Bilayer Coating Systems: Functional Interlayers and Top Layers for Enhanced Performance
Abstract
1. Introduction
- Deposition of sublayers to improve adhesion, strength, and corrosion resistance using electroless and electroplating, application of oxide, phosphate or chromate conversion coatings;
- Surface modification of coatings through impregnation, hydrophobization, and vacuum-arc deposition to enhance corrosion resistance and tribological performance.
2. Functional Sublayers Deposited on Various Metallic Substrates to Improve Adhesion, Mechanical Strength, and Corrosion Resistance of Coatings
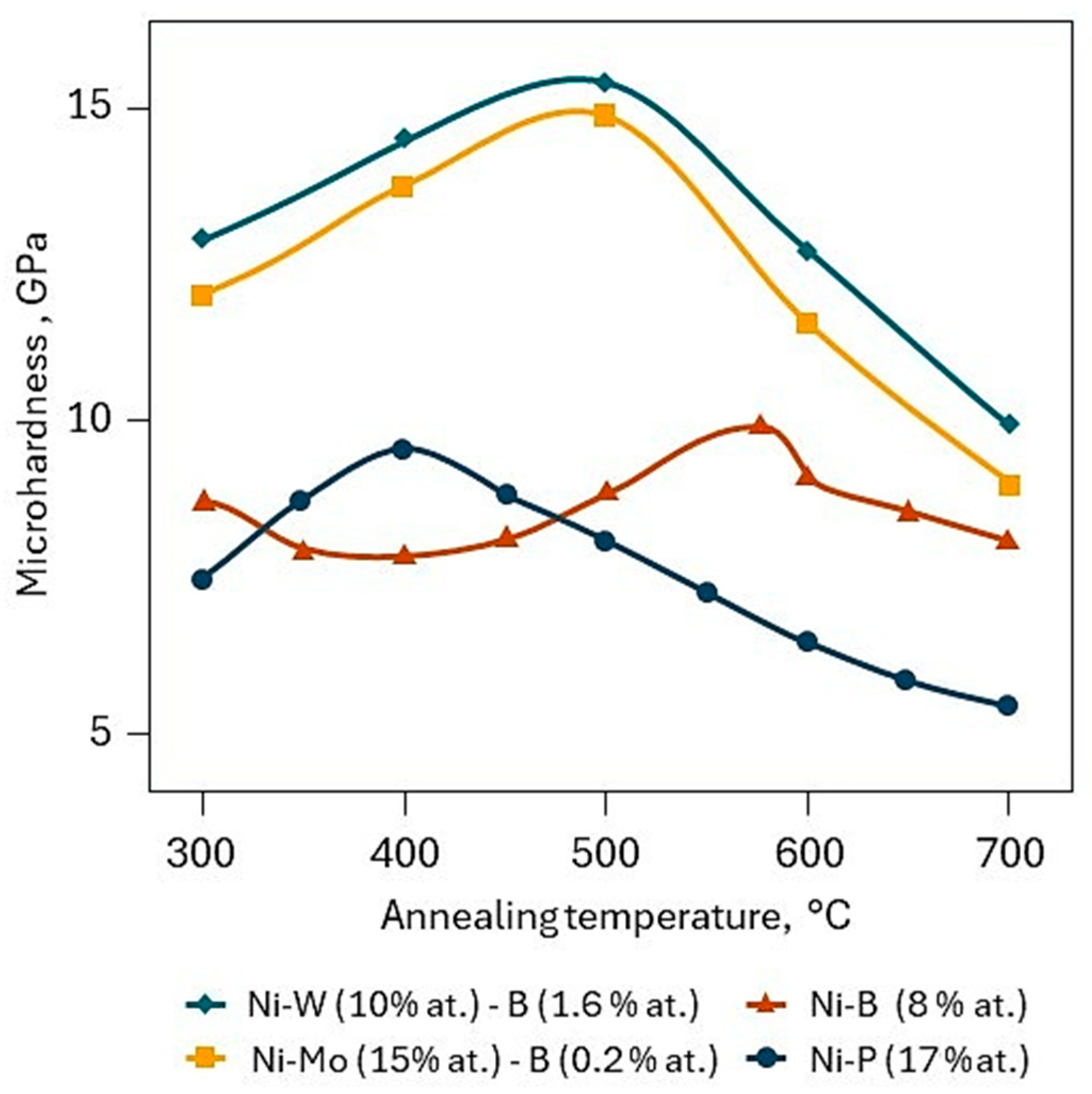
2.1. Ni–P Support Layers for Chemical Vapor Deposition (CVD) W–C Coatings
2.2. Conversion Coatings
2.2.1. Phosphate Conversion Coatings (PCCs)
2.2.2. Chromate Conversion Coatings (CCCs)
- Porosity—CCCs are porous before drying. Thicker coatings generally contain fewer pores.
- Hardness—higher chromating solution temperatures produce harder coatings;
- Thickness—determined by the solution composition, particularly pH. Lower pH values result in thicker coatings. Thick CCCs are less wear-resistant initially, but their wear resistance increases upon drying [44].
- Adhesion and plasticity—CCCs are generally sufficiently plastic, and cracks formed during deformation can self-heal to some extent [46].
- Transparent CCCs—formed using trivalent chromium compounds. These coatings allow the natural color of the metal to show through, making them suitable for industries focused on appearance, such as automotive and electronics. They provide corrosion resistance and are suitable for outdoor and marine applications [48,49,50,51].
- Yellow CCCs—formed using hexavalent chromium compounds, which impart a bright yellow color to the metal. These coatings are used for decorative purposes, such as in plumbing and equipment, and offer superior corrosion resistance, making them versatile for various applications. The formation of transparent CCCs is considered more environmentally friendly compared to yellow CCCs.
- Change to phosphate and oxide (chemical) conversion coatings in comparison with CCCs and less toxic substances are used to apply phosphate and oxide-conversion coatings. A moderate level of energy consumption characterizes these coating methods;
- Use other anti-corrosion coating technologies: electroless and electroplating, chemical heat treatment, hydrophobization, and vacuum arc spraying. However, in most cases, these coating methods consume a lot of energy;
- Create recyclable coatings. Here, it is possible to use polymer materials suitable for recycling; the development of formulations with the possibility of recovery; the use of biodegradable components in some types of coatings.
2.2.3. Oxide-Conversion Coatings (OCCs)
- Electrochemical—oxidation by oxygen generated on the metal surface as a result of an electrochemical process. Usually, products are treated at the anode in an alkaline solution with added oxidizers. Due to the complexity of the process, this method has found limited application [55].
- Chemical—oxidation of metal in liquid media. This type of oxidation is widely used to obtain protective and decorative coatings of black or dark blue color. The process is carried out in concentrated caustic solutions with added oxidizers such as sodium or potassium nitrates. The protective ability of such OCs is significantly increased if phosphoric acid and nitrates of certain metals are added to the solution [55].
- Steel (steel blackening)—a process of forming an iron oxide film 1 ÷ 10 µm thick on the surface of steel and cast iron. Stainless steels are not oxidized, while alloyed and high-alloy steels are more difficult to oxidize. Types of blackening include: alkaline—conducted in alkaline solutions with oxidizers at 135 ÷ 150 °C; acidic—conducted in acidic solutions chemically or electrochemically [55]. Since alkaline bluing uses caustic solutions at temperatures significantly above the boiling point of water, and the process is long, it is difficult to call it convenient or environmentally friendly. Therefore, research continues to improve steel blackening methods. Iron dissolution rate depends on chemical composition and microstructure: high-carbon steels oxidize faster than low-carbon steels. Steel composition affects the OC color: low-carbon steels produce deep black coatings, while high-carbon steels yield black with a grayish tint. OC on steel has a fine-crystalline, microporous structure. To enhance shine and protective properties, the coating is impregnated with oil (mineral or vegetable), which fills the pores, improving corrosion resistance, wear resistance, and deepening the black color. Oiled OCs on steel are used for corrosion protection, decorative finishing, and as an anti-glare coating on tools [56,57,58].
- Copper—highly resistant to corrosion due to its position in the electrochemical series. Oxide-conversion coatings on copper and its alloys are used for blackening, increasing the light absorption of optical components, decorative finishing, and improving adhesion for bonding. Copper OCs can be obtained by:
- Thermal (hot oxidation)—copper and its alloys are heated in the presence of an oxidizer (e.g., molten nitrate or oxygen-rich atmosphere). Temperature and time determine the thickness and color of the thermal OC: higher temperatures produce thicker, darker coatings. This method forms thick, durable OCs but requires high temperatures and precise control [34,36,59,60].
- Electrochemical (anodizing)—copper or copper alloy acts as the anode. Electrolytes are usually alkaline solutions, most often sodium hydroxide. Current density, voltage, temperature, and duration determine color and thickness. For example, in 0.25 N NaOH at 40 °C, current density 1 ÷ 2 A/dm2, and 10 min, light-colored anodized OC forms; 2 ÷ 4 A/dm2 produces darker, nearly blue coatings. Increasing temperature to 60 °C expands the current density range for dark shades to 2 ÷ 6 A/dm2. At 2 ÷ 3 A/dm2, brown films with bluish-green tints can be obtained in NaOH solutions of 0.25 ÷ 1.0 N. Anodized OCs have good adhesion and corrosion resistance [37,61,62,63].
- Chemical oxidation—copper is treated with solutions containing oxidizers, commonly persulfate or copper-ammonia solutions. Persulfate gives black coatings superior mechanical and anti-corrosion properties compared to copper-ammonia solutions. Alloys with less than 90% copper may require lower persulfate concentration or pre-coppering. Copper alloys with 50 ÷ 65% copper produce black films with a bluish tint, 2 ÷ 3 µm thick [60,64,65,66,67]. Chemical oxidation is simpler than electrochemical, but coating quality depends on solution composition and treatment conditions. Post-treatment with oil, wax, or lacquer improves corrosion resistance and appearance. The choice of method depends on coating requirements and production conditions.
- Zinc is a chemically reactive metal. Under conditions of high humidity and in chemically aggressive environments, zinc coatings corrode relatively rapidly, which inevitably deteriorates the appearance of the product. In this regard, the formation of an oxide-conversion coating (OCC) on the zinc surface significantly slows down the corrosion process, improves the adhesion of paint and varnish materials, and provides a decorative appearance to the zinc product (serving as one of the methods for blackening zinc). There are two main approaches to zinc oxidation. The thermal method involves cleaning and degreasing the surface, followed by treatment with a solution composed of equal parts of 25% copper acetate and 30% acetic acid. The sample is then heated to 300 °C for 2 min, and the procedure is repeated twice. The chemical method, in turn, employs a solution with the following composition (g/L): phosphoric acid, 2 ÷ 10; sodium nitrate, 70 ÷ 100. The treatment duration for zinc products is 30 ÷ 40 min at a temperature of 80 ÷ 100 °C. As a result of these processes, a smooth and matte OCC with good electrical insulating properties and a thickness of up to 40 µm is formed on the zinc surface. To further enhance corrosion resistance, it is recommended to carry out an additional treatment with oil [37,68,69,70].
- Aluminum is also known as a highly reactive metal; however, its surface is naturally covered by a protective passive film of Al2O3 with a thickness of 2 ÷ 5 nm, which significantly retards corrosion processes under atmospheric conditions. Nevertheless, due to its limited thickness, this oxide film does not provide sufficient corrosion resistance or adequate physical and mechanical properties in aggressive environments. To improve these characteristics, the thickness of the oxide film must be artificially increased, that is, through oxidation. In this context, three main oxidation methods are employed to form oxide-conversion coatings (OCCs) on aluminum surfaces: chemical oxidation, anodizing, and plasma electrolytic oxidation (PEO) [36,37,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
- Titanium belongs to the class of transition metals and exhibits remarkable stability in many environments, maintaining its resistance at room temperature and in air up to 550 °C. This corrosion resistance is attributed to the presence of a thin but dense oxide film on its surface. The thickness of this film reaches 5 ÷ 20 nm, which is slightly greater than that of aluminum, but is significantly stronger on titanium. The natural oxide layer on titanium primarily consists of rutile (β–TiO2) and anatase (α–TiO2). At temperatures above 600 °C, titanium actively reacts with oxygen, forming pure rutile. To enhance the protective capability of the natural oxide film on titanium, as well as improve its antifriction and physicomechanical properties, anodizing or PEO processes are employed [87,88,89,90,91,92,93,94].
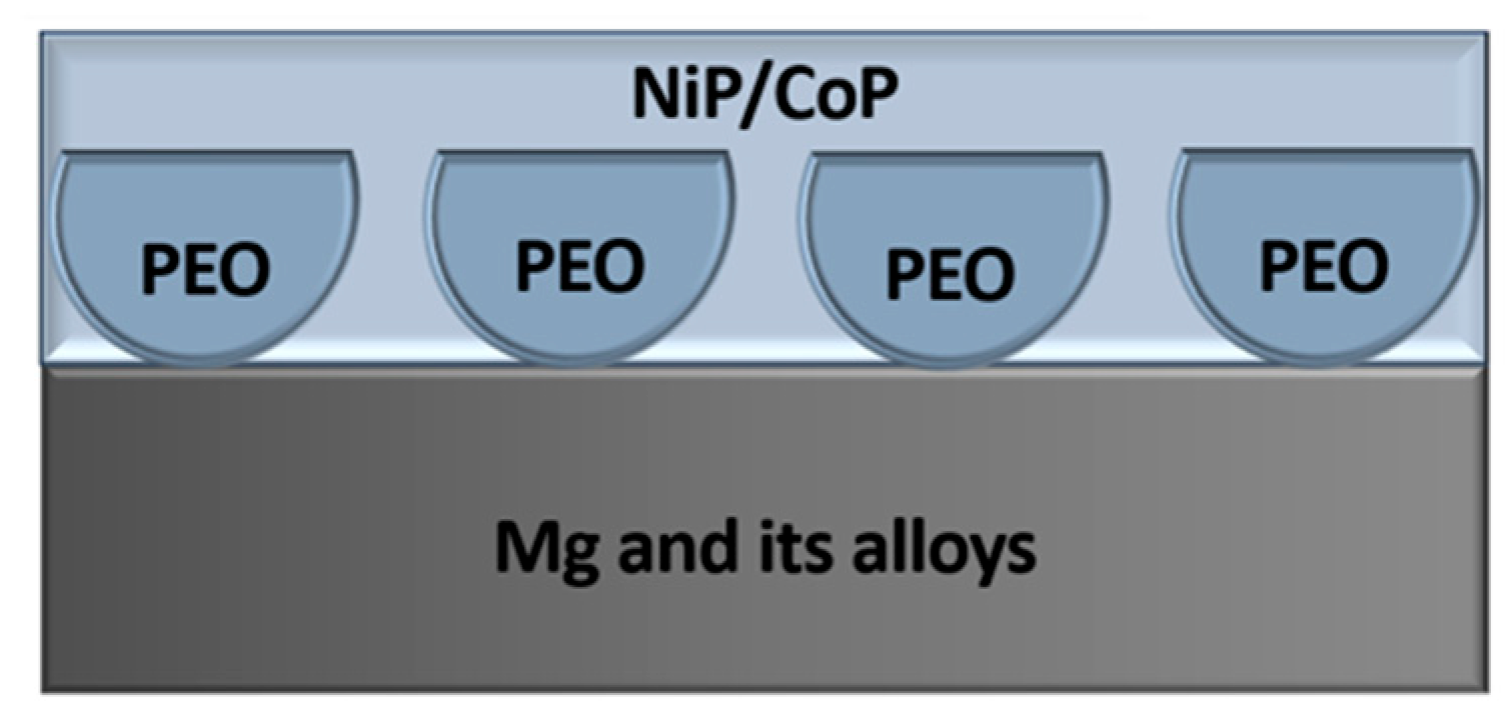
- Magnesium is the eighth most abundant element on Earth, providing ample resources for the use of Mg and its alloys across various engineering sectors. The advantages of magnesium alloys include high strength, low weight, and non-toxicity to both the environment and the human body [95,96,97,98,99,100]. However, magnesium possesses a highly electronegative electrode potential and exhibits a poor protective capability of its surface films due to their defective nature [99,100,101,102,103,104]. To ensure effective protection of magnesium and its alloys, chemical oxidation formulations have been developed, in which the main components are chromium salt compounds. As a result, oxychromate oxide-conversion coatings (OCCs) with a thickness of several micrometers are formed on the magnesium surface, with their color depending on the composition of the solution and the alloy used [105]. Due to the toxicity of such solutions, alternative methods have been developed to improve the corrosion resistance of oxide layers on magnesium parts and components. Currently, anodizing, the Dow-17 process, and PEO are the most widely employed techniques [98,100,106,107,108,109,110,111,112,113,114,115,116].
- Chemical Oxidation of Aluminum
- Anodizing Aluminum, Titanium, Magnesium
- Aluminum
- Hard anodizing involves electrolytes composed of acid mixtures—sulfuric acid combined with oxalic, acetic, boric, or orthophosphoric acids, chromic trioxide, and various organic compounds. The electrolyte temperature is typically 20 ÷ 30 °C. This method is widely used in modern industry to form thin, durable anodic OCCs on aluminum and its alloys [134,135,136,137].
- Warm anodizing is performed at 15 ÷ 20 °C. Aluminum is treated until a light milky film forms, then rinsed with cold water and dyed with aniline-based solutions. This process produces esthetically pleasing surfaces but provides limited protection under harsh conditions, offering lower corrosion, chemical, and mechanical resistance. Such coatings, however, serve well as substrates for paint finishes [138,139,140,141].
- Titanium
- Stationary mode—anodizing is carried out at a constant current density of 1 ÷ 1.5 A/dm2 with voltage increasing from 5 to 25 V. The resulting anodic coating has a thickness of 6 ÷ 12 µm.
- Pulse mode—the direct current source provides short pulses of 0.1 ÷ 0.3 s at a frequency of 120 pulses per minute, exceeding the working current by 5 ÷ 8 times. To obtain a coating thickness of 15 ÷ 20 µm, a current source capable of providing up to 50 A/dm2 is required. At the end of the process, the voltage rises to 250 V, and bath cooling is necessary. This procedure produces layer-by-layer compaction of the coating, resulting in low porosity. Coatings obtained from sulfuric acid electrolytes have a specific electrical resistance of σ = 3.7 × 10−8 Ω × cm [88,145,146,147,148,149].
- Magnesium
- Plasma Electrolytic Oxidation (PEO) of Aluminum, Titanium, Magnesium
- Electrolytes that do not contain components forming insoluble oxides, such as sulfuric acid, phosphoric acid, or alkaline solutions. In these electrolytes, the coating grows into the metal through its oxidation.
- Electrolytes containing cations or anions that form insoluble oxides or hydrolysis products, including aluminate, silicate-alkaline solutions, and solutions containing soluble phosphates, bicarbonates, and molybdates. After thermolysis, these electrolyte components are incorporated into the coating within the discharge zones, contributing to an increase in the coating thickness.
- Outer (technological) layer—loose; in alkaline electrolytes with liquid glass, it consists of mullite (Al2O3 × 2SiO2).
- Inner (working) layer—dense, with high microhardness, composed of aluminum oxide (Al2O3).
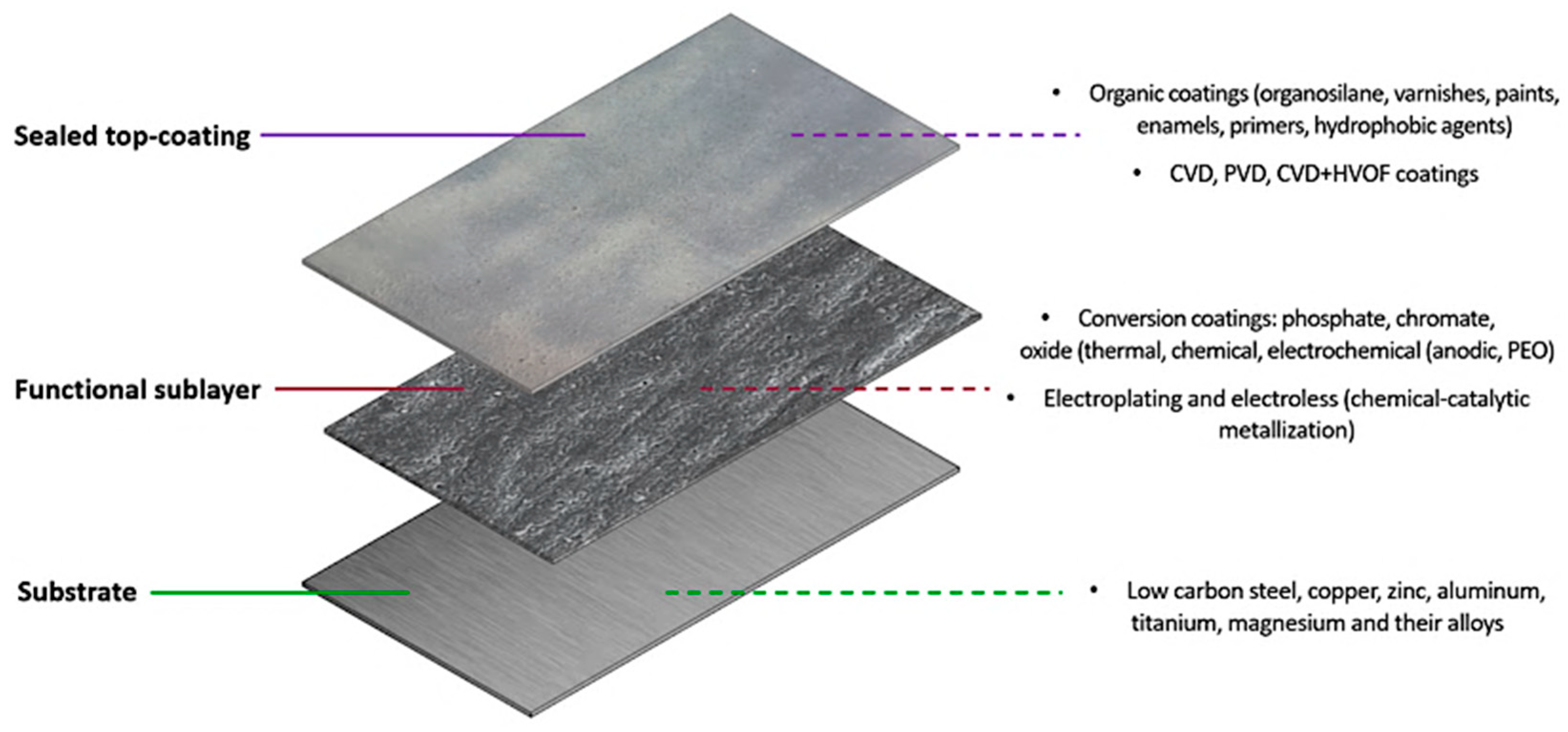
3. Overlayers on Functional Coatings to Enhance Mechanical and Corrosion Properties
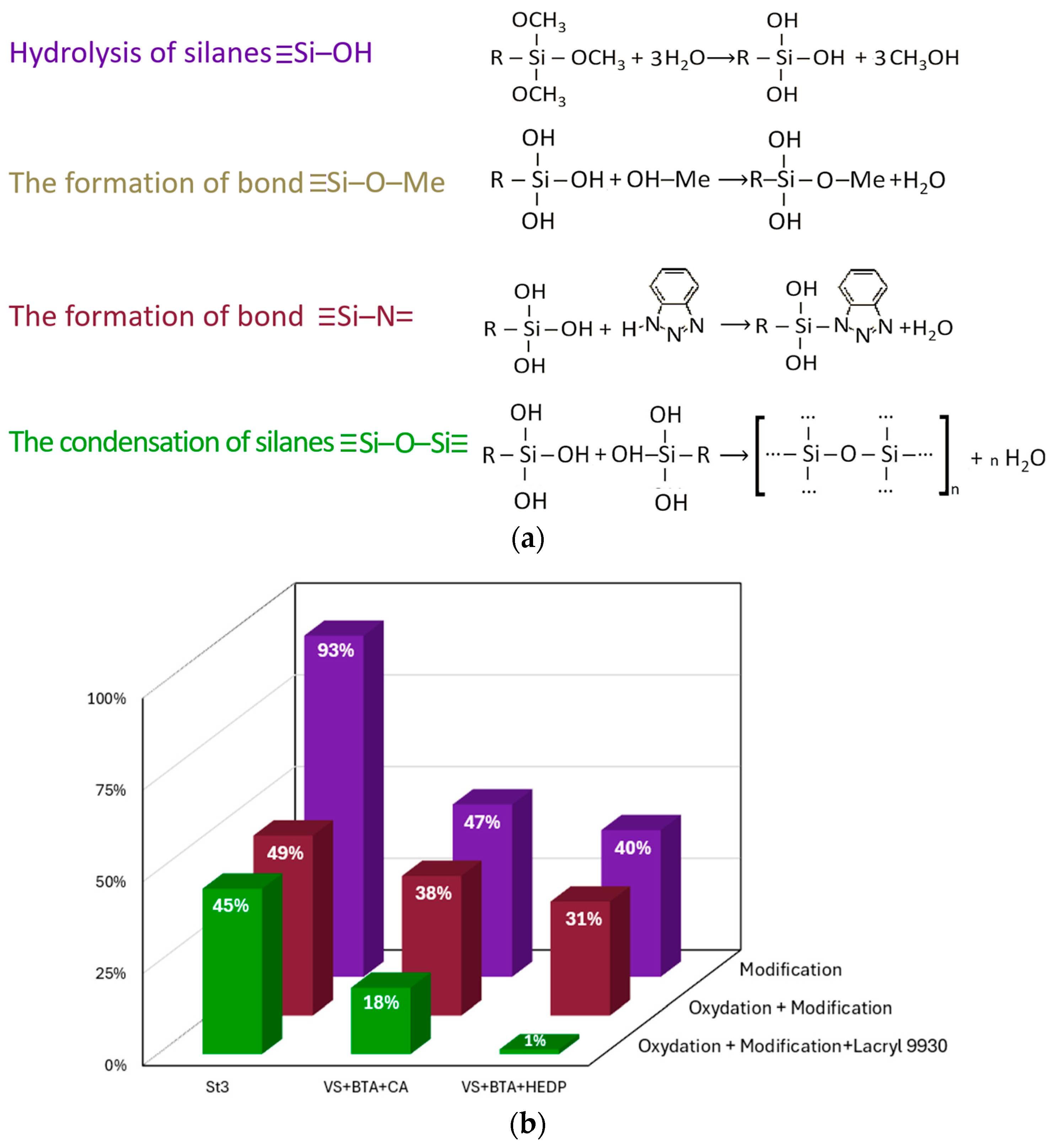
3.1. Sealing of Coatings with Organic Materials
3.2. Sealing of Functional Sublayers via Additional Coatings
3.3. Top-Coating Approaches for Improving Mechanical Performance
- 180 pits were observed on uncoated blades,
- 150 pits on blades with a standard TiN coating,
- No localized corrosion on blades coated using the proposed method.
- Consisted of four pairs of CrN/AlCrN layers with a total thickness of 2.44 µm.
- The structure featured decreasing layer thicknesses.
- Hardness: 18 GPa; Elastic modulus: 487 GPa; Porosity: 8.48%; Critical load: 47 N.
- Consisted of eight pairs of CrN/AlCrN layers with a total thickness of 2.55 µm.
- Layers had uniform thickness.
- Hardness: 24.8 GPa; Elastic modulus: 670 GPa; Porosity: 2.09%; Critical load: 52 N.
- M2 exhibited the best wear resistance under cryogenic cooling, as its graded structure is optimized for extreme operating conditions.
- M4 demonstrated higher thermal stability and improved hardness and elasticity, making it suitable for applications requiring high mechanical and thermal resilience.
4. Analysis of Bilayer Coating Systems
4.1. Advantages of Bilayer Coatings in Comparison with Single-Layer
- Improved adhesion because of the intermediate layer;
- Increased corrosion resistance owing to the combined action of the layers;
- Wear resistance exceeds that of single-layer equivalents;
- Sealing the pores of the base layer with a top coat;
- Adaptability to specific operating conditions.
- Increased durability of the coating, which reduces the frequency of repairs;
- Improved protection against corrosion and mechanical damage;
- Saving on maintenance in the long run;
- Reduced equipment downtime due to a longer service life;
- Optimization of the cost of replacement parts;
- Increased operational efficiency in difficult conditions;
- Reducing the overall life cycle costs of the product by using less expensive materials for the base layer;
- Improved operational characteristics that allow the equipment to be used in extreme conditions;
- Application possibilities in a wider range of operating conditions;
- Increased reliability of protected structures.
- The additional coloring of anodized coatings improves the decorative effect, anti-corrosion characteristics, wear, ultraviolet radiation, and fingerprints, so it is used in the automotive industry and in the manufacture of household appliances [214];
4.2. Analysis of the Failure Mechanisms of Bilayer Systems
- The formation of cracks in the upper layer because of the mismatch of the mechanical properties of the layers;
- Peeling of the coating from the substrate because of inadequate adhesion between the layers;
- Porosity of the coating, leading to the penetration of aggressive media;
- Deformation under thermal loads due to different expansion coefficients of the layers.
- Incompatibility of the layer materials in terms of physical and mechanical properties;
- Incorrect technology of applying intermediate layers;
- Corrosion of the outer layers promotes the penetration of the electrolyte to the porous functional sublayer;
- Mechanical stresses during heat treatment.
- Pretreatment of the surface is a critical step to create an optimal contact surface and achieve maximum adhesive strength of the joint;
- Use additional heat treatment to relieve internal stresses, as well as optimize the composition of the sublayers to improve adhesion;
- Control the parameters of the application process of each layer. It is necessary to carefully consider the selection of intermediate layers, taking into account the gradual changes in properties from the substrate to the outer coating;
- Apply consistent treatment with organic compounds to seal pores or use hydrophobizers to protect against moisture penetration in the top layer of the coating.
4.3. Economic Efficiency and Scalability of Industrial Functional Sublayer Production
- Conversion intermediate layers exhibit excellent adhesion to a variety of metal substrates. The production process is economically advantageous, featuring optimized energy consumption and a streamlined technological sequence. Scaling this conversion coating technology for any production volume is straightforward and maintains quality. Optimizing conversion coating production maximizes economic efficiency, particularly in large-scale manufacturing.
- Intermediate layers of ni–p coatings exhibit excellent mechanical bonding strength and high-temperature stability. The equipment for applying these coatings is more cost-effective to purchase and operate. Ni–p coating processes offer significant flexibility in scaling production and can be easily adapted to any volume. Production capacity is most efficiently utilized with average output volumes.
- Intermediate layers of PEO coatings exhibit significant surface roughness, which, on the one hand, reduces corrosion resistance in aggressive environments, and on the other hand, leads to wear resistance due to microrelief, as well as improved adhesion to subsequent layers. The process of forming intermediate layers in the form of PEO coatings is accompanied by significant energy consumption. The production line for applying PEO coatings requires serious investments in technological equipment. It is possible to scale production facilities based on PEO technology only if the technological parameters are strictly observed and the process is constantly monitored. The production potential of PEO coating technology is revealed most fully in the conditions of small-scale production.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchheit, R.G. Chapter 21—Corrosion Resistant Coatings and Paints. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 449–468. [Google Scholar] [CrossRef]
- Gabe, D.R.; Clarke, M. 12.1—Electroplating. In Corrosion, 3rd ed.; Shreir, L.L., Jarman, R.A., Burstein, G.T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1994; pp. 12:3–12:50. [Google Scholar] [CrossRef]
- Wani, F.M.; Katiyar, J.M.; Sehgal, R. Chapter 5—Hard ceramic coatings. In High-Temperature Tribology of Ceramics and Ceramic Matrix Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2025; pp. 135–155. [Google Scholar] [CrossRef]
- Advanced Ceramic Coatings. Fundamentals, Manufacturing, and Classification; Gupta, R.K., Motallebzadeh, A., Kakooei, S., Nguyen, T.A., Behera, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Future Development of Thermal Spray Coatings: Types, Designs, Manufacture and Applications; Espallargas, N., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kim, G.E.; Champagne, V.K.; Trexler, M.; Sohn, Y. 20—Processing nanostructured metal and metal-matrix coatings by thermal and cold spraying. In Nanostructured Metals and Alloys; Whang, S.H., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 615–662. [Google Scholar] [CrossRef]
- Zhang, B. Amorphous and Nano Alloys Electroless Depositions. Technology, Composition, Structure and Theory; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chiorcea-Paquim, A.-M.; Brett, C.M.A. Electrodeposition in Deep Eutectic Solvents: Perspectives towards Advanced Corrosion Protection. Appl. Mater. Today 2025, 44, 102746. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition. Principles, Technology and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Noyes Publications: New York, NY, USA, 2010. [Google Scholar]
- Hussain, C.M.; Verma, C.; Aslam, J.; Aslam, R.; Zehra, M. 21—Corrosion protective coatings. In Handbook of Corrosion Engineering. Modern Theory, Fundamentals and Practical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2023; pp. 283–321. [Google Scholar]
- Bunshah, R.F. Handbook of Hard Coatings. Deposition Technolgies, Properties and Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Metallurgical Coatings and Thin Films; Sartwell, B.D., McGuire, G.E., Hofmann, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Handbook of Modern Coating Technologies: Fabrication Methods and Functional Properties; Aliofkhazraei, M., Ali, N., De Hosson, J.T.M., Chipara, M., Laidani, N.B., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
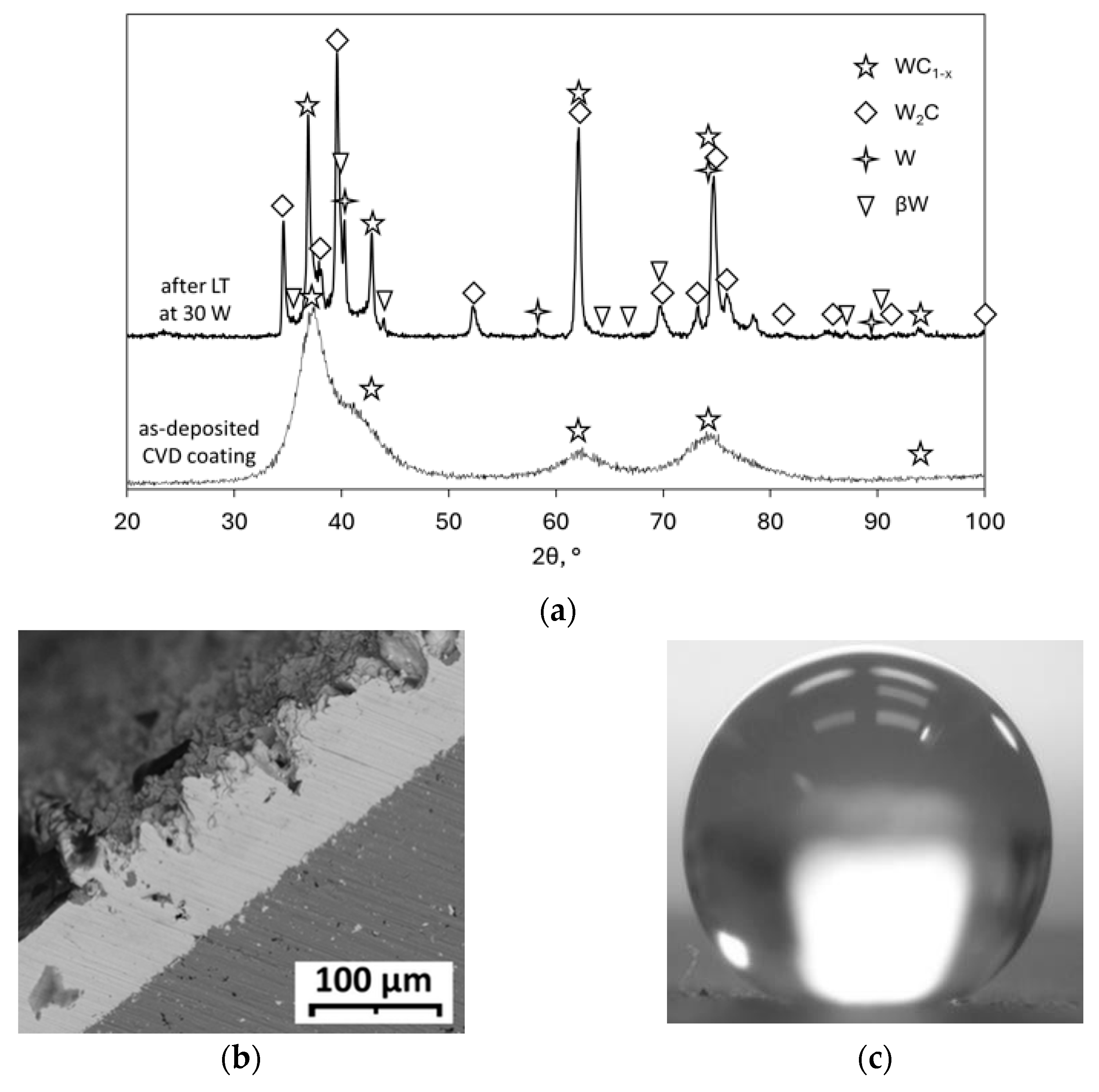
- Dushik, V.V.; Rozhanskii, N.V.; Lifshits, V.O.; Rybkina, T.V.; Kuzmin, V.P. The Formation of Tungsten and Tungsten Carbides by CVD Synthesis and the Proposed Mechanism of Chemical Transformations and Crystallization Processes. Mater. Lett. 2018, 228, 164–167. [Google Scholar] [CrossRef]
- Dushik, V.V.; Redkina, G.V.; Rozhanskii, N.V.; Rybkina, T.V.; Shaporenkov, A.A.; Mashchenko, V.E. Corrosion and Electrochemical Behavior of β-W CVD Coatings in NaCl Solution. Prot. Met. Phys. Chem. Surf. 2020, 56, 1321–1324. [Google Scholar] [CrossRef]
- Dushik, V.V.; Lakhotkin, Y.V.; Kuzmin, V.P.; Rybkina, T.V.; Rozhanskii, N.V.; Rychkov, B.A. The Corrosion and Electrochemical Behavior of Tungsten-Based CVD Coatings in Alkaline Aqueous Solutions. Prot. Met. Phys. Chem. Surf. 2018, 54, 1315–1319. [Google Scholar] [CrossRef]
- Dushik, V.V.; Rozhanskii, N.V.; Zalavutdinov, R.K. IR Study of the Transformation of WF6 on a W Substrate. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2019, 13, 919–924. [Google Scholar] [CrossRef]
- Högberg, H.; Tägtström, P.; Lu, J.; Jansson, U. Chemical Vapour Deposition of Tungsten Carbides on Tantalum and Nickel Substrates. Thin Solid Films 1996, 272, 116–123. [Google Scholar] [CrossRef]
- Zhuk, Y.; Lakhotkin, Y.; Aleksandrov, S. Alloyed Tungsten Produced by Chemical Vapour Deposition. Patent US20090117372A1, 7 May 2009. 16p. [Google Scholar]
- Riedel, W. Electroless Nickel Plating; ASM International, Finishing Publications LTD Stevenage: Hertfordshire, UK, 1991. [Google Scholar]
- Krutskikh, V.M.; Drovosekov, A.B.; Ivanov, V.M. Studies of Chemical–Catalytic Formation of Ni–Re (Mo, W)–B Alloys. Russ. J. Electrochem. 2016, 52, 873–884. [Google Scholar] [CrossRef]
- Functionally Graded Materials, 1st ed.; Miyamoto, Y., Kaysser, W.A., Rabin, B.H., Kawasaki, A., Ford, R.G., Eds.; Springer: New York, NY, USA, 1999. [Google Scholar]
- Sathish, M.; Radhika, N.; Saleh, B. A Critical Review on Functionally Graded Coatings: Methods, Properties, and Challenges. Compos. Part B 2021, 225, 109278. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of Functionally Graded Thermal Sprayed Coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Dushik, V.V.; Ruban, E.A.; Drovosekov, A.B.; Shaporenkov, A.A.; Rozhanskii, N.V. Synergetic Effect in Ni–P–W and W–C Multilayer Coating Systems Obtained by Chemical-Catalytic Metallization and Chemical-Vapor Deposition. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2023, 17, 1364–1371. [Google Scholar]
- Dushik, V.V.; Ruban, E.A.; Shaporenkov, A.A.; Drovosekov, A.B.; Rozhanskii, N.V.; Gladkikh, N.A. Mechanical Properties and Corrosion–Electrochemical Behavior of Multilayer Coatings of the Ni–P and W–C System Obtained by the Electroless Plating Method and Chemical Vapor Deposition. Part 1. Structure and Mechanical Properties of Coatings. Prot. Met. Phys. Chem. Surf. 2023, 58, 1301–1306. [Google Scholar] [CrossRef]
- GOST 9.402–2004; Unified System of Corrosion and Ageing Protection. Paint Coatings. Metal Surface Preparation for Painting. IPK Publishing Standards: Moscow, Russia, 2006.
- Kuzenkov, Y.A. Protection of Copper-Containing Aluminum Alloys Conversion Coatings and Inhibitory Pigments. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 11 December 2008. (In Russian). [Google Scholar]
- Freemann, D.B. Phosphating and Metal Pretreatment; Woodhead-Faulkner: Cambridge, UK, 1986. [Google Scholar]
- Raush, W. The Phosphating of Metals; Finishing Publications Ltd.: Metals Park, OH, USA, 1990. [Google Scholar]
- Khain, I.I. Theory and Practice of Metal Phosphating; Khimiya: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Burokas, V.; Martushene, A.; Ruchinskene, A.; Sudavichyus, A.; Bikul’chyus, G. Deposition of Amorphous Phosphate Coatings on Aluminum. Prot. Mater. 2006, 42, 373–378. (In Russian) [Google Scholar] [CrossRef]
- Grigoryan, N.S.; Akimova, E.F.; Vahramyan, T.A. Phosphating; Globus: Moscow, Russia, 2008. (In Russian) [Google Scholar]
- Sankara, T.S.N. Surface Pretreatment by Phosphate Conversion Coatings—A Review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Grilihes, S.Y. Oxidation and phosphating of metals; Khimiya: Moscow, Russia, 1971. (In Russian) [Google Scholar]
- Yampolskij, A.M.; Ilyin, V.A. A Short Handbook of Electroplating; Mashinostroenie: Leningrad, Russia, 1962. (In Russian) [Google Scholar]
- Kuzenkov, Y.A.; Konovalov, A.S.; Grafov, O.Y.; Luchkin, A.Y. Modification of Ultrathin Conversion Coatings for AMg3 Aluminum Alloy and Their Interaction with Painting System. Corros. Prot. Mater. Methods Res. 2023, 2, 37–48. (In Russian) [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. Part IV. Passivation and the role of mono- and diphosphonates. Int. J. Corros. Scale Inhib. 2017, 6, 384–427. [Google Scholar] [CrossRef]
- Zimina, Y.M. Chromate-Free Conversion Coatings on Magnesium-Containing Aluminum Alloys. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 27 January 2011. (In Russian). [Google Scholar]
- Osborne, J.H. Observations on Chromate Conversion Coatings from a Sol–Gel Perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E.J.; Mahajan, S. Encyclopedia of Materials: Science and Technology. MRS Bull. 2004, 49, 512–513. [Google Scholar] [CrossRef][Green Version]
- Degarmo, E.P.; Black, J.T.; Kohser, R.A. Materials and Processes in Manufacturing, 9th ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Corrosion Science: A Retrospective and Current Status in Honor of Robert P. Frankenthal Proceedings of an International Symposium P. Frankenthal; Frankel, G.S., Frankenthal, R.P., Eds.; Electrochemical Society: Pennington, CA, USA, 2002. [Google Scholar]
- Gharbi, O.; Ogle, K.; Han, J. On the Chemistry of Conversion Coatings. Encycl. Solid-Liq. Interfaces 2024, 3, 532–546. [Google Scholar] [CrossRef]
- Joseph, E. Coating and Surface Treatment Systems for Metals; ASM International: Almere, The Netherlands; Finishing Publications: Stevenage, UK, 1997. [Google Scholar]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Mu, S.; Li, N.; Zou, Z. Investigation of a Transparent Chromate (III) Passive Film on Electroless Ni–P Coating by XPS and Electrochemical Methods. Electrochim. Acta 2009, 54, 6718–6724. [Google Scholar] [CrossRef]
- Hazan, J.; Coddet, C.; Keddam, M. Study of Chromate Coatings on Zinc by Means of D.C, A.C and Gravimetric Methods in Alkaline Electrolyte—Correlation to Humid-Storage Test and to Cr Vi Content of the Conversion Film. Corros. Sci. 1990, 31, 313–318. [Google Scholar] [CrossRef]
- Liu, Y.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Chromate Conversion Coatings on Aluminium–Copper Alloys. Corros. Sci. 2005, 47, 341–354. [Google Scholar] [CrossRef]
- Liao, S.; Yu, B.; Zhang, X.; Lu, X.; Zhou, P.; Zhang, C.; Chen, X.B.; Zhang, T.; Wang, F. New Design Principles for the Bath towards Chromate- and Crack-Free Conversion Coatings on Magnesium Alloys. J. Magnes. Alloys 2021, 9, 505–519. [Google Scholar] [CrossRef]
- Ferguson, D. Non-Polluting Replacement for Chromate Conversion coating & Zinc Phosphating in Powder Coating Applications. Plat. Surf. Finish. 2003, 90, 66–75. [Google Scholar]
- Eckles, W.; Frischauf, R. Alternatives to the Hexavalent Chromates: An Evolution of Trivalent Chromate Technologies. Plat. Surf. Finish. 2007, 94, 24–26. [Google Scholar]
- Ferreira, M.G.S.; Zheludkevich, M.L.; Tedim, J.; Yasakau, K.A. 9—Self-healing nanocoatings for corrosion control. In Corrosion Protection and Control Using Nanomaterials. A Volume in Woodhead Publishing Series in Metals and Surface Engineering; Saji, V.S., Cook, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 213–263. [Google Scholar] [CrossRef]
- Grilikhes, S.Y.; Tikhonov, K.I. Electrolytic and Chemical Coatings. Theory and Practice; Khimiya: Leningrad, Russia, 1990. (In Russian) [Google Scholar]
- Vershok, D.B. Oxidation of Low-Carbon Steel in Solutions of Ammonium Nitrate. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 26 May 2006. [Google Scholar]
- Bulgakov, D.S. The Effect of the Composition of Nitrate Solutions on the Oxidation of Low-Carbon Steels and the Protective Properties of Oxide Coatings. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 16 June 2011. [Google Scholar]
- Chugunov, D.O. Surface Modification Low Carbon Emissions They Have Become Complexes of Phosphonic Acids for Enhancing Its Passivity by Organic Inhibitors. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 3 June 2021. (In Russian). [Google Scholar]
- Djebian, R.; Boudjema, B.; Kabir, A.; Sedrati, C. Physical Characterization of CuO Thin Films Obtained by Thermal Oxidation of Vacuum Evaporated Cu. Solid State Sci. 2020, 101, 106147. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, F.; Fernandes, F.; Schouteden, K.; Ustarroz, J.; Locquet, P.J.; Taurino, J. Tailoring the Glucose Oxidation Activity of Anodized Copper Thin Films. Catal. Sci. Technol. 2025, 15, 3022–3035. [Google Scholar] [CrossRef]
- Brudzisz, A.; Giziński, D.; Liszewska, M.; Wierzbicka, E.; Tiringer, U.; Taha, S.A.; Zając, M.; Orzechowska, S.; Jankiewicz, B.; Taheri, P.; et al. Low-Voltage Anodizing of Copper in Sodium Bicarbonate Solutions. Electrochim. Acta 2023, 443, 141918. [Google Scholar] [CrossRef]
- Kumar, S.K.; Murugesan, S.; Suresh, S. Anodization Assisted Preparation of Diverse Nanostructured Copper Oxide Films for Solar Selective Absorber. Opt. Mater. 2023, 135, 113304. [Google Scholar] [CrossRef]
- Al-Jubbori, M.A.; Ayed, O.; Ajaj, K. Effect of Gamma and Ultraviolet Irradiation on the Optical Properties of Copper Oxide Nanostructured Thin Films by Chemical Spray Pyrolysis. Radiat. Phys. Chem. 2025, 226, 112190. [Google Scholar] [CrossRef]
- Rakshit, S.; Maji, D.; Mondal, K.G.; Roy, T.; Jana, P.C.; Kar, B.S.; Datta, R. Structural, Optical and Magnetic Properties of Chemically Grown Copper Oxide Nanoparticles: An Insight into Anticancer Activities. Mater. Chem. Phys. 2024, 325, 129794. [Google Scholar] [CrossRef]
- Imran, M.; Asghar, G.; Tariq, G.H.; Faridi, A.W.; Bano, S.; Shifa, M.S.; Ullah, S. Investigation of Annealing Effects on Physical Properties of Chemically Prepared Copper Oxide Thin Films. Results Opt. 2023, 10, 100331. [Google Scholar] [CrossRef]
- Angayarkanni, S.; Neyvasagam, K. Structural and Optical Studies of Copper Oxide Nanoparticles Synthesized by Chemical Precipitation Method. Mater. Today Proc. 2021, 47, 1149–1154. [Google Scholar] [CrossRef]
- Grasza, K.; Łusakowska, E.; Skupinski, P.; Sakowska, H.; Mycielski, A. Thermal Annealing of ZnO Substrates. Superlattice Microst. 2007, 42, 290–293. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Abdulmunem, O.M.; Ali, M.J.M.; Hassan, E.S. Optical and Structural Characterization of Aluminium Doped Zinc Oxide Thin Films Prepared by Thermal Evaporation System. Opt. Mater. 2020, 109, 110374. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Kharina, I.V.; Isupova, L.A.; Litvak, G.S.; Moroz, E.M.; Kryukova, G.N.; Rudina, N.A.; Tanashev, Y.Y.; Parmon, V.N. Synthesis of Aluminum Oxides from the Products of the Rapid Thermal Decomposition of Hydrargillite in a Centrifugal Flash Reactor: III. Properties of Aluminum Hydroxides and Oxides Obtained via the Mild Rehydration of the Products of the Centrifugal Thermal Activation of Kydrargillite. Kinet. Catal. 2007, 48, 327–335. [Google Scholar] [CrossRef]
- Chukin, G.D. The structure of Aluminum Oxide and Hydrodesulfurization Catalysts. Reaction Mechanisms; Paladin Printing House, Printa LLC: Moscow, Russia, 2010. [Google Scholar]
- Smirnov, V.K.; Bodryj, A.B.; Irisova, K.N.; Ponjatkova, Z.J.; Pashkina, L.P. Method of Producing Powdered Aluminium Hydroxide (Versions) and Aluminium Oxide. Patent RU2432318C1, 27 October 2011. 13p. [Google Scholar]
- Cirik, E.; Genel, K. Effect of anodic oxidation on fatigue performance of 7075-T6 alloy. Surf. Coat. Technol. 2008, 202, 5190–5201. [Google Scholar] [CrossRef]
- Zhang, P.; Zuo, Y. Effects of Pore Parameters on Performance of Anodic Film on 2024 Aluminum Alloy. Mater. Chem. Phys. 2019, 231, 9–20. [Google Scholar] [CrossRef]
- Fiore, V.; Di Franco, F.; Miranda, R.; Santamaria, M.; Badagliacco, D.; Valenza, A. Effects of Anodizing Surface Treatment on the Mechanical Strength of Aluminum Alloy 5083 to Fibre Reinforced Composites Adhesive Joints. Int. J. Adhes. Adhes. 2021, 108, 102868. [Google Scholar] [CrossRef]
- Guo, F.; Cao, Y.; Wang, K.; Zhang, P.; Cui, Y.; Hu, Z.; Xie, Z. Effect of the Anodizing Temperature on Microstructure and Tribological Properties of 6061 Aluminum Alloy Anodic Oxide Films. Coatings 2022, 12, 314. [Google Scholar] [CrossRef]
- Baxevani, A.; Lamprou, E.; Mavropoulos, A.; Stergioudi, F.; Michailidis, N.; Tsoulfaidis, I. Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS). Alloys 2025, 4, 17. [Google Scholar] [CrossRef]
- Moon, S.; Jeong, Y. Generation Mechanism of Microdischarges during Plasma Electrolytic Oxidation of Al in Aqueous Solutions. Corros. Sci. 2009, 51, 1506–1512. [Google Scholar] [CrossRef]
- Li, K.; Li, W.; Zhang, G.; Guo, P. Preparation of Black PEO Layers on Al–Si Alloy and the Colorizing Analysis. Vacuum 2015, 111, 131–136. [Google Scholar] [CrossRef]
- Van Truong, P.; Van Bo, N.; Van Minh, N.; Anh, N.V.; Suresh Kumar, G.; Shkir, M. Investigation of Corrosion and Wear Resistance of PEO Coated D16T Aluminium Alloys in the Marine Tropical Climate Conditions. Mater. Chem. Phys. 2022, 290, 126587. [Google Scholar] [CrossRef]
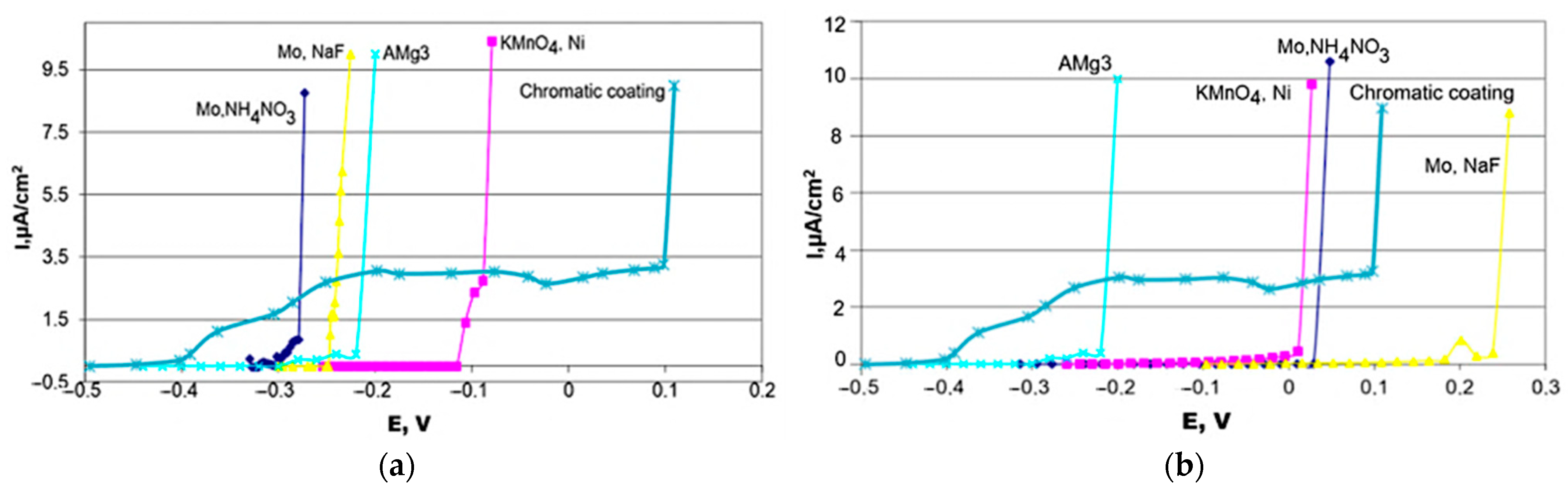
- Gnedenkov, A.S.; Kononenko, Y.I.; Sinebryukhov, S.L.; Filonina, V.S.; Vyaliy, I.E.; Nomerovskii, A.D.; Ustinov, A.Y.; Gnedenkov, S.V. The Effect of Smart PEO-Coatings Impregnated with Corrosion Inhibitors on the Protective Properties of AlMg3 Aluminum Alloy. Materials 2023, 16, 2215. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Du, S.; Cheng, B.; Pan, Y.; Lu, H. Influence of the Dual-Current Method on the Properties and Growth Mechanisms of Micro-Arc Oxidation Coatings. Mater. Chem. Phys. 2024, 328, 130031. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Vyaliy, I.E.; Gnedenkov, A.S.; Kharchenko, U.V.; Sinebryukhov, S.L.; Gnedenkov, S.V. Corrosion properties of the composite coatings formed on PEO pretreated AlMg3 aluminum alloy by dip-coating in polyvinylidenefluoride-polytetrafluoroethylene suspension. Polymers 2024, 16, 2945. [Google Scholar] [CrossRef]
- Sukuroglu, S. Characterization, Corrosion, Adhesion and Wear Properties of Al2O3 and Al2O3:TiB2 Composite Coating on Al 7075 Aluminum Alloy by One-Step Micro-Arc Oxidation Method. Mater. Today Commun. 2025, 46, 112493. [Google Scholar] [CrossRef]
- Averyanov, E.E. Questions of the Theory of Formation and Organization of Anodic Oxides. Ph.D. Thesis, Kazan State University, Kazan, Russia, 26 October 2004. (In Russian). [Google Scholar]
- Averyanov, E.E. Anodic Oxidation of Titanium and Its Alloys. Chem. Chem. Technol. 2004, 47, 35–37. (In Russian) [Google Scholar]
- Manjaiah, M.; Laubscher, R.F. Effect of Anodizing on Surface Integrity of Grade 4 Titanium for Biomedical Applications. Surf. Coat. Technol. 2017, 310, 263–272. [Google Scholar] [CrossRef]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.P.; Pinton, A.M.; Rocha, L.A.L.; Fernandes, J.C.S. Corrosion Mechanisms in Titanium Oxide Based Films Produced by Anodic Treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. 5—Plasma Electrolytic Oxidation Treatment of Aluminium and Titanium Alloys. Surface Engineering of Light Alloys. Aluminium, Magnesium and Titanium Alloys; Dong, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Molaei, M.; Babaei, K. The Effects of Nano-and Micro-Particles on Properties of Plasma Electrolytic Oxidation (PEO) Coatings Applied on Titanium Substrates: A Review. Surf. Interfaces 2020, 21, 100659. [Google Scholar] [CrossRef]
- Makurat-Kasprolewicz, B.; Ossowska, A. Recent Advances in Electrochemically Surface Treated Titanium and Its Alloys for Biomedical Applications: A Review of Anodic and Plasma Electrolytic Oxidation Methods. Mater. Today Commun. 2023, 34, 105425. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Biocompatibility of Plasma Electrolytic Oxidation Coated Titanium Alloy for Biomedical Applications. Bionanoscience 2025, 15, 232. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesium: Properties–Applications–Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of Corrosion-Resistant Conversion Coatings for Magnesium and Its Alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Abbott, T.B. Mg: Industrial and Research Developments over the Last 15 Years. Corrosion 2015, 71, 120–127. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Luchkina, V.A. Protection of the Mg90 Alloy with Organic Solutions Corrosion Inhibitors. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 10 March 2022. (In Russian). [Google Scholar]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer Hybrid Coatings on Magnesium Alloy to Improve Biodegradation and Biocompatibility Properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Liu, H.G.; Cao, F.Y.; Song, G.L.; Zheng, D.G.; Shi, Z.M.; Dargusch, M.S.; Atrens, A.J. Review of the Atmospheric Corrosion of Magnesium Alloys. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Birbilis, N.; Taylor, C.D.; Williams, K.S.; Samin, A.J.; Medhekar, N.V. Aqueous Electrochemistry of the Magnesium Surface: Thermodynamic and Kinetic Profiles. Corros. Sci. 2019, 147, 53–68. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Castellote-Alvarez, R.; Escobar, A.M.; Llorca-Isern, N. Robust and Superhydrophobic Coating Highly Resistant to Wear and Efficient in Water/Oil Separation. Surf. Coat. Technol. 2019, 364, 330–340. [Google Scholar] [CrossRef]
- Jiang, B.; Xiang, Q.; Atrens, A.; Song, J.; Pan, F. Influence of Crystallographic Texture and Grain Size on the Corrosion Behaviour of as-Extruded Mg Alloy AZ31 Sheets. Corros. Sci. 2017, 126, 374–380. [Google Scholar] [CrossRef]
- Semenchenko, I.Y.; Medvedeva, M.S. Features of the Technological Process of Mechanical Processing of Magnesium Parts. Sci. Educ. Today 2017, 16, 26–28. (In Russian) [Google Scholar]
- Buzzard, R.W.; Wilson, H. Anodic Coating of Magnesium Alloys. Natl. Bur. Stand. 1937, 18, 83–87. [Google Scholar] [CrossRef]
- Kosterina, M.L. Micro-Arc Oxidation Parameters Effects on Properties and Thickness of Coatings on AZ91D Magnesium Alloy. Review of Articles Published in Chinese Journals. Theory Pract. Corros. Prot. 2023, 28, 28–42. (In Russian) [Google Scholar] [CrossRef]
- Mei, H.; Song, H.; Feng, K.; Chen, Y.; Yau, H.; Luo, C.; Lio, D.; Guan, H.; Luc, C.; Hu, Z. Corrosion protection of folic acid and lauric acid modified films prepared by anodic oxidation on WE43 magnesium alloys. Appl. Surf. Sci. 2023, 638, 158014. [Google Scholar] [CrossRef]
- Kang, Y.-X.; Li, Z.-L.; Chen, W.-D.; Guo, C.-X. Optimization of Anodizing Conditions and Hole Sealing Treatments for Enhanced Anti-Corrosion Properties of Magnesium Alloys. Ceram. Int. 2024, 50, 25667–25678. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, S.; Chen, J.; Zhang, M.; Cui, Y.; Cao, Y.; Xiong, S.; Wang, S.; Yang, B. The Sequential Migration of Rare Earth Elements (REE) in WE43 Magnesium Alloy during Anodic Oxidation Treatment. Colloids Surf. A 2024, 698, 134562. [Google Scholar] [CrossRef]
- Moreno, J.; Merlo, J.L.; Renno, A.C.; Canizo, J.; Buchelly, F.J.; Pastore, J.I.; Katunar, M.R.; Cere, S. In vitro Characterization of Anodized Magnesium Alloy as a Potential Biodegradable Material for Biomedical Applications. Electrochim. Acta 2023, 437, 141463. [Google Scholar] [CrossRef]
- Takeda, O.; Uda, T.; Okabe, T.H. Chapter 2.7—Rare Earth, Titanium Group Metals, and Reactive Metals Production. In Treatise on Process Metallurgy. Industrial Processes; Seetharaman, S., Guthrie, R., McLean, A., Seetharaman, S., Sohn, H.Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2024; pp. 697–750. [Google Scholar] [CrossRef]
- Gerasimov, M.V.; Zhulikov, V.V. Coating for the Protection of Magnesium and Its Alloys from Corrosion and a Method for Its Production. Patent RU2757642C1, 19 October 2021. 9p. [Google Scholar]
- Snezhko, L.A.; Rudnev, V.S. Anodic-Spark Oxidation of Magnesium; Technika Publishing House, Tuma Group: Moscow, Russia, 2014. (In Russian) [Google Scholar]
- Vladimirov, B.V.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Rossiyskaya, A.D.; Suminov, I.V.; Epelfeld, A.V. Microarc Oxidation of Magnesium Alloys (Review). Electron. Process. Mater. 2014, 50, 195–232. (In Russian) [Google Scholar] [CrossRef]
- Nazarenko, A.D.; Dumanitskiy, M.A.; Girn, A.V.; Trushkina, T.V. Microarc Oxidation of Magnesium Alloys. Curr. Probl. Aviat. Cosmonaut. 2017, 1, 77–79. (In Russian) [Google Scholar]
- Elaish, R.; Curioni, M.; Gowers, K.; Kasuga, A.; Habazaki, H.; Hashimoto, T.; Skeldon, P. Effects of Fluoride Ions in the Growth of Barrier-Type Films on Aluminium. Electrochim. Acta 2017, 245, 854–862. [Google Scholar] [CrossRef]
- Kim, M.; Brewer, L.N.; Kubacki, G.W. Microstructure and Corrosion Resistance of Chromate Conversion Coating on Cold Sprayed Aluminum Alloy 2024. Surf. Coat. Technol. 2023, 460, 129423. [Google Scholar] [CrossRef]
- Ikubanni, P.P.; Oki, M.; Adediran, A.A.; Akintola, S.A.; Adeleke, A.A. Scanning and Transmission Electron Microscopy Examinations of Composite Hybrid Chromate and Chromate Phosphate Conversion Coatings Exposed in Hot 100% Relative Humidity Environments. Hybrid Adv. 2023, 3, 100067. [Google Scholar] [CrossRef]
- Bierwagen, G.; Twite, R.L. Review of Alternatives to Chromate for Corrosion Protection of Aluminum Aerospace Alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Bierwagen, G.; Brown, R.; Battochi, D.; Hayes, S. Active Metal-Based Corrosion Protective Coating Systems for Aircraft Requiring No-Chromate Pretreatment. Prog. Org. Coat. 2010, 68, 48–61. [Google Scholar] [CrossRef]
- Machkova, M.; Matter, E.A.; Kozhukharov, S.; Kozhukharov, V. Effect of the Anionic Part of Various Ce(III) Salts on the Corrosion Inhibition Efficiency of AA2024 Aluminium Alloy. Corros. Sci. 2013, 69, 396–405. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.-N.; Zhuang, J.-J.; Song, R.-X.; Lu, X.-Y.; Su, X.-P. Effects of Current Density on Microstructure and Properties of Plasma Electrolytic Oxidation Ceramic Coatings Formed on 6063 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]
- Nyrkov, N.P.; Shuvalov, D.A.; Zaitseva, E.R. Protective Submicron Conversion Coatings on Aluminum Alloys. Bull. Sci. 2019, 4, 72–77. (In Russian) [Google Scholar]
- Li, J.; Wei, H.; Zhao, K.; Wang, M.; Chen, D.; Chen, M. Effect of Anodizing Temperature and Organic Acid Addition on the Structure and Corrosion Resistance of Anodic Aluminum Oxide Films. Thin Solid Films 2020, 713, 138359. [Google Scholar] [CrossRef]
- Chumnanwat, S.; Watanabe, Y.; Taniguchi, N.; Higashi, H.; Kodama, A.; Seto, T.; Otani, Y.; Kumita, M. Pore Structure Control of Anodized Alumina Film and Sorption Properties of Water Vapor on CaCl2-Aluminum Composites. Energy 2020, 208, 118370. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ağaoğlu, G.H.; Yüksel, B.; Orhan, G. Evaluation of the Electrochemical Corrosion Behavior of Anodic Aluminum Oxide Produced by the Two-Step Anodization Process. Anti-Corros. Methods Mater. 2020, 67, 509–518. [Google Scholar] [CrossRef]
- Bogoyavlensky, A.F. On the Theory of Anodic Oxidation of Aluminum. Russ. J. Appl. Chem. 1972, 4, 712–717. (In Russian) [Google Scholar]
- Takahashi, H.; Nagayama, M. The Determination of the Porosity of Anodic Oxide Films on Aluminium by the Pore Filling Method. Corr. Sci. 1978, 18, 911–925. [Google Scholar] [CrossRef]
- Shcherbachev, D.R.; Sorokin, I.N.; Tsvetkov, D.V.; Nazarov, N.G. Modeling of the Kinetics of Barrier Anode Oxide Growth. Electrochem 1991, 27, 1114–1122. (In Russian) [Google Scholar]
- Savelyeva, E.A. Problems of Theory and Technology of Functional Anodizing of Aluminum Alloys; SSTU: Saratov, Russia, 1998. [Google Scholar]
- Kuskov, V.N.; Kolenchin, N.F.; Safronov, A.V. The Structure of the Oxide Coating with Anodized Aluminum and Its Alloys. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2014, 16, 175–178. (In Russian) [Google Scholar]
- Tantserev, A.A. Electrochemical Formation of Non-Ferrous Oxide Coatings on Aluminum and Its Alloys. Ph.D. Thesis, Yuri Gagarin State Technical University of Saratov, Saratov, Russia, 20 May 2016. (In Russian). [Google Scholar]
- Bakhti, H.; Weyrich, T.; Es-Souni, M.; Laghrissi, A.; Es-Souni, M. Non-Fouling Polymer Films on Hard-Anodized Aluminum Substrates: Nanomechanical Properties and Modelling. Prog. Org. Coat. 2021, 161, 106553. [Google Scholar] [CrossRef]
- Rueda, F.C.G.; González, J.T.; Hernández-López, J.M. Differences between the Untreated and Treated Diffusion Zone in the Alclad 2024-T3 Aluminum Alloy and Hard Anodic Films. Surf. Coat. Technol. 2022, 429, 127939. [Google Scholar] [CrossRef]
- Zhao, W.; Qiu, J.; Sakai, E.; Wu, H.; Zhang, G.; Feng, H.; Guo, S.; Wu, H. Enhanced Bonding of Polyphenylene Sulfide-Aluminum Alloy Composites using Combined Mild and Hard Anodizing Techniques. Surf. Coat. Technol. 2024, 483, 130773. [Google Scholar] [CrossRef]
- Wang, Y.; He, B.; Zhao, Y.; Zhang, T.; Wang, F. Advanced Metal Ion Dipping-Steam Sealing for Enhanced Corrosion Resistance of Hard Anodized Aluminum in Semiconductor Plasma Etching Environments. Mater. Lett. 2025, 400, 139164. [Google Scholar] [CrossRef]
- Ono, S.; Okura, M.; Tanaka, H.; Fujita, M.; Asoh, H. Rapid Room-Temperature Sealing of Anodic Oxide Films on Aluminum using Lithium Hydroxide. Electrochim. Acta 2025, 538, 146965. [Google Scholar] [CrossRef]
- Cartigny, V.; Veys-Renaux, D.; Desenne, P.; Rocca, E. Rapid Sealing of an Alumina Nanoporous Network Grown by Anodizing and Dye-Filled. Surf. Coat. Technol. 2019, 364, 369–376. [Google Scholar] [CrossRef]
- Brady, P.V. Alumina Surface Chemistry at 25, 40, and 60 °C. Geochim. Cosmochim. Acta 1994, 58, 1213–1217. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Ashrafizadeh, F. A Comparative Study of Corrosion Performance of Sealed Anodized Layers of Conventionally Colored and Interference-Colored Aluminium. Surf. Coat. Technol. 2012, 206, 4628–4633. [Google Scholar] [CrossRef]
- Feschet-Chassot, E.; Chennell, P.; Cueff, R.; Mailhot-Jensen, B.; Sautou, V. Anodic Alumina Oxide Surfaces Prepared by Dual Hard and Mild Anodization at Subzero Temperature: Surface Microscopic Characterization and Influence on Wettability. Surf. Interfaces 2020, 19, 100473. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskuła, M. Porous Anodic Alumina Formed by Anodization of Aluminum Alloy (AA1050) and High Purity Aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of High Quality Anodic Aluminum Oxide (AAO) on Low Purity Aluminum—A Comparative Study with the AAO Produced on High Purity Aluminum. Electrochim. Acta 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Othsuka, T.; Otsuki, T. Effect of Ultra-Violet Light Irradiation on Anodic Oxide Films on Titanium in Sulfuric Acid Solution. J. Electroanal. Chem. 1999, 473, 272–278. [Google Scholar] [CrossRef]
- Xia, Z.; Nanjo, H.; Tetsuka, H.; Ebina, T.; Izumisawa, M.; Fujimura, M.; Onagawa, J. Crystallization of the Anodic Oxide on Titanium in Sulphuric Acids Solution at a Very Low Potential. Electrochem. Commun. 2007, 9, 850–856. [Google Scholar] [CrossRef]
- Capek, D.; Gigandet, M.-P.; Masmoudi, M.; Wery, M.; Banakh, O. Long-Time Anodisation of Titanium in Sulphuric Acid. Surf. Coat. Technol. 2008, 202, 1379–1384. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure Commercial Titanium Color Anodizing and Corrosion Resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Fushimi, K.; Kurauchi, K.; Yamamoto, Y.; Nakanishi, T.; Hasegawa, Y.; Othsuka, T. Growth and Degradation of an Anodic Oxide Film on Titanium in Sulphuric Acid Observed by Ellipso-microscopy. Electrochim. Acta 2014, 144, 56–63. [Google Scholar] [CrossRef]
- Ferdjani, S.; David, D.; Beranger, G. Anodic Oxidation of Titanium in Phosphoric Acid Baths: Phosphorus Incorporation into the Oxide. J. Alloys Compd. 1993, 200, 191–194. [Google Scholar] [CrossRef]
- Marino, C.E.B.; de Oliveira, E.M.; Rocha-Filho, R.C.; Biaggio, S.R. On the Stability of Thin-Anodic-Oxide Films of Titanium in Acid Phosphoric Media. Corros. Sci. 2001, 43, 1465–1476. [Google Scholar] [CrossRef]
- Krasicka-Cydzik, E. Gel-Like Layer Development during Formation of Thin Anodic Films on Titanium in Phosphoric Acid Solutions. Corros. Sci. 2004, 46, 2487–2502. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Phosphoric Acid Anodization of Ti-6Al-4V-Structural and Corrosion Aspects. Corros. Sci. 2007, 49, 542–558. [Google Scholar] [CrossRef]
- Biestek, T.; Weber, J. Electrolytic and Chemical Conversion Coatings, 1st ed.; Portcullis Press: Redhill, UK, 1976. [Google Scholar]
- Mirzoev, R.A.; Davydov, A.D. Anodic Processes of Electrochemical and Chemical Processing of Metals; Polytechnic Publishing House: St. Petersburg, Russia, 2013. (In Russian) [Google Scholar]
- Yabuki, A.; Sakai, M. Anodic Films Formed on Magnesium in Organic, Silicate-Containing Electrolytes. Corros. Sci. 2008, 51, 793–798. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Y.; Wang, H.; Zhang, Z. Anodization of AZ91 Magnesium Alloy in Alkaline Solution Containing Silicate and Corrosion Properties of Anodized Films. Trans. Nonferrous Met. Soc. China 2008, 18, 722–727. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Wei, Z.; Zhang, Z. Anodizing of AZ91D Magnesium Alloy using Environmental Friendly Alkaline Borate-Biphthalate Electrolyte. Trans. Nonferrous Met. Soc. China 2012, 22, 1778–1785. [Google Scholar] [CrossRef]
- Wu, C.S.; Zhang, Z.; Cao, F.H.; Zhang, L.J.; Zhang, J.Q.; Cao, C.N. Study on the Anodizing of AZ31 Magnesium Alloys in Alkaline Borate. Solutions. Appl. Surf. Sci. 2007, 253, 3893–3898. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Yang, F.; Zhang, Z. Environmental Friendly Anodizing of AZ91D Magnesium Alloy in Alkaline Borate–Benzoate Electrolyte. J. Alloys Compd. 2011, 509, 6440–6446. [Google Scholar] [CrossRef]
- Hiramoto, S.; Shishido, T.; Yamamoto, A.; Maruyama, N.; Somekawa, H.; Mukai, T. Precipitation Control of Calcium Phosphate on Pure Magnesium by Anodization. Corros. Sci. 2008, 50, 2906–2913. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Y.; Wang, H.; Zhang, Z.; Salman, S.A.; Okido, M. 8—Anodization of magnesium (Mg) alloys to improve corrosion resistance. In Corrosion Prevention of Magnesium Alloys; Song, G.-L., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 197–231. [Google Scholar] [CrossRef]
- Tagnite Excellence in Magnesium Surface Protection. DOW 17 Coating. Available online: https://www.tagnite.com/antiquated-coatings/dow-17-coating/ (accessed on 3 July 2025).
- Gordienko, P.S.; Gnedenkov, S.V. Microarc Oxidation of Titanium and Its Alloys; Dalnauka Publishing: Vladivostok, Russia, 1997. [Google Scholar]
- Gordienko, P.S.; Dostovalov, V.A.; Efimenko, A.V. Microarc Oxidation of Metals and Alloys; Publishing House of the Far Eastern Federal University: Vladivostok, Russia, 2013. [Google Scholar]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Sergienko, V.I. Composite Multifunctional Coatings on Metals and Alloys Formed by Plasma Electrolytic Oxidation; Dalnauka Publishing: Vladivostok, Russia, 2013. [Google Scholar]
- Borisov, A.M.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Suminov, I.V.; Apelfeld, A.V. Microarc Oxidation in Slurry Electrolytes: A Review. Surf. Eng. Appl. Electrochem. 2016, 52, 50–78. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Gladkova, A.A.; Dub, A.V. Plasma Electrolytic Treatment of Aluminum and Titanium Alloys; NUST MISiS: Moscow, Russia, 2017. [Google Scholar]
- Strekalina, D.M. Model Ideas about the Formation of the MDO Method Wear-Resistant Decorative Coatings on VT6 Alloy. Ph.D. Thesis, National Research Technological University “MISIS”, Moscow, Russia, 24 November 2016. (In Russian). [Google Scholar]
- Monakhova, E.P. The Effect of the Stages of Coating Formation during Plasma Electrolytic Treatment of ML5 AND MA2 Alloys on Their Corrosion Resistance. Ph.D. Thesis, National Research Technological University “MISIS”, Moscow, Russia, February 2021. (In Russian). [Google Scholar]
- Denisenko, A.V. Synthesis of Nanostructured Materials Based on Titanium Dioxide and Copper for Catalytic Processes. Ph.D. Thesis, Mendeleev University of Chemical Technology, Moscow, Russia, 11 February 2021. (In Russian). [Google Scholar]
- Rykov, E.V. The Effect of the Composition and Properties of Aluminum Alloys and the Parameters of Coatings Obtained by Microarc Oxidation on the Performance Characteristics of Space Technology Parts. Ph.D. Thesis, Moscow Aviation Institute (National Research University), Moscow, Russia, 23 July 2023. (In Russian). [Google Scholar]
- Khabibullina, Z.V. Mechanisms and Kinetics of Growth of Anticorrosive Coatings in Various Time Intervals of PEO Plates Made of D16T Alloy. Ph.D. Thesis, National Research Technological University “MISIS”, Moscow, Russia, 27 September 2024. (In Russian). [Google Scholar]
- Shapagina, N.A.; Dushik, V.V. Coatings Based on Refractory Materials for Corrosion and Wear Applications. Materials 2024, 17, 5936. [Google Scholar] [CrossRef]
- Shapagina, N.A.; Dushik, V.V. Application of Electrophoretic Deposition as an Advanced Technique of Inhibited Polymer Films Formation on Metals from Environmentally Safe Aqueous Solutions of Inhibited Formulations. Materials 2023, 16, 19. [Google Scholar] [CrossRef]
- Mayne, J.E.O.; Bhuiyan, M.S.H. Mechanisms of Protection by Paints. MATS 2016, 1–12. [Google Scholar] [CrossRef]
- Gladkikh, N.A.; Dushik, V.V.; Shaporenkov, A.A.; Shapagin, A.V.; Makarychev, Y.B.; Gordeev, A.V.; Marshakov, A.I. Water Suspension Containing Organosilan, Corrosion Inhibitor and Polycondensation Promoter and Method for Producing Protective Films on Surface of Tungsten and Coatings on Its Basis from Water Suspension Containing Organosilan, Corrosion Inhibitor and Polycondensation Promoter. Patent RU2744336C1, 5 March 2021. 14p. [Google Scholar]
- Gladkikh, N.A. Inhibition of Local Dissolution of Metals by Organosilane-Based Compositions. Ph.D. Thesis, The Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, Moscow, Russia, 10 October 2020. (In Russian). [Google Scholar]
- Gladkikh, N.; Makarychev, Y.; Chirkunov, A.; Shapagin, A.; Petrunin, M.; Maksaeva, L.; Maleeva, M.; Yurasova, T.; Marshakov, A. Formation of Polymer-Like Anticorrosive Films based on Organosilanes with Benzotriazole, Carboxylic and Phosphonic Acids. Protection of Copper and Steel against Atmospheric Corrosion. Prog. Org. Coat. 2020, 141, 105544. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Petrunin, M.; Maksaeva, L.; Rybkina, A.; Marshakov, A.; Kuznetsov, Y. Synthesis of Thin Organic Layers Containing Silane Coupling Agents and Azole on the Surface of Mild Steel. Synergism of Inhibitors for Corrosion Protection of Underground Pipelines. Prog. Org. Coat. 2019, 132, 481–489. [Google Scholar] [CrossRef]
- Rouzmeh, S.S.; Naderi, R.; Mahdavian, M. Steel Surface Treatment with Three Different Acid Solutions and Its Effect on the Protective Properties of the Subsequent Silane Coating. Prog. Org. Coat. 2017, 112, 133–140. [Google Scholar] [CrossRef]
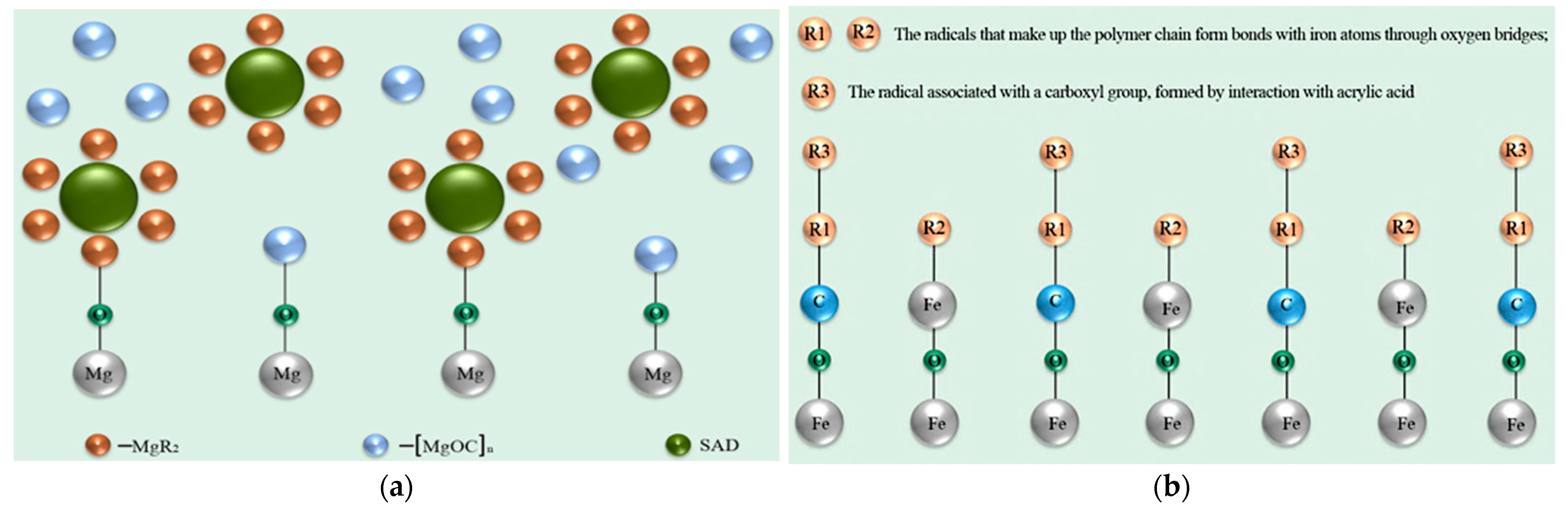
- Makarychev, Y.B.; Chirkunov, A.A.; Grafov, O.Y. Conversion Coatings for Magnesium and Steel Alloys Based on Styrene-Acrylic Dispersion. Int. J. Adhes. Adhes. 2024, 134, 103761. [Google Scholar] [CrossRef]
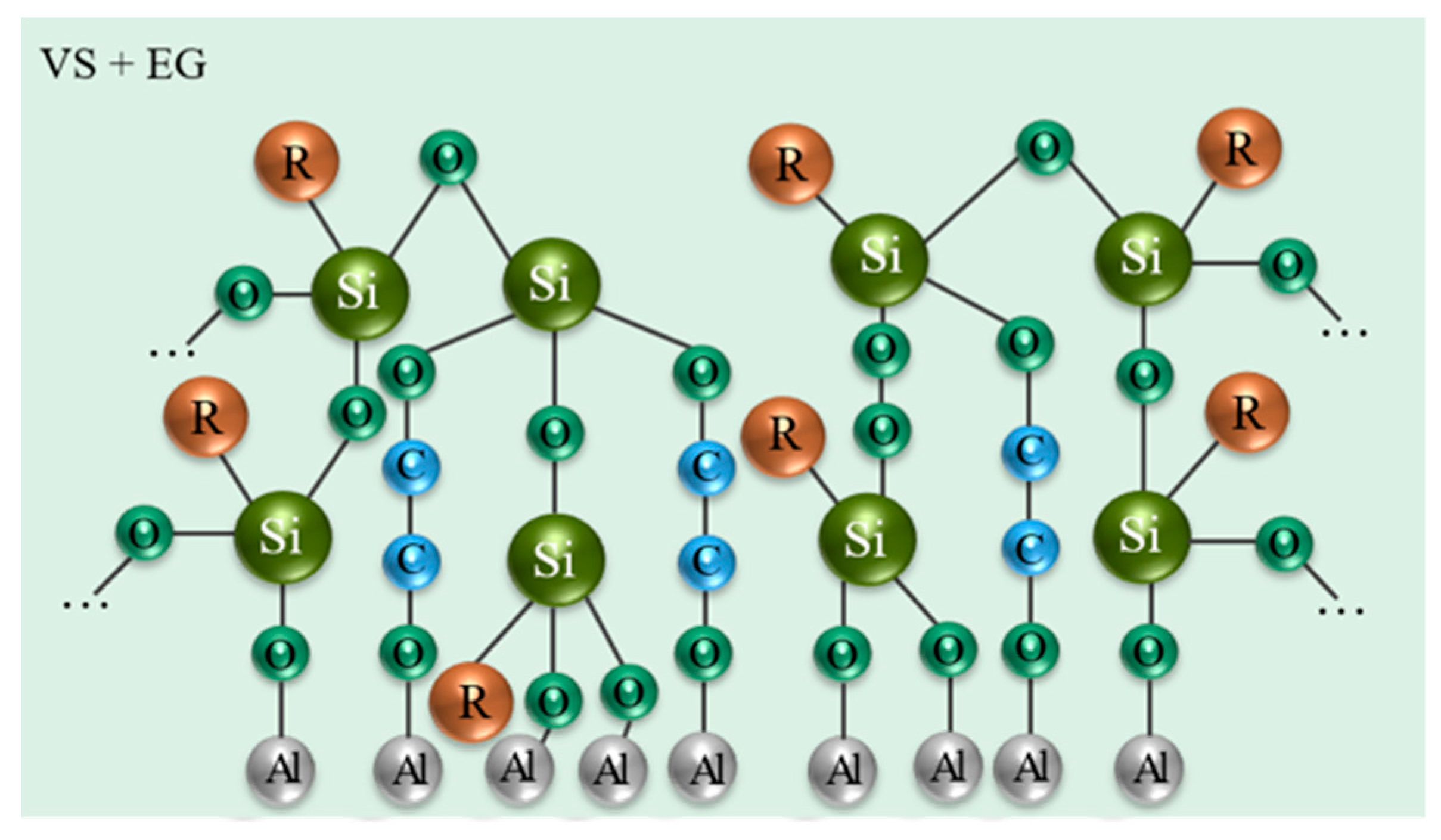
- Makarychev, Y.B.; Kuzenkov, Y.A.; Chugunov, D.O.; Grafov, O.Y.; Aliev, A.D. Vapor-Phase Deposition of Polymer Siloxane Coatings on Aluminum and Magnesium Alloys. Prog. Org. Coat. 2023, 183, 107755. [Google Scholar] [CrossRef]
- Franco, M.; Anoop, S.; Uma Rani, R.; Sharma, A.K. Porous Layer Characterization of Anodized and Black-Anodized Aluminium by Electrochemical Studies. ISRN Corros. 2012, 2012, 323676. [Google Scholar] [CrossRef]
- Pustov, I.A.; Zolotarev, A.S.; Gladkikh, N.A.; Kalita, V.I.; Komlev, D.I.; Radyuk, A.A.; Ivannikov, A.Y. Structure and Corrosion-Electrochemical Behavior of the Systems of Fe-Based Amorphousplasma Coating-Steel Substrate. Phys. Chem. Mater. Treat. 2015, 3, 35–43. [Google Scholar]
- Liu, X.Q.; Zheng, Y.G.; Chang, X.C.; Hou, W.L.; Wang, J.Q.; Tang, Z.; Burgess, A. Microstructure and Properties of Fe-Based Amorphous Metallic Coating Produced by High Velocity Axial Plasma Spraying. Alloys Compd. 2009, 484, 300–307. [Google Scholar] [CrossRef]
- Nielsen, P.T.; Mathiesen, T.; Nielsen, S.E. Corrosion Properties of Selected Coatings Produced by Atmospheric Plasma Spraying, HVOF and Laser Remelt Methods. In Proceedings of the from the International Thermal Spray Conference, Busan, Republic of Korea, 13–15 May 2013; pp. 85–90. [Google Scholar] [CrossRef]
- Ilichev, L.L.; Nasyrov, S.G.; Klevtsov, G.V. Corrosion Resistance of Ion-Plasma Coatings in Hydrogen Sulfide Environments. Fundam. Res. 2006, 8, 65–66. (In Russian) [Google Scholar]
- Paul, S.; Harvey, M.D.F. Corrosion Testing of HVOF Coatings in High Temperature Environments for Biomass Applications. In Proceedings of the International Thermal Spray Conference, Houston, TX, USA, 21–24 May 2012; pp. 184–189. [Google Scholar] [CrossRef]
- Vyaliy, I.E. Hydrophobic Coatings on Aluminum and Magnesium Alloys Formed Using Plasma Electrolytic Oxidation. Ph.D. Thesis, Institute of Chemistry, Far Eastern Branch Russian Academy of Sciences, Vladivostok, Russia, 25 November 2021. (In Russian). [Google Scholar]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S.; Vyaliy, I.E. Wettability and Electrochemical Properties of the Highly Hydrophobic Coatings on PEO-Pretreated Aluminum Alloy. Surf. Coat. Technol. 2016, 307 Pt C, 1241–1248. [Google Scholar] [CrossRef]
- Chirkunov, A.A.; Rakoch, A.G.; Monakhova, E.V.; Gladkova, A.A.; Khabibullina, Z.V.; Ogorodnikova, V.A.; Serdechnova, M.; Blawert, C.; Kuznetsov, Y.I.; Zheludkevich, M.L. Corrosion Protection of Magnesium Alloy by PEO-Coatings Containing Sodium Oleate. Int. J. Corros. Scale Inhib. 2019, 8, 1170–1188. [Google Scholar] [CrossRef]
- Rykalina, U.S.; Semiletov, A.M.; Dushik, V.V.; Ruban, E.A.; Kuznetsov, Y.I. Hydrophobization of Tungsten Carbide Coatings. Int. J. Corros. Scale Inhib. 2025, 14, 980–994. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Bhushan, A.; Dewan, A.; Gupta, A.; Hasija, V.; Mukherjee, B.; Wadhwa, S.; Xu, D.; Zhou, H. System and Method for Machine Learning Based Line Assignment. Patent US20170249697A1, 31 August 2017. 17p. [Google Scholar]
- Redkina, G.V.; Rozhkov, A.S.; Sergienko, A.S.; Grafov, O.Y. Corrosion Resistance of Laser-Nanostructured Galvanized Steel Modified with Alkylphosphonic Acid. Int. J. Corros. Scale Inhib. 2024, 13, 1936–1960. [Google Scholar] [CrossRef]
- Kuzina, E.A.; Teplonogova, M.A.; Emelyanenko, A.M.; Boinovoch, L.B. Superhydrophobic Epoxy Coatings via Pulsed Laser Processing. Prog. Org. Coat. 2025, 204, 109272. [Google Scholar] [CrossRef]
- Zabala, B.; Fernandez, X.; Rodriguez, J.C.; Lipez-Ortega, A.; Fuentes, E.; Bayon, R.; Lgartua, A.; Girot, F. Mechanism-Based Wear Models for Plastic Injection Moulds. Wear 2019, 440–441, 203105. [Google Scholar] [CrossRef]
- Gripon, L.; Belyamani, I.; Legros, B.; Seaudeau-Pirouley, K.; Lafranche, E.; Cauret, L. Brominated Flame Retardants Extraction from Waste Electrical and Electronic Equipment-Derived Retardants Extraction from Waste Electrical and Electronic Equipment-Derived ABS Using Supercritical Carbon Dioxide. Waste Manag. 2021, 131, 313–322. [Google Scholar] [CrossRef]
- Meng, X.; Ding, L.; Xiao, H.; Li, C.; Zhang, D.; Wang, Y.; Chen, W. A Study of Microstructures and Corrosion Behavior of HVOF/PVD Duplex Coating against 10 vol% HCl Solution. Surf. Coat. Technol. 2024, 476, 130227. [Google Scholar] [CrossRef]
- Chen, W.L.; Mao, T.J.; Zhang, B.Y.; Zhang, S.H.; Meng, X.N. Designs and Preparation of Advanced HVOF-PVD Duplex Coating by Combination of HVOF and Arc Ion Plating. Surf. Coat. Technol. 2016, 304, 125–133. [Google Scholar] [CrossRef]
- Chen, W.L.; Fang, B.; Zhang, D.D.; Meng, X.N.; Zhang, S.H. Thermal Stability and Mechanical Properties of HVOF/PVD Duplex Ceramic Coatings Produced by HVOF and Cathodic Vacuum Arc. Ceram. Int. 2017, 43, 7415–7423. [Google Scholar] [CrossRef]
- Murugan, K.; Ragupathy, A.; Balasubramanian, V.; Sridhar, K. Optimizing HVOF Spray Process Parameters to Attain Minimum Porosity and Maximum Hardness in WC-10Co-4Cr Coatings. Surf. Coat. Technol. 2014, 247, 90–102. [Google Scholar] [CrossRef]
- Liu, M.M.; Hu, H.X.; Zheng, Y.G. Effects of Three Sealing Methods of Aluminum Phosphate Sealant on Corrosion Resistance of the Fe-Based Amorphous Coating. Surf. Coat. Technol. 2017, 309, 579–589. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Doleker, K.M.; Demirel, B.; Turk, A.; Varol, R. Effect of Shot Peening on the Oxidation Behavior of Thermal Barrier Coatings. In Properties and Characterization of Modern Materials; Advanced Structured Materials; Springer: Singapore, 2015; Volume 354, pp. 314–322. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Doleker, K.M.; Ozgurluk, Y. State of the Art Thermal Barrier Coating (TBC) Materials and TBC Failure Mechanisms. In Properties and Characterization of Modern Materials; Advanced Structured Materials; Springer: Singapore, 2017; pp. 441–452. [Google Scholar] [CrossRef]
- Doleker, K.M.; Ozgurluk, Y.; Kahraman, Y.; Karaoglanli, A.C. Oxidation and Hot Corrosion Resistance of HVOF/EB-PVD Thermal Barrier Coating System. Surf. Coat. Technol. 2021, 409, 129862. [Google Scholar] [CrossRef]
- Johar, M.; Moradizadeh, L.; Gupta, A.; Chellehbari, Y.M.; Li, X.; Shahgaldi, S. Development of Novel Nb and Ta Multilayer Coatings for Corrosion Protection of Ti-Based Bipolar Plates for Proton Exchange Membrane Fuel Cells. Corros. Sci. 2025, 245, 112707. [Google Scholar] [CrossRef]
- Xiang, T.; Chen, D.; Lv, Z.; Yang, Z.; Yang, L.; Li, C. Robust Superhydrophobic Coating with Superior Corrosion Resistance. J. Alloys Compd. 2019, 798, 320–325. [Google Scholar] [CrossRef]
- Tabakov, V.P.; Smirnov, M.Y.; Tulisov, A.N. Development of Multilayer Coatings Based on the Assessment of Their Crack Resistance. New Mater. Prod. Technol. 2010, 58, 32–36. (In Russian) [Google Scholar]
- Paderov, A.N.; Veksler, Y.G. Method of Application of Multi-Layer on Metal Articles. Patent RU2228387C1, 10 May 2004. 8p. [Google Scholar]
- Zhang, Y.; Outeiro, J.C.; Nouveau, C.; Marcon, B.; Denguir, L.A. Performance of New Cutting Tool Multilayer Coatings for Machining Ti-6Al-4V Titanium Alloy under Cryogenic Cooling Conditions. Adv. Ind. Manuf. Eng. 2025, 10, 100165. [Google Scholar] [CrossRef]
- Olejnik, S.V.; Kuzenkov, J.A.; Kuznetsov, J.I.; Rudnev, V.S.; Jarovaja, T.P.; Nedozorov, P.M. Method of Anticorrosion Processing of Aluminium Alloys. Patent RU2228387C1, 10 September 2014. 10p. [Google Scholar]
- Mikov, D.A.; Kutyrev, A.E.; Petrova, V.A. Hydrophobic Structures for Additional Protection of Aluminum Alloys in Fuel Systems of Products of Aircraft Equipment. VIAM Works 2015, 8, 66–72. [Google Scholar] [CrossRef]
- What Is Anodizing? Process, Types, Applications, and Benefits. Available online: https://www.machining-custom.com/ru/blog/what-is-anodizing.html/ (accessed on 30 October 2025).
- Bemporad, E.; Sebastiani, M.; Casadei, F.; Carassiti, F. Modelling, Production and Characterisation of Duplex Coatings (HVOF and PVD) on Ti–6Al–4V Substrate for Specific Mechanical Applications. Surf. Coat. Technol. 2007, 201, 7652–7662. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Muktanova, N.; Zhurerova, L.G. Application of HVOF technology for WC-Based Wear-Resistant Coatings—An Overview. NNC RK Bull. 2023, 93, 4–14. [Google Scholar] [CrossRef]
- Flame Supersonic Coating Equipment, Application of Coatings. Available online: https://mashprom.ru/press/publication/_aview_b7/ (accessed on 30 October 2025).
- PVD/PACVD Coatings for Pumping Equipment. Available online: https://ritm-magazine.com/en/node/11031/ (accessed on 30 October 2025).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapagina, N.A.; Dushik, V.V. Bilayer Coating Systems: Functional Interlayers and Top Layers for Enhanced Performance. Materials 2025, 18, 5217. https://doi.org/10.3390/ma18225217
Shapagina NA, Dushik VV. Bilayer Coating Systems: Functional Interlayers and Top Layers for Enhanced Performance. Materials. 2025; 18(22):5217. https://doi.org/10.3390/ma18225217
Chicago/Turabian StyleShapagina, Natalia A., and Vladimir V. Dushik. 2025. "Bilayer Coating Systems: Functional Interlayers and Top Layers for Enhanced Performance" Materials 18, no. 22: 5217. https://doi.org/10.3390/ma18225217
APA StyleShapagina, N. A., & Dushik, V. V. (2025). Bilayer Coating Systems: Functional Interlayers and Top Layers for Enhanced Performance. Materials, 18(22), 5217. https://doi.org/10.3390/ma18225217







