Differential Corrosion Behavior of High-Aluminum 304 Stainless Steel in Molten Nitrate Salts: The Roles of Rolling and Heat Treatment
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Procedure
2.2.1. Treatment Processes of Steels
2.2.2. Corrosion Experiment
2.3. Microstructural Characterization
3. Results
3.1. Molten Salt Corrosion Resistance Performance
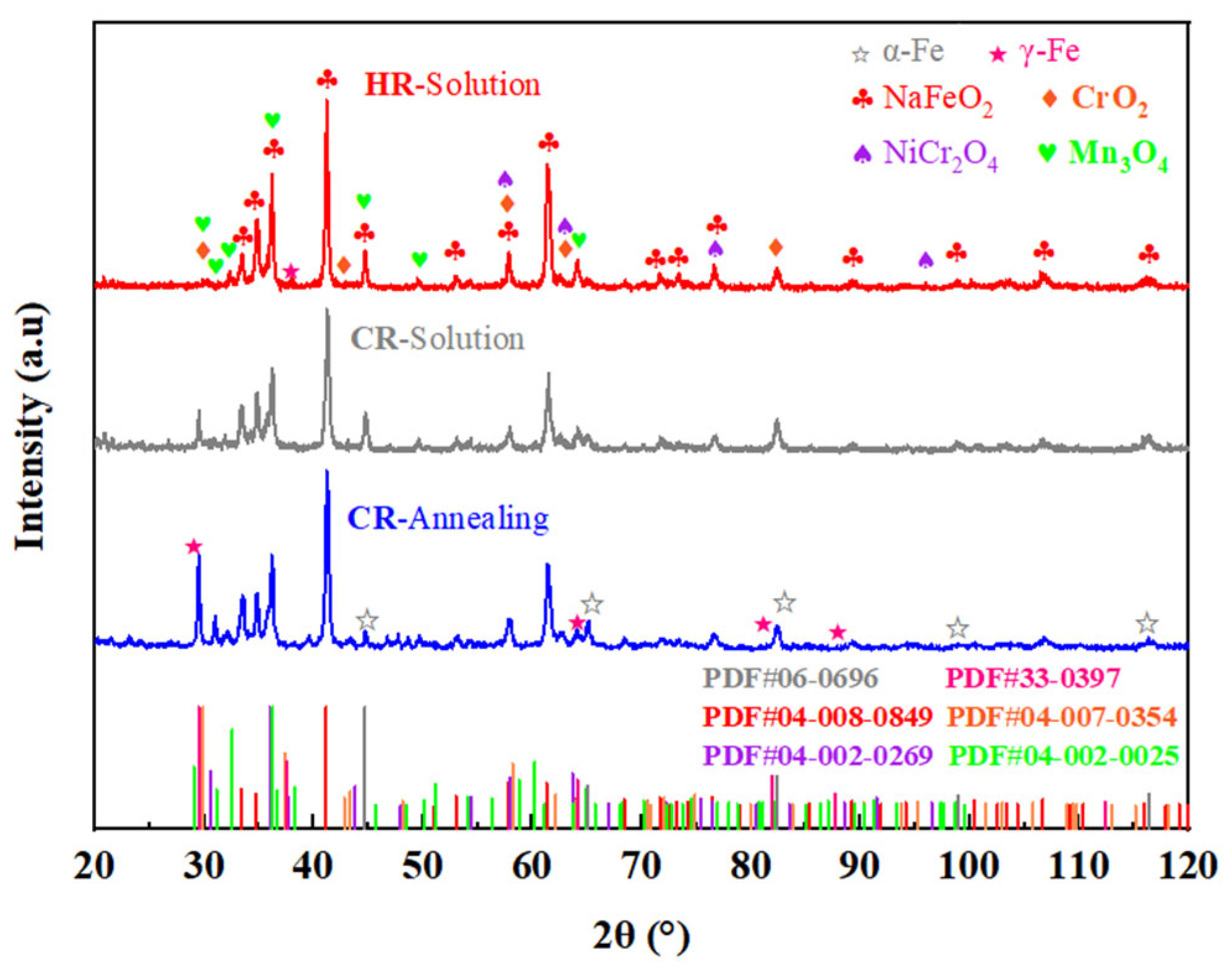
3.2. Phase Analysis of Corrosion Products
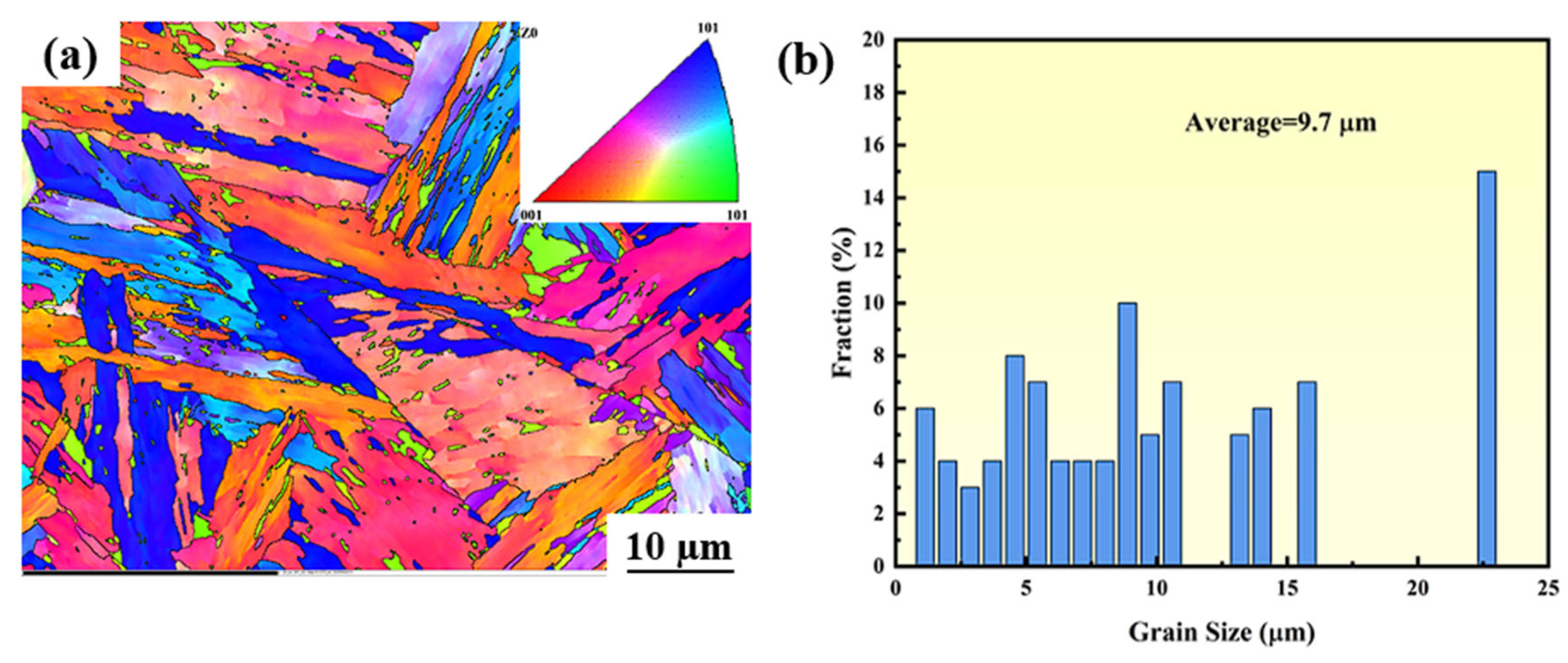
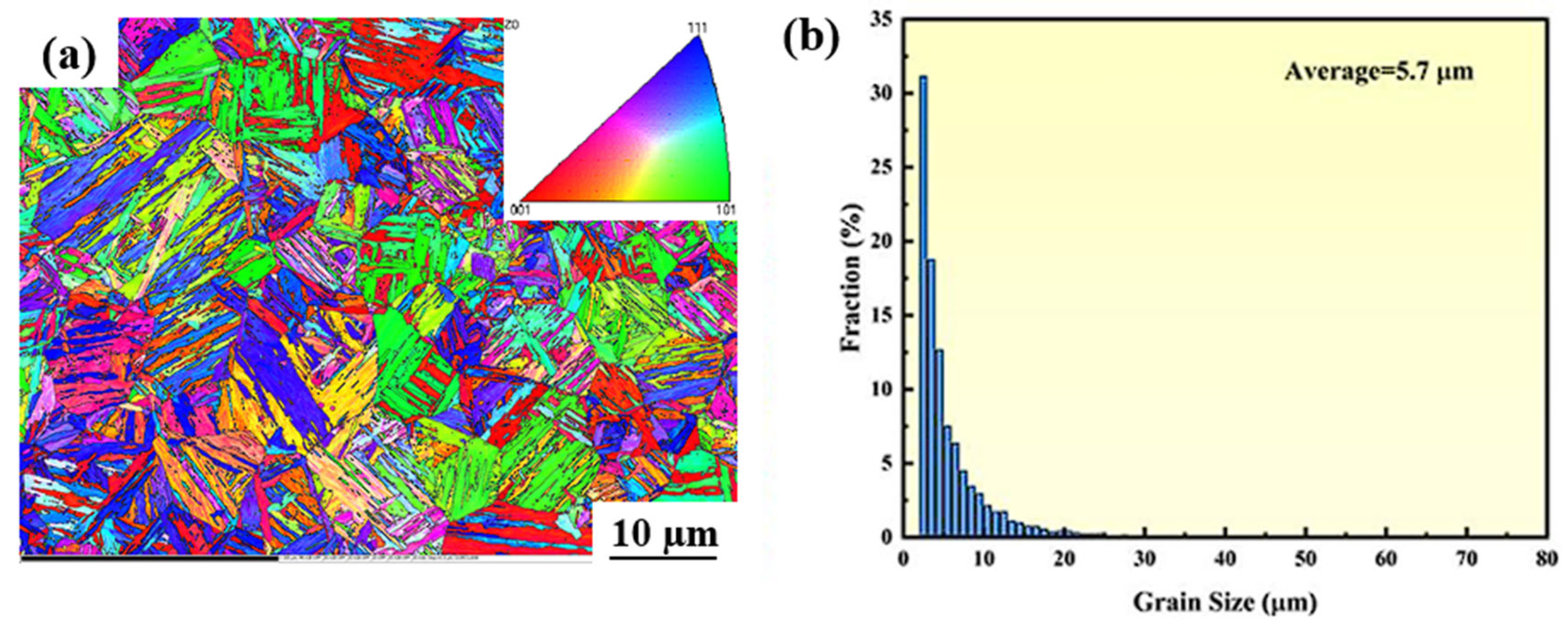
3.3. The Microstructure of the Surface Before Corrosion
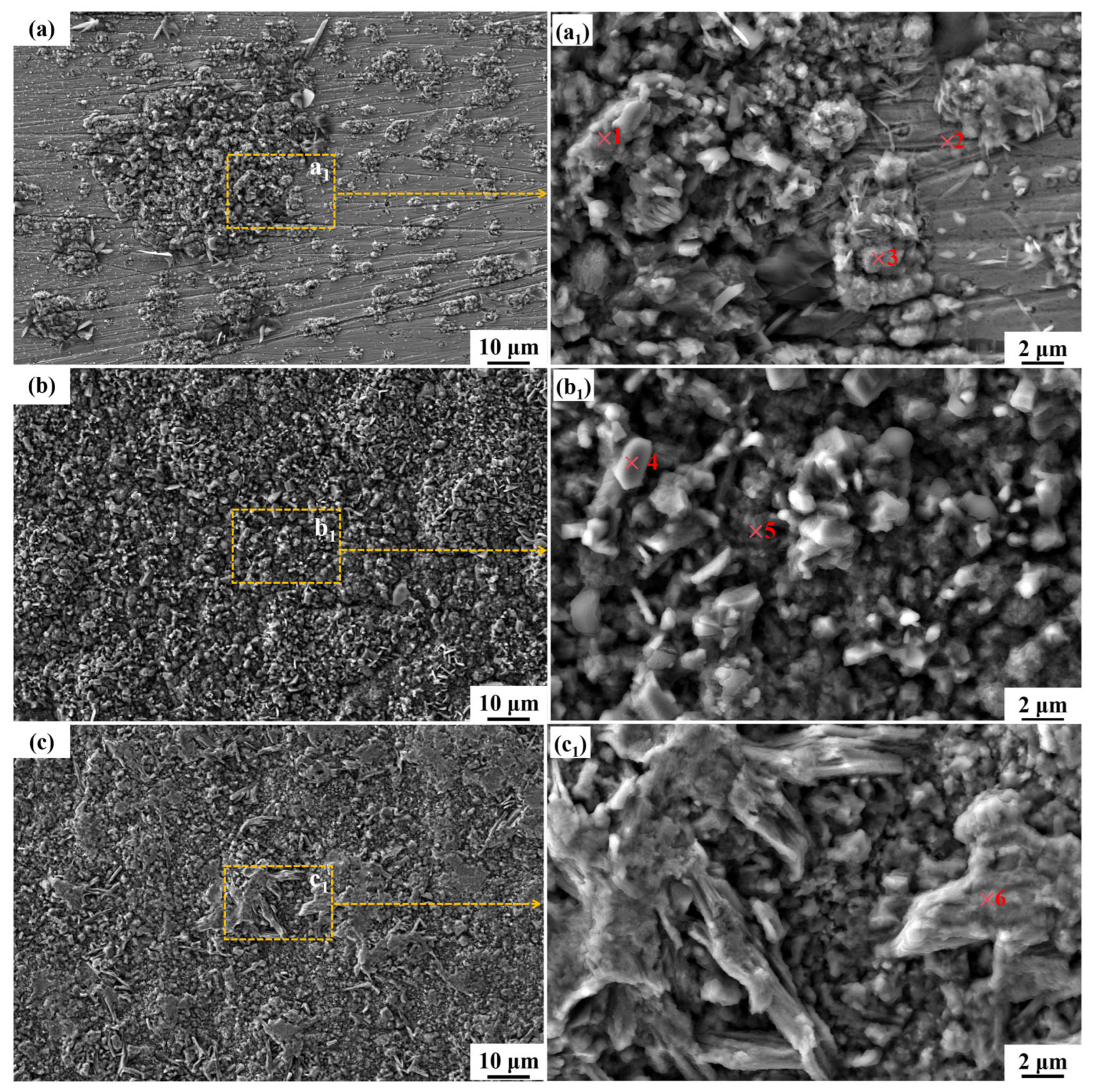
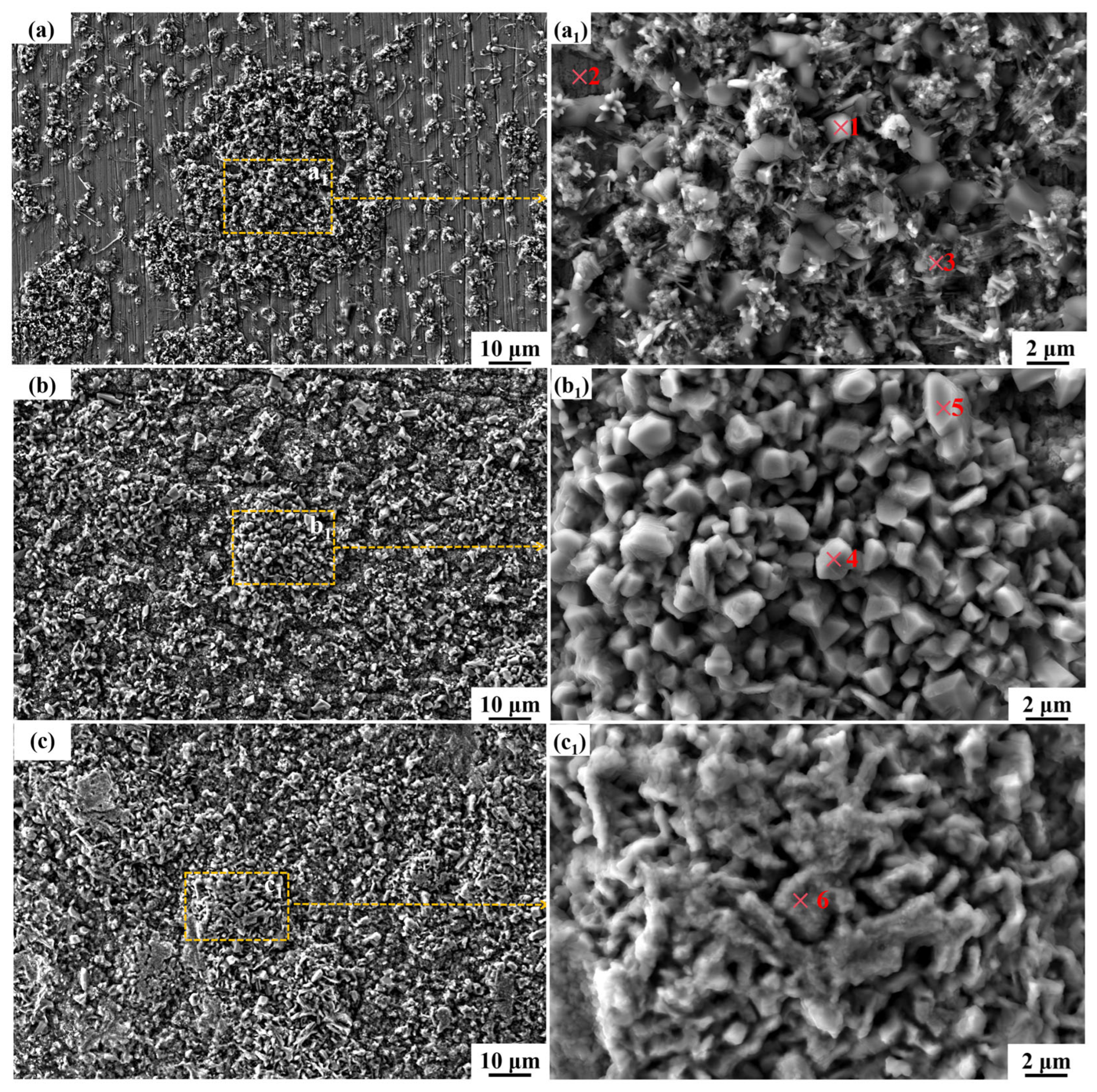
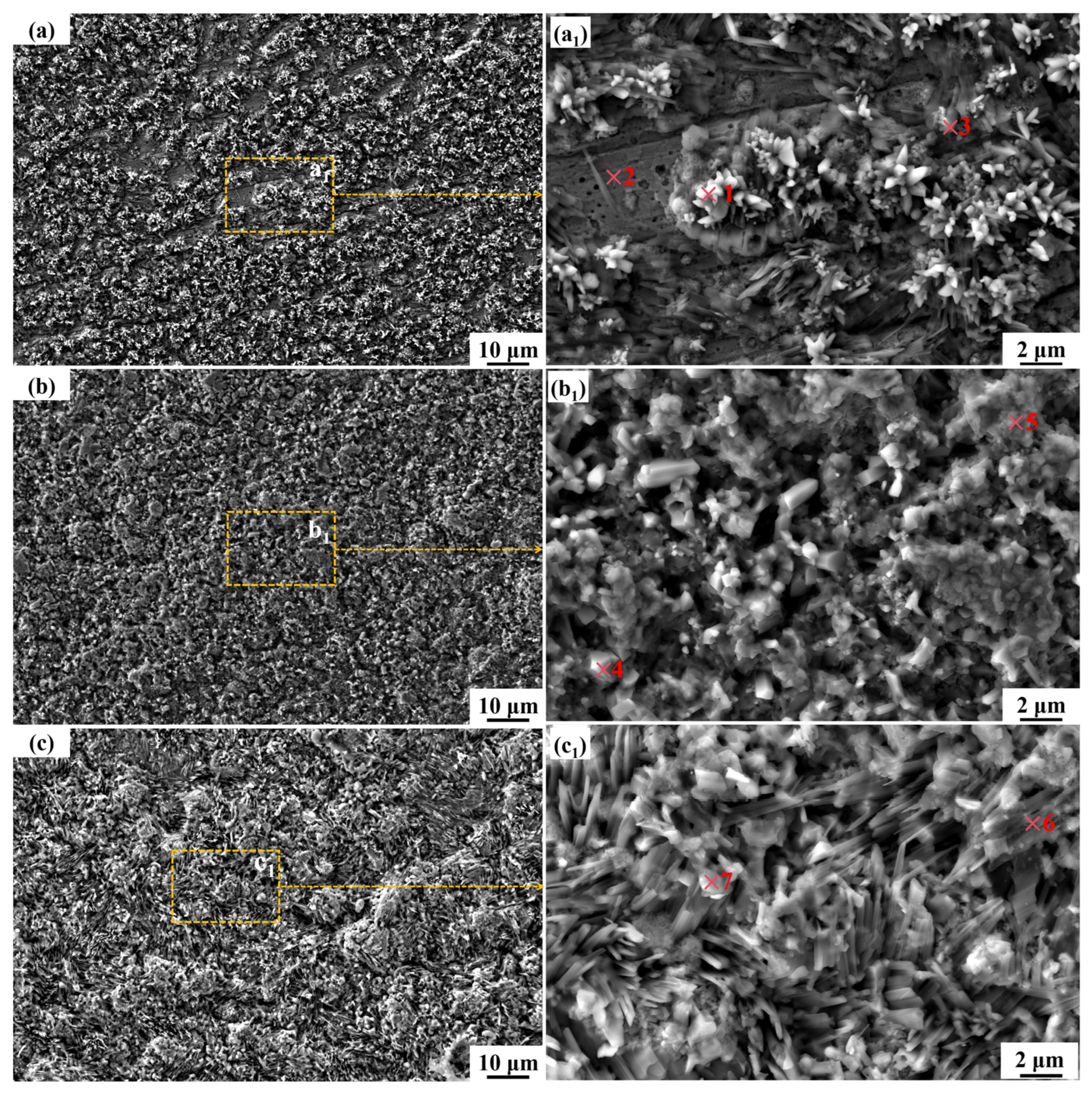
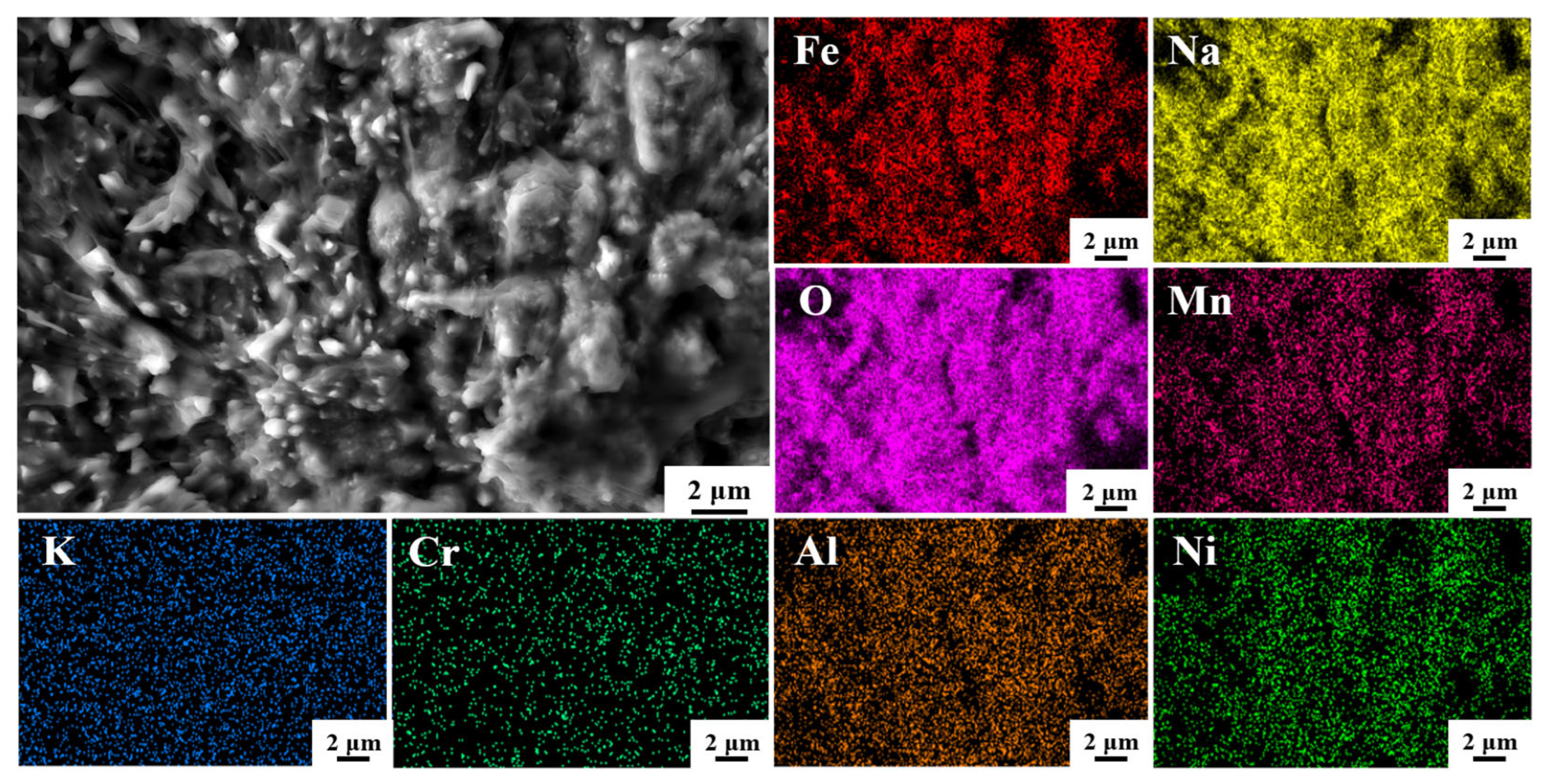
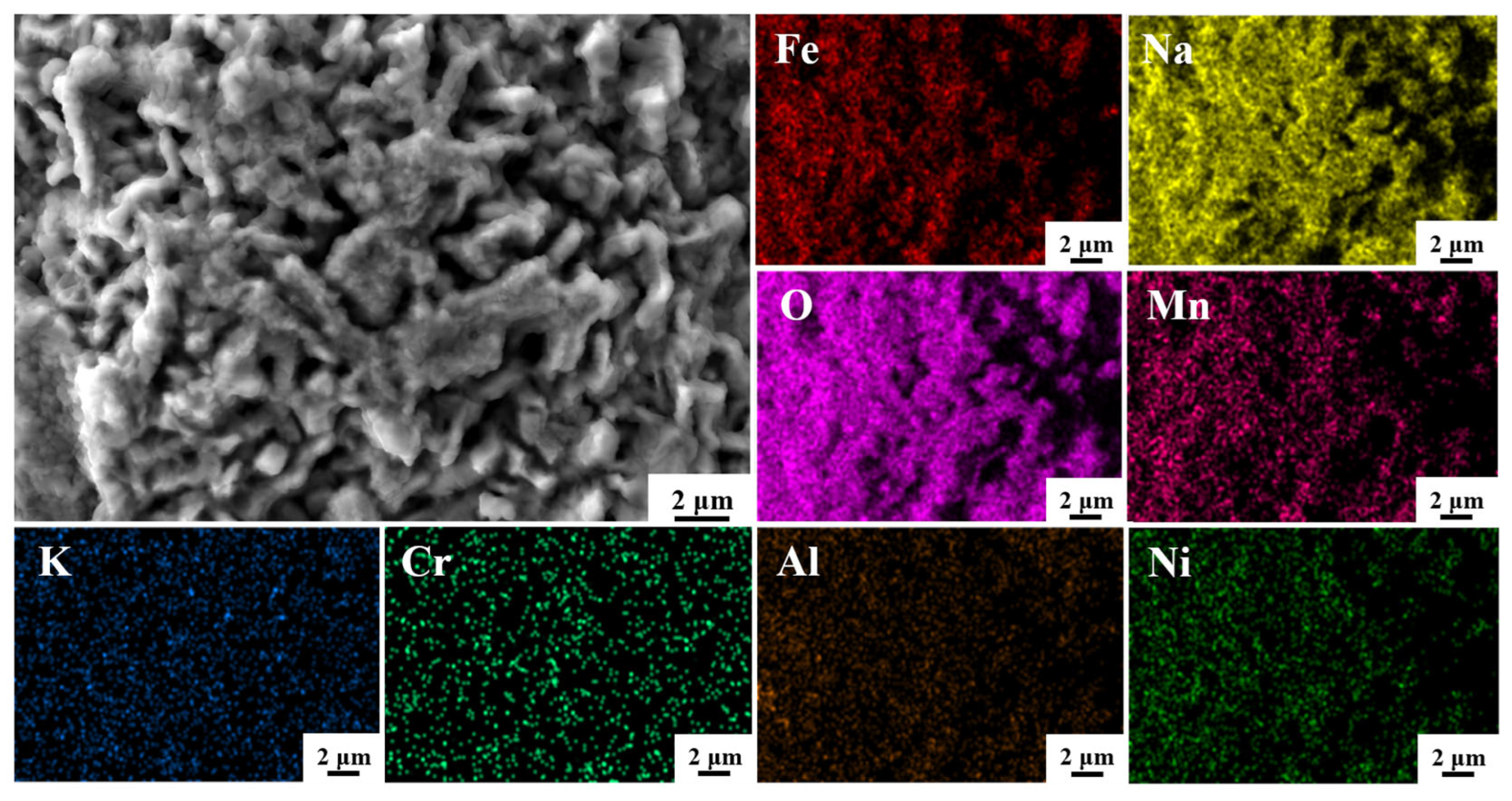
3.4. The Microstructure and Composition of the Corroded Surface
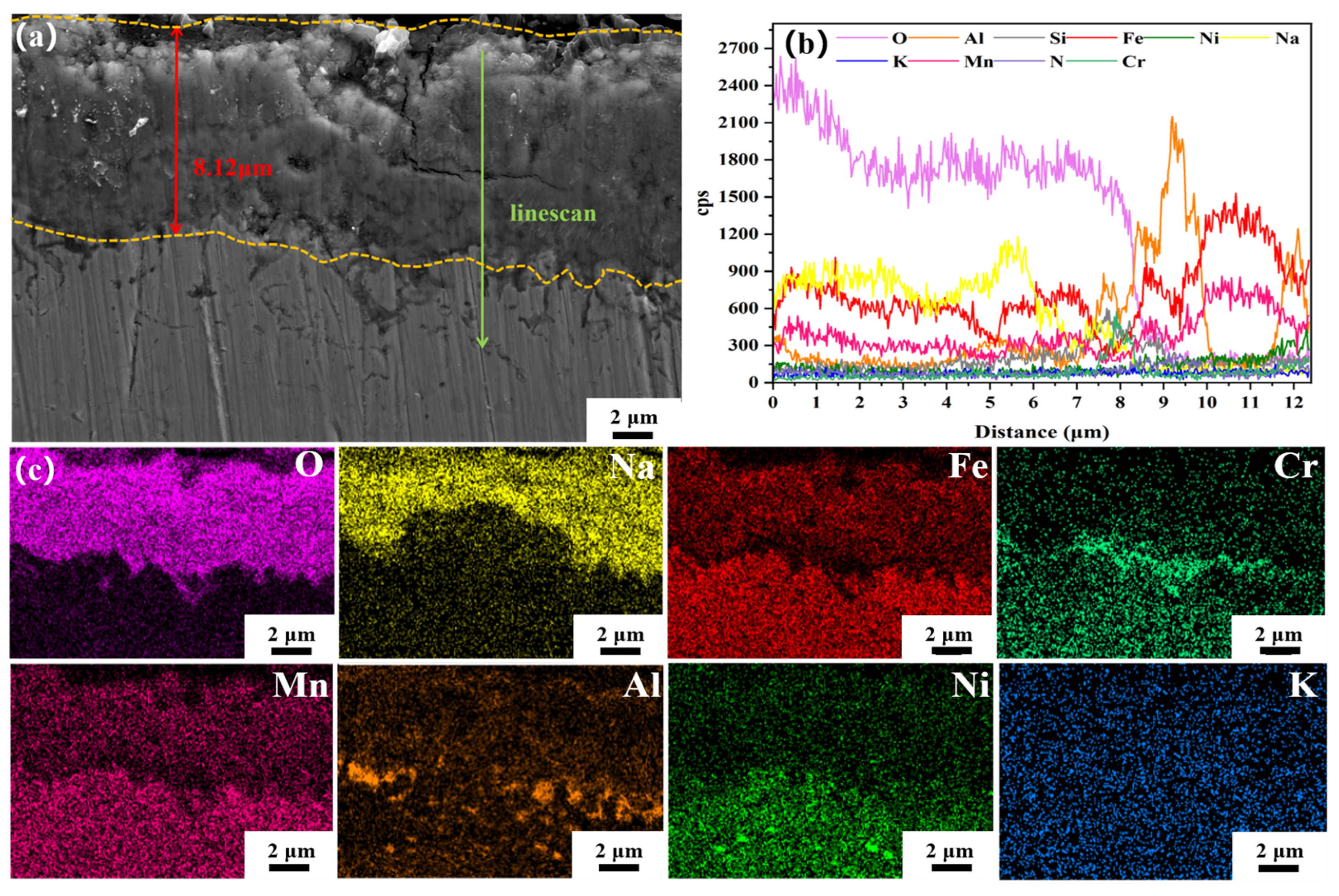
3.5. The Microstructure and Composition of the Corroded Cross-Section
4. Discussion
4.1. The Influence of Different Processes on the Microstructure and Corrosion Performance
4.2. Corrosion Mechanism of High-Al 304SS in Binary Molten Nitrate Salts
4.3. Comparative Analysis of Corrosion Rates for Various Alloys Across Diverse Environmental Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nunes, V.M.B.; Queirós, C.S.; Lourenço, M.J.V.; Santos, F.J.V.; Nietode Castro, C.A. Molten salts as engineering fluids—A review: Part I. Molten alkali nitrates. Appl. Energy 2016, 183, 603–611. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.B.T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Breidenbach, N.; Eck, M.; Laing, D.; Kaesche, S. Material aspects of Solar Salt for sensible heat storage. Appl. Energy 2013, 111, 1114–1119. [Google Scholar] [CrossRef]
- Alami, A.H.; Olabi, A.G.; Mdallal, A.; Rezk, A.; Radwan, A.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Concentrating solar power (CSP) technologies: Status and analysis. Int. J. Thermofluids 2023, 18, 100340. [Google Scholar] [CrossRef]
- Alam, M.I.; Nuhash, M.M.; Zihad, A.; Zihad, A.; Nakib, T.H.; Ehsan, M.M. Conventional and Emerging CSP Technologies and Design Modifications: Research Status and Recent Advancements. Int. J. Thermofluids 2023, 20, 100406. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, C.; Wang, G.; Wang, G.; Wu, Y.; Lu, Y. Molten salt stress corrosion of metal alloys—An updated review. Sol. Energy Mater. Sol. Cells 2025, 282, 113379. [Google Scholar] [CrossRef]
- Du, J.; Xiang, K.; Zhao, L.; Lan, X.; Liu, P.; Liu, Y. Corrosion Inhibition of 13Cr Stainless Steel in HCl/HAc/HF Acid Solution. Int. J. Electrochem. Sci. 2019, 14, 8919–8930. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.; Chen, H.; Liu, Z.; Dai, W.; Yang, X.; Yang, W.; Deng, Y.; Li, X. Stress corrosion cracking behavior and mechanism of 2205 duplex stainless steel under applied polarization potentials. Corros. Sci. 2024, 231, 111978. [Google Scholar] [CrossRef]
- Byun, T.S.; Hashimoto, N.; Farrell, K. Temperature dependence of strain hardening and plastic instability behaviors in austenitic stainless steels. Acta Mater. 2004, 52, 3889–3899. [Google Scholar] [CrossRef]
- Qu, X.; Li, X.; Zhang, L.; Yi, D.; Wang, J.; Wen, C.; Zhao, Z.; Gu, X.; Lin, Y.; Liu, B.; et al. Effect of construction angles on the microstructure and mechanical properties of LPBF-fabricated 15-5 PH stainless steel. Mater. Sci. Eng. A 2024, 900, 146423. [Google Scholar] [CrossRef]
- Fernández, A.G.; Galleguillos, H.; Fuentealba, E.; Pérez, F.J. Corrosion of stainless steels and low-Cr steel in molten Ca(NO3)2–NaNO3–KNO3 eutectic salt for direct energy storage in CSP plants. Sol. Energy Mater. Sol. Cells 2015, 141, 7–13. [Google Scholar] [CrossRef]
- Bin, W.; Li, N.; Zhang, Y.; Ming, H.; Shu, M.; Sun, Y.; Hou, D.; Li, Y.; Wang, J.; Han, E. Effect of solution annealing and Al addition on the corrosion behavior of austenitic stainless steel in supercritical carbon dioxide at high temperature. Corros. Sci. 2024, 234, 112131. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Xu, Y.; Li, J.; Xiao, X.; Jiang, L. Effect of aluminum on microstructure, mechanical properties and pitting corrosion resistance of ultra-pure 429 ferritic stainless steels. Mater. Des. (1980–2015) 2015, 65, 682–689. [Google Scholar] [CrossRef]
- Hua, L.; Du, Y.; Qian, D.; Sun, M.; Wang, F. Influence of Prior Cold Rolling on Bainite Transformation of High Carbon Bearing Steel. Metall. Mater. Trans. A 2025, 56, 640–654. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Wang, C.; Li, Y.; Wang, J.; Yuan, J.; Hu, J.; Huang, M.; Xu, W. Optimizing crack initiation energy in austenitic steel via controlled martensitic transformation. J. Mater. Sci. Technol. 2024, 198, 231–242. [Google Scholar] [CrossRef]
- Li, Y.; Martín, D.S.; Wang, J.; Wang, C.; Xu, W. A review of the thermal stability of metastable austenite in steels: Martensite formation. J. Mater. Sci. Technol. 2021, 91, 200–214. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, C.; Yuan, Y.; Xu, J.; Wang, P.; Wang, K. Numerical characterization and formation process study of rail light bands in high-speed turnout areas. Eng. Fail. Anal. 2025, 168, 109083. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, J.; Wu, L.; Wang, W.; Sun, X.; Meng, X.; Huang, Y. Unraveling the relationship between severe plastic deformation and corrosion responses of AZ31 Mg alloys. Corros. Sci. 2025, 24, 112881. [Google Scholar] [CrossRef]
- Wang, W.; Mu, W.; Han, M.; Zhang, Y.; Wang, N.; Zhang, W.; LI, Z.; Weng, Z.; Liaw, P. An integration study of corrosion and mechanical behaviors of Ti-/Zr-/Hf-doped cobalt-based high-entropy alloys. Mater. Des. 2025, 256, 114230. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, X.; Lan, R.; Ran, G.; Li, J.; Jiang, Y. Mechanisms of inclusion-induced pitting of stainless steels: A review. J. Mater. Sci. Technol. 2024, 168, 143–156. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, J.; Grosdidier, T.; Dong, C.; Yang, D. Improved pitting corrosion resistance of AISI 316L stainless steel treated by high current pulsed electron beam. Surf. Coat. Technol. 2006, 201, 1393–1400. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion properties of plasma nitrided AISI 410 martensitic stainless steel in 3.5% NaCl and 1% HCl aqueous solutions. Corros. Sci. 2006, 48, 2036–2049. [Google Scholar] [CrossRef]
- Deng, J.; Sierla, S.; Sun, J.; Vyatkin, V.V. Physics-informed generative regression for industrial process modeling in steel strip rolling. Expert Syst. Appl. 2025, 282, 127713. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Jang, J.H.; Lee, T.-H.; Kim, S.; Lee, C.; Moon, J. Enhancement of the resistance to localized corrosion of type 304 borated stainless steels through hot rolling. Corros. Sci. 2021, 192, 109798. [Google Scholar] [CrossRef]
- Tao, H.; Zhou, C.; Zheng, Y.; Hong, Y.; Zheng, J.; Zhang, L. Anomalous evolution of corrosion behaviour of warm-rolled type 304 austenitic stainless steel. Corros. Sci. 2019, 154, 268–276. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, K.; Ma, J.; Huang, H.; Chang, S.; Lin, H. Improvement of Corrosion and Wear Resistance of CoCrNiSi0.3 Medium-Entropy Alloy by Sputtering CrN Film. Materials 2023, 16, 1482. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, S.; Hu, L.; Wu, Y.; Huang, Y.; Le, P.; Dai, S.; Li, W.; Xue, N.; Xu, F.; et al. Influence of Heat Treatment Condition on the Microstructure, Microhardness and Corrosion Resistance of Ag-Sn-In-Ni-Te Alloy Wire. Materials 2024, 17, 2785. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, R.; Qin, J.; Kang, Y.; Yu, Z.; Yan, J.; Zhang, S.; Zhang, W. Effect of cold rolling rates on microstructure and corrosion behavior of selective laser melted 316L austenitic stainless steel bipolar plates. J. Mater. Res. Technol. 2025, 35, 3245–3256. [Google Scholar] [CrossRef]
- Shi, Z.; Fang, C.; Li, J.; Bandaru, S.; Liu, M.; Zhao, L.; Zhang, X. Multi-Dimensional Design of Slippery Liquid-Infused Coatings Empowering Long-Term Corrosion Protection for Sintered Nd-Fe-B Magnets in Harsh Environments. Small 2025, 21, 2–6. [Google Scholar] [CrossRef]
- Dong, J.; Wang, E.; Wei, S.; Kang, F.; Chen, H.; Jiang, F.; Liu, Z.; Li, J.; Jiang, W. Synergistic enhancement of cryogenic strength–toughness in CoCrFeNiV0.4 high-entropy alloys via Gd microalloying and thermomechanical processing. Mater. Sci. Eng. A 2025, 945, 149078. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, Y.; Gu, T.; Guo, Y.; Xu, F.; Hou, H. Cooperative effect of strength and ductility processed by thermomechanical treatment for Cu–Al–Ni alloy. Mater. Sci. Eng. A 2022, 849, 143485. [Google Scholar] [CrossRef]
- Sfikas, A.K.; Lekatou, A.G.; Emmanouilidou, S.; Tsirka, K. Corrosion Behavior of As-Cast and Heat-Treated Al–Co Alloys in 3.5 wt% NaCl. Materials 2024, 17, 655. [Google Scholar] [CrossRef]
- Zou, Y.; Tang, S.; Guo, S.; Song, X. Tool wear analysis in turning inconel-657 using various tool materials. Mater. Manuf. Process. 2024, 39, 1363–1368. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Deng, Y.; Cao, Y.; He, Y.; Liu, Y.; Deng, Y. Fatigue behavior of high-strength steel wires considering coupled effect of multiple corrosion-pitting. Corros. Sci. 2024, 244, 112633. [Google Scholar] [CrossRef]
- Chen, Y.T.; Xu, J.L.; Huang, J.; Luo, J.M.; Lian, L.; Zheng, Y.F. Microstructure, mechanical properties, and corrosion resistance of biomedical Ti-Zn alloys prepared by spark plasma sintering. Intermetallics 2025, 185, 108926. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, J.; Yu, H.; Sheng, J.; La, P. Effect of Mg Addition on Molten Chloride Salt Corrosion Resistance of 310S Stainless Steel with Aluminum. Materials 2024, 14, 1109. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, J.; Zheng, Y.; Yu, H.; Yang, P.; La, P. Corrosion Behavior and Mechanism of High-Aluminum Inconel 625 in Chlorinated Salts. Materials 2025, 15, 144. [Google Scholar] [CrossRef]
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- Zhao, X.; Wang, A.; Wang, J.; Ling, C.; Gui, X. Study on the effect of solid solution treatment on the microstructure evolution and properties of 304 stainless steel bellows. Results Eng. 2025, 26, 104565. [Google Scholar] [CrossRef]
- Purwitasari, A.; Oskay, C.; Heinzel, A.; Fetzer, R.; Weisenburger, A.; Müller, W. Corrosion of austenitic stainless steels in liquid Pb with 2E-7 wt% oxygen at 600 and 700 °C. Corros. Sci. 2025, 244, 112651. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, R.; Xu, S.; Wen, C.; Ge, F.; Gao, M.; Si, Y.; Diao, S.; Li, B. Corrosion behavior of aluminum-containing austenitic steels exposed to high-temperature molten chloride salt. Sol. Energy Mater. Sol. Cells 2025, 280, 113269. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Shi, H.; Zhang, L.; Huan, Y.; Zhang, G.; Zhao, Y.; Guo, X. Mechanisms of defect-promoted oxidation and carburization in austenitic stainless steels and the beneficial role of Al on defect elimination. Corros. Sci. 2025, 246, 112755. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Ren, M.; Jiang, H.; Li, L. Microstructure and mechanical behavior of an AISI 304 austenitic stainless steel prepared by cold- or cryogenic-rolling and annealing. Mater. Sci. Eng. A 2018, 724, 260–268. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Z.; Jin, G.; Gao, M. Effect of temperature on galvanic corrosion of Al 6061-SS 304 in nitric acid. Energy Rep. 2022, 8, 112–123. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nucl. Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- Hu, J.; Chai, Z.; Zhang, Z.; Jia, C.; Wang, L.; Li, Y.; Xu, W. Two-stage rolling and partial annealing enables heterogeneous structure and strength-ductility enhancement for austenitic stainless steel. Mater. Sci. Eng. A 2024, 912, 147006. [Google Scholar] [CrossRef]
- Hong, Y.; Yin, G.; Zheng, Y.; Zhang, W.; Wei, Z. Mechanisms of synergistically enhanced strength and plasticity in 316L stainless steel fabricated by laser powder bed fusion via cold rolling and annealing treatments. Mater. Sci. Eng. A 2025, 935, 148364. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H. Effects of strain and strain-induced α′-martensite on passive films in AISI 304 austenitic stainless steel. Mater. Sci. Eng. C 2014, 34, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Deng, Z.; Chen, B.; Wang, Y. High-temperature dynamic corrosion mechanisms of austenitic stainless and carbon steels in nitrates for concentrating solar power. Sol. Energy Mater. Sol. Cells 2024, 266, 112690. [Google Scholar] [CrossRef]
- Kumar, S.; Swaminathan, S.; Hesse, R.; Goldbeck, H.; Ding, W.; Bonk, A.; Bauer, T. Understanding the effect of oxide ions on Solar Salt chemistry and corrosion mechanism of 316 L stainless steel at 600 °C. Corros. Sci. 2025, 249, 112849. [Google Scholar] [CrossRef]
- García-Martín, G.; Lasanta, M.I.; Encinas-Sánchez, V.; Miguel, M.T.; Pérez, F. Evaluation of corrosion resistance of A516 Steel in a molten nitrate salt mixture using a pilot plant facility for application in CSP plants. Sol. Energy Mater. Sol. Cells 2017, 161, 226–231. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, C.; Ma, L.; Wang, G.; Wu, Y.; Lu, Y. Comparative investigation on dynamic hot corrosion behavior of 347H in quaternary molten salt and its nanofluids. Sol. Energy Mater. Sol. Cells 2025, 280, 113263. [Google Scholar] [CrossRef]
- Castro-Quijada, M.; Jullian, D.; Walczak, M.; Pineda, P.; Videla, Á. Quaternary nitrate and chloride molten salts for the next concentrating solar power plants: Corrosion considerations for the use of AISI 304L steel. Sol. Energy Mater. Sol. Cells 2024, 274, 112971. [Google Scholar] [CrossRef]
- Pérez, F.J.; Otero, E.; Hierro, M.P.; Gómez, C.; Pedraza, F.; Segovia, J.; Roḿan, E. High temperature corrosion protection of austenitic AISI 304 stainless steel by Si, Mo and Ce ion implantation. Surf. Coat. Technol. 1998, 108–109, 127–131. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, K.H.; Shao, Z.; Wang, F.; Zhao, S.; Suo, N. Effects of Mo content on microstructure and corrosion resistance of arc ion plated Ti–Mo–N films on 316L stainless steel as bipolar plates for polymer exchange membrane fuel cells. J. Power Sources 2014, 253, 201–204. [Google Scholar] [CrossRef]
- Lai, X.; Yin, H.; Li, P.; Liu, B.; Gao, L.; Tang, Z. The corrosion behavior of 304 stainless steel in NaNO3–NaCl–NaF molten salt and vapor. RSC Adv. 2022, 12, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Navas, M.; Uranga, N.; Paiva, T.; Figueira, I.; Diamantino, T.C. High-temperature corrosion performance of austenitic stainless steels type AISI 316L and AISI 321H, in molten Solar Salt. Sol. Energy 2019, 177, 408–419. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Ma, L.; Wu, Y.; Lu, Y. Dynamic hot corrosion behavior of austenitic stainless steels in binary nitrate-carbonate molten salts at 600 °C. Sol. Energy Mater. Sol. Cells 2025, 285, 113533. [Google Scholar] [CrossRef]
- Fernández, Á.G.; Cabeza, L.F. Molten salt corrosion mechanisms of nitrate based thermal energy storage materials for concentrated solar power plants: A review. Sol. Energy Mater. Sol. Cells 2019, 194, 160–165. [Google Scholar] [CrossRef]
- Oskay, C.; Grégoire, B.; Meißner, T.M.; Burek, B.; Solimani, A.; Galetz, M. In-depth corrosion mechanisms of Fe- and Ni-based alloys in molten solar salt with varying extents of chloride and sulfate impurities. Corros. Sci. 2025, 247, 112775. [Google Scholar] [CrossRef]









| Ingredients | Element Content (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | P | S | Al | Mn | Ni | Cr | Fe | |
| 304SS | ≤0.08 | ≤1.00 | ≤0.045 | ≤1.00 | / | ≤2.00 | 8.00 | 18.0 | Basal |
| High-Al 304SS | 0.04 | 0.72 | <0.035 | <0.03 | 2.88 | 1.9 | 8.19 | 12.71 | Basal |
| Name | K Value | Weight wt.% |
|---|---|---|
| NaFeO2 | 3.94 | 54.00 |
| Mn3O4 | 2.64 | 19.60 |
| NiCr2O4 | 4.60 | 13.30 |
| CrO2 | 3.92 | 5.10 |
| CrO3 | 3.18 | 8.00 |
| Location | Element Content (At.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Na | Al | Si | K | Cr | Mn | Fe | Ni | |
| Point 1 | 0 | 53.36 | 23.58 | 1.03 | 0.19 | 0.23 | 0 | 0 | 21.61 | 0 |
| Point 2 | 0.97 | 24.27 | 2.57 | 15.16 | 1.50 | 0.22 | 3.03 | 0 | 41.86 | 10.42 |
| Point 3 | 0.50 | 62.79 | 12.52 | 2.08 | 0.80 | 0.43 | 0 | 0 | 20.88 | 0 |
| Point 4 | 0 | 52.86 | 23.24 | 1.53 | 0.16 | 0.08 | 0 | 0 | 22.13 | 0 |
| Point 5 | 0.29 | 52.74 | 27.47 | 1.52 | 0.50 | 0.30 | 0 | 0 | 16.91 | 0.27 |
| Point 6 | 0 | 57.50 | 17.95 | 0.16 | 0.01 | 0.47 | 0 | 0 | 23.91 | 0 |
| Location | Element Content (At.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Na | Al | Si | K | Cr | Mn | Fe | Ni | |
| Point 1 | 3.89 | 52.59 | 20.9 | 1.80 | 0.75 | 2.16 | 0 | 0 | 17.91 | 0 |
| Point 2 | 7.38 | 14.90 | 1.92 | 11.28 | 1.40 | 0.21 | 2.53 | 0 | 52.46 | 7.92 |
| Point 3 | 0.26 | 60.65 | 4.00 | 1.01 | 0.47 | 0.30 | 0 | 0 | 33.31 | 0 |
| Point 4 | 0 | 51.46 | 24.72 | 0.62 | 0.06 | 0.04 | 0 | 0 | 23.10 | 0 |
| Point 5 | 0 | 52.97 | 21.98 | 1.07 | 0.17 | 0.06 | 0 | 0 | 23.75 | 0 |
| Point 6 | 0 | 64.62 | 19.26 | 0.12 | 0.07 | 0.16 | 0 | 0 | 15.77 | 0 |
| Location | Element Content (At.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Na | Al | Si | K | Cr | Mn | Fe | Ni | |
| Point 1 | 0 | 57.80 | 9.90 | 1.66 | 1.30 | 0.04 | 0 | 0 | 29.30 | 0 |
| Point 2 | 0.42 | 10.68 | 0.83 | 6.69 | 1.13 | 0.09 | 9.14 | 0.10 | 66.16 | 4.76 |
| Point 3 | 0 | 61.58 | 12.90 | 5.14 | 2.78 | 0.49 | 0 | 0 | 17.11 | 0 |
| Point 4 | 0 | 50.12 | 23.10 | 1.29 | 0.15 | 0.05 | 0 | 0 | 25.29 | 0 |
| Point 5 | 0 | 51.99 | 26.79 | 0.45 | 0.20 | 0.24 | 0 | 0 | 20.33 | 0 |
| Point 6 | 0 | 56.28 | 32.81 | 0.08 | 0.08 | 0.04 | 0 | 0 | 10.71 | 0 |
| Point 7 | 0.55 | 61.84 | 14.92 | 0.25 | 0.14 | 0.18 | 0 | 0 | 22.21 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Zhou, K.; Li, Z.; Xin, L.; Huang, D.; Zhan, F.; Yang, P.; Yu, H.; La, P. Differential Corrosion Behavior of High-Aluminum 304 Stainless Steel in Molten Nitrate Salts: The Roles of Rolling and Heat Treatment. Materials 2025, 18, 4513. https://doi.org/10.3390/ma18194513
Tang W, Zhou K, Li Z, Xin L, Huang D, Zhan F, Yang P, Yu H, La P. Differential Corrosion Behavior of High-Aluminum 304 Stainless Steel in Molten Nitrate Salts: The Roles of Rolling and Heat Treatment. Materials. 2025; 18(19):4513. https://doi.org/10.3390/ma18194513
Chicago/Turabian StyleTang, Weijie, Kan Zhou, Zhenguo Li, Lifu Xin, Dexian Huang, Faqi Zhan, Penghui Yang, Haicun Yu, and Peiqing La. 2025. "Differential Corrosion Behavior of High-Aluminum 304 Stainless Steel in Molten Nitrate Salts: The Roles of Rolling and Heat Treatment" Materials 18, no. 19: 4513. https://doi.org/10.3390/ma18194513
APA StyleTang, W., Zhou, K., Li, Z., Xin, L., Huang, D., Zhan, F., Yang, P., Yu, H., & La, P. (2025). Differential Corrosion Behavior of High-Aluminum 304 Stainless Steel in Molten Nitrate Salts: The Roles of Rolling and Heat Treatment. Materials, 18(19), 4513. https://doi.org/10.3390/ma18194513








