Effect of Solution Temperature on the Microstructure and Properties of AlSi37Cu0.7Mg0.9Ni0.2 Alloy Prepared by Rapid Solidification and Hot Extrusion
Abstract
1. Introduction
2. Experimental Materials and Methods
3. Results and Discussion
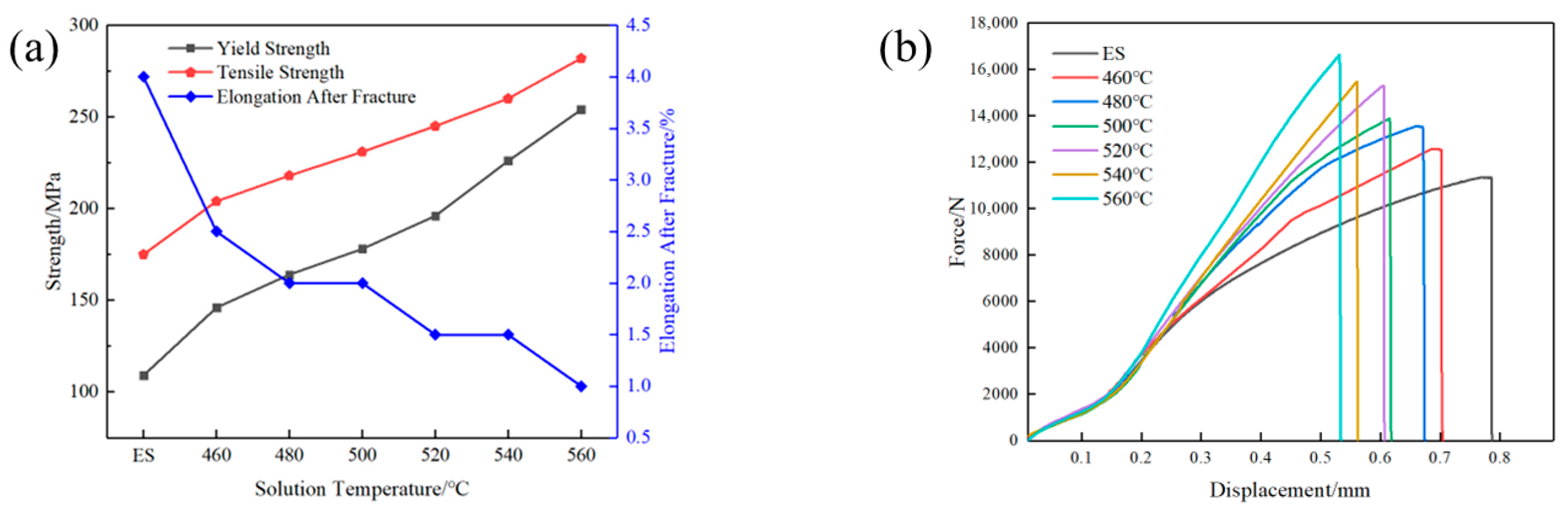
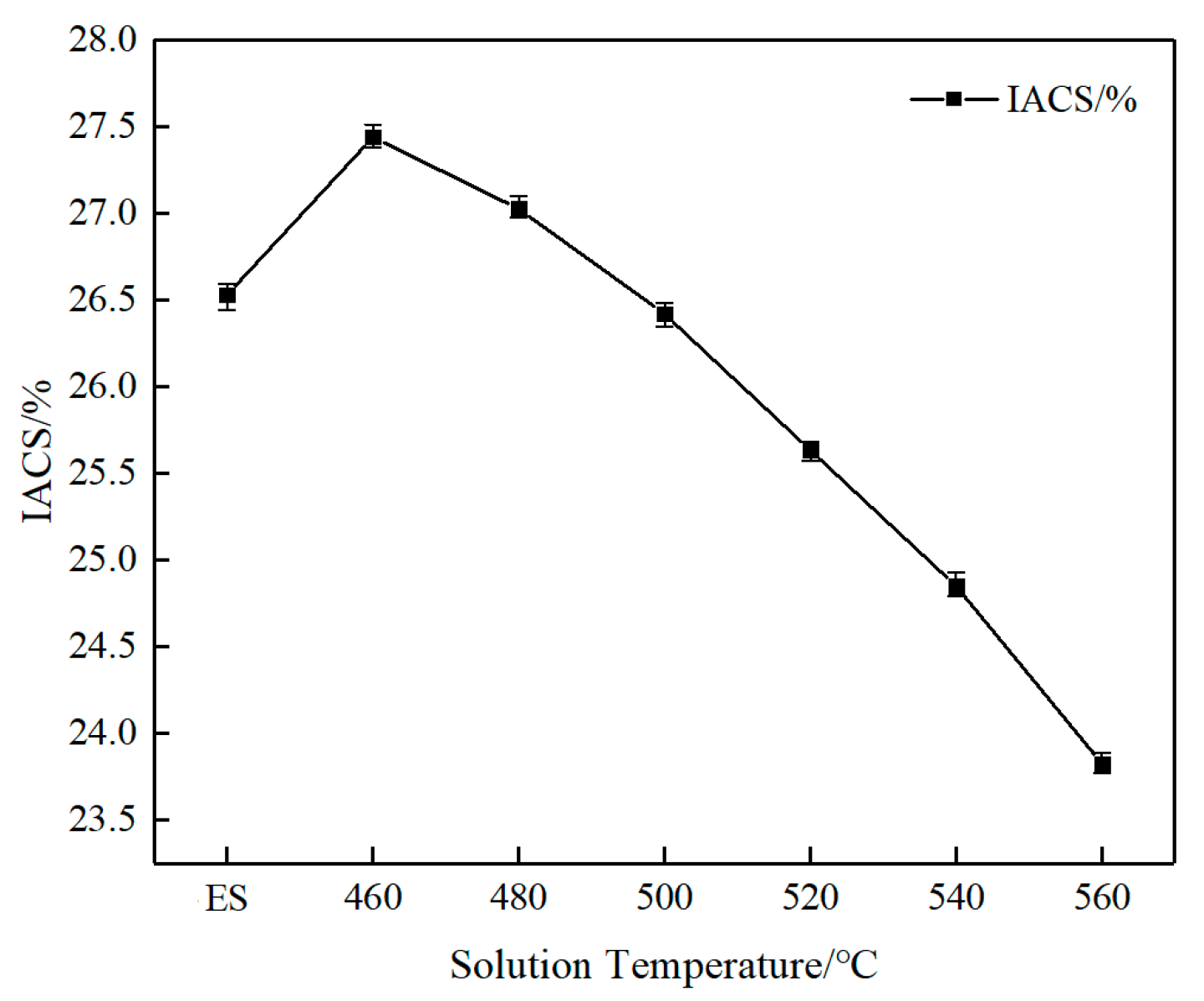
3.1. Mechanical and Electrical Conductivity Properties
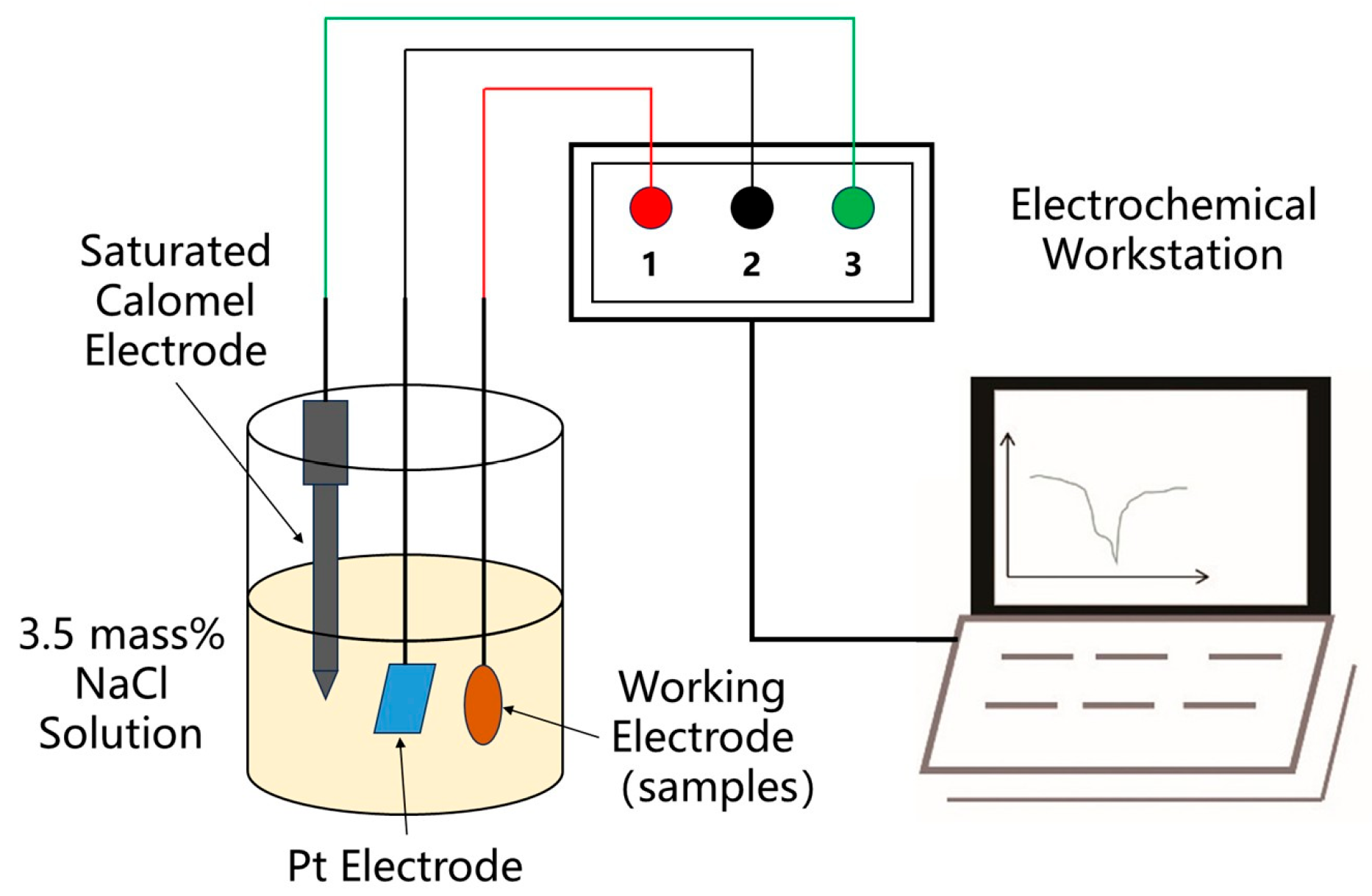
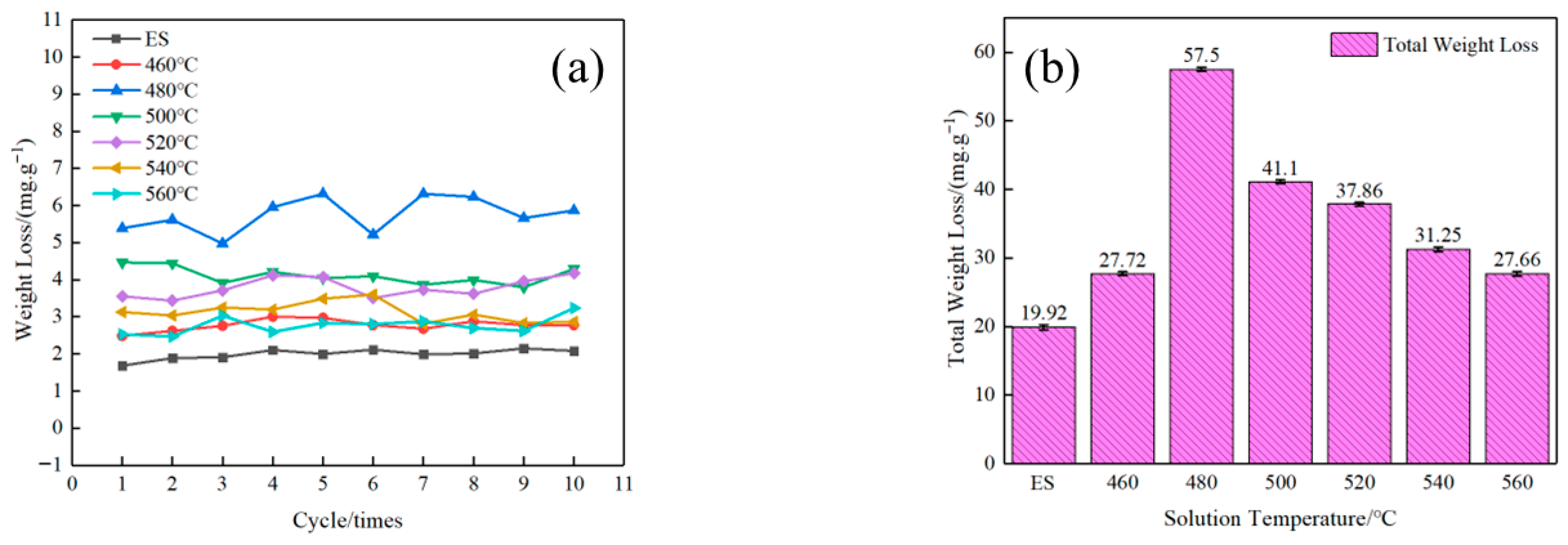
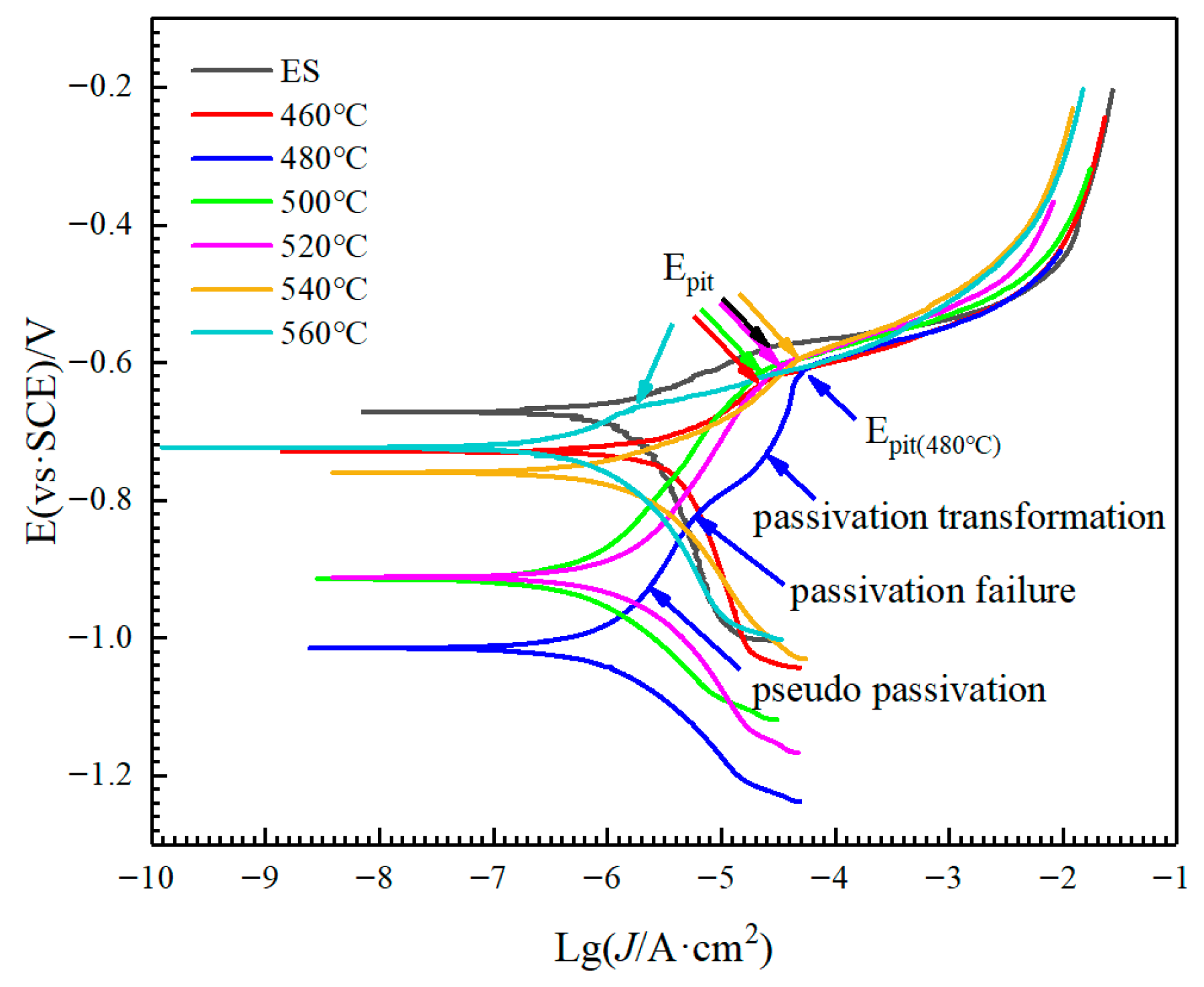
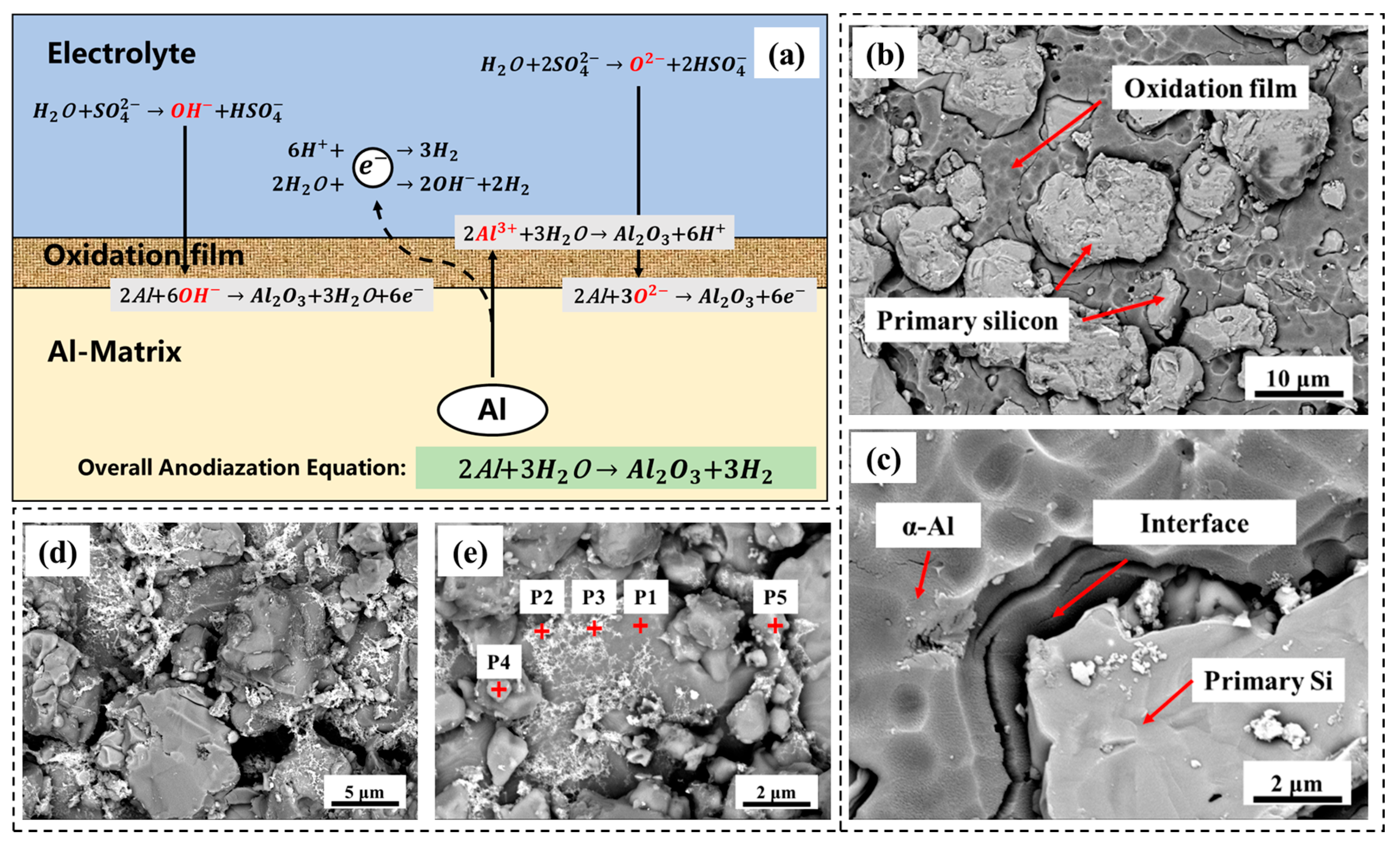
3.2. Anodic Oxidation Corrosion and Electrochemical Performance
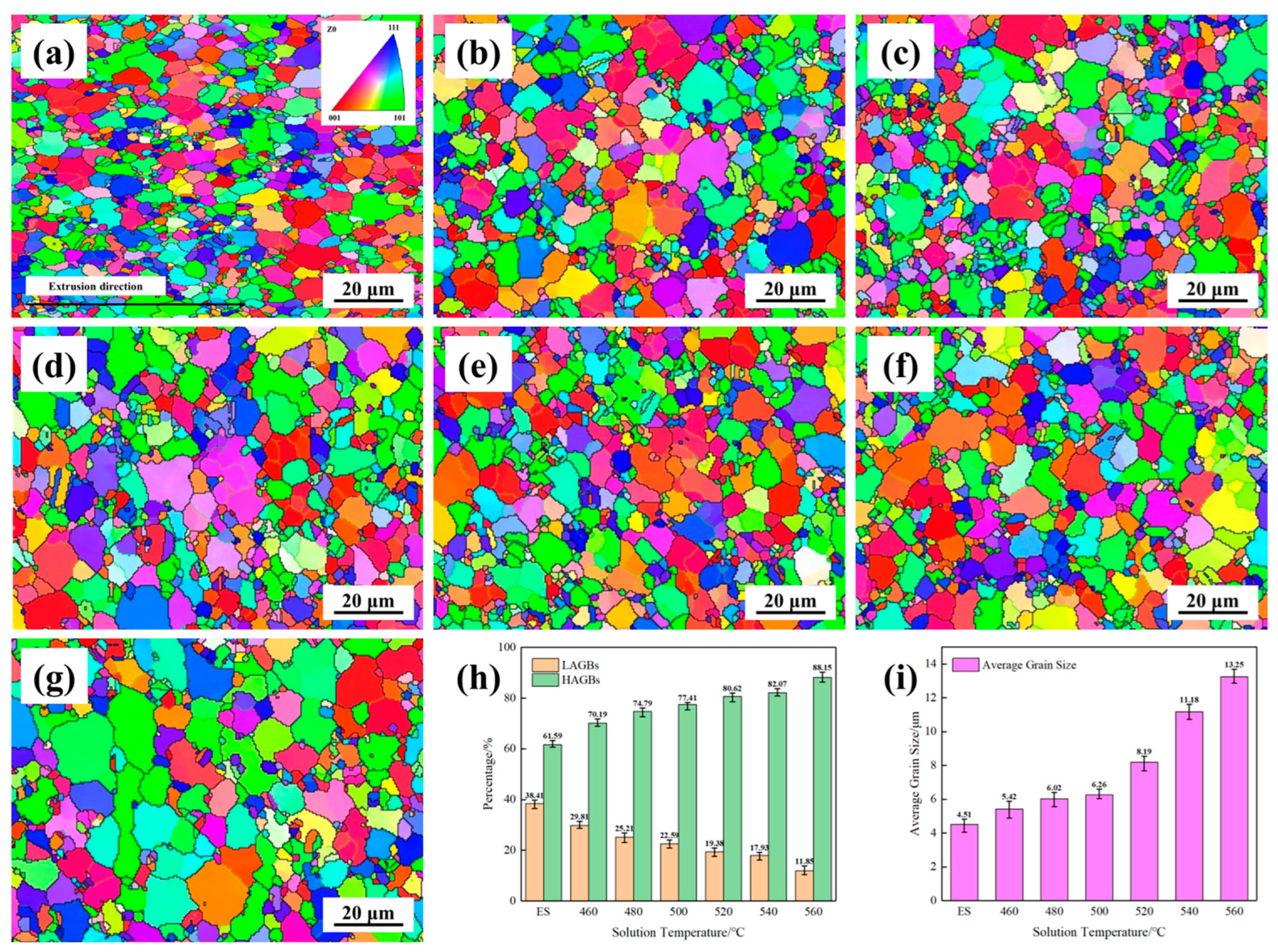
3.3. Microstructure
4. Conclusions
- (1)
- As the solution temperature increased, the Vickers hardness, yield strength, and tensile strength of AlSi37Cu0.7Mg0.9Ni0.2 alloy gradually increased from 95HV, 109 MPa, and 175 MPa under the extruded state to 198.4HV, 254 MPa, and 282 MPa, respectively, at the solution temperature of 560 °C; the elongation at break gradually decreased from 4.0% to 1.0%. The conductivity of the alloy at the solution temperature of 460 °C (27.44% IACS) was higher than that of the extruded state (26.53% IACS), while the conductivity gradually decreased with the rise in solution temperature.
- (2)
- The extruded state alloy exhibited the best corrosion resistance through anodic oxidation, with a cumulative weight loss of 19.92 mg·g−1. As the solution temperature increased, the corrosion weight loss showed a trend of first increasing and then decreasing. The corrosion sensitivity of the alloy reached its maximum value with a cumulative weight loss of 57.50 mg·g−1 at 480 °C solution treatment. The self-corrosion potential of the electrochemical polarization curves was consistent with the results of corrosion weight loss.
- (3)
- With the increase in solution temperature, the fraction of low-angle grain boundaries (LAGBs) progressively decreased from 38.41% under the extruded state to 11.85% at 560 °C, accompanied by an increase in average grain size from 4.51 μm to 13.25 μm. Following solution treatment, the majority of alloying elements dissolved into the matrix to form supersaturated solid solutions, with the exception of insoluble phases such as Al3Ni. The re-precipitation of β″-Mg2Si strengthening phases and AlCuNi ternary compounds occurred, along with the formation of discontinuous Q′ (AlCuMgSi) phases distributed along grain boundaries during subsequent aging treatment. These precipitation characteristics, including their morphology, size distribution, and spatial arrangement, collectively govern the resultant mechanical properties and corrosion behavior of AlSi37Cu0.7Mg0.9Ni0.2 alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymański, W.; Bigaj, M.; Gawlik, M.; Mitka, M.; Szymanek, M. Consolidation by Continuous Rotary Extrusion of Aluminium Alloys Cast by the Melt Spinning Process. Arch. Metall. Mater. 2014, 59, 309–312. [Google Scholar] [CrossRef][Green Version]
- Varga, B.; Fazakas, E.; Hargitai, H.; Varga, L.K. Dilatometer study of rapidly solidified aluminium-silicon based alloys. J. Phys. Conf. Ser. 2009, 144, 012105. [Google Scholar] [CrossRef]
- Karakose, E.; Colak, H. Effect of cooling rate and Mg addition on the structural evaluation of rapidly solidified Al-20wt%Cu-12wt%Fe alloy. Mater. Charact. 2016, 121, 68–75. [Google Scholar] [CrossRef]
- Kalkanli, A.; Angi, S. Effect of cooling rate on microstructure and high temperature stability of rapidly solidified Al-Fe-V-Si alloys. Powder Metall. 2013, 42, 359–365. [Google Scholar] [CrossRef]
- Cooper, K.P.; Iii, H.N.J. Microstructural evolution in rapidly solidified Al-Cu-Si ternary alloys. J. Mater. Sci. 2001, 36, 5315–5323. [Google Scholar] [CrossRef]
- Katgerman, L.; Dom, F. Rapidly solidified aluminium alloys by melt spinning. Mater. Sci. Eng. A 2004, 375–377, 1212–1216. [Google Scholar] [CrossRef]
- Voděrová, M.; Novák, P.; Marek, I.; Vojtech, D. Microstructure and Mechanical Properties of Rapidly Solidified Al-Fe-X Alloys. Key Eng. Mater. 2013, 592–593, 639–642. [Google Scholar] [CrossRef]
- Kula, A.; Blaz, L.; Sugamata, M. Microstructure and Mechanical Properties of Rapidly Solidified Al-Fe-Ni-Mg Alloys. Mater. Sci. Forum 2011, 674, 165–170. [Google Scholar] [CrossRef]
- Uzun, O.; Yılmaz, F.; Kilicaslan, M.F. Formation of novel flower-like silicon phases and evaluation of mechanical properties of hypereutectic melt-spun Al-20Si-5Fe alloys with addition of V. Mater. Sci. Eng. A 2014, 607, 368–375. [Google Scholar] [CrossRef]
- Kasprzak, W.; Chen, D.L.; Shaha, S.K. Heat Treatment Development for a Rapidly Solidified Heat Resistant Cast Al-Si Alloy. J. Mater. Eng. Perform. 2013, 22, 1839–1847. [Google Scholar] [CrossRef]
- Jin, S.; Li, W.; Xu, X.; Wang, K.; Wan, B.; Zhang, L. Extremely improved the yield strength of 6061 aluminum alloy by melt spinning and hot extrusion. J. Alloys Compd. 2025, 1020, 179599. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, H.; Zhou, K. Effect of minor addition of vanadium on mechanical properties and microstructures of as-extruded near eutectic Al-Si-Mg alloy. Mater. Sci. Eng. A 2014, 602, 41–48. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, H.; Yang, J.; Zhou, K. Effect of Si content on dynamic recrystallization of Al–Si–Mg alloys during hot extrusion. J. Mater. Sci. Technol. 2014, 30, 1271–1277. [Google Scholar] [CrossRef]
- Jiang, S.; Jiang, T.; Yu, W.; Li, Y.; Zhang, W.; Sun, Y.; Zhao, H. Optimization of Microstructure and Properties of High-Silicon Aluminum Alloys for Electronic Packaging Based on Semi-Solid Thixotropic Forming Process. J. Mater. Res. Technol. 2025, 36, 1194–1201. [Google Scholar] [CrossRef]
- Hou, J.; Li, R.; Xu, C.; Li, T.; Shi, Z. A comparative study on microstructure and properties of pulsed laser welding and continuous laser welding of Al-25Si-4Cu-Mg high silicon aluminum alloy. J. Manuf. Process. 2021, 68, 657–667. [Google Scholar] [CrossRef]
- Gu, Y.; Han, L.; Lin, J.; Sun, B.; Zhang, Y.; Gao, T.; Fu, B.; Yu, B. Surface modification mechanism of laser-assisted grinding process for high silicon aluminum alloy: A molecular dynamics study. Surf. Interfaces 2025, 56, 105753. [Google Scholar] [CrossRef]
- Shui, Y.; Liu, J.; Huang, W.; Zhao, N.; Ren, P.; Zhao, C. Corrosion mechanism and fatigue behavior of aluminum alloy with high silicon content. Mater. Chem. Phys. 2023, 296, 127211. [Google Scholar] [CrossRef]
- Murakami, Y.; Furushima, R.; Shiga, K.; Miyajima, T.; Omura, N. Mechanical property prediction of aluminium alloys with varied silicon content using deep learning. Acta Mater. 2025, 286, 120683. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Liu, C.F.; Guo, Z.P.; Tong, G.; Ma, S.; Bi, Y.; Zhang, Y.; Xiong, S. The characterization of Fe-rich phases in a high-pressure die cast hypoeutectic aluminum-silicon alloy. J. Mater. Sci. Technol. 2020, 51, 54–62. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, N.; Xia, C.; Zhang, Z.; Cheng, L.; Zhou, D. Effect of brazing and artificial aging on the microstructure and mechanical properties of a high-strength four-layer laminar aluminum alloy. Vacuum 2025, 239, 114388. [Google Scholar] [CrossRef]
- Tai, C.L.; Lin, Y.X.; Tseng, C.Y.; Chung, T.-F.; Yang, Y.-L.; Tsao, T.-C.; Lu, S.-Y.; Chiu, P.-H.; Su, T.-C.; Chen, C.-Y.; et al. Effects of different combinations of artificial ageing and warm forming on Ω phase and S phase evolutions in an Al-5.1Cu-1.0Mg-0.4Ag high strength aluminum alloy. Mater. Sci. Eng. A 2024, 897, 146254. [Google Scholar] [CrossRef]
- Deng, Z.; He, H.; Liu, K.; Tao, X.; Shang, Z.; Gong, Z.; Wang, X. The influence of natural aging on the precipitation behavior of the low-alloy content Al-Zn-Mg aluminum alloys during subsequent artificial aging and related mechanisms. Mater. Sci. Eng. A 2024, 891, 16. [Google Scholar] [CrossRef]
- Tao, X.; He, H.; Li, K.; Ding, Q.; Wang, T.; Mo, Y.; Jin, Q.; Gong, Z. Influence of temperature on the precipitation phase transformation behavior of RR350 aluminum alloy during artificial aging and related mechanisms. J. Alloys Compd. 2024, 970, 172550. [Google Scholar] [CrossRef]
- Mao, X.D.; Gu, N.J.; Huang, D.N.; Cao, H.; Song, X.; Zhuang, L. Hot deformation behaviors and hot processing maps of AlSi37Cu0.7Mg0.9Ni0.2 aluminum alloy prepared by rapid solidification and hot pressure. J. Mater. Res. Technol. 2025, 35, 6485–6496. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. National Standard of the People’s Republic of China: Beijing, China, 2021.
- Li, H.; Huang, Y.; Liu, Y. Dynamic recrystallization mechanisms of as-forged Al-Zn-Mg-(Cu) aluminum alloy during hot compression deformation. Mater. Sci. Eng. A 2023, 878, 17. [Google Scholar] [CrossRef]
- Li, J.-C.; Wu, X.-D.; Liao, B.; Lin, X.-M.; Cao, L.-F. Simulation of low proportion of dynamic recrystallization in 7055 aluminum alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 1902–1915. [Google Scholar] [CrossRef]
- Luo, R.; Cao, Y.; Bian, H.; Chen, L.; Peng, C.-T.; Cao, F.; Ouyang, L.; Qiu, Y.; Xu, Y.; Chiba, A.; et al. Hot workability and dynamic recrystallization behavior of a spray formed 7055 aluminum alloy. Mater. Charact. 2021, 178, 111203. [Google Scholar] [CrossRef]
- Ding, S.; Khan, S.A.; Yanagimoto, J. Flow behavior and dynamic recrystallization mechanism of A5083 aluminum alloys with different initial microstructures during hot compression. Mater. Sci. Eng. A 2020, 787, 139522. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; He, K.; Zhang, H.; Dong, K.; Cai, Y.; Han, E.-H. Role of oxidants in the anodic oxidation of 2024 aluminum alloy by dominating anodic and cathodic processes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703 Pt 1, 135229. [Google Scholar] [CrossRef]
- Yang, J.-j.; Yin, S.-y.; Fan, C.-h.; Ou, L.; Wang, J.-h.; Peng, H.; Wang, B.-w.; Luo, D.; Dong, S.-y.; Zhang, Z.-y. Effect of voltage on structure and properties of 2024 aluminum alloy surface anodized aluminum oxide films. Surf. Coat. Technol. 2024, 479, 130508. [Google Scholar]
- Su, K.; Zhang, J.; Cui, G.; Li, H.; Ji, D. Effects of sealing and prior shot peening treatments on fatigue behavior of sulphuric acid anodic oxidation coated 6082-T6 aluminium alloy. Int. J. Fatigue 2024, 183, 108251. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Wang, X. Effect of different sealing treatments of oxide films on corrosion resistance of anodized ZL101A aluminum alloy in simulated marine atmospheric environment. Int. J. Electrochem. Sci. 2022, 17, 220851. [Google Scholar] [CrossRef]
- Wu, J.; Wu, H.; Wu, L.; Yao, W.; Chen, Y.; Sun, L.; Ma, Y.; Jiang, B.; Wang, J.; Atrens, A.; et al. Preparation technology and properties of a thin anodic oxide composite film on the surface of an aluminum alloy foil. Surf. Coat. Technol. 2022, 447, 128825. [Google Scholar] [CrossRef]
- Araujo, J.V.S.; Chen, J.; Costa, I.; Zhou, X. Localized corrosion in anodized aluminium alloys: The role of constituent particle-induced defects in anodic films. Corros. Sci. 2025, 252, 112961. [Google Scholar] [CrossRef]
- Löffler, A.; Gröbner, J.; Hampl, M.; Engelhardt, H.; Schmid-Fetzer, R.; Rettenmayr, M. Solidifying incongruently melting intermetallic phases as bulk single phases using the example of Al2Cu and Q-phase in the Al–Mg–Cu–Si system. J. Alloys Compd. 2012, 515, 123–127. [Google Scholar] [CrossRef]
- Dong, X.; Yang, S.; Li, N.; Zhong, H.; Wang, X.; Dong, H. The evolution and dissolution mechanism of θ-Al2Cu phase and the analysis of precipitation of a novel AlCu phase during elevated aging. Mater. Charact. 2024, 217, 114454. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Frederick, A.; Wang, Y.; Wang, Y.; Allard, L.; Koehler, M.; Shin, S.; Hu, A.; Feng, Z. In-situ formation of Al3Ni nano particles in synthesis of Al 7075 alloy by friction stir processing with Ni powder addition. J. Mater. Process. Technol. 2023, 311, 117803. [Google Scholar] [CrossRef]





| Si | Fe | Cu | Mg | Ni | Mn | Al |
|---|---|---|---|---|---|---|
| 36.5–38 | 0.4 | 0.65–0.75 | 0.8–1.0 | 0.15–0.25 | ≤0.10 | Bal. |
| Number | Ecorr/V | Epit/V | Jcorr/(μA·cm−2) |
|---|---|---|---|
| ES | −0.67244 | −0.57936 | 0.3303 |
| 460 | −0.72933 | −0.63105 | 0.20069 |
| 480 | −1.0159 | −0.61579 | 0.10683 |
| 500 | −0.91501 | −0.62015 | 0.056329 |
| 520 | −0.91214 | −0.60589 | 0.13351 |
| 540 | −0.76026 | −0.59874 | 0.14685 |
| 560 | −0.72362 | −0.66028 | 0.080878 |
| Element | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| C | 2.95 | 4.80 | 8.51 | 13.61 | 13.43 |
| O | 0.57 | 1.81 | 9.99 | 3.09 | |
| Mg | 2.32 | 0.95 | 10.31 | ||
| Al | 92.16 | 41.21 | 42.07 | 5.21 | 28.27 |
| Si | 4.32 | 43.65 | 37.54 | 67.70 | 36.33 |
| Fe | 5.70 | ||||
| Ni | 2.88 | ||||
| Cu | 10.34 | 7.75 | 2.54 | ||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Chen, Z.; Gu, N.; Huang, D.; Zhuang, L. Effect of Solution Temperature on the Microstructure and Properties of AlSi37Cu0.7Mg0.9Ni0.2 Alloy Prepared by Rapid Solidification and Hot Extrusion. Materials 2025, 18, 5244. https://doi.org/10.3390/ma18225244
Mao X, Chen Z, Gu N, Huang D, Zhuang L. Effect of Solution Temperature on the Microstructure and Properties of AlSi37Cu0.7Mg0.9Ni0.2 Alloy Prepared by Rapid Solidification and Hot Extrusion. Materials. 2025; 18(22):5244. https://doi.org/10.3390/ma18225244
Chicago/Turabian StyleMao, Xiaodong, Zhenning Chen, Ningjie Gu, Dongnan Huang, and Linzhong Zhuang. 2025. "Effect of Solution Temperature on the Microstructure and Properties of AlSi37Cu0.7Mg0.9Ni0.2 Alloy Prepared by Rapid Solidification and Hot Extrusion" Materials 18, no. 22: 5244. https://doi.org/10.3390/ma18225244
APA StyleMao, X., Chen, Z., Gu, N., Huang, D., & Zhuang, L. (2025). Effect of Solution Temperature on the Microstructure and Properties of AlSi37Cu0.7Mg0.9Ni0.2 Alloy Prepared by Rapid Solidification and Hot Extrusion. Materials, 18(22), 5244. https://doi.org/10.3390/ma18225244





