Derivatization of Lignin via Ternary Eutectic Solvent Systems for Enhanced Functionalities Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TESS
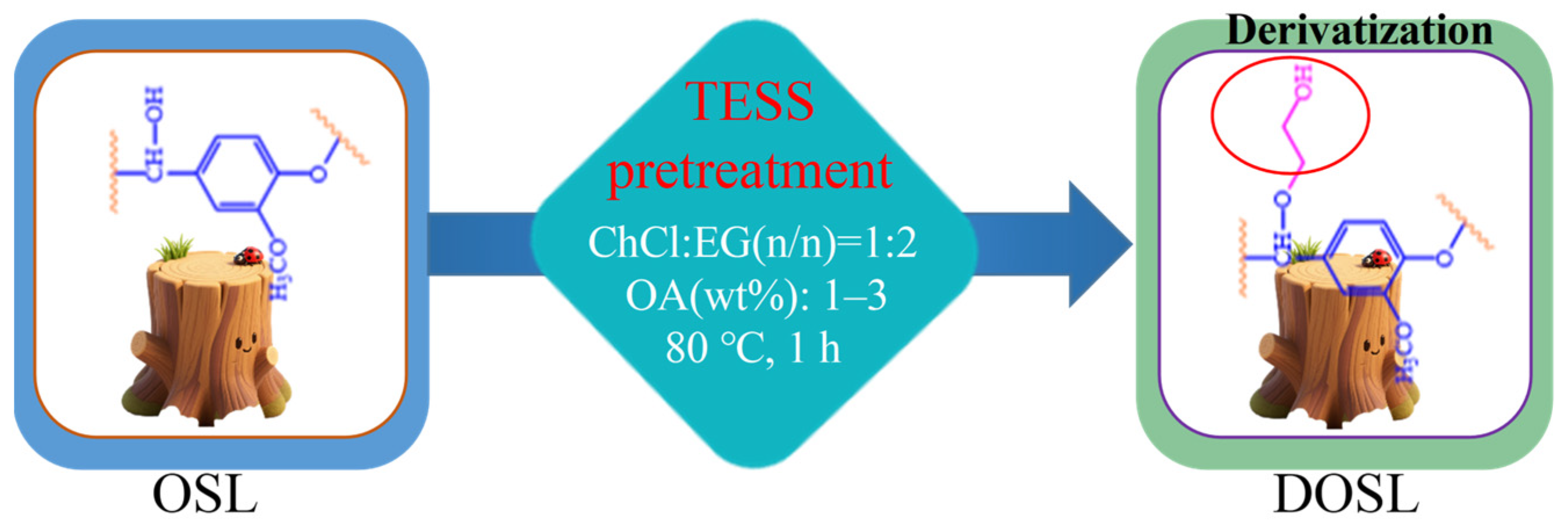
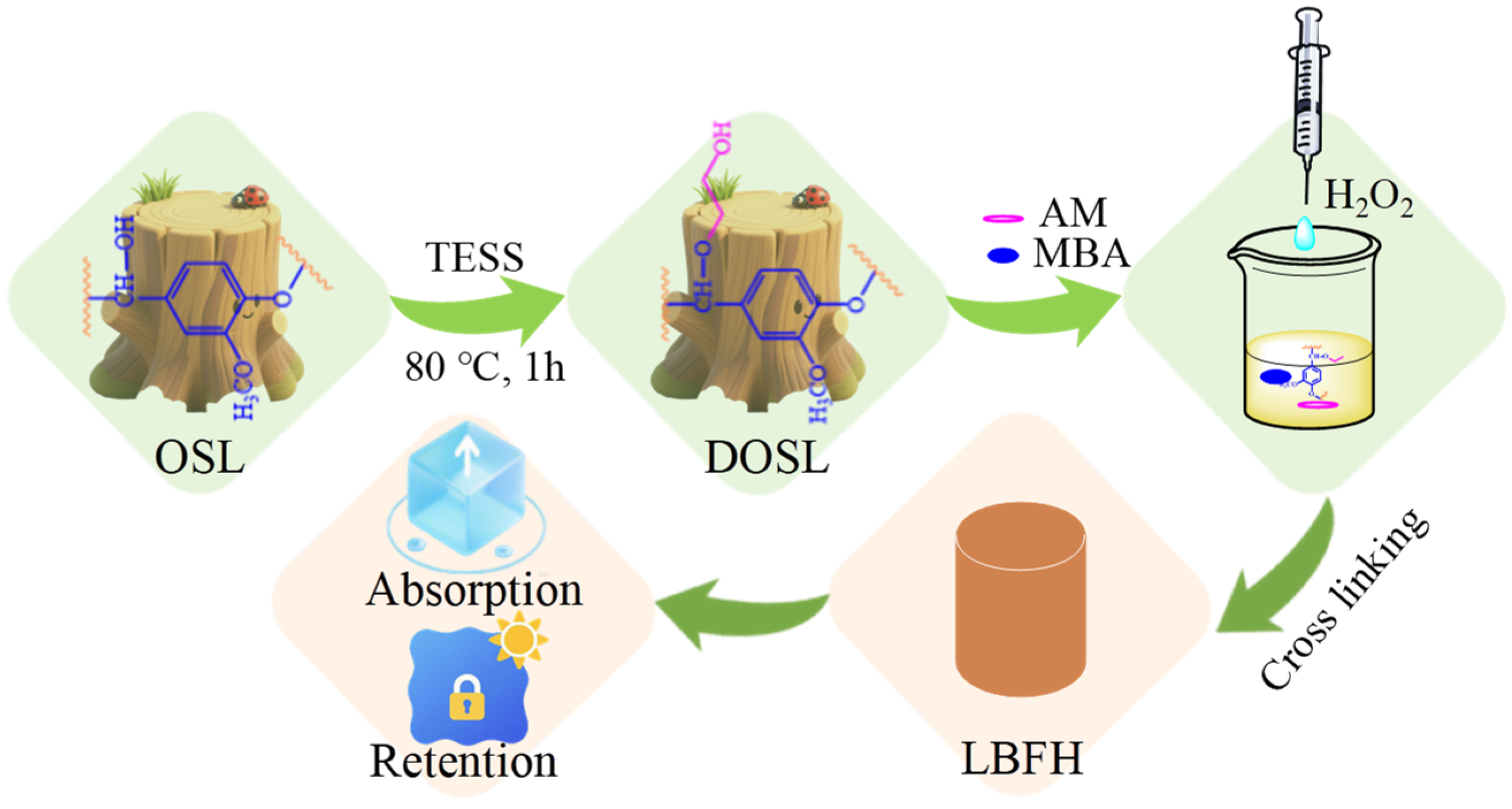
2.3. Synthesis of Derivated OSL (DOSL)
2.4. Synthesis of LBFH
2.5. Characterization of Samples
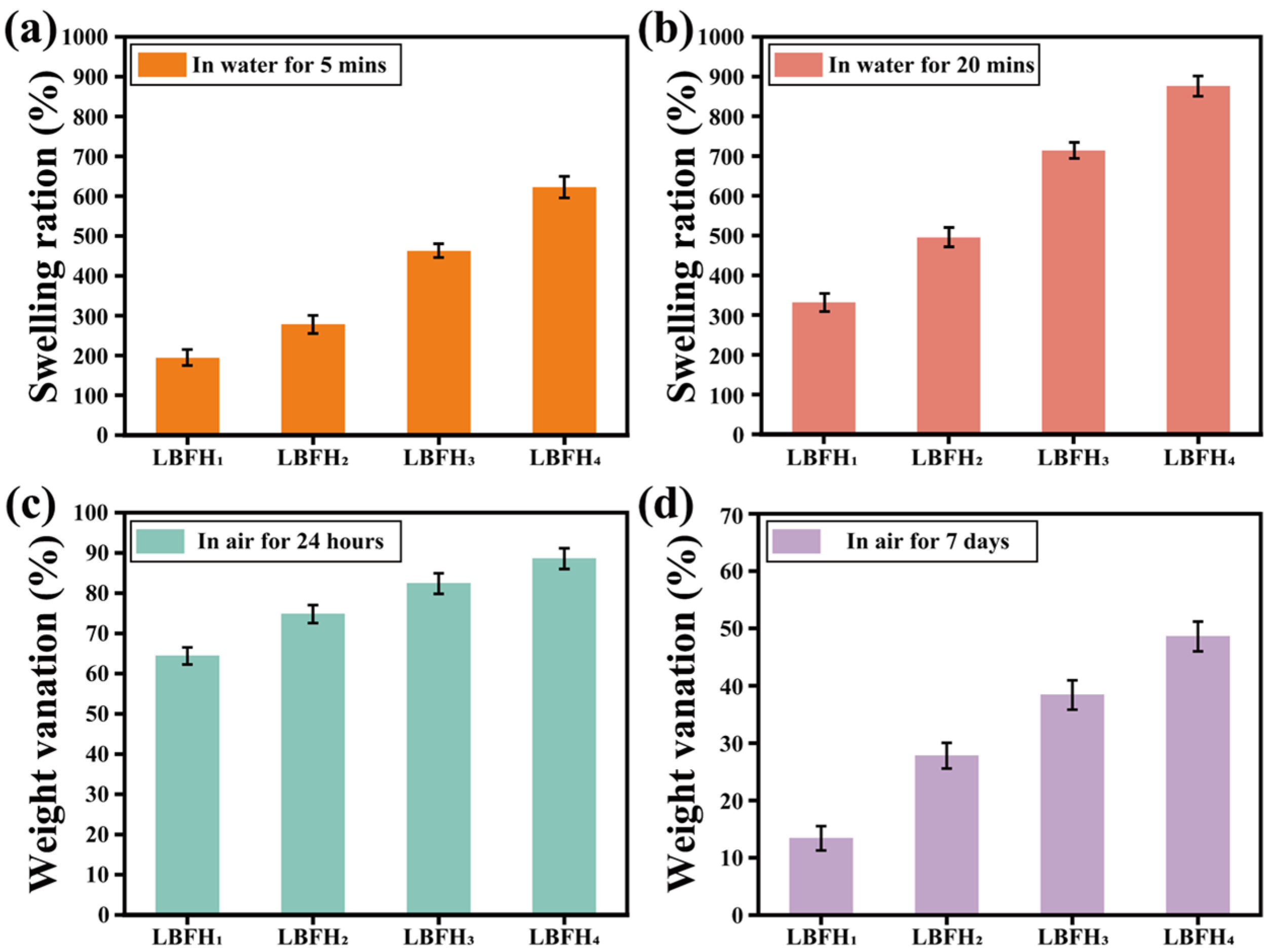
2.6. Water Absorption Swelling and Anti-Dehydratioan Performance
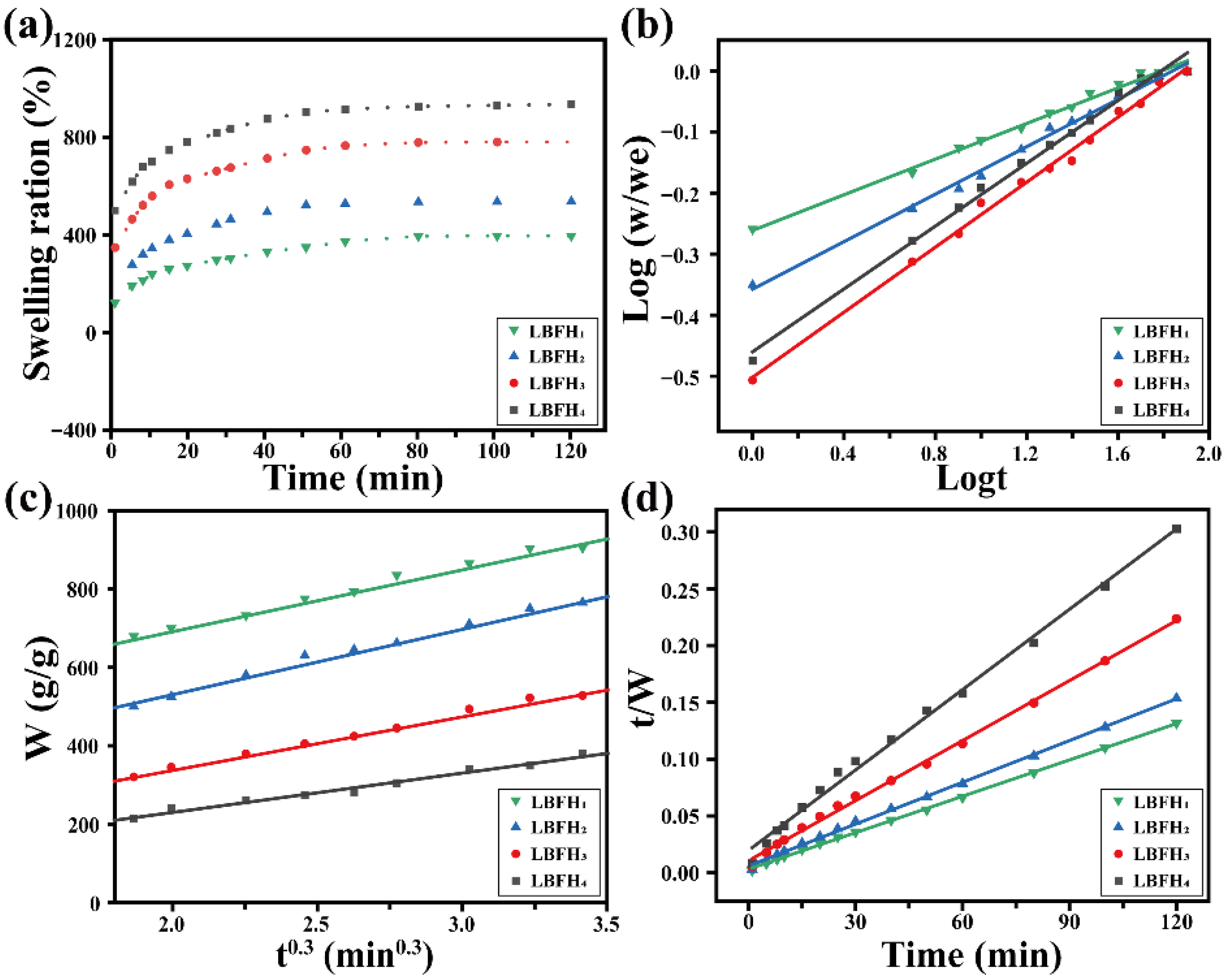
2.7. Swelling Kinetics of Hydrogel
3. Results
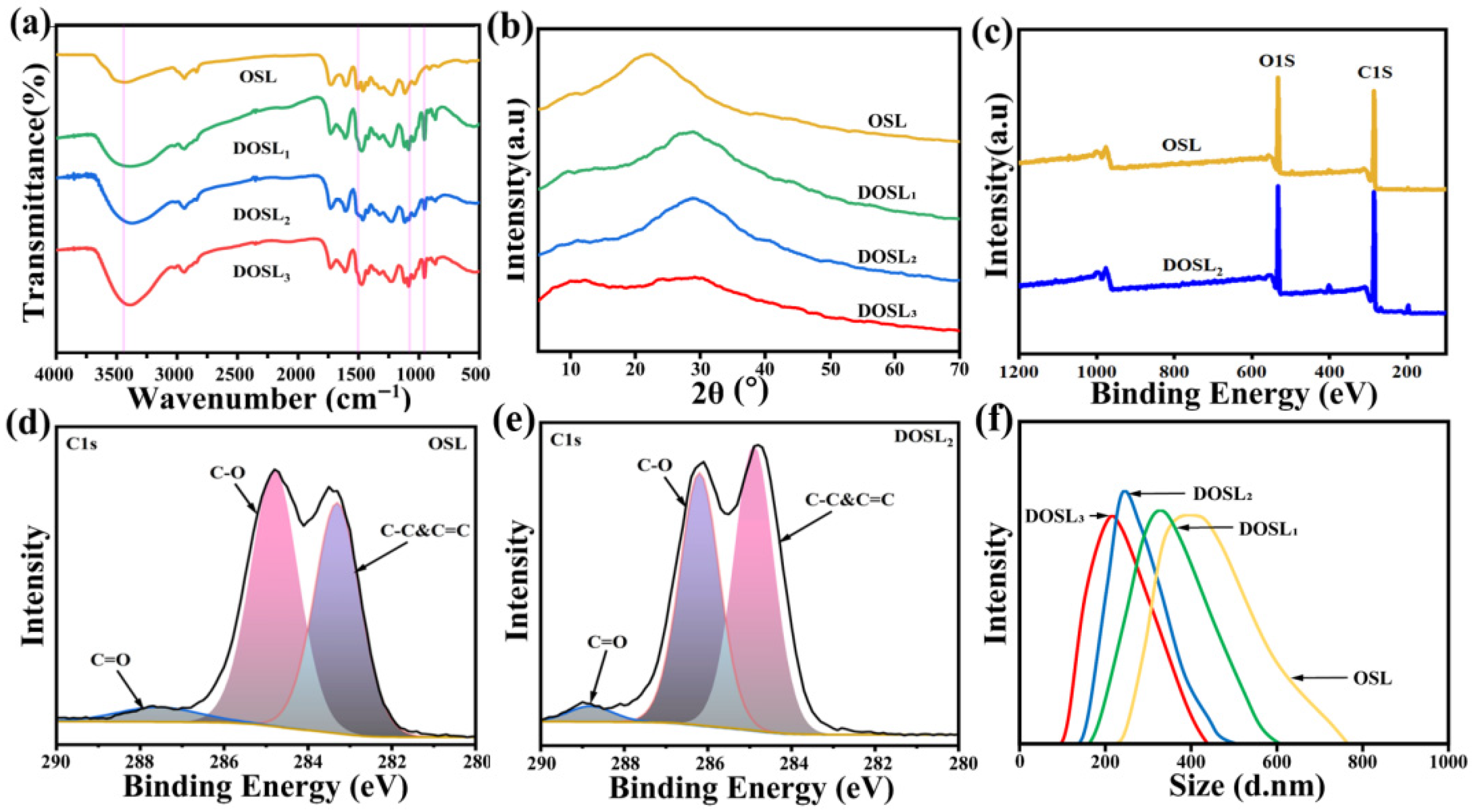
3.1. Characterization of OSL and DOSL
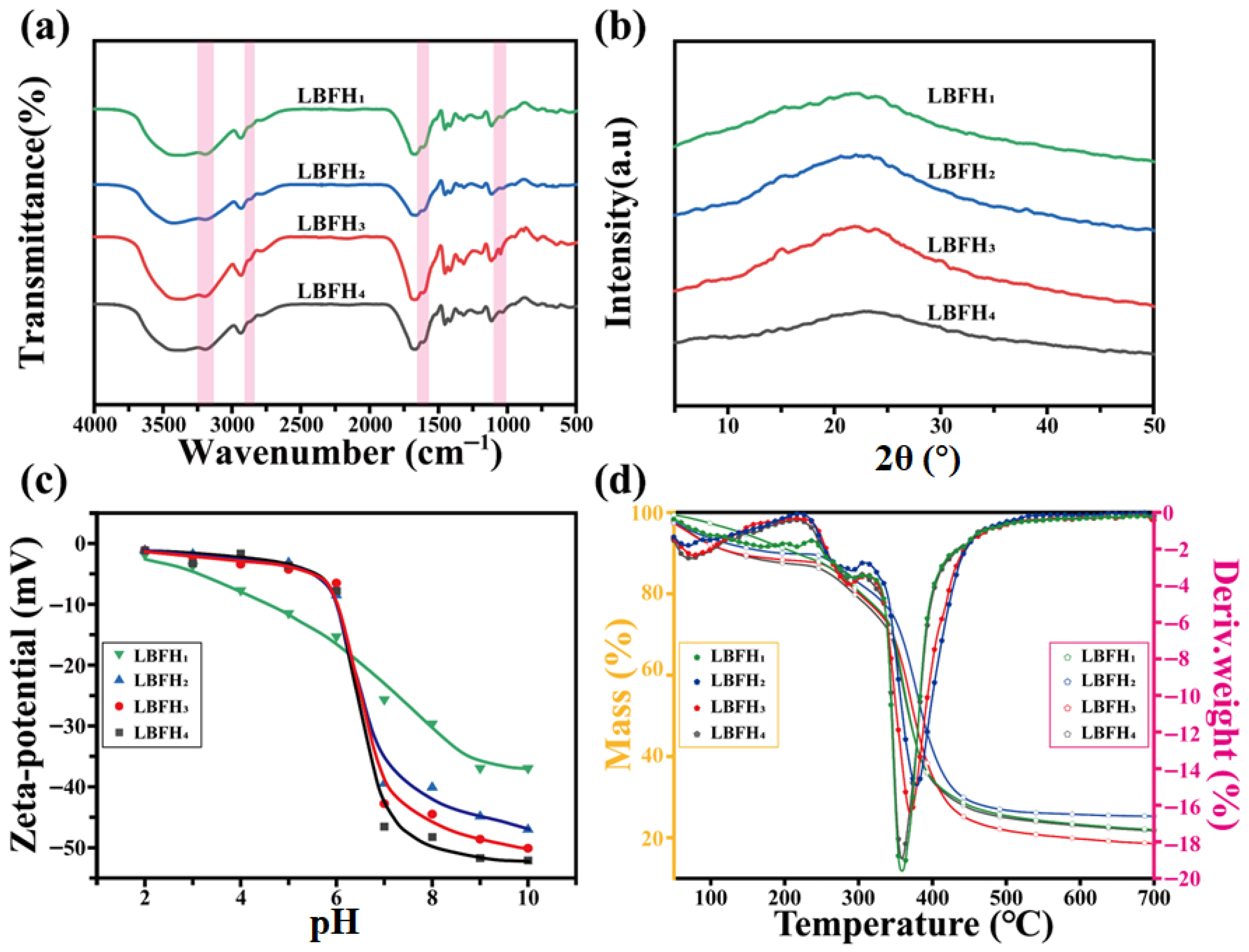
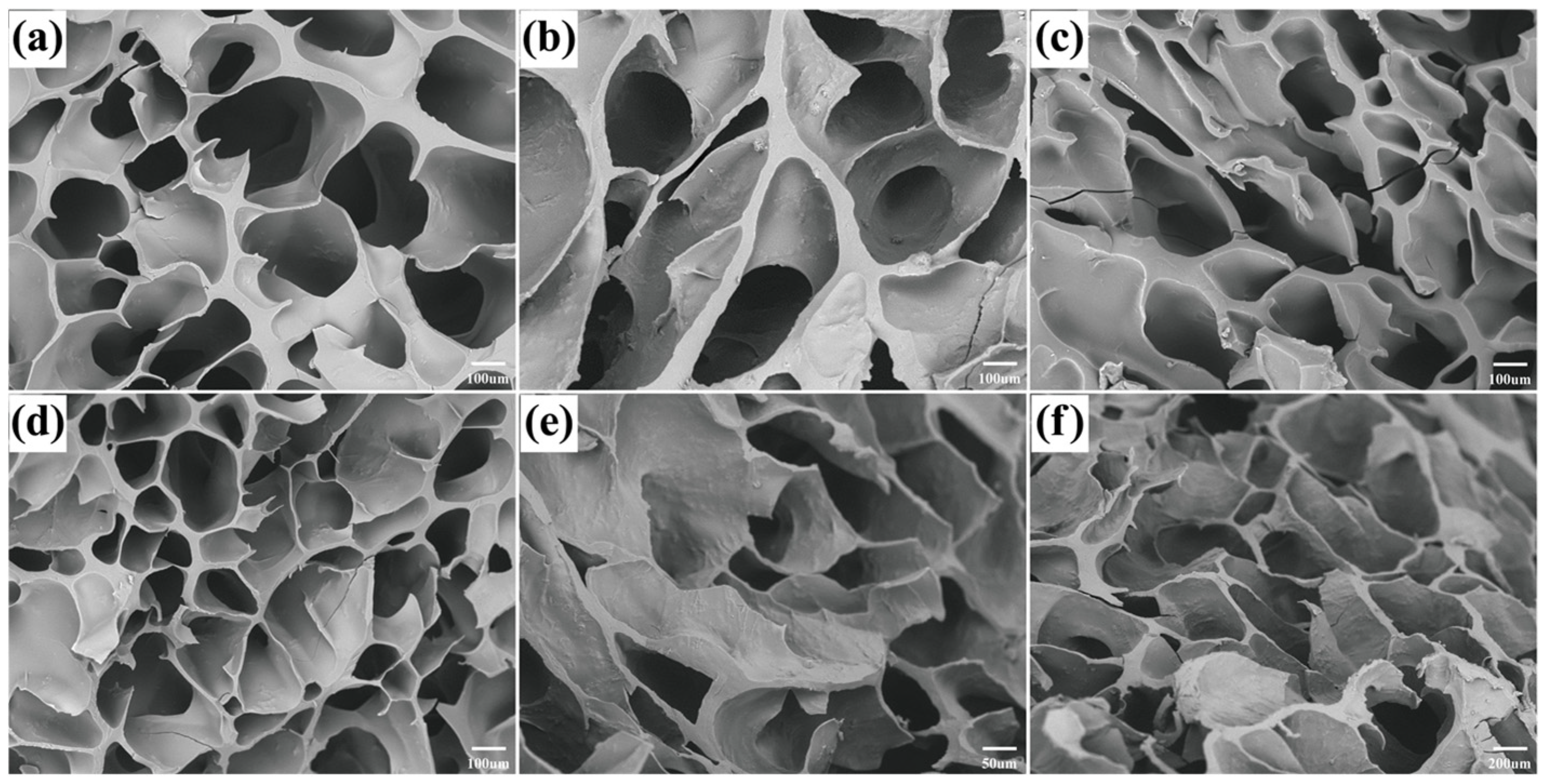
3.2. Characterization of LBFH
3.3. Characterization of Water Absorption Swelling and Anti-Dehydration Performance
3.4. Water Absorption and Swelling Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Li, P.; Chen, W.; Ren, J.; Wu, W. Preparation and Adsorption Properties of Lignin/Cellulose Hydrogel. Materials 2023, 16, 4260. [Google Scholar] [CrossRef]
- Huang, S.; Wu, L.; Li, T.; Xu, D.; Lin, X.; Wu, C. Facile preparation of biomass lignin-based hydroxyethyl cellulose super-absorbent hydrogel for dye pollutant removal. Int. J. Biol. Macromol. 2019, 137, 939–947. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Peresin, M.S.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A.-S. Lignin-based hydrogels with “super-swelling” capacities for dye removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Pan, X.; Ma, X.; Cao, S.; Ni, Y. Adhesive, Transparent Tannic Acid@ Sulfonated Lignin-PAM Ionic Conductive Hydrogel Electrode with Anti-UV, Antibacterial and Mild Antioxidant Function. Materials 2019, 12, 4135. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.; Hui, L.; Shang, Z.; Yuan, S.; Dai, L.; Liu, P.; Liu, X.; Ni, Y. Lignin-containing cellulose nanocrystals/sodium alginate beads as highly effective adsorbents for cationic organic dyes. Int. J. Biol. Macromol. 2019, 139, 640–646. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Mei, A.; Peng, L.; Sun, J. Novel chitosan/lignin hydrogel prepared by the Mannich reaction for Pb(II) and Cu(II) removal from aqueous solution. Int. J. Biol. Macromol. 2025, 285, 138177. [Google Scholar] [CrossRef]
- Jõul, P.; Ho, T.T.; Kallavus, U.; Konist, A.; Leiman, K.; Salm, O.-S.; Kulp, M.; Koel, M.; Lukk, T. Characterization of Organosolv Lignins and Their Application in the Preparation of Aerogels. Materials 2022, 15, 2861. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.I.; Nazir, S.; Restu, W.K.; Rajamanickam, R.; Selvasembian, R.; Hua, L.S.; Antov, P.; Yadav, K.K.; Abbas, M.; Farobie, O.; et al. Recent advances in lignin from forest residue for hydrogel application. Biomass-Convers. Biorefinery 2024, 15, 11475–11491. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, R.; Chahal, G.K. Synthesis of lignin-based hydrogels and their applications in agriculture: A review. Chem. Pap. 2021, 75, 4465–4478. [Google Scholar] [CrossRef]
- Ding, W.; Sun, H.; Li, X.; Li, Y.; Jia, H.; Luo, Y.; She, D.; Geng, Z. Environmental applications of lignin-based hydrogels for Cu remediation in water and soil: Adsorption mechanisms and passivation effects. Environ. Res. 2024, 250, 118442. [Google Scholar] [CrossRef]
- Yang, L.; Bao, L.; Zhong, Y.; Hao, C.; Chen, J.; Wu, J.; Wang, X. Fabrication of in situ metal-organic framework grown on sodium lignosulphonate hydrogel for removal of Pb2+, methylene blue and crystal violet from aqueous solution. J. Clean. Prod. 2024, 434, 139831. [Google Scholar] [CrossRef]
- Pandit, S.; Sharma, P.; Prakash, A.; Lal, B.; Bhuyan, R.; Ahmad, I.; Kuila, A. A comprehensive review on technical lignin, lignin hydrogels, properties, preparation, applications & challenges in lab to market transition. Ind. Crops Prod. 2024, 211, 118262. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Y.; Wang, J.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Liu, P.; Wang, J. Highly efficient uranium extraction by aminated lignin-based thermo-responsive hydrogels. J. Mol. Liq. 2022, 368, 120744. [Google Scholar] [CrossRef]
- Yan, S.; Chai, L.; Li, W.; Xiao, L.-P.; Chen, X.; Sun, R.-C. Tunning the properties of pH-responsive lignin-based hydrogels by regulating hydroxyl content. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128815. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Impact of the lignin type and source on the characteristics of physical lignin hydrogels. Sustain. Mater. Technol. 2022, 31, e00369. [Google Scholar] [CrossRef]
- Jiang, M.; Niu, N.; Chen, L. A template synthesized strategy on bentonite-doped lignin hydrogel spheres for organic dyes removal. Sep. Purif. Technol. 2022, 285, 120376. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Influence of lignin modifications on physically crosslinked lignin hydrogels for drug delivery applications. Sustain. Mater. Technol. 2022, 33, e00474. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Xiang, L.; Qi, J.; Jiang, Y.; Xie, J. Maleic acid modified lignin for preparation of ultra-flexible and UV shielding gelatin/lignin films. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133805. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, Y.; Liu, C.; Yu, G.; Li, B.; Mu, X.; Peng, H. Preparation of Concrete Water Reducer via Fractionation and Modification of Lignin Extracted from Pine Wood by Formic Acid. ACS Sustain. Chem. Eng. 2017, 5, 4214–4222. [Google Scholar] [CrossRef]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125133. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Tang, R.; Xue, D.; Sun, Y.; Li, X. Efficient dissolution of lignin in novel ternary deep eutectic solvents and its application in polyurethane. Int. J. Biol. Macromol. 2020, 164, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Barta, K.; Deuss, P.J. The Effect of Acidic Ternary Deep Eutectic Solvent Treatment on Native Lignin. ACS Sustain. Chem. Eng. 2022, 10, 12569–12579. [Google Scholar] [CrossRef]
- Liu, Y.; Deak, N.; Wang, Z.; Yu, H.; Hameleers, L.; Jurak, E.; Deuss, P.J.; Barta, K. Tunable and functional deep eutectic solvents for lignocellulose valorization. Nat. Commun. 2021, 12, 5424. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.-X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Bergrath, J.; Rumpf, J.; Burger, R.; Do, X.T.; Wirtz, M.; Schulze, M. Beyond Yield Optimization: The Impact of Organosolv Process Parameters on Lignin Structure. Macromol. Mater. Eng. 2023, 308, 2300093. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, Y.; Huang, C.; Yong, C.; Fu, Y. Deep eutectic solvent engineering: a novel ternary system for efficient lignocellulose extraction. Green Chem. 2025, 27, 1556–1569. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, R.; Chen, X.; Ni, S.; Xu, C.; Fu, Y.; Qin, M.; Zhang, Y. High-efficient fractionation of poplar chips by ternary deep eutectic solvents system for elevating enzymatic hydrolysis. Ind. Crops Prod. 2025, 225, 120489. [Google Scholar] [CrossRef]
- Suarez, I.J.; Sierra-Martin, B.; Fernandez-Barbero, A. Swelling of ionic and non-ionic minigels. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 343, 30–33. [Google Scholar] [CrossRef]
- Sun, X.-F.; Hao, Y.; Cao, Y.; Zeng, Q. Superadsorbent hydrogel based on lignin and montmorillonite for Cu(II) ions removal from aqueous solution. Int. J. Biol. Macromol. 2019, 127, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Arapova, O.V.; Bondarenko, G.N.; Chistyakov, A.V.; Tsodikov, M.V. Vibrational spectroscopy studies of structural changes in lignin under microwave irradiation. Russ. J. Phys. Chem. A 2017, 91, 1717–1729. [Google Scholar] [CrossRef]
- Lehto, J.; Louhelainen, J.; Kłosińska, T.; Drożdżek, M.; Alén, R. Characterization of alkali-extracted wood by FTIR-ATR spectroscopy. Biomass- Convers. Biorefinery 2018, 8, 847–855. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.-T.; Ko, F.K. X-Ray Diffraction Analysis of Kraft Lignins and Lignin-Derived Carbon Nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Bock, P.; Gierlinger, N. Infrared and Raman spectra of lignin substructures: Coniferyl alcohol, abietin, and coniferyl aldehyde. J. Raman Spectrosc. 2019, 50, 778–792. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Hou, P.; Gao, Z.; Liu, Y.; Zhao, J.; Huo, P. Preparation of dual cross-linked hydrogel electrolytes containing modified lignin for supercapacitors and sensors. Chem. Eng. J. 2024, 480, 148259. [Google Scholar] [CrossRef]
- Xu, C.; Liu, L.; Renneckar, S.; Jiang, F. Chemically and physically crosslinked lignin hydrogels with antifouling and antimicrobial properties. Ind. Crops Prod. 2021, 170, 113759. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, J.; Huang, W.; Yuan, Z.; Wei, B.; Wen, Y. Preparation of crosslinked lignin-polyacrylamide hydrogel with high resistance to temperature and salinity. Int. J. Biol. Macromol. 2025, 296, 139730. [Google Scholar] [CrossRef]
- Xue, B.; Wen, J.; Sun, R. Ethanol organosolv lignin as a reactive filler for acrylamide-based hydrogels. J. Appl. Polym. Sci. 2015, 132, 42638. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Effect of the formulation parameters on the absorption capacity of smart lignin-hydrogels. Eur. Polym. J. 2020, 129, 109631. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Huang, W.; Xie, J.; Song, Z.; Guo, S.; Wang, E. Preparation of Peanut Shell Cellulose Double-Network Hydrogel and Its Adsorption Capacity for Methylene Blue. J. Renew. Mater. 2023, 11, 3001–3023. [Google Scholar] [CrossRef]
- Tally, M.; Atassi, Y. Optimized synthesis and swelling properties of a pH-sensitive semi-IPN superabsorbent polymer based on sodium alginate-g-poly(acrylic acid-co-acrylamide) and polyvinylpyrrolidone and obtained via microwave irradiation. J. Polym. Res. 2015, 22, 181. [Google Scholar] [CrossRef]
| Samples ID | ChCl:EG (n/n) | OA (wt%) | Temperature (°C) | Rotation Speed (r/min) |
|---|---|---|---|---|
| TESS1 | 1:2 | 1 | 80 | 300 |
| TESS2 | 1:2 | 2 | 80 | 300 |
| TESS3 | 1:2 | 3 | 80 | 300 |
| Samples ID | OSL (g) | TESS | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| DOSL1 | 1.0 | TESS1 | 80 | 1 | 48.42 |
| DOSL2 | 1.0 | TESS2 | 80 | 1 | 53.57 |
| DOSL3 | 1.0 | TESS3 | 80 | 1 | 52.01 |
| Samples ID | OSL (g) | TESS2 (mL) | AM (g) | MBA (mg) | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| LBFH1 | 10 | 5 | 0.2 | 4.0 | 60 | 6 | 82.42 |
| LBFH2 | 20 | 5 | 0.2 | 4.0 | 60 | 6 | 83.57 |
| LBFH3 | 30 | 5 | 0.2 | 4.0 | 60 | 6 | 86.01 |
| LBFH4 | 40 | 5 | 0.2 | 4.0 | 60 | 6 | 85.97 |
| Samples ID | C (%) | H (%) | O (%) | Mw (g/mol) | Mn (g/mol) | PDI (Mw/Mn) |
|---|---|---|---|---|---|---|
| OSL | 78.06 | 6.12 | 15.82 | 5818 | 2806 | 2.07 |
| DOSL1 | 74.24 | 7.02 | 18.74 | 3740 | 2108 | 1.77 |
| DOSL2 | 70.06 | 6.61 | 23.33 | 2692 | 1660 | 1.62 |
| DOSL3 | 71.26 | 6.62 | 22.12 | 2162 | 1466 | 1.47 |
| Samples ID | Fickian Model | Schott Model | ||||
|---|---|---|---|---|---|---|
| n | k | R2 | We,cal (g/g) | We,exp (g/g) | R2 | |
| LBFH1 | 0.2822 | 0.4628 | 0.9942 | 934.24 | 914.56 | 0.9943 |
| LBFH2 | 0.2654 | 0.3648 | 0.9838 | 781.25 | 764.24 | 0.9976 |
| LBFH3 | 0.2852 | 0.2642 | 0.9876 | 536.82 | 524.64 | 0.9984 |
| LBFH4 | 0.2904 | 0.2840 | 0.9928 | 395.97 | 388.42 | 0.9992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Qin, T.; Yin, X.; Zhang, Z. Derivatization of Lignin via Ternary Eutectic Solvent Systems for Enhanced Functionalities Hydrogel. Materials 2025, 18, 5283. https://doi.org/10.3390/ma18235283
Li F, Qin T, Yin X, Zhang Z. Derivatization of Lignin via Ternary Eutectic Solvent Systems for Enhanced Functionalities Hydrogel. Materials. 2025; 18(23):5283. https://doi.org/10.3390/ma18235283
Chicago/Turabian StyleLi, Fengfeng, Tianci Qin, Xiuxin Yin, and Zhili Zhang. 2025. "Derivatization of Lignin via Ternary Eutectic Solvent Systems for Enhanced Functionalities Hydrogel" Materials 18, no. 23: 5283. https://doi.org/10.3390/ma18235283
APA StyleLi, F., Qin, T., Yin, X., & Zhang, Z. (2025). Derivatization of Lignin via Ternary Eutectic Solvent Systems for Enhanced Functionalities Hydrogel. Materials, 18(23), 5283. https://doi.org/10.3390/ma18235283







