1. Introduction
The rapid urbanization and urban renewal processes have generated a large amount of construction and demolition waste (CDW), especially in developing countries [
1,
2,
3]. In China, the annual production of construction waste exceeded 3 billion tons [
4]. To manage these CDW, a significant amount of land is required, which also leads to adverse environmental impacts [
5]. At the same time, the construction industry consumes a large amount of natural aggregates (NA). Globally, about 50 billion tons of NA are consumed annually, leading to significant impacts on the ecological environment [
6,
7]. Hence, utilizing CDW to produce recycled aggregate (RA) as a substitute for NA not only addresses the issues caused by CDW but also mitigates the problems associated with the extraction of NA [
8,
9]. However, due to the adhered mortar, RA exhibits higher water absorption, lower strength, and lower density. As a result, RAC performs worse than NAC [
10,
11,
12].
To enhance the performance of RA and RAC, numerous studies have been conducted. Removing the old mortar of RA is an effective approach. By placing RA in a specialized machine, RA can be forced to move quickly, and the movement of RA causes impact and friction between the RA and the machine, as well as between the RAs themselves. This process helps to remove or partially remove the adhered old mortar [
13,
14]. According to [
15,
16], heating RA can improve the effectiveness of removing the old mortar. Soaking RA in various acid solutions, including acetic acid, tannic acid, hydrochloric acid and sulfuric acid, is another method to remove the old mortar [
17,
18,
19]. Acid solutions, while dissolving the old mortar, can also cause damage to the NA. The removal of adhered mortar through mechanical or thermal methods results in increased energy consumption and carbon emissions, whereas the use of acid soaking for mortar removal poses risks of environmental pollution [
20,
21]. Therefore, the development of efficient strengthening methods with minimal environmental impact is of great importance.
Surface treatment represents another approach to strengthening RA. Since this method does not require the removal of adhered mortar, it results in a lower environmental impact and has consequently attracted significant attention from researchers. A commonly used surface treatment method for enhancing the performance of RA involves encapsulating them with high-strength or dense materials. Typical encapsulating materials include cement-based compounds and polymers, such as polyvinyl alcohol (PVA). The cement-based material, prepared as a slurry, is applied to the RA surface by immersion or spraying. After hardening, a dense coating layer with relatively high strength is formed, which improves the surface smoothness and, consequently, the overall performance of the RA. However, the hardened cementitious layer is not effective in preventing moisture ingress; as a result, this method does not significantly reduce, and may even increase, the water absorption of RA [
21,
22,
23]. Polymer materials are typically applied to modify recycled aggregates (RA) in solution form. The polymer molecules form a dense film on the RA surface, effectively improving surface roughness and reducing water absorption. However, if the thickness of the film is not properly controlled, the polymer layer may interfere with the bonding between the RA and the new cement matrix, which could negatively affect the performance of the RAC [
24,
25,
26,
27]. Another surface treatment technique involves the penetration of active substances into the surface layer of the adhered old mortar on recycled aggregates (RA). The active substances react with the mortar, and the resulting products fill the internal pores, thereby enhancing the strength of the RA and reducing its water absorption. Typical strengthening agents include nanomaterials, water glass and CO
2 [
7,
20,
28,
29,
30,
31,
32,
33]. However, nanomaterials are costly, and CO
2 treatment requires specialized equipment, limiting their practical application. In contrast, water glass treatment—especially by immersion—has been widely adopted, due to its simplicity and applicability. Nonetheless, although water glass treatment can partially mitigate the high water absorption of RA, capillary pores within the old mortar remain channels for moisture ingress.
Owing to the energy consumption, carbon emissions, and environmental pollution associated with the removal of old mortar, methods for strengthening old mortar have received more attention. Nevertheless, further research is warranted to develop optimized treatment strategies that synergistically improve the performance of both RA and RAC, particularly through combined treatment methods utilizing multiple reinforcing agents. Furthermore, the mechanism by which combined treatment methods affect RCA and RAC requires further investigation. In this paper, an inorganic–organic combined modification method, using water glass (WG) and sodium methyl silicate (SMS), was proposed, aiming to enhance the strength and durability of RAC. The influences and mechanisms of the combined method on RA and RAC were verified. This work provides an effective approach for enhancing the performance of RA and RAC and offers a better understanding of the mechanisms through which the treatment method influences both the RA and the RAC.
3. Results and Discussion
3.1. Morphology Analysis of RCA
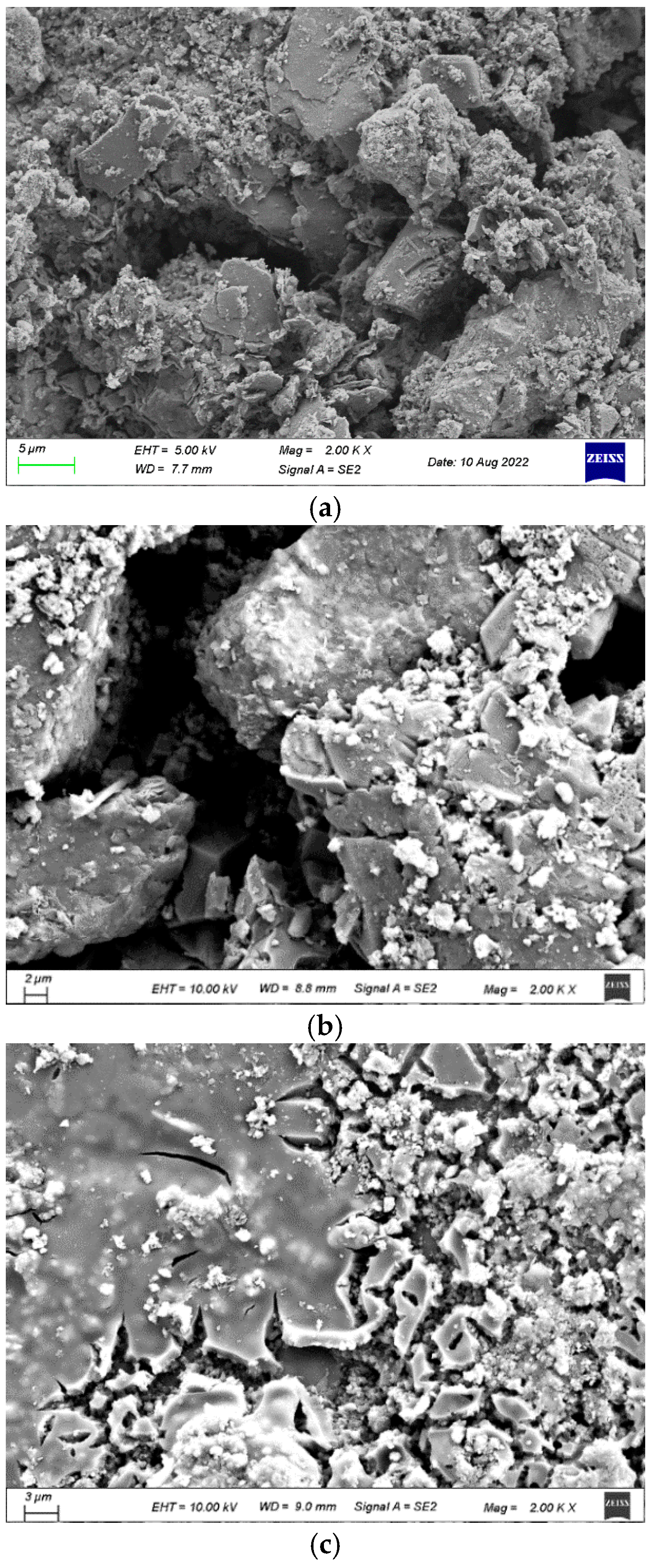
The treated RCAs are shown in
Figure 7 and
Figure 8. In terms of macro morphology, the surfaces of the RCAs did not form a distinct coating layer, and the treated RCAs remained rough and porous. It meant that during the spraying process, the WG solution and SMS solution mainly infiltrated the RCA.
In terms of micro morphology, pores and cracks could be clearly observed in the SMS-solution-treated RCA, showing no significant difference from the raw RCA. As reported in [
41], a transparent molecular film formed through the reaction between SMS and RCA, which could not be observed. For the WG-solution-treated RCA, a gel layer could be observed, which was consistent with [
42]. Nevertheless, the gel layer did not completely cover the recycled aggregate, which still permitted the ingress of water into the RCA.
3.2. Crushing Value and Water Absorption of RCA
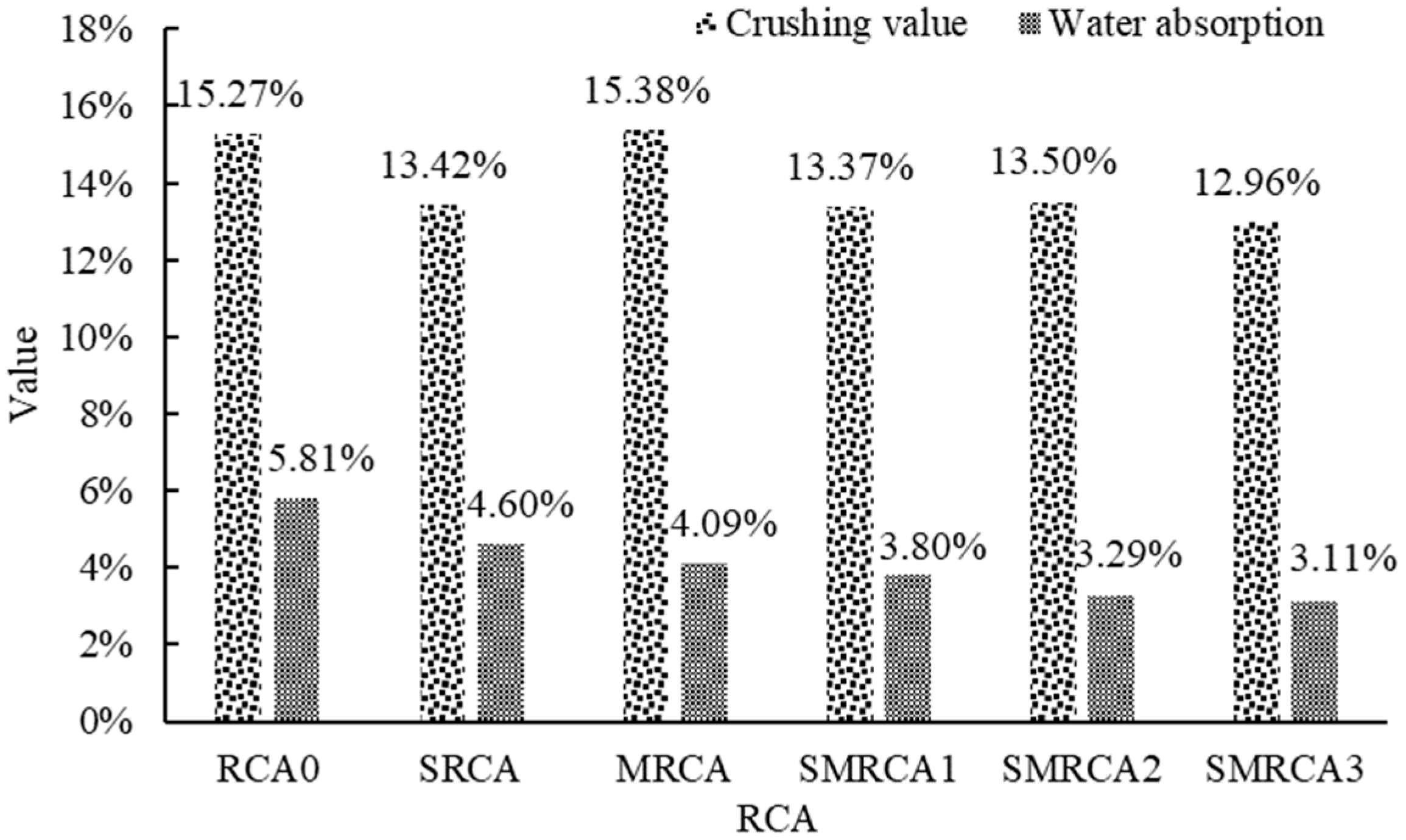
The crushing value results and water absorption results are shown in
Figure 9. Apparently, the strength of the RCA was improved by the WG solution, and the crushing value decreased by 12.11% when the 40% WG solution was used. During the spraying process, the WG solution penetrated the RCA, and then C-S-H was generated, due to the reaction between the WG and the old mortar. The pores and cracks of the old mortar could be filled, thereby improving the RCA’s strength. Similar results were also reported in [
43]. For the SMS-solution-treated RCA, the crushing value was not significantly affected. This is because only a molecular film was formed on the treated RCA’s surface, which did not affect its strength.
Compared with the untreated RCA, the water absorption of the WG-solution-treated RCA decreased by 20.83%. As reported in [
1], this phenomenon is attributed to the formation of C-S-H through the reaction between WG and the old mortar, as well as the formation of the gel layer on the surface. Furthermore, the interaction between the C-S-H gel, formed by the reaction of the water glass with the old mortar, and water molecules restricts the free movement of water within the pores, which further reduces the water absorption of the treated RCA [
44]. The water absorption of the MRCA decreased by 29.60%. This is because SMS is a waterproof material which could form a hydrophobic layer on the cement-based material [
45]. When WG and SMS were used in combination, the water absorption was further reduced. Specifically, for SMRCA1, SMRCA2 and SMRCA3, the water absorption decreased by 34.60%, 43.37% and 46.47%, respectively. This synergistic effect demonstrates that WG and SMS collaboratively enhance the water-blocking performance, effectively inhibiting moisture infiltration. Mechanistically, WG reacts to form calcium silicate hydrate (C-S-H) and gel, filling pores and cracks within the RCA. Simultaneously, SMS creates a hydrophobic layer on the RCA surface. As the concentration of the SMS solution increased, the water absorption of the treated RCA decreased. As the SMS solution concentration increased, the water absorption of the treated RCA progressively decreased. At a 10% SMS concentration, the hydrophobic layer achieved optimal coverage, resulting in a significant reduction in water absorption. Beyond this concentration, the improvement in water absorption became marginal, indicating the saturation of the hydrophobic effect.
3.3. Contact Angle
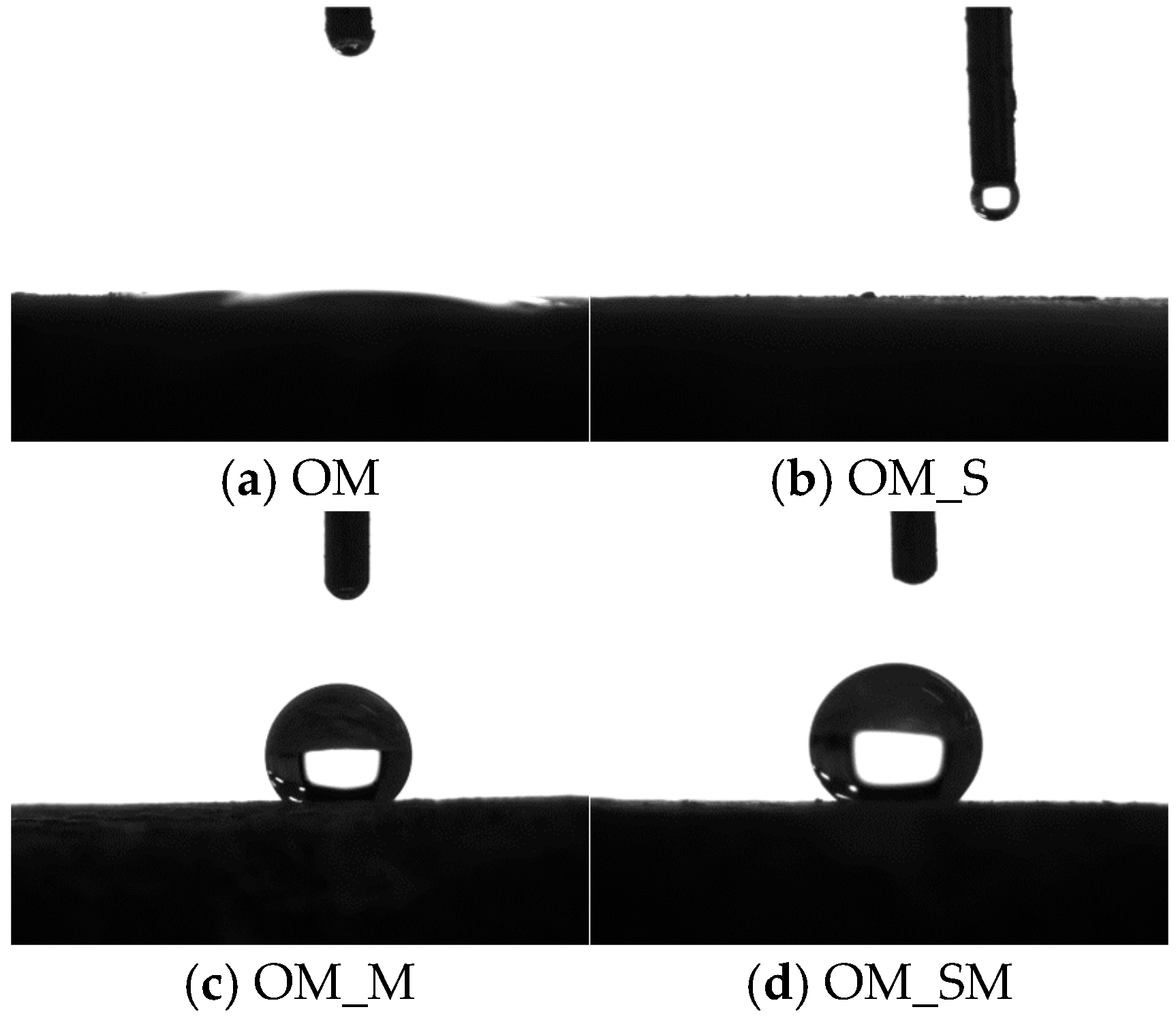
The contact angle results are presented in
Figure 10. The results show that both the untreated old mortar and WG-solution-treated old mortar were hydrophilic, while the SMS-solution-treated old mortar and combined-treated old mortar were hydrophobic. This is because the reaction between SMS and the old mortar lead to the formation of a hydrophobic film on the surface of the old mortar. The contact angles of OM_M and OM_SM were greater than 120°. According to the Young–Laplace equation (Equation (5)) [
46], the capillary suction was negative, indicating that water could not penetrate the old mortar, which consequently reduced the water absorption of the treated RCA. For the WG-solution-treated RCA, the reduction in water absorption was due to the sealing effect of the gel layer, as shown in
Figure 9.
3.4. Compressive Strength and Splitting Tensile Strength of Concrete
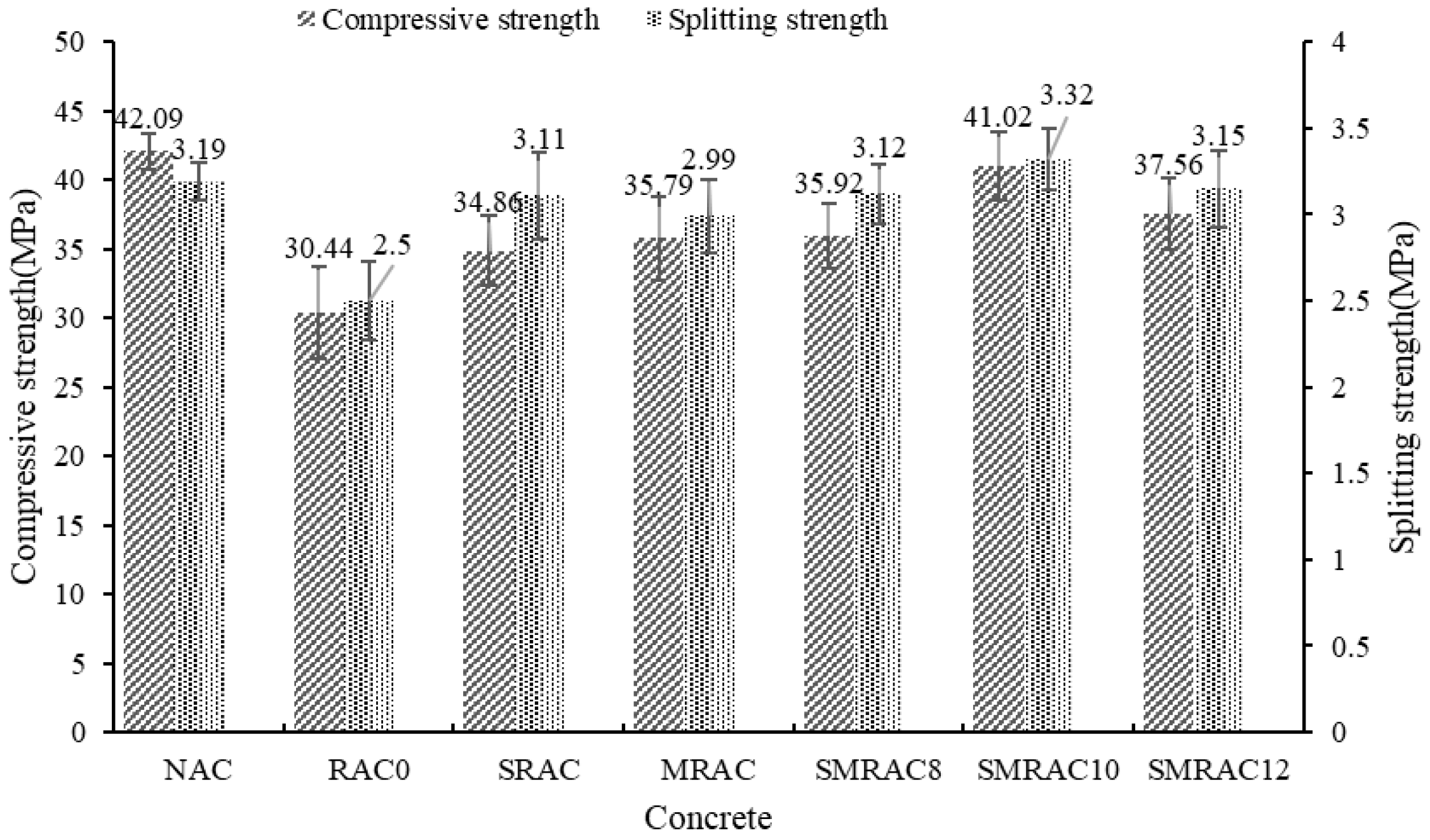
The compressive strength and splitting tensile strength results are shown in
Figure 11. When the NCA was replaced by the untreated RCA, the compressive strength and splitting tensile strength decreased by 27.7% and 21.6%, respectively. Similar results were also reported in [
47]. This means that the use of RCA negatively affected the strength of the RAC. According to the failure mode, both interface failure and RCA failure were observed, particularly at the interface between the old mortar and the new mortar. Therefore, improving the strength of the RCA and the interface are beneficial for enhancing the strength of the RAC.
When the RCA was treated with the WG solution, the compressive strength and splitting tensile strength of the SRAC increased by 14.5% and 24.4%, respectively. A similar result was also reported in [
48]. On one hand, the strength of the RCA was enhanced through the WG solution treatment. On the other hand, in the RAC, the water content was reduced due to the lower water absorption of the treated RCA. Therefore, the strength of the SRAC was higher than the RAC0. According to Alqarni A S [
49], the enhancement induced by the WG solution treatment can be attributed to the reaction between the WG and the old mortar, which forms a dense layer on the surface of the RCA.
For MRAC, the SMS-solution-treated RCA was used to replace the NCA, and the compressive strength and splitting tensile strength increased by 17.6% and 19.6%, respectively. According to the RCA test results, the SMS solution could not enhance the strength of the RCA; hence, the strength enhancement of MRAC was due to the improvement of the interface between the MRCA and the new mortar.
When the WG solution and SMS solution were used in combination to treat the RCA, the compressive strength and splitting tensile strength of the SMRAC increased by up to 34.8% and 32.8%, respectively. This means that the strength performance of the SMRAC2 was approaching or even surpassing that of the NAC. The enhancement of the strength was due to the combined reinforcement effect of the WG solution and SMS solution. The WG solution could improve the strength and reduce the water absorption of the RCA, while the SMS solution could enhance the interface between the RCA and the new mortar, and thus lead to a significant improvement in the RAC’s strength.
3.5. Capillary Water Absorption of Concrete
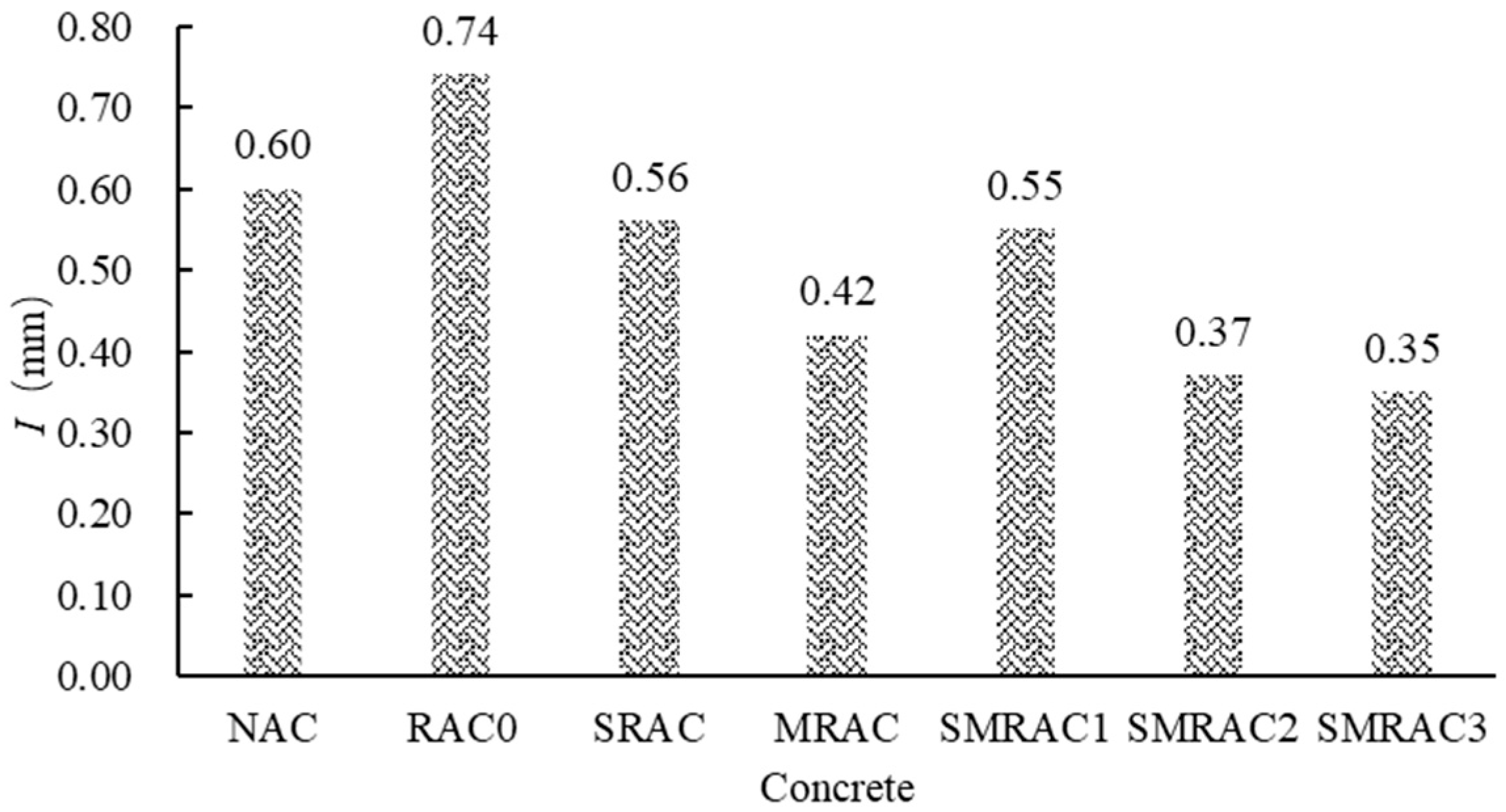
The results of the capillary water absorption (
I) are shown in
Figure 12. In concrete, during the water transmission process, the NCAs, with their low water absorption rate, block the pathways for moisture transmission, resulting in lower capillary water absorption of the NAC. Compared to the NAC,
I of the RAC0 increased by 23.33%. This is because the water absorption of the untreated RCA (RCA0) is higher than that of the NCA. When the RCA0 was used in concrete to replace the NCA, more water could penetrate the RAC0, which negatively affected the durability of the RAC.
When the SRCA was used to replace the NCA, the I of the SRAC (ISRAC) showed a significant decrease. Compared to the RAC0, the ISRAC decreased by 24.32%. This result is attributed to the reduction in water absorption of RCA caused by the WG solution treatment. When MRCA was used in concrete, the I of the MRAC (IMRAC) showed a further decrease, which was lower than that of the NAC. Compared to RAC0, the IMRAC decreased by 43.24%. This is because the SMS solution can form a hydrophobic layer near the interface between the MRCA and the new mortar, effectively preventing the water transmission.
When the RCAs treated with WG and SMS were used in concrete, including SMRAC1, SMRAC2 and SMRAC3, the I of the SMRAC3 decreased by 52.70% compared to the RAC0 and by 41.67% compared to the NAC. This indicates that the combined treatment method can effectively combine the moisture-blocking effects of both the WG solution and the SMS solution. It is worth noting that ISMRAC1 was essentially equal to ISRAC1, indicating that the 8% SMS solution cannot form an effective hydrophobic layer near the interface between the MRCA and the new mortar. When the 10% SMS solution was used, ISMRAC2 showed a noticeable decrease. For the SMRAC3, compared to ISMRAC2, ISMRAC3 did not show a further significant decrease. This indicates that the 10% SMS solution was already effective in forming a good hydrophobic layer near the interface between the MRCA and the new mortar. Therefore, the 10% SMS solution is recommended.
3.6. Coulomb Electric Flux
The results of the coulomb electrical flux (CEF) are shown in
Figure 13. The CEF of the NAC was minimal, indicating that its ability to resist chloride ion penetration was the strongest. When the NA was replaced by the untreated RCA, the CEF of the RAC0 showed the highest value. This is because the adhered mortar of the RCA is porous, making it easier for water and chloride ions to penetrate the RAC0. When SRCA was used, the CEF of the SRAC was slightly affected. This is due to the modification effect of the WG solution, which could enhance the surface layer of the old mortar. However, it has little impact on the overall permeability of the concrete. According to the result for the MRAC, the influence of the SMS-solution-treated RCA on CEF was also insignificant. This is because the structure of the treated RCA was not changed and only a molecular film was formed. Under the saturation conditions, the hydrophobic molecular film could not effectively prevent the transmission of water. When the RCAs were treated with the WG solution combined with the SMS solution, the CEF of the SMRAC2 exhibited the most significant decrease, dropping by 19.67%. Nevertheless, it remained 41.73% higher than that of the NAC. This is due to the improvement of the pore structure within the SMRAC2, leading to the decrease in CEF. However, the porous old mortar of the treated RCA still cannot effectively prevent the penetration of chloride ions in a saturated state.
3.7. Nanoindentation
Figure 14 presents the nanoindentation results, revealing two distinct types of interface transition zones (ITZs). The first type exhibited a traditional weakening pattern, such as I_0, I_S. The modulus within the weakening ITZ was lower than that of the matrix mortar; similar results were also reported in [
32]. The second type showed a strengthening pattern, such as I_M, I_SM8, I_SM10 and I_SM12. The modulus within the strengthened ITZ was higher than that of the matrix mortar. Mechanistically, when the SMS-modified RCA was incorporated into the new concrete system, the SMS coating not only formed a durable bond with the RCA substrate but also underwent chemical interactions with the fresh mortar. These reactions generated expansive products that effectively filled interfacial voids, resulting in the microstructural densification of the ITZ and consequent mechanical improvement. The I_SM10 specimen exhibited the maximum ITZ modulus, demonstrating that the optimal SMS solution concentration for performance enhancement is 10%. This phenomenon can be attributed to the concentration-dependent formation mechanism of hydration products. Increased SMS concentration (up to 10%) promotes the formation of expansive products within the ITZ, effectively reducing porosity through controlled volumetric expansion. Nevertheless, exceeding the critical concentration threshold (>10%) induces excessive products that generate internal stress concentrations, ultimately initiating microcracks that compromise the interfacial integrity and mechanical properties.
3.8. FTIR
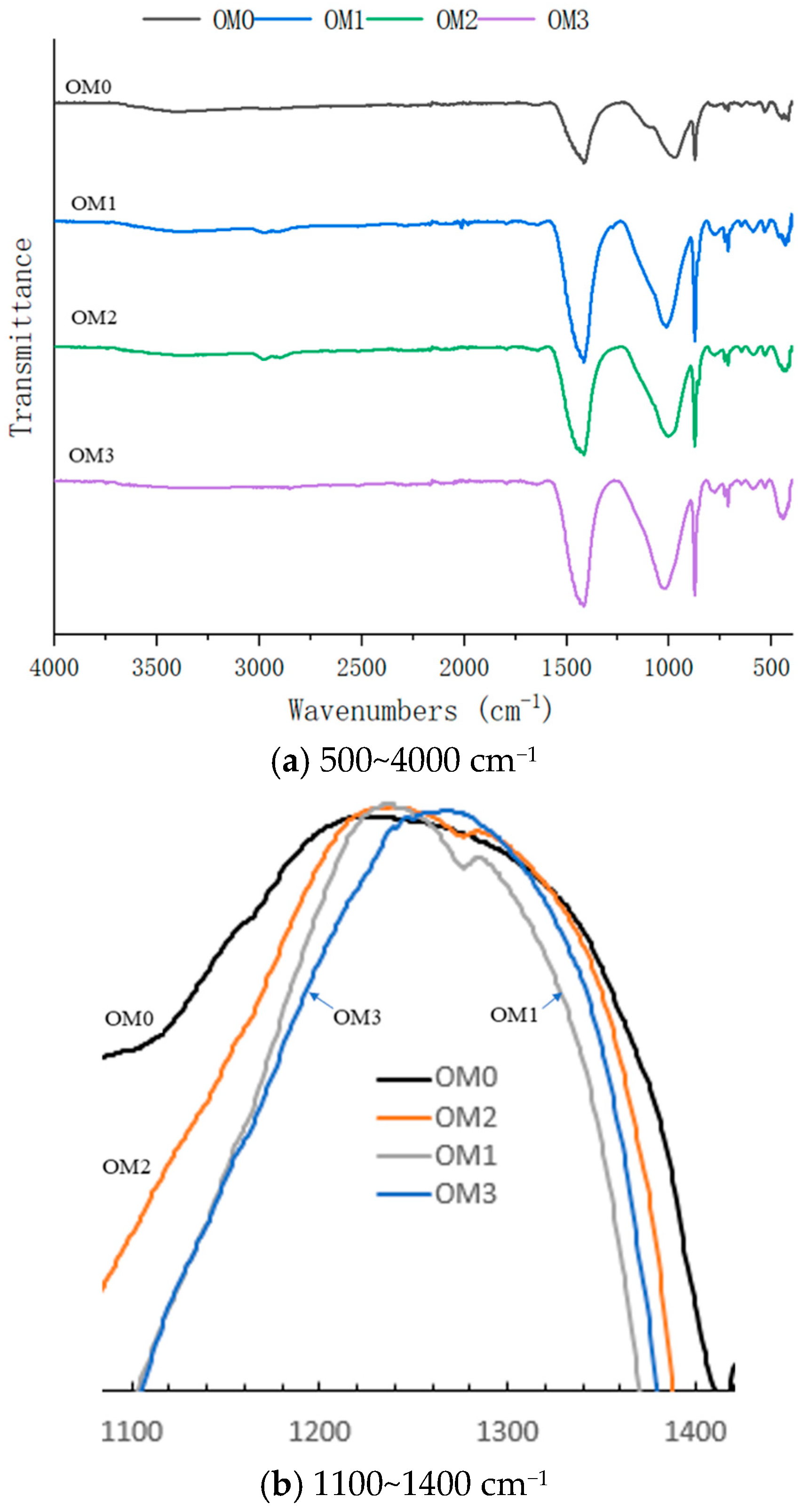
The FTIR results are shown in
Figure 15 and
Figure 16. As shown in
Figure 15a, the Si-O absorption peak appears around 1016 cm
−1, while the C-O absorption peaks are detected at approximately 1410 cm
−1 and 870 cm
−1, with no significant H-O absorption peak [
50,
51]. This suggests that Ca(OH)
2 in the old mortar has reacted adequately with CO
2 from the air [
52,
53]. The infrared spectra of different old mortars are essentially identical and similar to that of common cement concrete. This indicates that neither the WG solution nor the SMS solution significantly influenced the functional groups of the old mortar. Unlike other samples, the old mortar treated with the SMS solution showed an absorption peak around 2960 cm
−1 and 1270 cm
−1, as shown in
Figure 15. This was the absorption peak of the methyl group [
54], which was formed through the interaction between SMS and the old mortar. This chemical modification is responsible for imparting hydrophobicity to the treated old mortar.
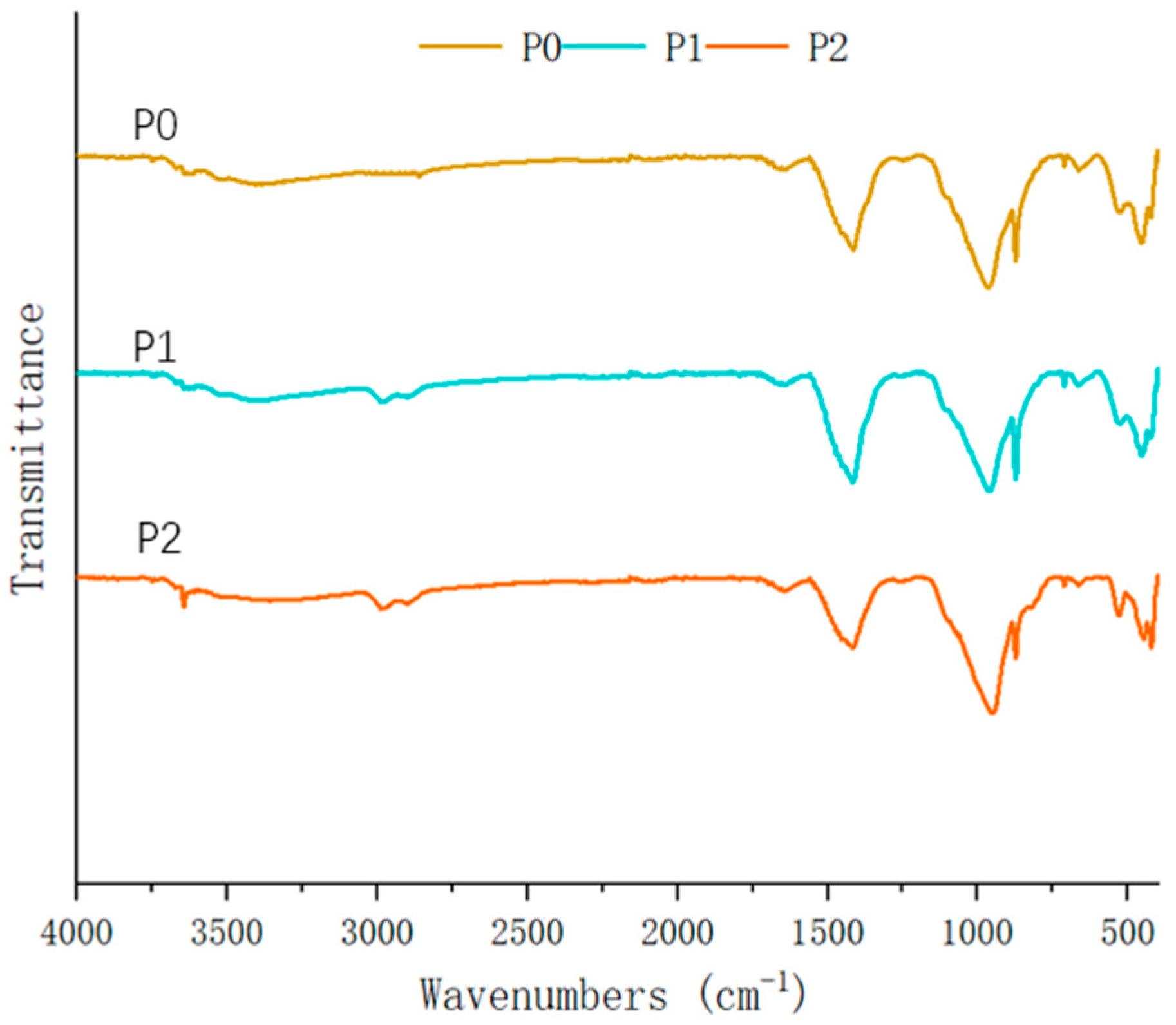
The FTIR analysis of new cement pastes (
Figure 16) revealed spectral characteristics that are fundamentally consistent with the reference sample (P0), indicating that the SMS solution’s interaction with the cement hydration products preserved the essential functional group chemistry of the hardened matrix. Notably, the characteristic absorption band near 2960 cm
−1 appeared, which meant that the methyl group also existed in P1 and P2. In new cement paste systems, the predominant reaction involves the SMS solution interacting with CaO to form calcium methyl silicate, a chemical transformation that fails to impart hydrophobic properties to the cementitious matrix [
45,
55].