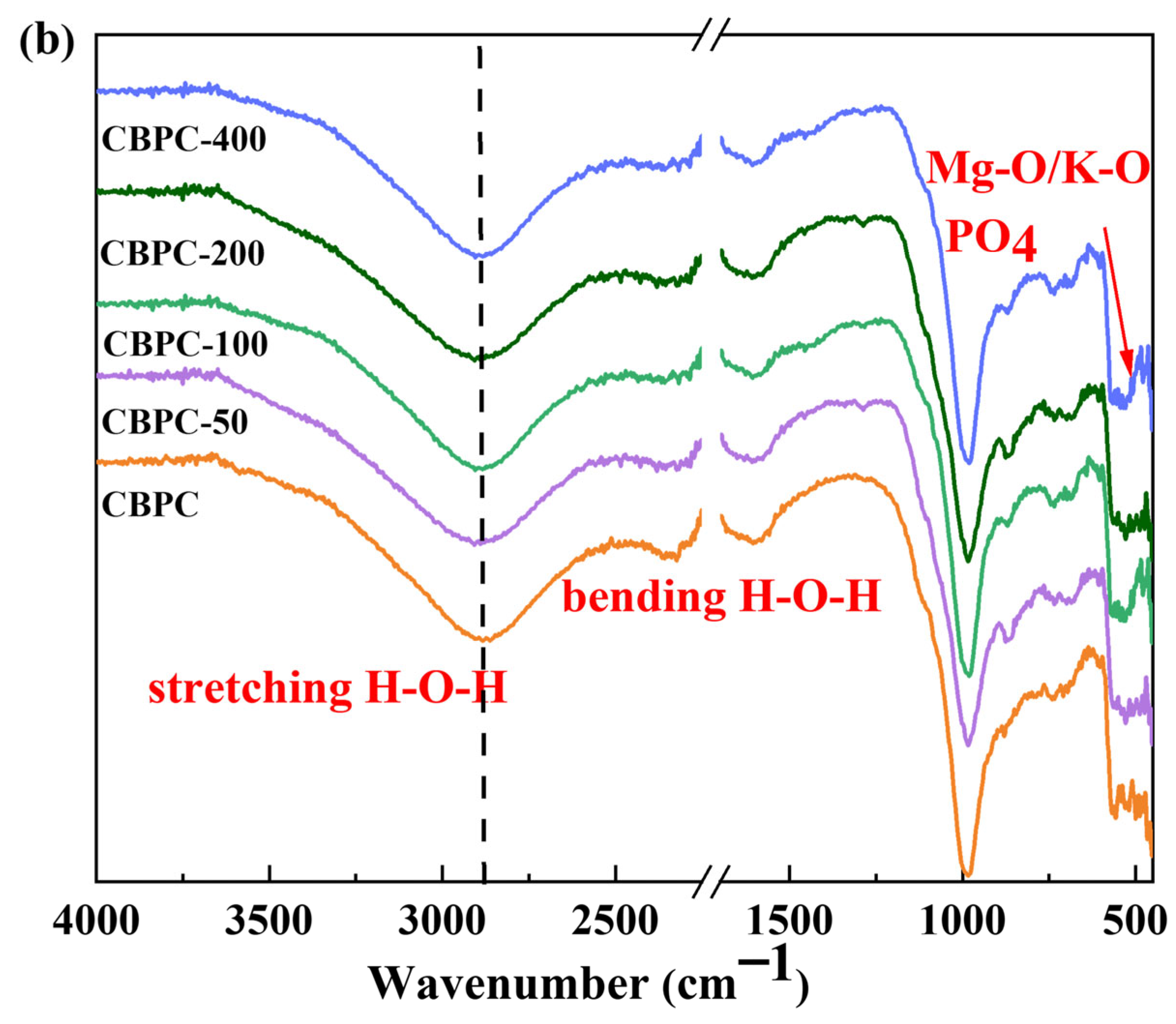
3.4. FTIR Analysis
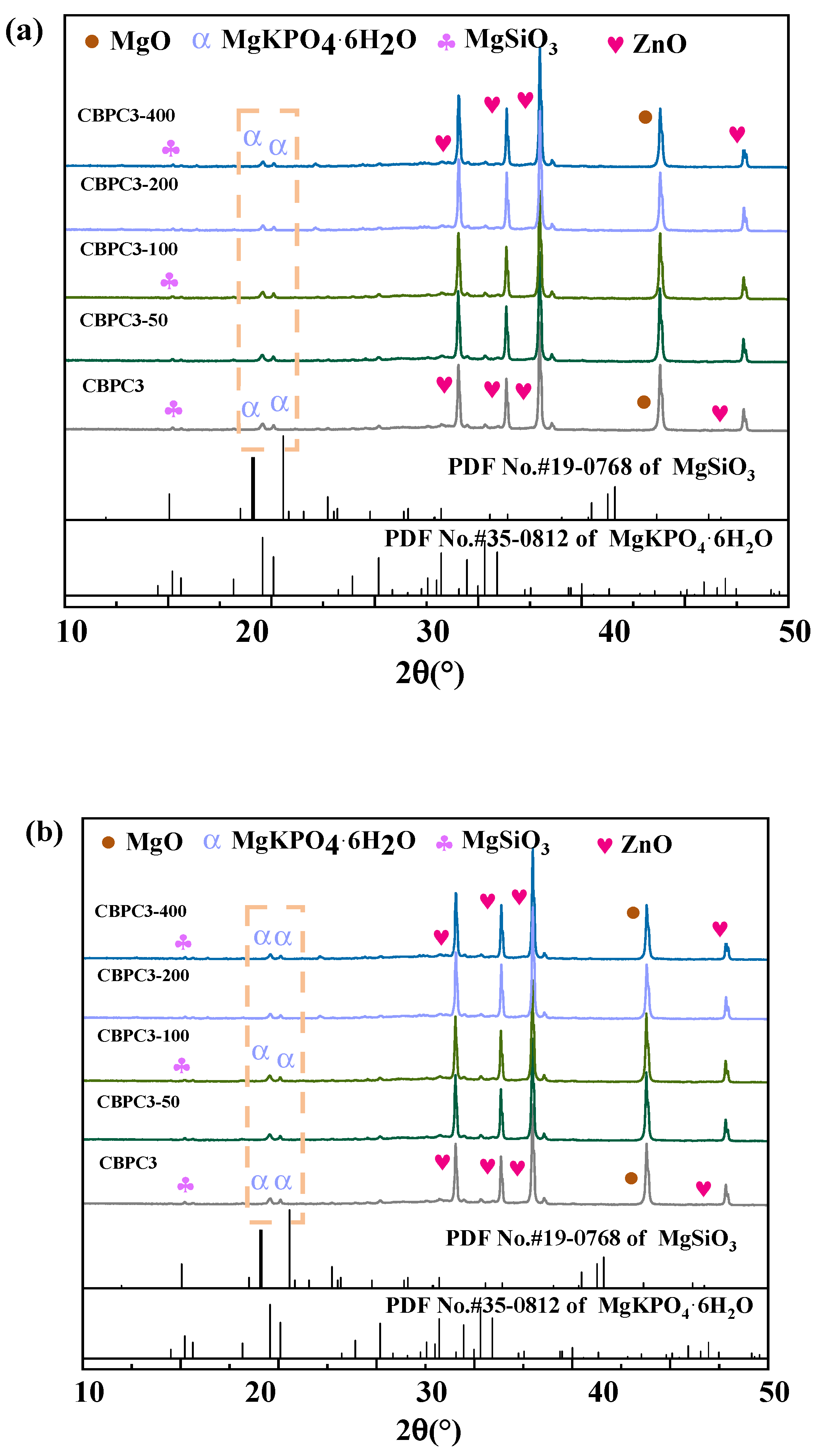
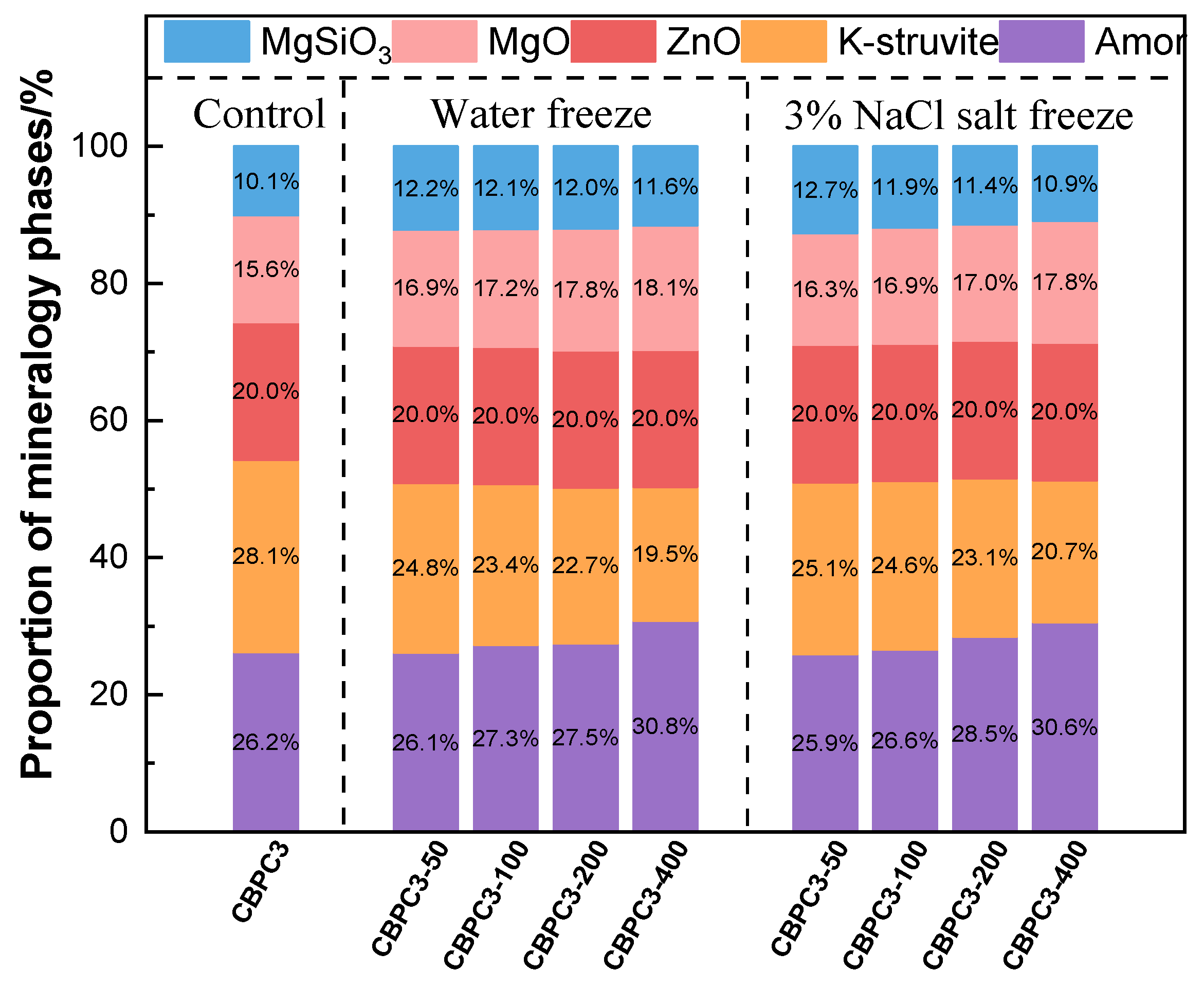
3.5. XRD Analysis
3.6. TG/DTG Analysis
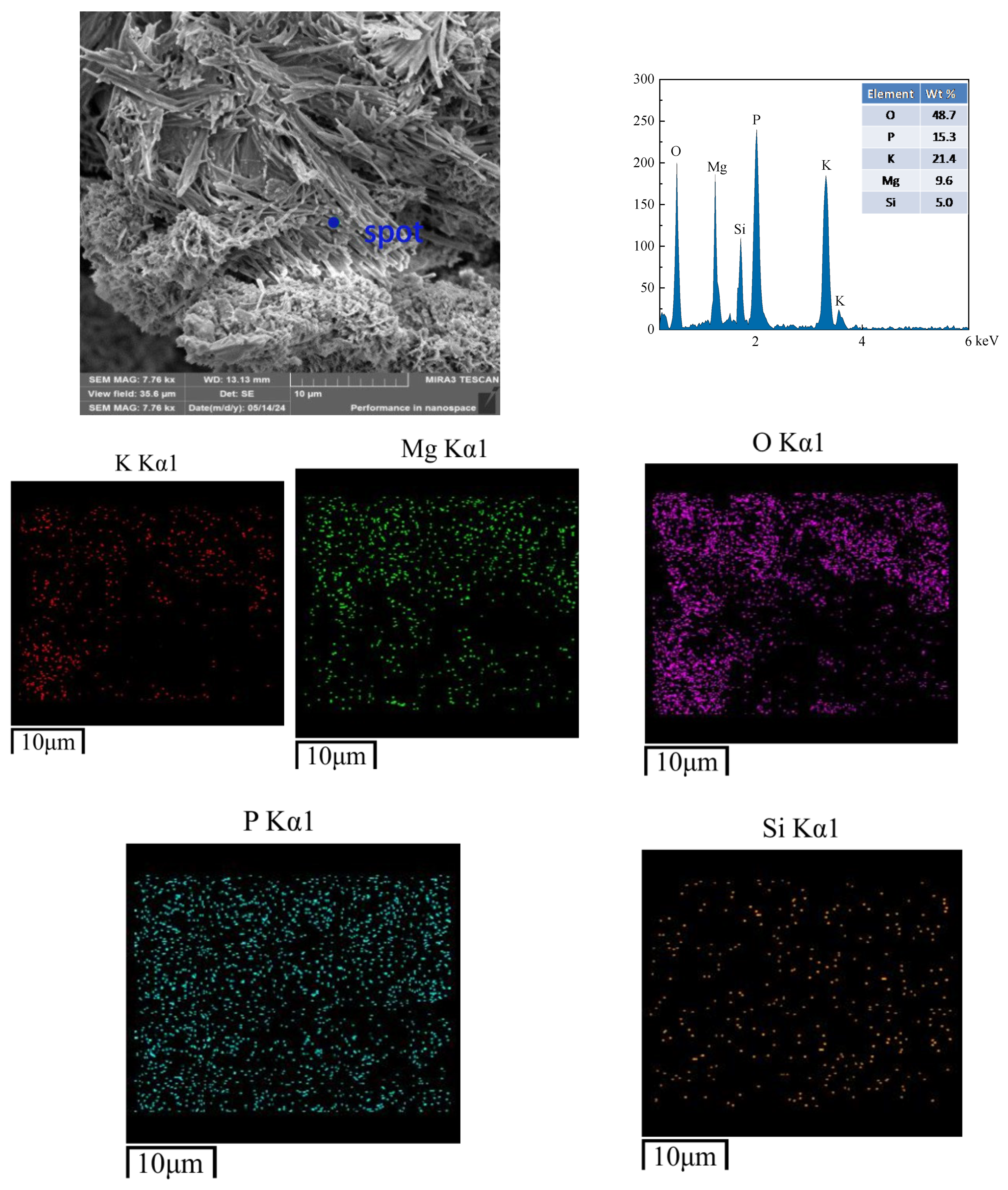
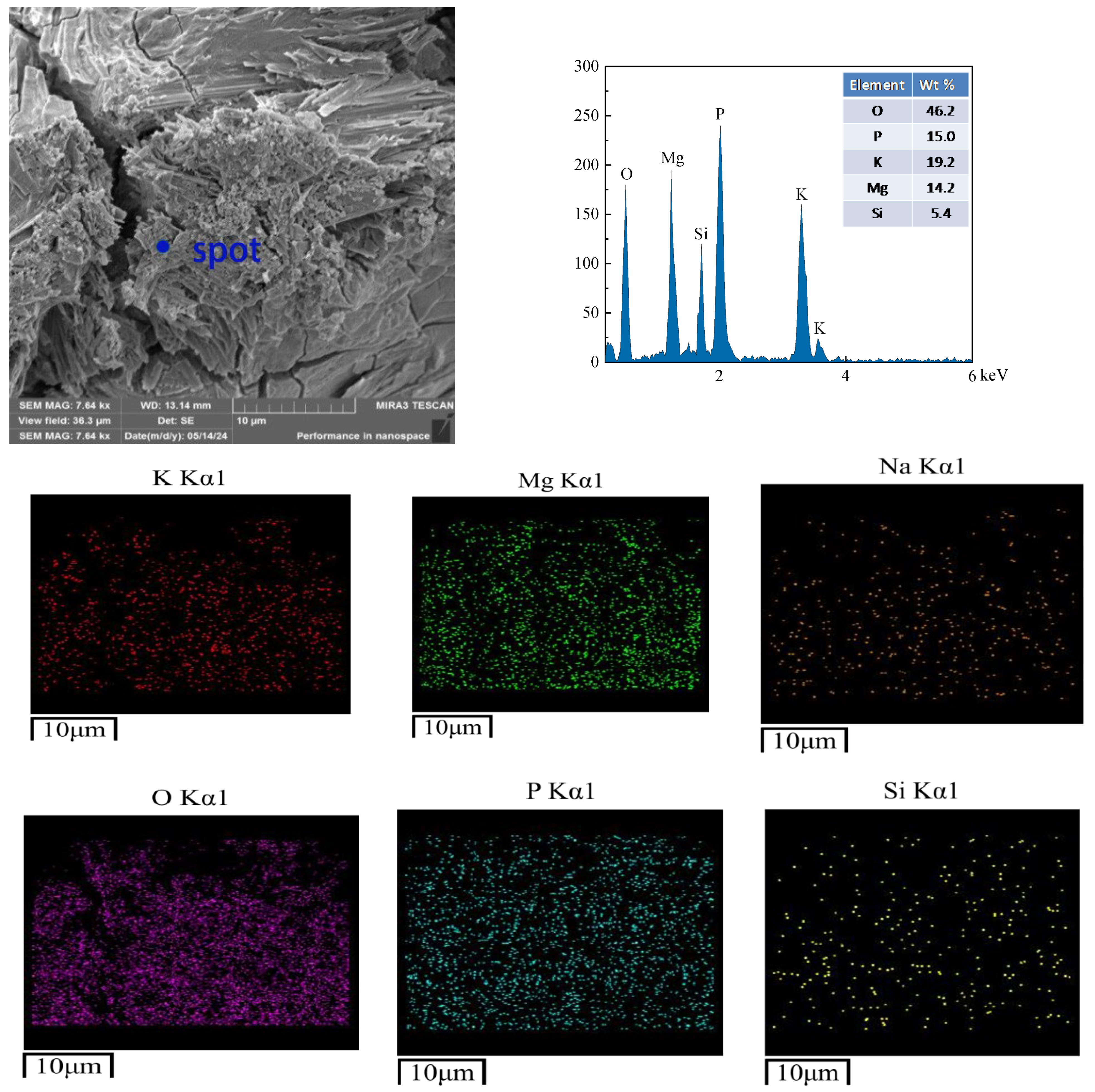
3.7. SEM Analysis
4. Conclusions
- (1)
- Among the tested mixes, the CBPC3 sample with a 15% silica fume content demonstrates optimal overall performance. It exhibits the lowest mass loss (0.75% in water and 0.54% in salt solution after 400 cycles) and the highest compressive strength retention under both freeze–thaw environments, indicating that this proportion most effectively enhances the microstructure density and freeze–thaw resistance of the CBPC matrix.
- (2)
- The compressive strength results reveal that the compressive strength of CBPC cement initially increases and then decreases with an increasing number of freeze–thaw cycles in different media. The compressive strengths decrease by 29.91% and 25.16% under water freeze–thaw and salt freeze–thaw cycling, respectively. The higher compressive strength under salt freeze–thaw cycling conditions is attributed to the accumulation of NaCl crystals.
- (3)
- The results regarding the external pH and ion concentrations of CBPC cement after freeze–thaw cycling show that the pH value increases with the number of freeze–thaw cycles under different media conditions. Simultaneously, the ion concentrations of K+, Mg2+, and PO43− all increase, while the ion concentrations of Na+ and Cl− decrease during salt freeze–thaw cycling. Freeze–thaw cycling in different media causes irreversible erosion to the hydration products of CBPC cement.
- (4)
- XRD/Rietveld quantitative analysis demonstrates that the content of MgKPO4·6H2O decreases sequentially with an increasing number of freeze–thaw cycles, while the content of MgSiO3 exhibits a different trend. After 400 water freeze–thaw cycles, the content of MgKPO4·6H2O decreases from 28.1% to 19.5%, whereas after 400 salt freeze–thaw cycles, it gradually decreases from 28.1% to 20.7%. Additionally, the crystallinity of MgKPO4·6H2O gradually decreases. The results from TG/DTG and FTIR analyses are consistent with the quantitative analysis results and align with the macroscopic performance variations.
- (5)
- The microstructural results of the products reveal that freeze–thaw cycling induces numerous cracks in the hydration products of CBPC cement, resulting in an overall non-dense structure. The elements detected by EDS are consistent with those in the phase products. Furthermore, the microstructural changes are in line with the macroscopic and phase variations.
- (6)
- Future prospects: Based on the findings of this study, future research can be directed towards the following: (i) investigating the performance of the optimal mix (15% SF) under coupled deterioration conditions, such as freeze–thaw cycles combined with mechanical loading or chemical corrosion; (ii) exploring the long-term durability (beyond 400 cycles) and field application performance in real cold environments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dabarera, A.; Fernández, R.; Provis, J.L. A systematic review of engineering properties of magnesium potassium phosphate cement as a repair material. Front. Mater. 2024, 11, 1451079. [Google Scholar] [CrossRef]
- Shi, J.; Li, W.; Zheng, K.; Yang, K.; Zhou, G. Experimental investigation into stressing state characteristics of large-curvature continuous steel box-girder bridge model. Constr. Build. Mater. 2018, 178, 574–583. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Gao, P.; Yang, J.; Zhen, S.; Wang, H.; Du, T. The Effect of Magnesium Chloride on the Macroscopic and MI-Croscopic Properties of Phosphate Cement-Based Materials. Coatings 2022, 12, 370. [Google Scholar] [CrossRef]
- Wu, F.; Su, J.; Wei, J.; Guo, H.; Liu, C. Injectable bioactive calcium–magnesium phosphate cement for bone regeneration. Biomed. Mater. 2008, 3, 44105. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, P.; Wang, D.; Wang, H. The Corrosion Resistance of Reinforced Magnesium Phosphate Cement Reactive Powder Concrete. Materials 2022, 15, 5692. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qian, J.; You, C.; Fan, Y.; Li, Z.; Wang, H. Bond behavior and interfacial micro-characteristics of magnesium phosphate cement onto old concrete substrate. Constr. Build. Mater. 2018, 167, 166–176. [Google Scholar] [CrossRef]
- Arora, A.; Singh, B.; Kaur, P. Novel material i.e., magnesium phosphate cement (MPC) as repairing material in roads and buildings. Mater. Today Proc. 2019, 17, 70–76. [Google Scholar] [CrossRef]
- Yang, N.; Shi, C.; Yang, J.; Chang, Y. Research Progresses in Magnesium Phosphate Cement–Based Materials. J. Mater. Civ. Eng. 2014, 26, 04014071. [Google Scholar] [CrossRef]
- Qiu, H.; Yu, J.; Chen, H.; Kuang, D.; He, R. Investigation on a sustainable magnesium phosphate cement (MPC) with recycled waste MPC powders. J. Environ. Chem. Eng. 2024, 12, 114160. [Google Scholar] [CrossRef]
- Pang, B.; Liu, R. Study on solidification mechanism of magnesium phosphate cement on heavy metals Cu2+. J. Environ. Chem. Eng. 2023, 11, 110891. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef]
- Zoqi, M.J.; Doosti, M.R. Solidification/Stabilization of Lead Contaminated Soil Using Magnesia Phosphate cement and Ordinary Portland cement. J. Environ. Health Eng. 2021, 8, 257–270. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Alfocea-Roig, A.; Huete-Hernández, S.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Magnesium phosphate cement incorporating sheep wool fibre for thermal insulation applications. J. Build. Eng. 2023, 76, 107043. [Google Scholar] [CrossRef]
- Rostami, N. Development of Novel Magnesium Phosphate Bone Cement. Master’s Thesis, University of Toledo, Toledo, OH, USA, 2014. [Google Scholar]
- Hamada, H.M.; Abed, F.; Katman, H.Y.B.; Humada, A.M.; Al Jawahery, M.S.; Majdi, A.; Yousif, S.T.; Thomas, B.S. Effect of silica fume on the properties of sustainable cement concrete. J. Mater. Res. Technol. 2023, 24, 8887–8908. [Google Scholar] [CrossRef]
- Hussein, Y.M.; Elrahman, M.A.; Elsakhawy, Y.; Tayeh, B.A.; Tahwia, A.M. Development and performance of sustainable structural lightweight concrete containing waste clay bricks. J. Mater. Res. Technol. 2022, 21, 4344–4359. [Google Scholar] [CrossRef]
- Ma, H.; Huang, K.; Su, M.; Fu, H.; Li, S.; Feng, J. Study on Micro-mesoscale Characterization and Impact Load Mechanical Properties of Ultra-High-Performance Concrete After Sustained Elevated Temperature. J. Mater. Eng. Perform. 2024, 34, 14618–14636. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Rodier, L.; Savastano, H.; Lefrán, M.; Rojas, M.F. A Comparative Study on the Pozzolanic Activity Between Bamboo Leaves Ash and Silica Fume: Kinetic Parameters. Waste Biomass Valorization 2019, 11, 1627–1634. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B. Effect of silica fume and basalt fiber on the mechanical properties and microstructure of magnesium phosphate cement (MPC) mortar. Constr. Build. Mater. 2018, 190, 466–478. [Google Scholar] [CrossRef]
- Khoso, S.; Shahzaib, K.; Aziz, A.; Hussain, K. Experimental investigation on the properties of cement concrete partially replaced by silica fume and fly ash. Istraz. Proj. Privredu 2016, 14, 345–350. [Google Scholar] [CrossRef]
- Kumar, R.R. Evaluation of mechanical and carbonation properties of self-compacting treated recycled coarse aggregates with two-stage mixing approaches. Clean. Waste Syst. 2022, 3, 100060. [Google Scholar] [CrossRef]
- Xu, D.; Tang, J.; Hu, X.; Zhou, Y.; Yu, C.; Han, F.; Liu, J. Influence of silica fume and thermal curing on long-term hydration, microstructure and compressive strength of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2023, 395, 132370. [Google Scholar] [CrossRef]
- Ma, S.; Li, J.; Wang, Y.; Wei, B. Study on the mechanism of the difference in flotation performance between fine-grained crystalline SiO2 and amorphous SiO2. Physicochem. Probl. Miner. Process. 2023, 59, 174567. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, X.; Gao, J.; Guo, Z. Chemical Structure of Si–O in Silica Fume from Ferrosilicon Production and Its Reactivity in Alkali Dissolution. ISIJ Int. 2019, 59, 1098–1104. [Google Scholar] [CrossRef]
- Scott, S.; Galeczka, I.M.; Gunnarsson, I.; Arnórsson, S.; Stefánsson, A. Silica polymerization and nanocolloid nucleation and growth kinetics in aqueous solutions. Geochim. Et Cosmochim. Acta 2024, 371, 78–94. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Li, Y.; Jia, Y.; Cheeseman, C.R. Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement. Processes 2020, 8, 163. [Google Scholar] [CrossRef]
- Dong, D.; Huang, Y.; Pei, Y.; Zhang, X.; Cui, N.; Zhao, P.; Hou, P.; Lu, L. Effect of spherical silica fume and fly ash on the rheological property, fluidity, setting time, compressive strength, water resistance and drying shrinkage of magnesium ammonium phosphate cement. J. Build. Eng. 2023, 63, 105484. [Google Scholar] [CrossRef]
- Gao, M.; Chen, B.; Lang, L.; Muhammad, R.A. Influence of Silica Fume on Mechanical Properties and Water Resistance of Magnesium–Ammonium Phosphate Cement. J. Mater. Civ. Eng. 2020, 32, 04019368. [Google Scholar] [CrossRef]
- Yao, P.; Lin, X.; Wu, Y.; Ji, T.; Liang, Y. Influence of slag on water resistance of magnesium silicon potassium phosphate cement. J. Build. Eng. 2024, 97, 110862. [Google Scholar] [CrossRef]
- Pehlivan, A. Mechanical properties of magnesium phosphate cement incorporating basalt fibers. Cem. Wapno Beton 2021, 26, 233–241. [Google Scholar] [CrossRef]
- Pang, B.; Yang, Y.; Cui, Y. Corrosion resistance behavior of MgO-SiO2-KH2PO4 under sulfate environments. Ceram. Int. 2025, 51, 8156–8167. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Shi, J.; Zhang, A. Influencing mechanism of silica fume on early-age properties of magnesium phosphate cement-based coating for hydraulic structure. J. Build. Eng. 2022, 54, 104623. [Google Scholar] [CrossRef]
- JGJ 63-2006; Ministry of Construction of the People’s Republic of China. Standard of Water for Concrete. China Architecture & Building Press: Beijing, China, 2006.
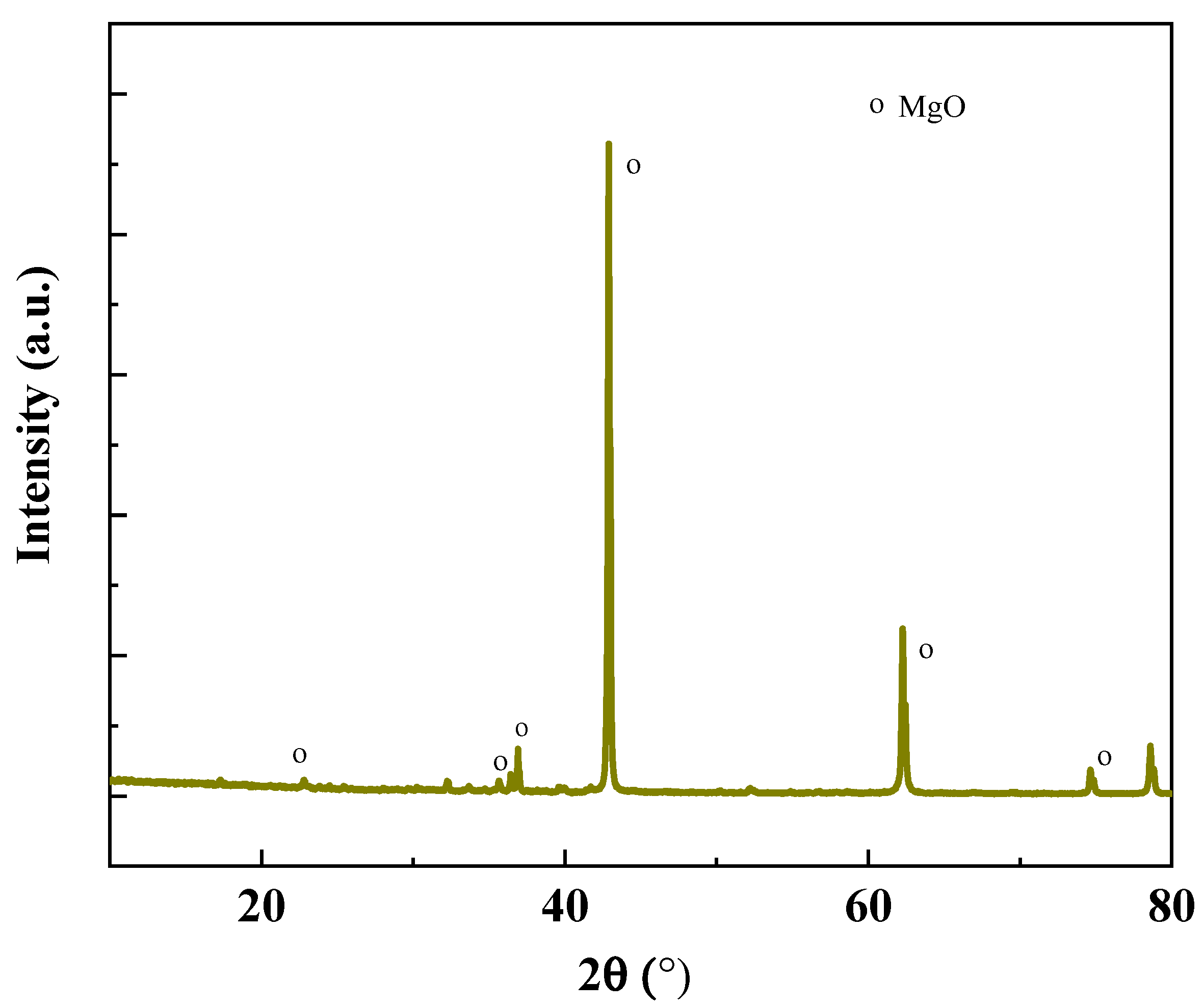
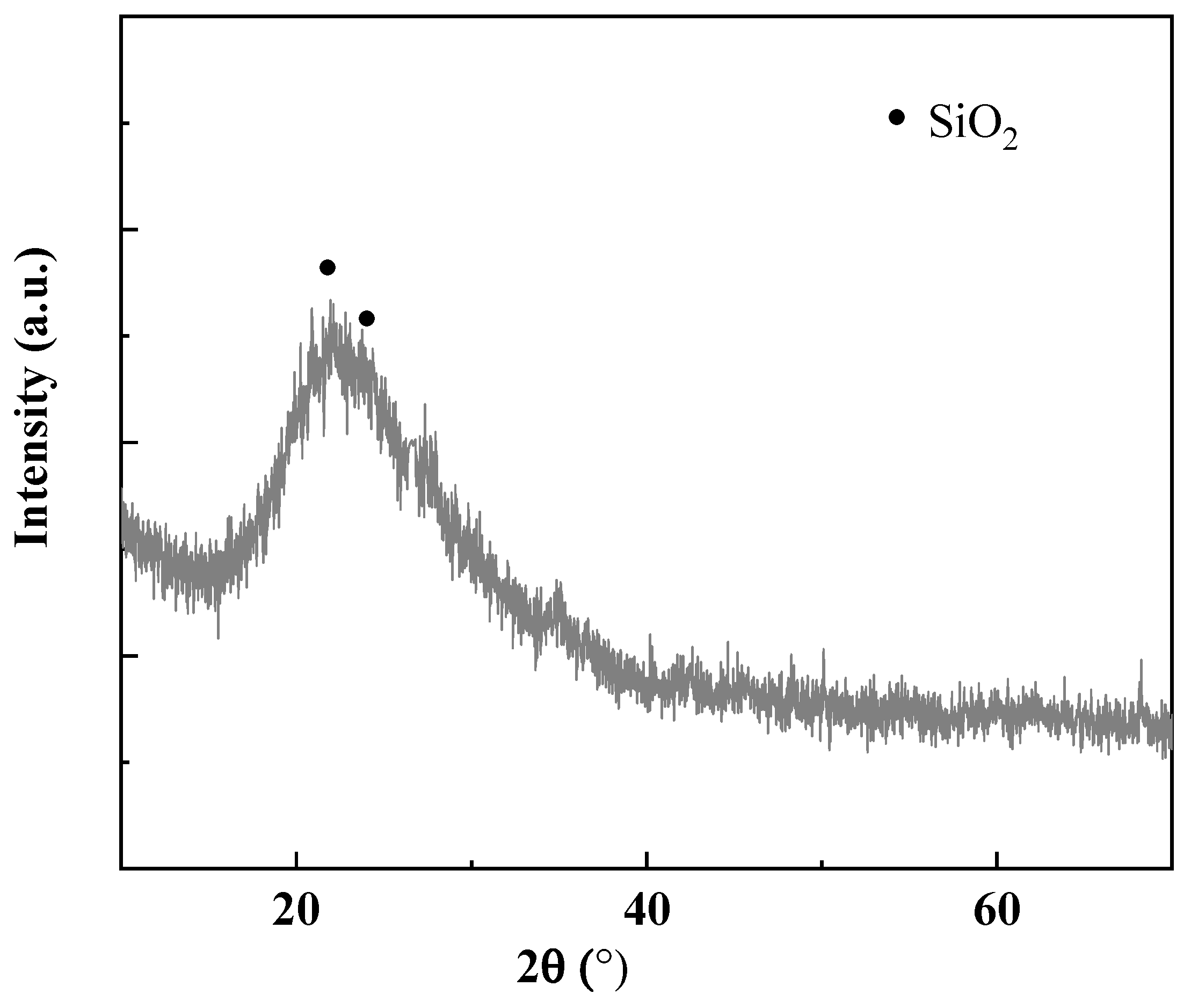
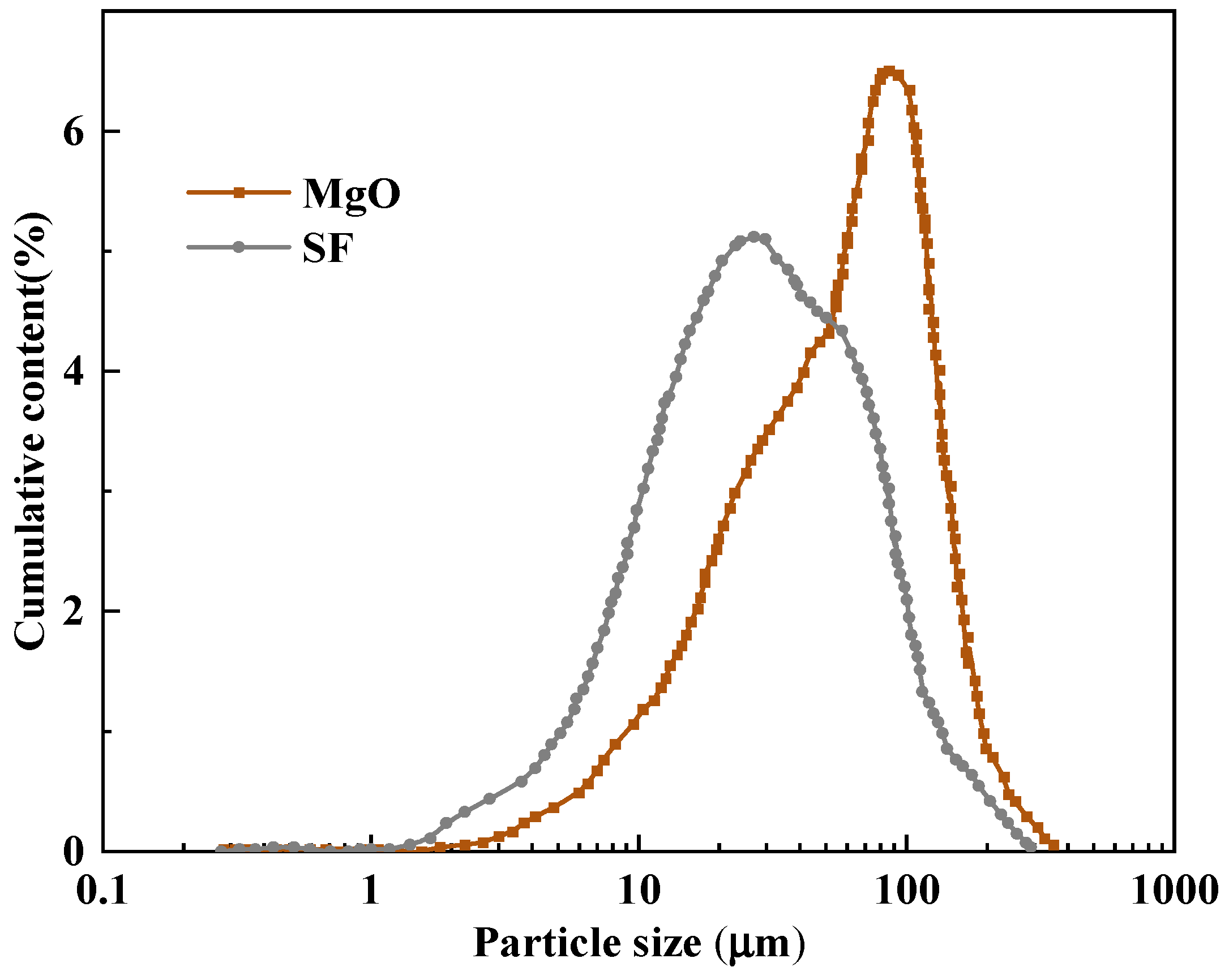



| Oxide | MgO | CaO | SiO2 | Fe2O3 | Na2O | Al2O3 | K2O |
| Content (%) | 93.1 | 2.1 | 2.2 | 0.4 | 0.03 | 0.19 | 0.25 |
| Oxide | SiO2 | CaO | Fe2O3 | K2O | Na2O | SO3 | C | Loss |
| Content (%) | 95.6 | 1.3 | 0.7 | 0.45 | 0.2 | 0.55 | 0.6 | 0.6 |
| Sample | Water/Cement Ratio | Borax (% Cement) | Mg/P Ratio | SF (% by Mass of Cement) |
|---|---|---|---|---|
| CBPC1 | 0.18 | 5 | 4 | 5 |
| CBPC2 | 10 | |||
| CBPC3 | 15 | |||
| CBPC4 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, B.; Liu, R.; Yang, Y.; Cui, Y. Performance Evolution and Degradation Mechanism of Chemically Bonded Phosphate Ceramic Cement Under Freeze–Thaw Cycles. Materials 2025, 18, 5298. https://doi.org/10.3390/ma18235298
Pang B, Liu R, Yang Y, Cui Y. Performance Evolution and Degradation Mechanism of Chemically Bonded Phosphate Ceramic Cement Under Freeze–Thaw Cycles. Materials. 2025; 18(23):5298. https://doi.org/10.3390/ma18235298
Chicago/Turabian StylePang, Bo, Runqing Liu, Yuanquan Yang, and Yunpeng Cui. 2025. "Performance Evolution and Degradation Mechanism of Chemically Bonded Phosphate Ceramic Cement Under Freeze–Thaw Cycles" Materials 18, no. 23: 5298. https://doi.org/10.3390/ma18235298
APA StylePang, B., Liu, R., Yang, Y., & Cui, Y. (2025). Performance Evolution and Degradation Mechanism of Chemically Bonded Phosphate Ceramic Cement Under Freeze–Thaw Cycles. Materials, 18(23), 5298. https://doi.org/10.3390/ma18235298





