Effect of Temperature on Corrosion of HSLA Steels with Different Cr Contents in a Water-Saturated Supercritical CO2 Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results
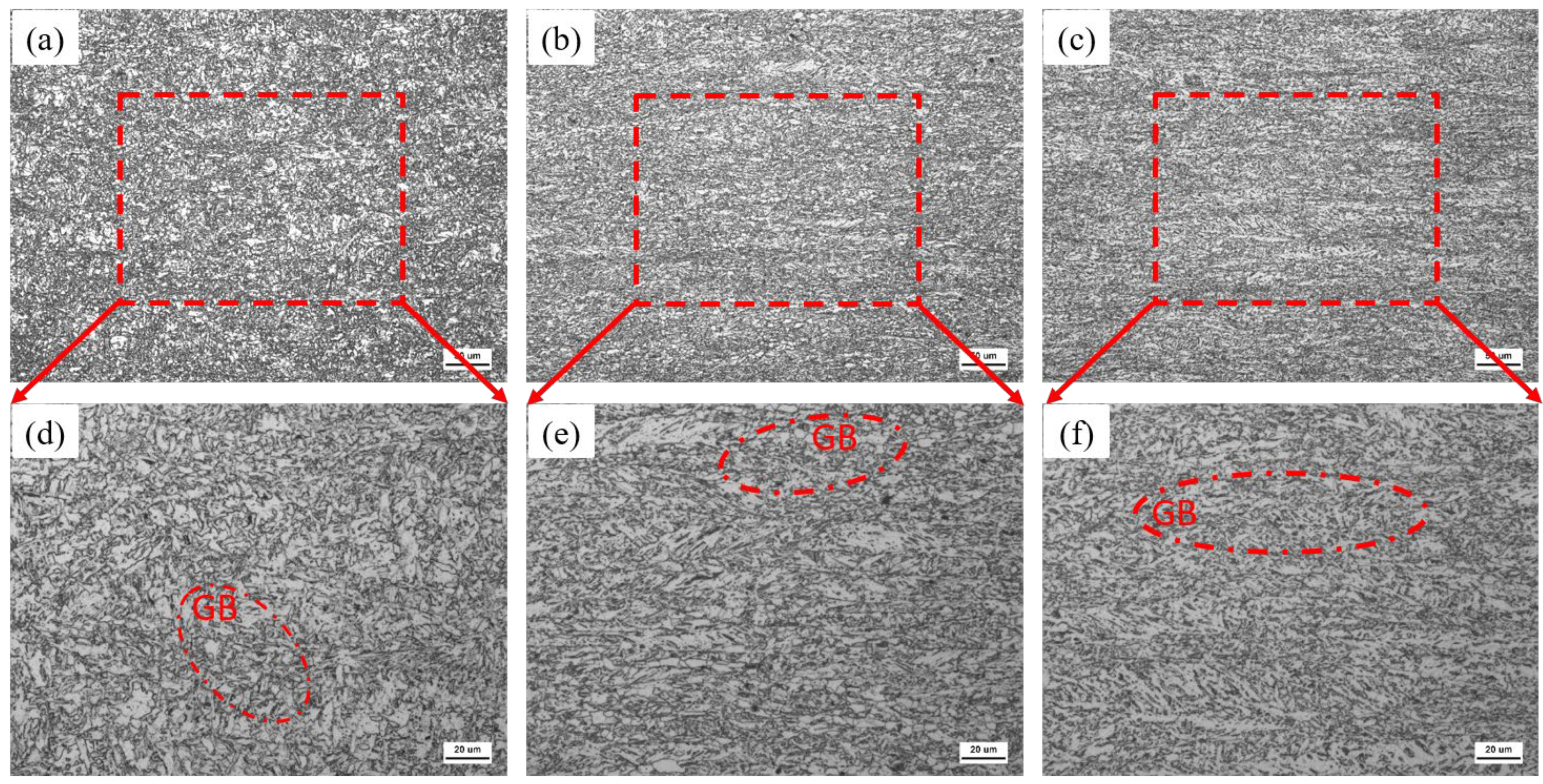
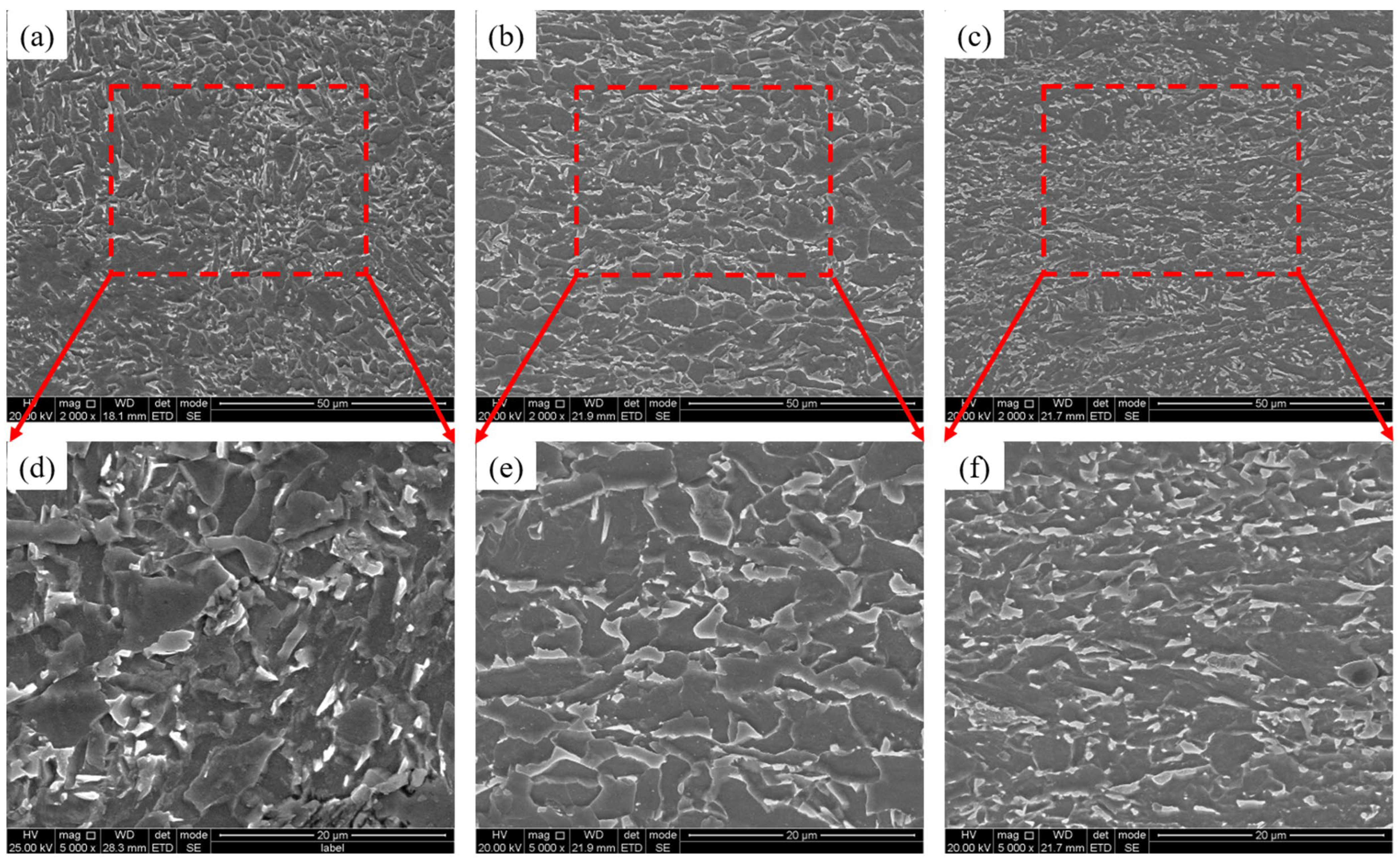
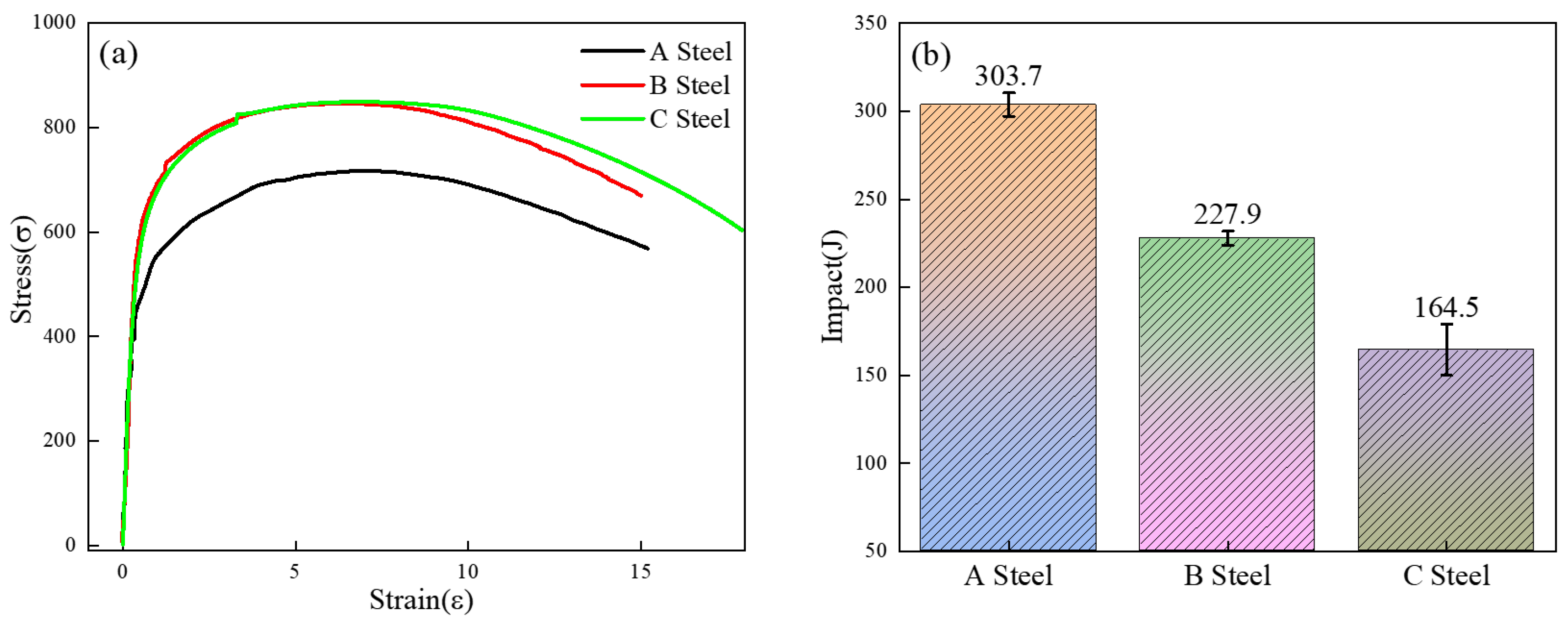
3.1. Microstructure and Mechanical Properties
3.2. Weight Loss and Corrosion Rate
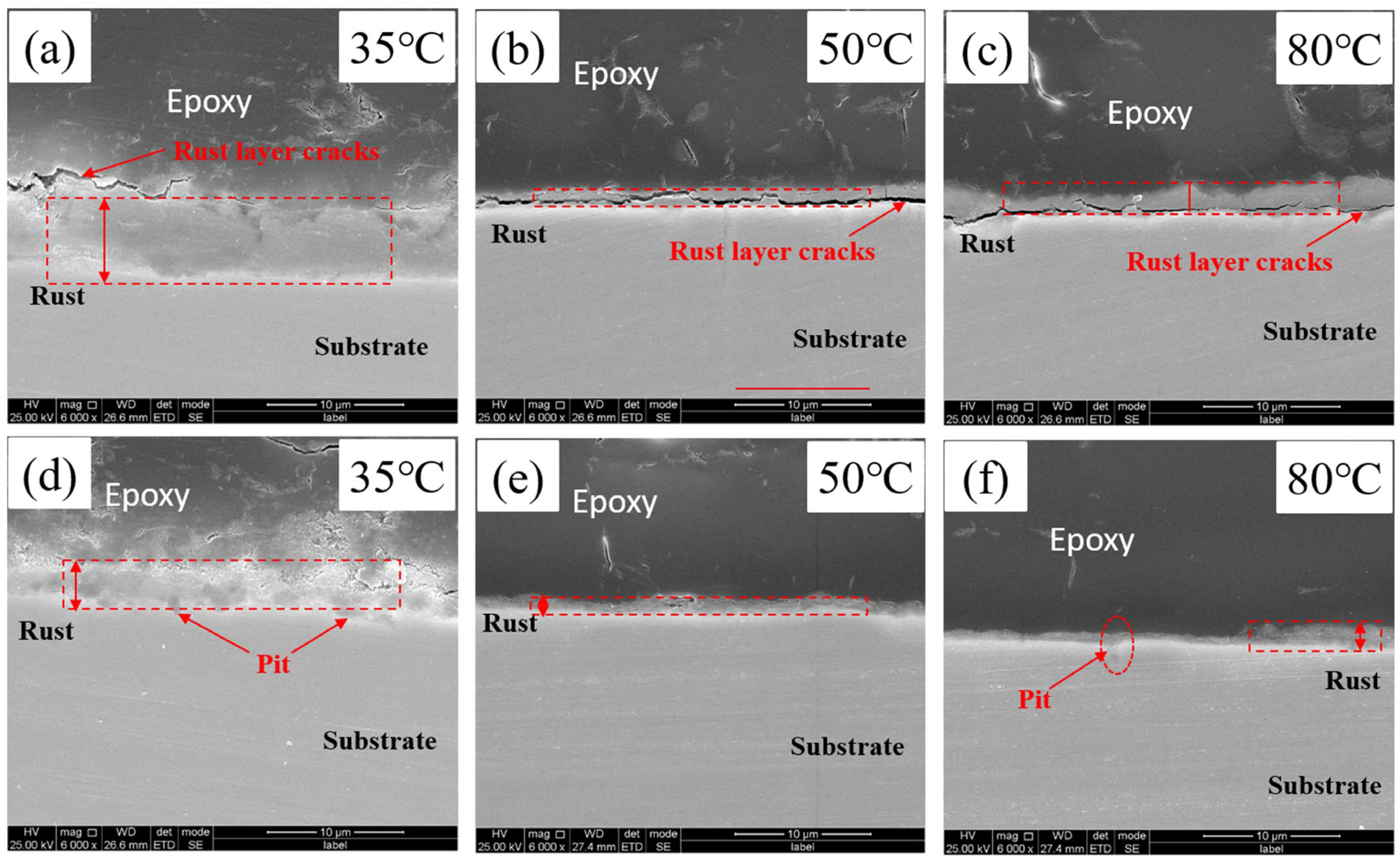
3.3. Rust Layer Morphology and Composition
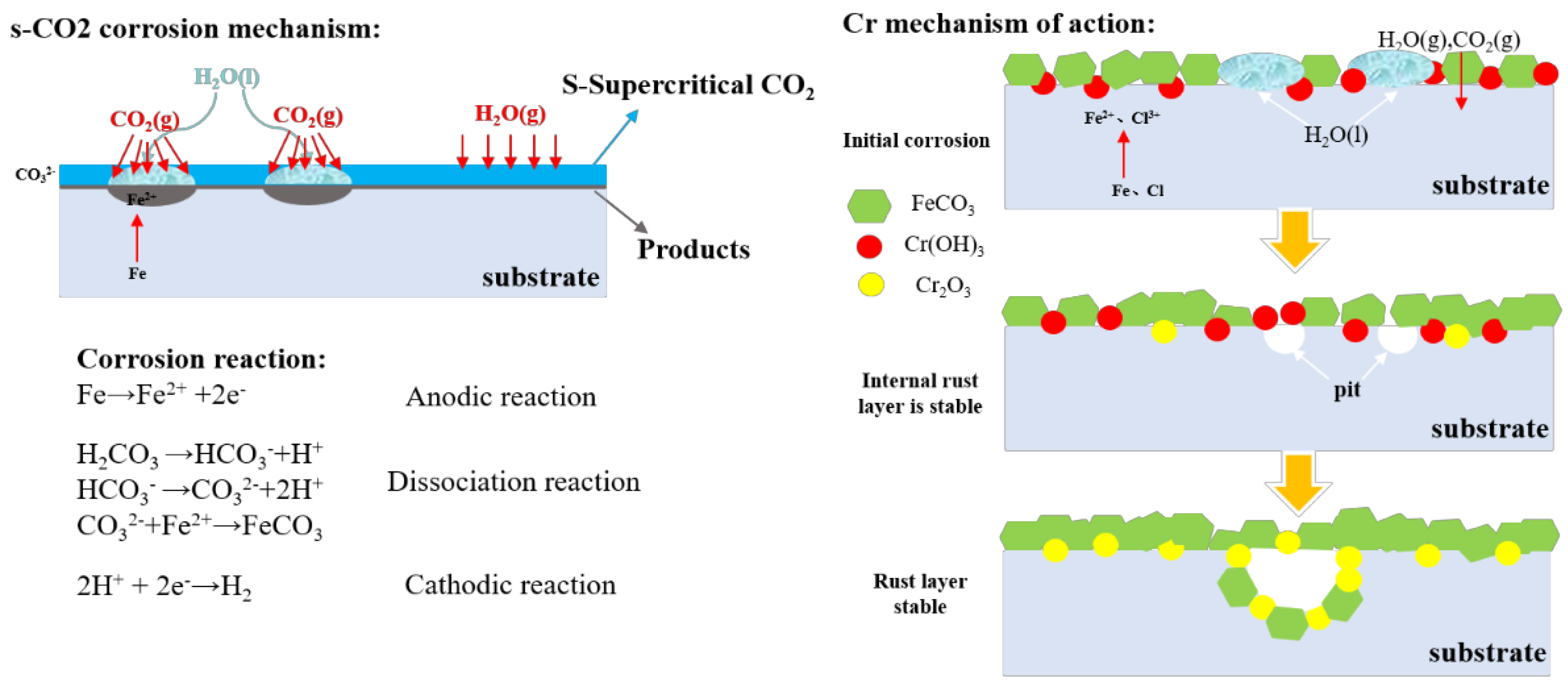
4. Discussion
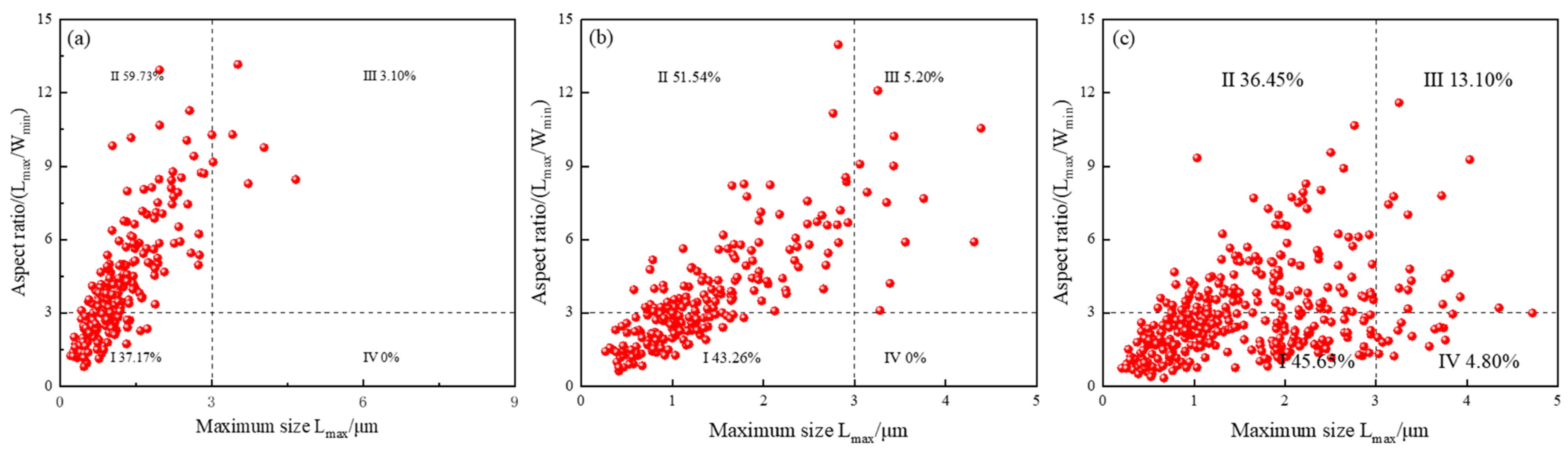
Fine Analysis of MA Microstructure
- Reduced H2O activity in S-CO2 phase at elevated temperatures;
- Diminished electrochemical reaction driving forces at higher thermal energy states.
| Temperature (°C) | Pressure (bar) | CO2 Density (kg/m3) | CO2 in Water (ppm) | Water in CO2 (ppm) |
|---|---|---|---|---|
| 35 | 80 | 489.818 | 2.231 × 104 | 3.381 × 103 |
| 50 | 80 | 219.217 | 1.757 × 104 | 3.197 × 103 |
| 80 | 80 | 186.314 | 1.329 × 104 | 1.196 × 104 |
- CO2 density dependence: 1: Density decreases exponentially from 489.82 kg/m3 at 35 °C (308.15 K) to 186.31 kg/m3 at 80 °C (353.15 K) under constant pressure (8 MPa); 2: Corresponds to a 62% reduction in CO2 phase density across the tested thermal range;
- Solubility behavior: 1: H2O solubility in CO2-rich phase: 3381 ppm at 35 °C vs. 3197 ppm at 50 °C (Δ = 5.4%); 2: CO2 solubility in aqueous phase decreases monotonically with temperature;
- Mass transport implications: 1: Despite similar molar solubilities at 35 °C and 50 °C, the volumetric H2O concentration in the CO2 phase exhibits strong temperature dependence; 2: Demonstrates a 38.4% reduction in dissolved H2O mass per unit volume despite only a 5.4% decrease in molar fraction.
- Electrochemical dissolution at CO2/Fe interfaces;
- Carbonic acid (H2CO3) formation via CO2 hydration;
- Dominance conditions: Prevails at higher S-CO2 densities (>400 kg/m3), where dissolved H2O mass concentration peaks.
- Local pH reduction to 3.2–4.1 (calculated using HSC Chemistry);
- Microenvironmental galvanic cell formation;
- Dominance conditions: Favored near critical point (30–35 °C/8 MPa), where minor T/P fluctuations trigger phase separation.
- ■
- Thermal energy provision lowers activation barriers for:
- ◆
- Charge transfer reactions: ;
- ◆
- Ionic transport through double layers;
- ■
- Increased frequency of energy-sufficient molecular collisions (Boltzmann distribution):
- ■
- Predicts +2.3× corrosion rate increase per 25 °C rise (35→60 °C) based on typical Ea ≈ 45 kJ/mol [38].
- ■
- Temperature-enhanced FeCO3 precipitation thermodynamics;
- ◆
- Nucleation rate (Classical nucleation theory);
- ◆
- Growth dominated by Fickian transport of Fe2+/CO32−;
- ■
- Above 60 °C, achieves critical nuclei density () for continuous film formation [39].
5. Conclusions
- (1)
- With the increase in Cr content, the basic type of the three test steels is granular bainite, but the microstructure is refined (25% reduction in average packet size). The MA group element content in particular increases (8.2% → 14.7%), but the size is refined. The strength of the test steel increases and the toughness decreases.
- (2)
- In the water-saturated supercritical CO2 environment, with the increase in Cr content, the corrosion rate shows a decreasing trend (0.38Cr:0.0379 mm/y→1.17Cr:0.0291 mm/y). The Cr element is involved in the supercritical CO2 reaction. Carbonic acid combined with hydration, and ultimately the formation of stable Cr2O3, improves the densification and stability of the rust layer.
- (3)
- In the water-saturated supercritical CO2 environment, with the increase in ambient temperature, the corrosion rate shows a decreasing trend; at 35 °C, the corrosion rate reaches its highest value. At 35 °C, the environmental state close to the critical point of CO2, the water phase is easy to precipitate from the supercritical CO2, and the supercritical CO2 density is at the maximum, 489.818 kg/m3. At this time, the CO2 has maximum solubility in the H2O phase, and corrosion also occurs in the form of H2O. The corrosion is dominated by the electrochemical corrosion of the aqueous phase. With the further increase in temperature, corrosion is dominated by chemical corrosion, and with the increase in temperature, promoting the formation and stabilization of corrosion film is conducive to further reducing the corrosion rate.
- (4)
- In the water-saturated supercritical CO2 environment, corrosion is mainly divided into two types, the first being water dissolved into the supercritical CO2 to form carbonic acid, which directly corrodes the surface of the experimental steel; this corrosion is generally uniform corrosion. This corrosion is mainly dependent on the amount of water dissolved; the corrosion is weak. The other is direct contact between the water phase from the supercritical CO2 precipitation and the steel surface, generally resulting in localized corrosion; this is the corrosion principle for electrochemical corrosion, and the steel is more affected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Kalam, M.A.; Fayaz, H.; Qazi, A. Carbon capture, utilization and storage opportunities to mitigate greenhouse gases. Heliyon 2024, 10, e25419. [Google Scholar] [CrossRef]
- Paltsev, S.; Morris, J.; Kheshgi, H.; Herzog, H. Hard-to-Abate Sectors: The role of industrial carbon capture and storage (CCS) in emission mitigation. Appl. Energy 2021, 300, 117322. [Google Scholar] [CrossRef]
- Shen, M.; Kong, F.; Tong, L.; Luo, Y.; Yin, S.; Liu, C.; Ding, Y. Carbon capture and storage (CCS): Development path based on carbon neutrality and economic policy. Carbon Neutrality 2022, 1, 37. [Google Scholar] [CrossRef]
- Kim, T.W.; Yoon, H.C.; Lee, J.Y. Review on carbon capture and storage (CCS) from source to sink; part 1: Essential aspects for CO2 pipeline transportation. Int. J. Greenh. Gas Control 2024, 137, 104208. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Chen, Z.F.; Li, Y.X. Integrity assessment of supercritical CO2 transport pipelines. Geoenergy Sci. Eng. 2023, 221, 211355. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.; Chen, P. China’s CO2 pipeline development strategy under carbon neutrality. Nat. Gas Ind. B 2023, 10, 502–510. [Google Scholar] [CrossRef]
- Liu, J.; Yao, D.; Chen, K.; Wang, C.; Sun, C.; Pan, H.; Meng, F.; Chen, B.; Wang, L. Effect of H2O content on the corrosion behavior of X52 steel in supercritical CO2 Streams Containing O2, H2S, SO2 and NO2 impurities. Energies 2023, 16, 6119. [Google Scholar] [CrossRef]
- Sun, C.; Yan, X.; Sun, J.; Pang, J.; Zhao, W.; Lin, X. Unraveling the effect of O2, NO2 and SO2 impurities on the stress corrosion behavior of X65 steel in water-saturated supercritical CO2 streams. Corros. Sci. 2022, 209, 110729. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, C.; Cui, G.; Xing, X.; Mu, J.; Li, Z. Analysis of internal corrosion of supercritical CO2 pipeline. Corrosion. Rev. 2021, 39, 219–241. [Google Scholar] [CrossRef]
- Li, Q.K.; Kutana, A.; Penev, E.S.; Yakobson, B.I. Iron corrosion in the “inert” supercritical CO2, ab initio dynamics insights: How impurities matter. Matter 2022, 5, 751–762. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Verma, C. A review on corrosion inhibitors for high-pressure supercritical CO2 environment: Challenges and opportunities. J. Pet. Sci. Eng. 2022, 215, 110695. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Xiao, B.; Luo, J.L.; Zhang, N. State of the art overview material degradation in high-temperature supercritical CO2 environments. Prog. Mater. Sci. 2023, 136, 101107. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Tong, D.; Wang, Y.; Yu, C.; Zhang, J. Aotmic-insights into the corrosion behavior of Fe-Cr alloys in supercritical CO2 environment. J. Supercrit. Fluids 2024, 209, 106271. [Google Scholar] [CrossRef]
- Li, K.; Han, X.; Zeng, Y. Effect of Cr on Corrosion Performance of Steels in Supercritical CO2 Environments. In Proceedings of the AMPP Annual Conference + Expo, San Antonio, TX, USA, 6–10 March 2022. [Google Scholar]
- Kowta, R.; Erickson, D.D.; Barker, R. Models for Calculating Corrosion Rates in Water-Saturated and Under Saturated CO2 Systems & Water Solubility in CO2 Systems at Supercritical Conditions. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2022. [Google Scholar]
- Hua, Y.; Barker, R.; Neville, A. Effect of temperature on the critical water content for general and localised corrosion of X65 carbon steel in the transport of supercritical CO2. Int. J. Greenh. Gas Control 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Sui, P.; Sun, J.; Hua, Y.; Liu, H.; Zhou, M.; Zhang, Y.; Wang, Y. Effect of temperature and pressure on corrosion behavior of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S. Int. J. Greenh. Gas Control 2018, 73, 60–69. [Google Scholar] [CrossRef]
- Baker, T.N. Microalloyed steels. Ironmak. Steelmak. 2016, 43, 264–307. [Google Scholar] [CrossRef]
- Fan, L.; Wang, T.; Fu, Z.; Zhang, S.; Wang, Q. Effect of heat-treatment on-line process temperature on the microstructure and tensile properties of a low carbon Nb-microalloyed steel. Mater. Sci. Eng. A 2014, 607, 559–568. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Gong, B.; Jiang, E.; Huang, Y.; Zhang, G.; Zhao, Y. Corrosion behavior of typical structural steels in 500 °C, 600 °C and high pressure supercritical carbon dioxide conditions. Corros. Sci. 2021, 192, 109801. [Google Scholar] [CrossRef]
- GB/T 229.1-2020; Metallic Materials—Charpy Pendulum Impact Test—Part 1: Test Methods. China Standards Press: Beijing, China, 2020.
- GB/T 228.1-2021; Metallic Materials-Tensile Testing-Part 1: Method of Test at Room Temperature. Standards Press of China: Beijing, China, 2021.
- Ma, Q.; Jia, S.; Liu, Q. Effects of Cr and Ni addition on critical crack tip opening displacement (CTOD) and supercritical CO2 corrosion resistance of high-strength low-alloy steel (HSLAs). Mater. Sci. Eng. A 2024, 908, 146772. [Google Scholar] [CrossRef]
- Li, R.; Ge, Z.; Wang, Z.; Zhou, Z.; Zhou, J.; Li, C. Effect of supercritical carbon dioxide (S-CO2) on the microstructure of bituminous coal with different moisture contents in the process of ScCO2 enhanced coalbed methane and CO2 geological sequestration. Energy Fuels 2022, 36, 3680–3694. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.Y.; Park, S.J.; Lee, T.H.; Jang, J.H.; Lee, C.H.; Hong, H.U. Effect of Mo and Cr additions on the microstructure, mechanical properties and pitting corrosion resistance of austenitic Fe-30Mn-10.5 Al-1.1 C lightweight steels. J. Alloys Compd. 2019, 775, 1136–1146. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Ding, R. Microstructure and mechanical properties of a novel medium Mn steel with Cr and Mo microalloying. Mater. Sci. Eng. A 2021, 825, 141926. [Google Scholar] [CrossRef]
- Caiza Vega, J.M.; Gamboa Rodríguez, F.A. Comparación del Rendimiento de Tres Métodos de Protección Anti Corrosiva Yes Timación de la Velocidad de Corrosión Según la Norma ASTM G1-03 en Placas de Acero ASTM A36; Escuela Superior Politécnica de Chimborazo: Riobamba, Ecuador, 2022. [Google Scholar]
- Sarrade, S.; Féron, D.; Rouillard, F.; Perrin, S.; Robin, R.; Ruiz, J.C.; Turc, H.A. Overview on corrosion in supercritical fluids. J. Supercrit. Fluids 2017, 120, 335–344. [Google Scholar] [CrossRef]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Fatah, A.; Mahmud, H.B.; Bennour, Z. Effect of supercritical CO2 treatment on physical properties and functional groups of shales. Fuel 2021, 303, 121310. [Google Scholar] [CrossRef]
- Jin, Z.; Xiong, C.; Zhao, T.; Du, Y.; Zhang, X.; Li, N.; Yu, Y.; Wang, P. Passivation and depassivation properties of Cr–Mo alloyed corrosion-resistant steel in simulated concrete pore solution. Cem. Concr. Compos. 2022, 126, 104375. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hassani, S.; Vu, T.N.; Nešić, S.; Abas, A.Z.B.; Nor, A.M.; Suhor, M.F. Effect of H2S on the corrosion behavior of pipeline steels in supercritical and liquid CO2 environments. Corrosion 2016, 72, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochim. et Cosmochim. Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, Y.; Pang, X.; Gao, K. Corrosion behaviors of steels under supercritical CO2 conditions. Corros. Rev. 2015, 33, 151–174. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J.-L. Advancing the mechanistic understanding of corrosion in supercritical CO2 with H2O and O2 impurities. Corros. Sci. 2023, 213, 110981. [Google Scholar] [CrossRef]
- Lim, J.Y.; McKrell, T.J.; Eastwick, G.; Ballinger, R.G. Corrosion of materials in supercritical carbon dioxide environments. In Proceedings of the NACE CORROSION, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Sun, H.; Wang, H.; Zeng, Y.; Li, K. Corrosion challenges in supercritical CO2 transportation, storage, and utilization—A review. Renew. Sustain. Energy Rev. 2023, 179, 113292. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Inomata, H.; Izumi, K. Chemical reactions of C1 compounds in near-critical and supercritical water. Chem. Rev. 2004, 104, 5803–5822. [Google Scholar] [CrossRef]
- Liang, Y.; Du, Y.; Tang, D. Research on AC corrosion behavior and corrosion product film evolution of X70 steel under the combined action of AC interference and CP. Corros. Sci. 2022, 197, 110085. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Li, Z.; Zhou, Y.; Li, W. Effect of temperature on corrosion behavior of X70 steel in high pressure CO2/SO2/O2/H2O environments. Corros. Eng. Sci. Technol. 2013, 48, 121–129. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Zhou, Y. Effect of pressure on corrosion behavior of X60, X65, X70, and X80 carbon steels in water-unsaturated supercritical CO2 environments. Int. J. Greenh. Gas Control. 2016, 51, 357–368. [Google Scholar] [CrossRef]
- Firouzdor, V.; Sridharan,, K.; Cao, G.; Anderson, M.; Allen, T.R. Corrosion of a stainless steel and nickel-based alloys in high temperature supercritical carbon dioxide environment. Corros. Sci. 2013, 69, 281–291. [Google Scholar] [CrossRef]
- Kang, Y.; Leng, X.; Zhao, L. Review on the corrosion behaviour of nickel-based alloys in supercritical carbon dioxide under high temperature and pressure. Crystals 2023, 13, 725. [Google Scholar] [CrossRef]







| Steels | C | Si | Mn | Cr | Ni+Mo | Cu | Nb | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.050 | 0.24 | 1.39 | 0.38 | 0.4 | 0.16 | 0.042 | 0.016 | 0.031 |
| B | 0.055 | 0.25 | 1.38 | 0.75 | 0.4 | 0.16 | 0.041 | 0.015 | 0.033 |
| C | 0.055 | 0.25 | 1.38 | 1.17 | 0.4 | 0.16 | 0.041 | 0.015 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Liu, S.; Ren, Y.; Peng, L.; Li, B.; Shang, C.; Jia, S. Effect of Temperature on Corrosion of HSLA Steels with Different Cr Contents in a Water-Saturated Supercritical CO2 Environment. Materials 2025, 18, 5243. https://doi.org/10.3390/ma18225243
Ma Q, Liu S, Ren Y, Peng L, Li B, Shang C, Jia S. Effect of Temperature on Corrosion of HSLA Steels with Different Cr Contents in a Water-Saturated Supercritical CO2 Environment. Materials. 2025; 18(22):5243. https://doi.org/10.3390/ma18225243
Chicago/Turabian StyleMa, Qilin, Shilin Liu, Yi Ren, Leng Peng, Ba Li, Chengjia Shang, and Shujun Jia. 2025. "Effect of Temperature on Corrosion of HSLA Steels with Different Cr Contents in a Water-Saturated Supercritical CO2 Environment" Materials 18, no. 22: 5243. https://doi.org/10.3390/ma18225243
APA StyleMa, Q., Liu, S., Ren, Y., Peng, L., Li, B., Shang, C., & Jia, S. (2025). Effect of Temperature on Corrosion of HSLA Steels with Different Cr Contents in a Water-Saturated Supercritical CO2 Environment. Materials, 18(22), 5243. https://doi.org/10.3390/ma18225243








