Oxygen-Vacancy-Rich V2O5@NC Composite with Enhanced Zinc-Storage Performance for Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization Instruments
2.2. Synthesis of V2O5 Nanoparticles (V2O5 NPs)
2.3. Synthesis of V2O5@NC Composite
2.4. Assembly of Aqueous Zinc-Ion Batteries
3. Results and Analysis
3.1. Material Characterization
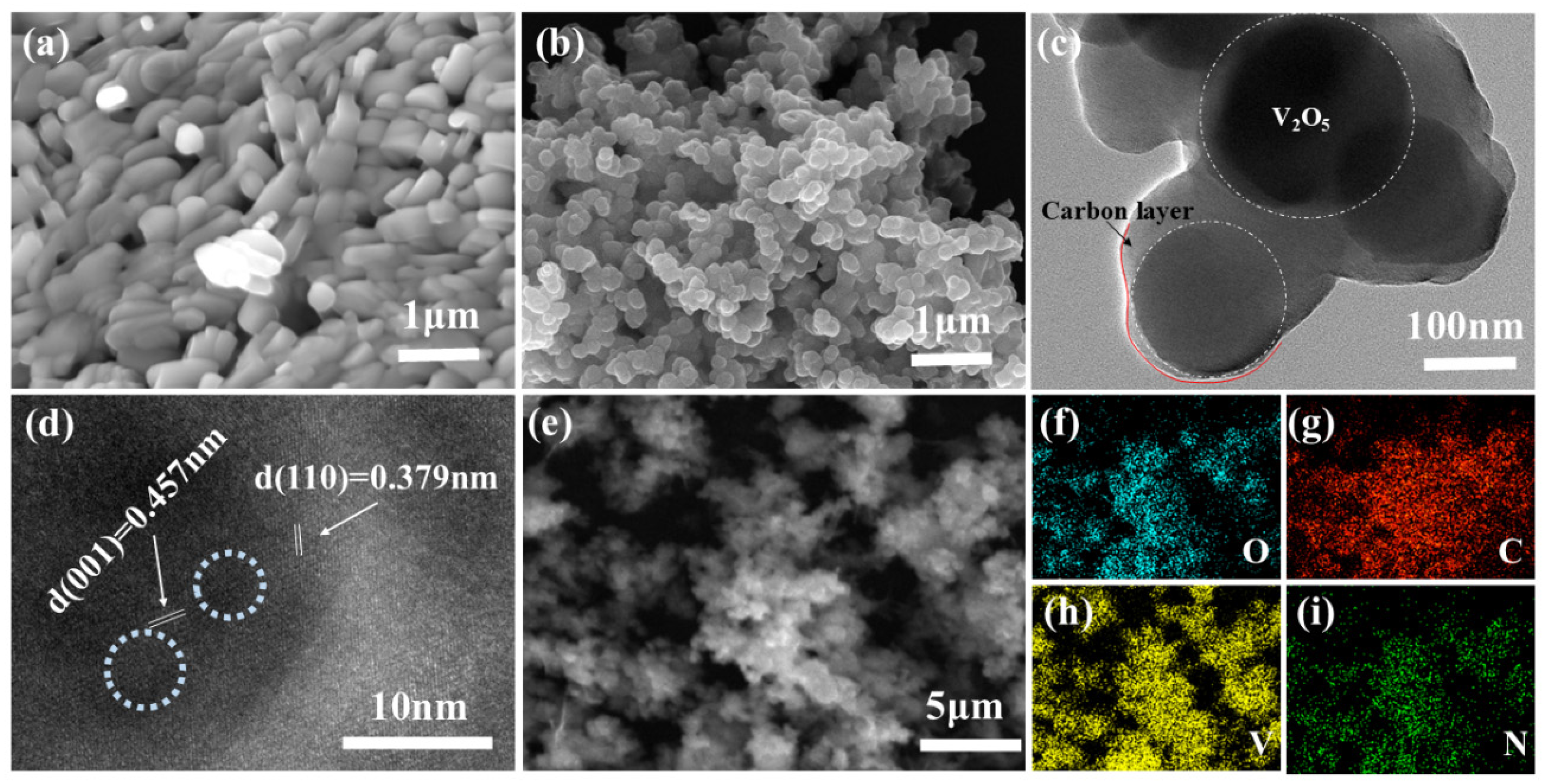
3.1.1. SEM and TEM Characterization
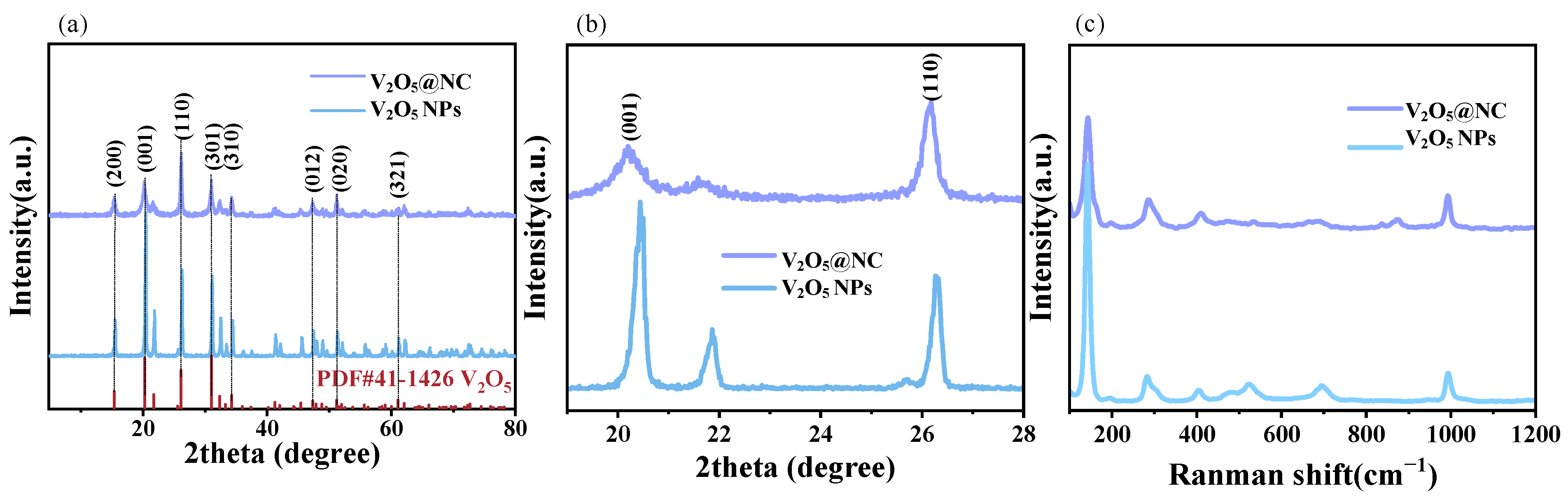
3.1.2. X-Ray Diffraction and Raman Spectroscopy Analysis
3.1.3. X-Ray Photoelectron Spectroscopy and Thermogravimetric Analysis
3.2. Electrochemical Performance of the V2O5@NC Electrode
3.2.1. Cyclic Voltammetry and Galvanostatic Charge–Discharge Tests
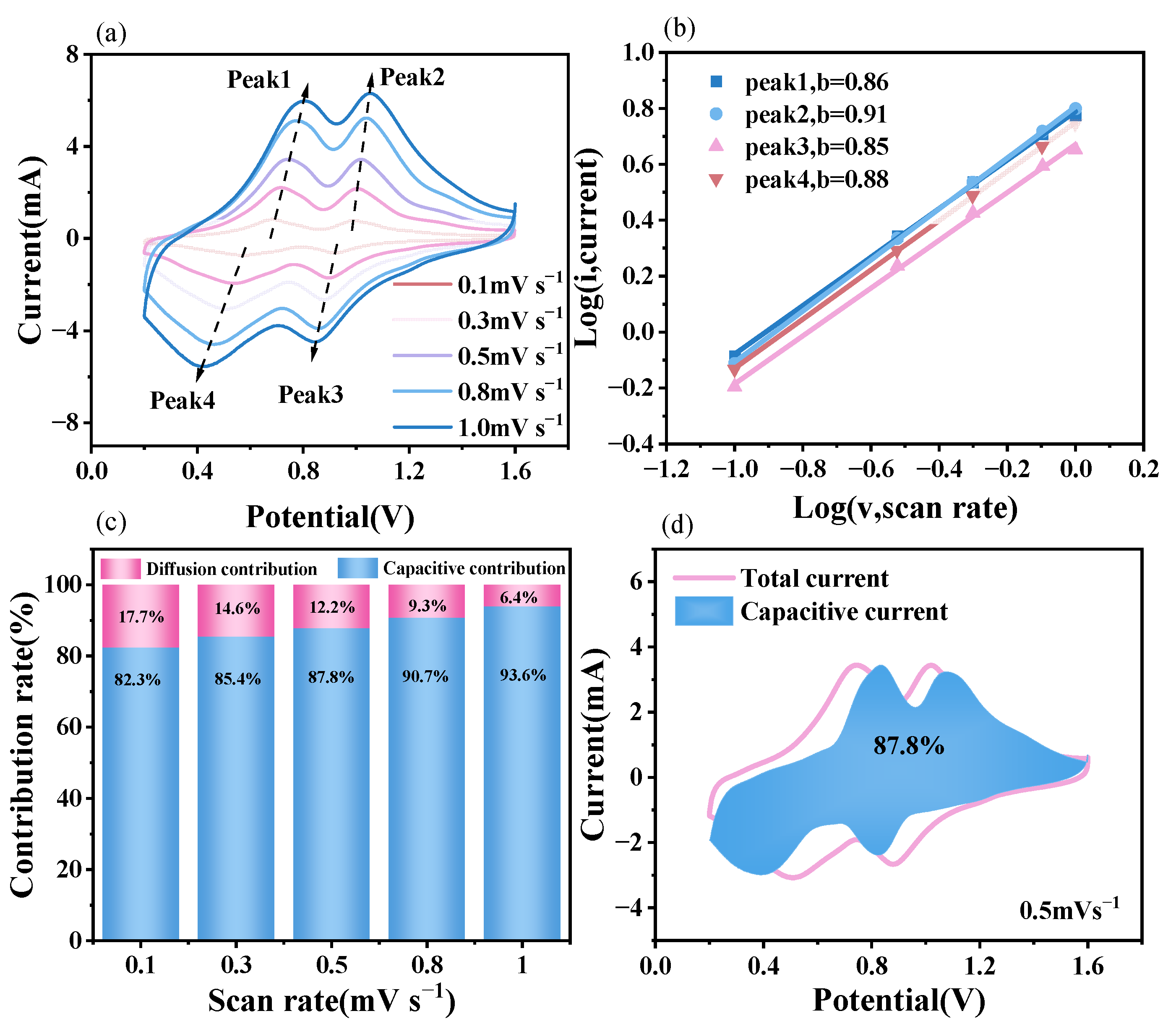
3.2.2. Electrochemical Kinetic Analysis
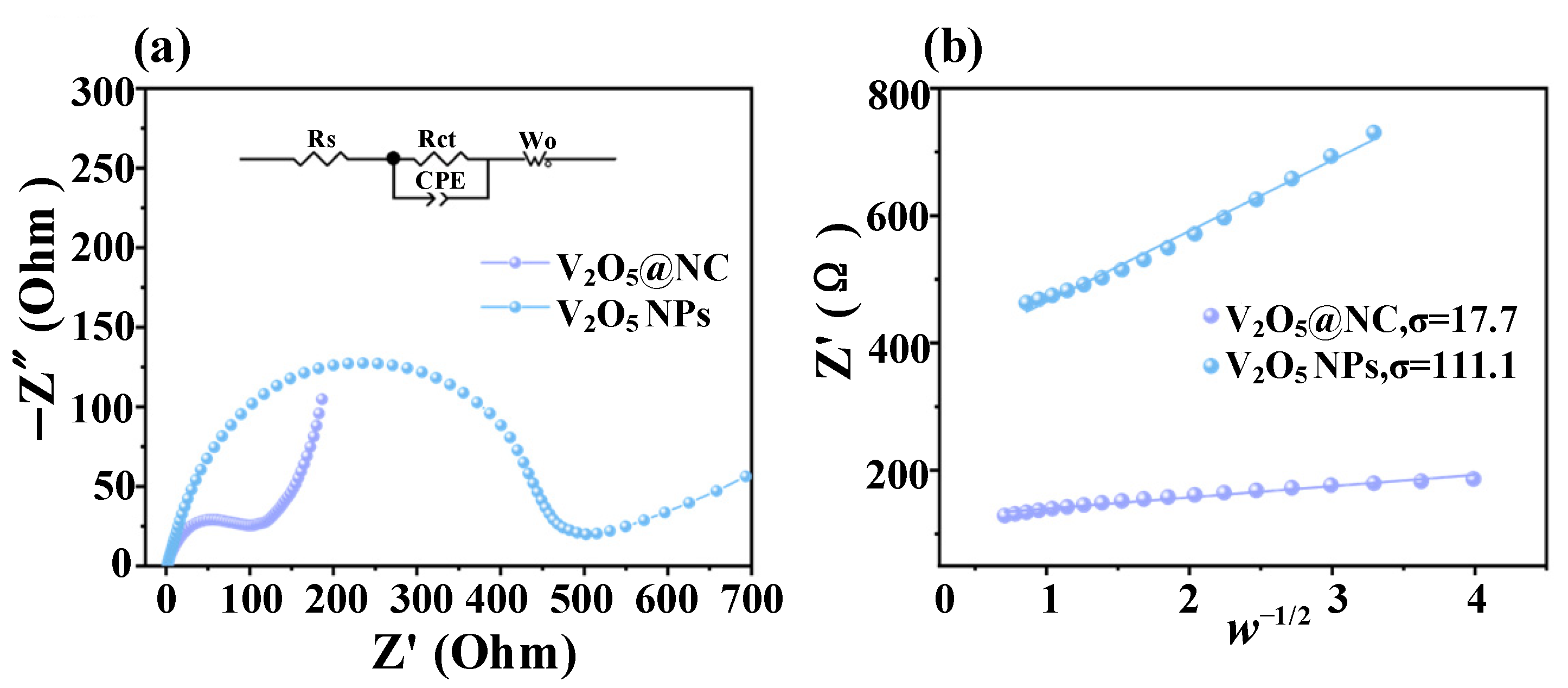
3.2.3. Electrochemical Impedance Spectroscopy (EIS) Analysis
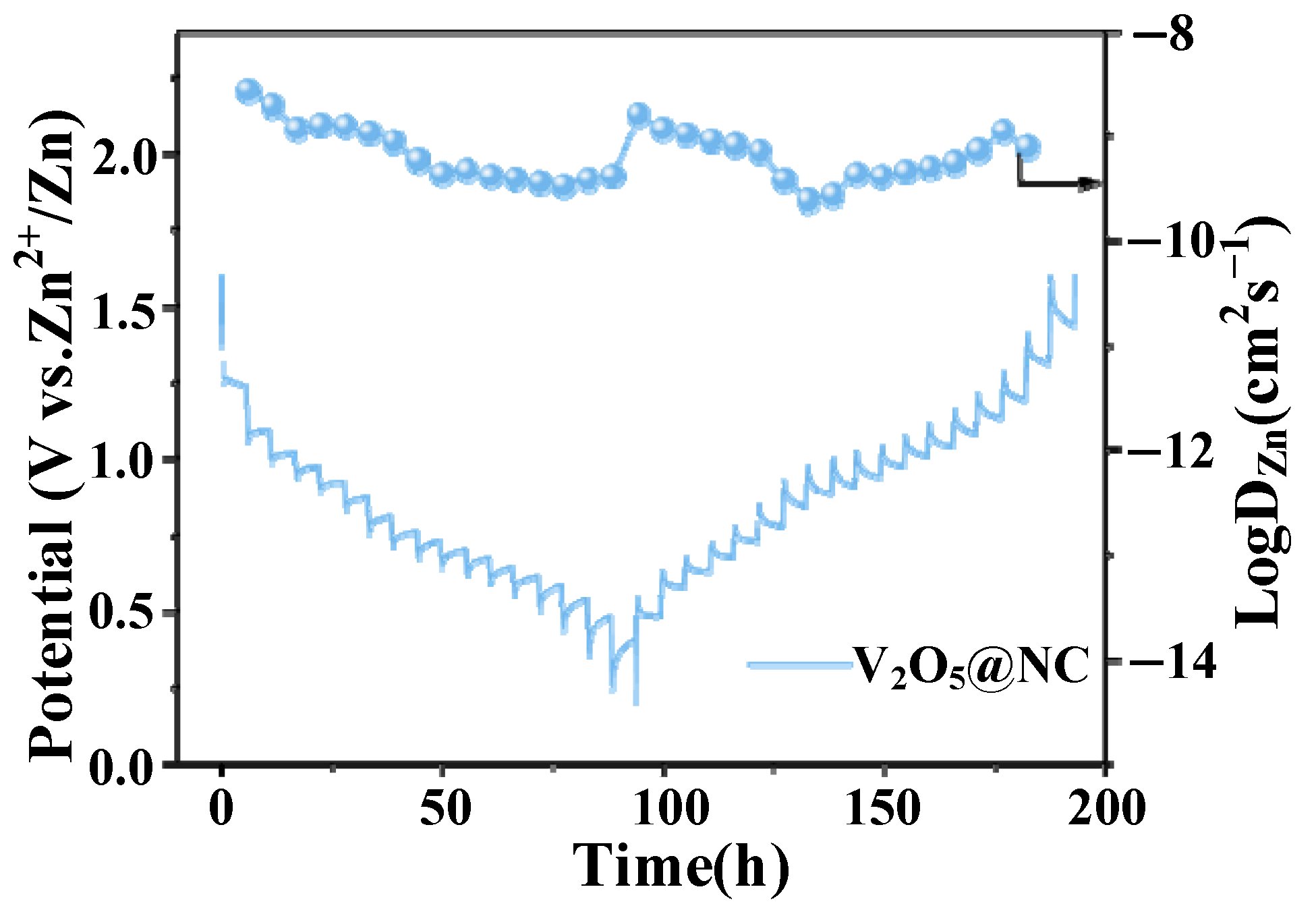
3.2.4. Galvanostatic Intermittent Titration Technique (GITT) Analysis
3.3. Comparative Performance Analysis of Vanadium-Based Cathodes for Aqueous Zn-Ion Batteries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, H.; Zhang, N.; Lu, Y.; Zhang, Z.; Li, M.; Liu, J.; Song, W.; Zhao, Y.; Miao, Z. Strategies toward the development of high-energy-density lithium batteries. J. Energy Storage 2024, 88, 111666–111679. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, B.; Liu, J.; He, X.; Xiao, Z.; Qin, H.; Liu, T.; Amine, K.; Ou, X. NaSICON-type materials for lithium-ion battery applications: Progress and challenges. Nano Energy 2024, 127, 109730. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, Y.; Hua, D.; Gu, H.; Wang, N. Creative method for efficiently leaching Ni, Co, Mn, and Li in a mixture of LiFePO4 and LiMO2 using only Fe(III). ACS Sustain. Chem. Eng. 2021, 9, 3979–3984. [Google Scholar] [CrossRef]
- Xu, J.; Dou, Y.; Wei, Z.; Ma, J.; Deng, Y.; Li, Y.; Liu, H.; Dou, S. Recent Progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries. Adv. Sci. 2017, 4, 1700146. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Xia, Q.; Lei, Y.; Huang, X.L.; Tebyetekerwa, M.; Zhao, X.S. Interface challenges and optimization strategies for aqueous zinc-ion batteries. J. Energy Chem. 2023, 77, 642–659. [Google Scholar] [CrossRef]
- Lu, G.; Yong, T.; Wang, Y.; Yang, Y.; Li, X. Discrete V2O5/NCQDs heterogeneous assemblies synergizing built-in fields and confinement for enhanced aqueous zinc ion storage. Appl. Phys. Lett. 2025, 126, 253903. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, S.; Xiao, Z.; Meng, F.; Li, Q.; Zhang, X.; Zhang, Z.; Zhi, L.; Tang, Z. Layered structure regulation for zinc-ion batteries: Rate capability and cycle ability enhancement by rotatable pillars. Adv. Energy Mater. 2023, 13, 2203810. [Google Scholar] [CrossRef]
- Fei, B.; Liu, Z.; Fu, J.; Guo, X.; Li, K.; Zhang, C.; Yang, X.; Cai, D.; Liu, J.; Zhan, H. In situ induced core–shell carbon-encapsulated amorphous vanadium oxide for ultra-long cycle life aqueous zinc-ion batteries. Adv. Funct. Mater. 2023, 33, 2215170. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. K2V6O16·2.7H2O nanorod cathode: An advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 15530–15539. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Qian, J.; You, Y.; Fan, Z.; Liu, X.; Tang, J.; He, W.; Sun, Z. Mn pre-intercalated hydrated vanadium pentoxide activated by nitrogen plasma for enhanced zinc ion storage. J. Energy Storage 2023, 63, 92–99. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Jiang, H.; Liu, Y.; Sun, J.; Hu, T.; Sun, J.; Meng, C.; Wang, J. On the origin of enhanced electrochemical kinetics in guest-ions pre-intercalated layered vanadium oxides: Interlayer spacing vs lattice distortion. Energy Storage Mater. 2024, 71, 327–343. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, Y.; Chen, S.; Zhang, X.; Li, Z.; Zhu, L.; Mo, L.E.; Hu, L. Hierarchical porous metallic V2O3@C for advanced aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 44109–44117. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z.; Sun, F.; Zhang, Y.; Li, H.; Liu, Y.; Ma, T. Oxygen-vacancy-reinforced vanadium oxide/graphene heterojunction for accelerated zinc storage with long life span. Small 2024, 20, 2306275. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, W.; Zhang, Y.; Liu, X.; Waqar, M.; Sun, J.; Liu, Y.; Dong, X.; Meng, C.; Pan, Z.; et al. Quench-tailored Al-doped V2O5 nanomaterials for efficient aqueous zinc-ion batteries. J. Energy Chem. 2022, 70, 52–58. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Yue, Y.; Pakornchote, T.; Bovornratanaraks, T.; Sawangphruk, M.; Zhang, X.; Qin, J. Revealing the impacts of oxygen defects on Zn2+ storage performance in V2O5. Mater. Today Energy 2021, 21, 100824. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, P.; Zhang, R.; Liu, Z.; Li, W.; Pan, Z.; Yang, H.; Shen, X.; Wang, J. Oxygen-defective V2O5 nanosheets boosting 3D diffusion and reversible storage of zinc ion for aqueous zinc-ion batteries. Appl. Surf. Sci. 2021, 562, 150196. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; He, P.; Zhang, Z.; Chen, T. Edge-rich vertical graphene nanosheets templating V2O5 for highly durable zinc ion battery. Carbon 2021, 172, 207–213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jiang, H.; Sun, J.; Feng, Z.; Hu, T.; Meng, C.; Pan, Z. Synergistic engineering of oxygen-defect and heterojunction boosts Zn2+ (De)intercalation kinetics in vanadium oxide for high-performance zinc-ion batteries. Chem. Eng. J. 2022, 435, 134949–134956. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Meng, J.; Yang, Z.; Wu, J.; Rong, Y.; Deng, L.; Shi, Y. Synergistic effects in V3O7/V2O5 composite material for high capacity and long cycling life aqueous rechargeable zinc ion batteries. J. Power Sources 2020, 474, 228569–228577. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Man, J.; Sun, J. A novel organic-inorganic hybrid V2O5@polyaniline as high-performance cathode for aqueous zinc-ion batteries. Mater. Lett. 2020, 272, 127813–127817. [Google Scholar] [CrossRef]
- Li, S.; Zhou, T.; Cheng, Y.; Li, X. Preparation of V2O5 composite cathode material based on in situ intercalated polyaniline and its high-performance aqueous zinc-ion battery applications. Materials 2025, 18, 2166. [Google Scholar] [CrossRef]
- Ji, F.; Yu, J.; Hou, S.; Hu, J.; Li, S. Doping engineering in manganese oxides for aqueous zinc-ion batteries. Materials 2024, 17, 3327. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, J.; Wu, T.; Li, R.; Zhao, X.; Pang, Y.; Pan, Z. Rational design of Ni-Doped V2O5@3D Ni core/shell composites for high-voltage and high-rate aqueous Zinc-Ion batteries. Materials 2024, 17, 215. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Lu, A. Using confined carbonate crystals for the fabrication of nanosized metal oxide@carbon with superior lithium storage capacity. J. Mater. Chem. A 2016, 4, 15030–15035. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, J.; Liu, Z.; He, H. Carbon-coated hydrated vanadium dioxide for high-performance aqueous zinc-ion batteries. J. Alloys Compd. 2022, 906, 164388. [Google Scholar] [CrossRef]
- Cui, J.; Ma, J.; Yuan, H. Direct synthesis of vanadium pentoxide powder with carbon recombination as aqueous zinc-ion battery cathode. J. Appl. Electrochem. 2024, 54, 289–299. [Google Scholar] [CrossRef]
- Nzengu, P.; Hlongwa, N.; Sekhosana, K.; Kebede, M. Enhancing vanadium pentoxide-based (V2O5) cathodes for high-performance aqueous zinc-ion batteries: Optimization of interlayer spacing, ion kinetics, voltage window. RSC Adv. 2025, 15, 34362–34398. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.; Dhinasekaran, D.; Subramanian, B.; Rajendran, A.R. Dual ion insertion and oxygen vacancy engineering in nanostructured V2O5 cathodes for enhanced Zn-ion battery performance and stability. J. Power Sources 2025, 636, 236593. [Google Scholar] [CrossRef]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Lei, H.; Wang, Z.; Liu, B.-T.; Cai, X.; Mai, W. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Cui, F.; Meng, J.; Chen, H.; Zeng, X. Enhanced rate and cycling performances of hollow V2O5 nanospheres for aqueous zinc ion battery cathode. Appl. Surf. Sci. 2020, 507, 145137. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Sun, S.; Bergh, W.; Huang, K. Synergistic H+/Zn2+ dual ion insertion mechanism in high-capacity and ultra-stable hydrated VO2 cathode for aqueous Zn-ion batteries. Energy Storage Mater. 2020, 29, 60–70. [Google Scholar] [CrossRef]
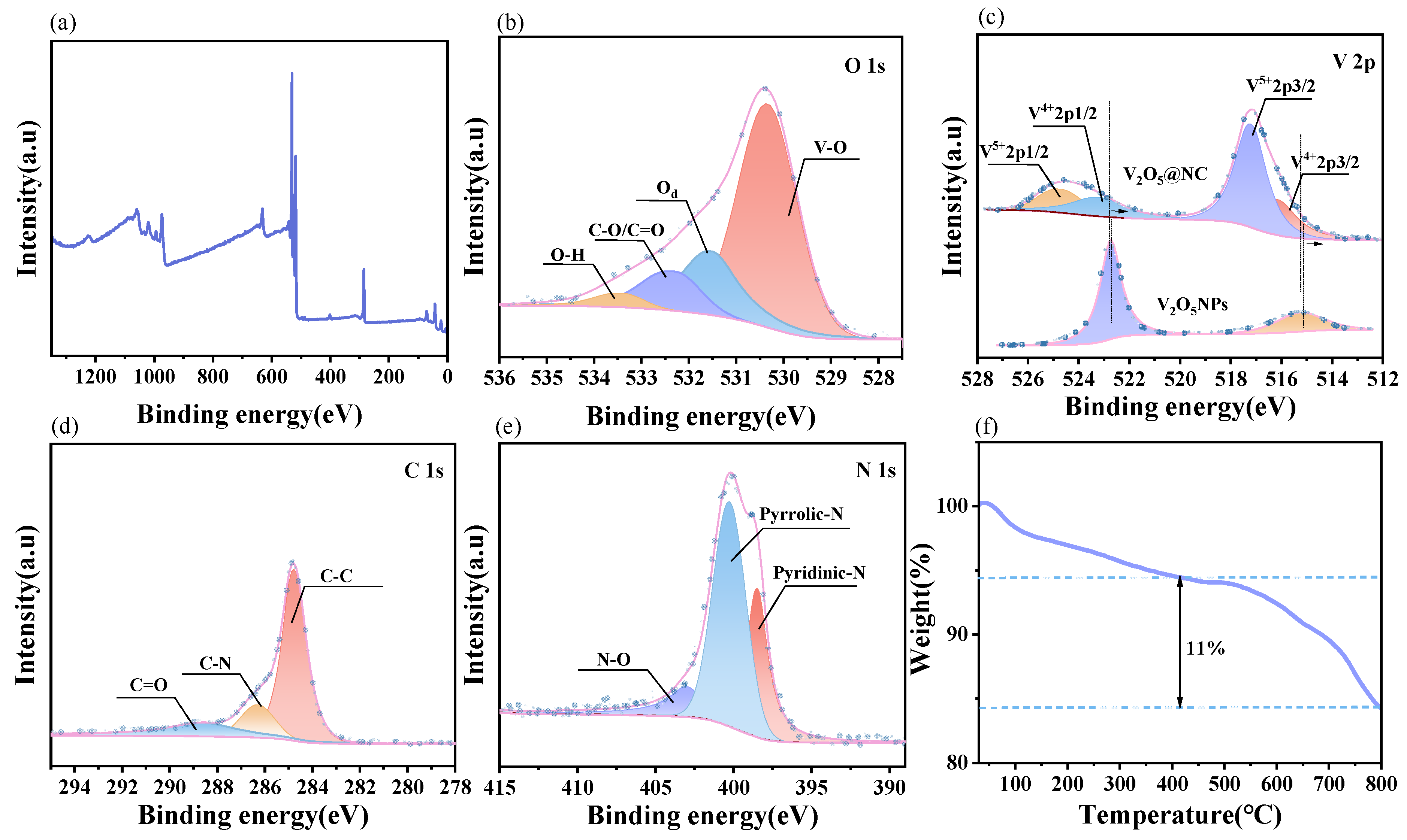

| Sample | V–O (Lattice Oxygen) | Od (Oxygen Vacancy) | C–O/C=O | O–H | Total O (%) |
|---|---|---|---|---|---|
| Pristine V2O5 | 72.1% | 6.7% | 14.3% | 6.9% | 100 |
| V2O5@NC composite | 59.4% | 15.3% | 17.1% | 8.2% | 100 |
| Sample | Rs (Ω) | Rct (Ω) | CPE-T | CPE-P | Wo-R (Ω) | Wo-T (s) | Wo-P |
|---|---|---|---|---|---|---|---|
| V2O5@NC | 12.5 | 94.0 | 3.12 × 10−4 | 0.89 | 98.5 | 8.6 × 10−3 | 0.71 |
| V2O5 NPs | 15.8 | 437.0 | 2.76 × 10−4 | 0.87 | 195.3 | 1.1 × 10−2 | 0.73 |
| Ref. | Cathode Material | Modification Strategy | Specific Capacity | Rate Capability | Cycling Stability |
|---|---|---|---|---|---|
| This paper | V2O5@NC | Oxygen-vacancy & N-doped carbon dual regulation | 437 @ 0.1 A g−1 | 252 @ 3 A g−1 | 89.3% after 2000 cycles |
| [21] | V3O7/V2O5 | Synergistic phase interaction | 176 @ 5 A g−1 | high | 96.2% after 1120 cycles |
| [35] | H-VO2 | H+/Zn2+ dual-ion insertion | 410 @ 0.1 A g−1 | 200 @ 5 A g−1 | 88% after 200 cycles |
| [14] | V2O3@C | Porous conductive carbon hybrid | 350 @ 0.1 A g−1 | 250 @ 2 A g−1 | 90% after 4000 cycles |
| [22] | V2O5@PANI | Organic–inorganic composite | 361 @ 0.1 A g−1 | — | 93.8% after 1000 cycles |
| [23] | PANI–V2O5 (in situ intercalated) | Interlayer expansion by PANI chains | 450 @ 0.1 A g−1 | 220 @ 2 Ag−1 | 96.7% after 300 cycles@ 1 A g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Liang, P.; Li, S.; Cheng, Y.; Li, X. Oxygen-Vacancy-Rich V2O5@NC Composite with Enhanced Zinc-Storage Performance for Aqueous Zinc-Ion Batteries. Materials 2025, 18, 5216. https://doi.org/10.3390/ma18225216
Zhou T, Liang P, Li S, Cheng Y, Li X. Oxygen-Vacancy-Rich V2O5@NC Composite with Enhanced Zinc-Storage Performance for Aqueous Zinc-Ion Batteries. Materials. 2025; 18(22):5216. https://doi.org/10.3390/ma18225216
Chicago/Turabian StyleZhou, Taoyun, Pingyuan Liang, Shilin Li, Yun Cheng, and Xinyu Li. 2025. "Oxygen-Vacancy-Rich V2O5@NC Composite with Enhanced Zinc-Storage Performance for Aqueous Zinc-Ion Batteries" Materials 18, no. 22: 5216. https://doi.org/10.3390/ma18225216
APA StyleZhou, T., Liang, P., Li, S., Cheng, Y., & Li, X. (2025). Oxygen-Vacancy-Rich V2O5@NC Composite with Enhanced Zinc-Storage Performance for Aqueous Zinc-Ion Batteries. Materials, 18(22), 5216. https://doi.org/10.3390/ma18225216




