Optimization of Plasma-Sprayed CeScYSZ Thermal Barrier Coating Parameters and Investigation of Their CMAS Corrosion Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Design of Key Plasma Spraying Parameters
2.3. CMAS Corrosion Experiment
2.4. Coating Microstructure and Characterization Techniques
3. Results and Discussion
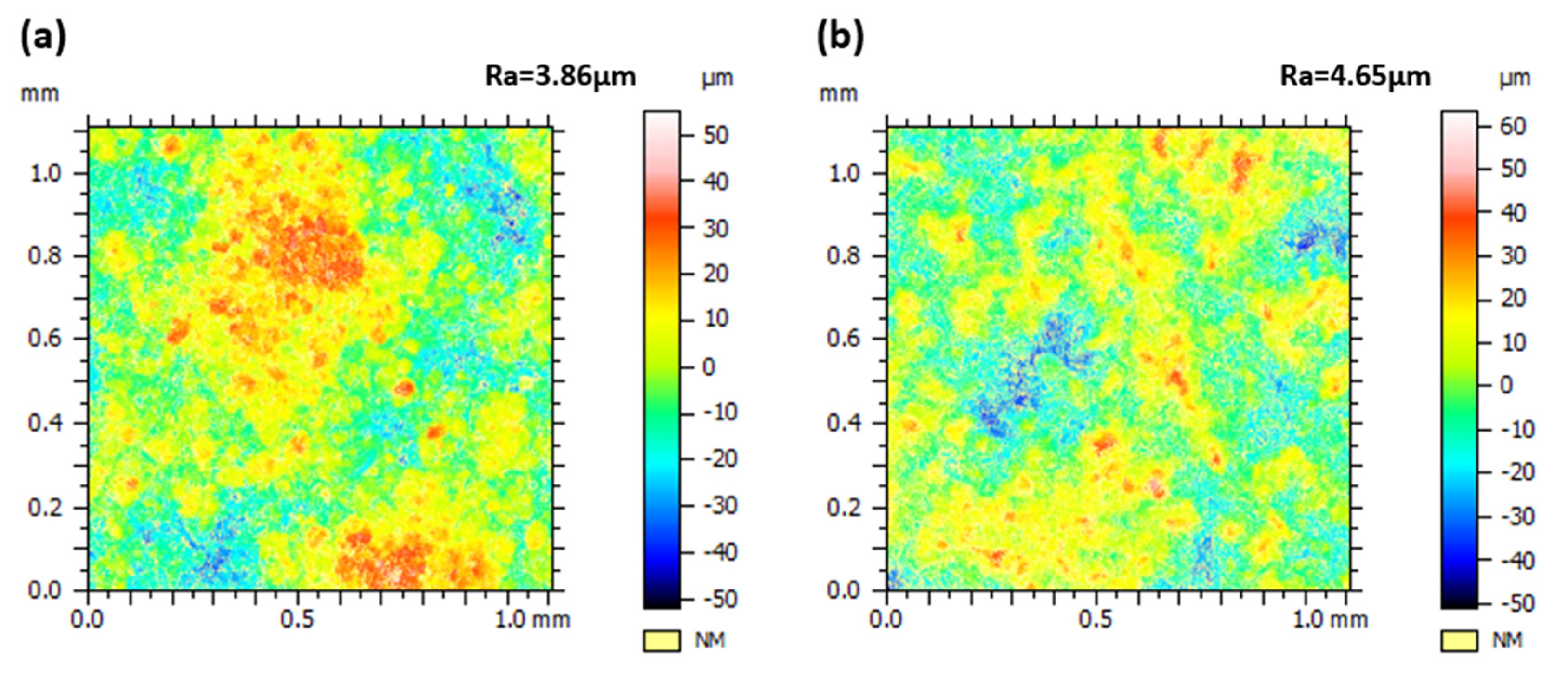
3.1. Influence of Process Parameters on Coating Microstructure
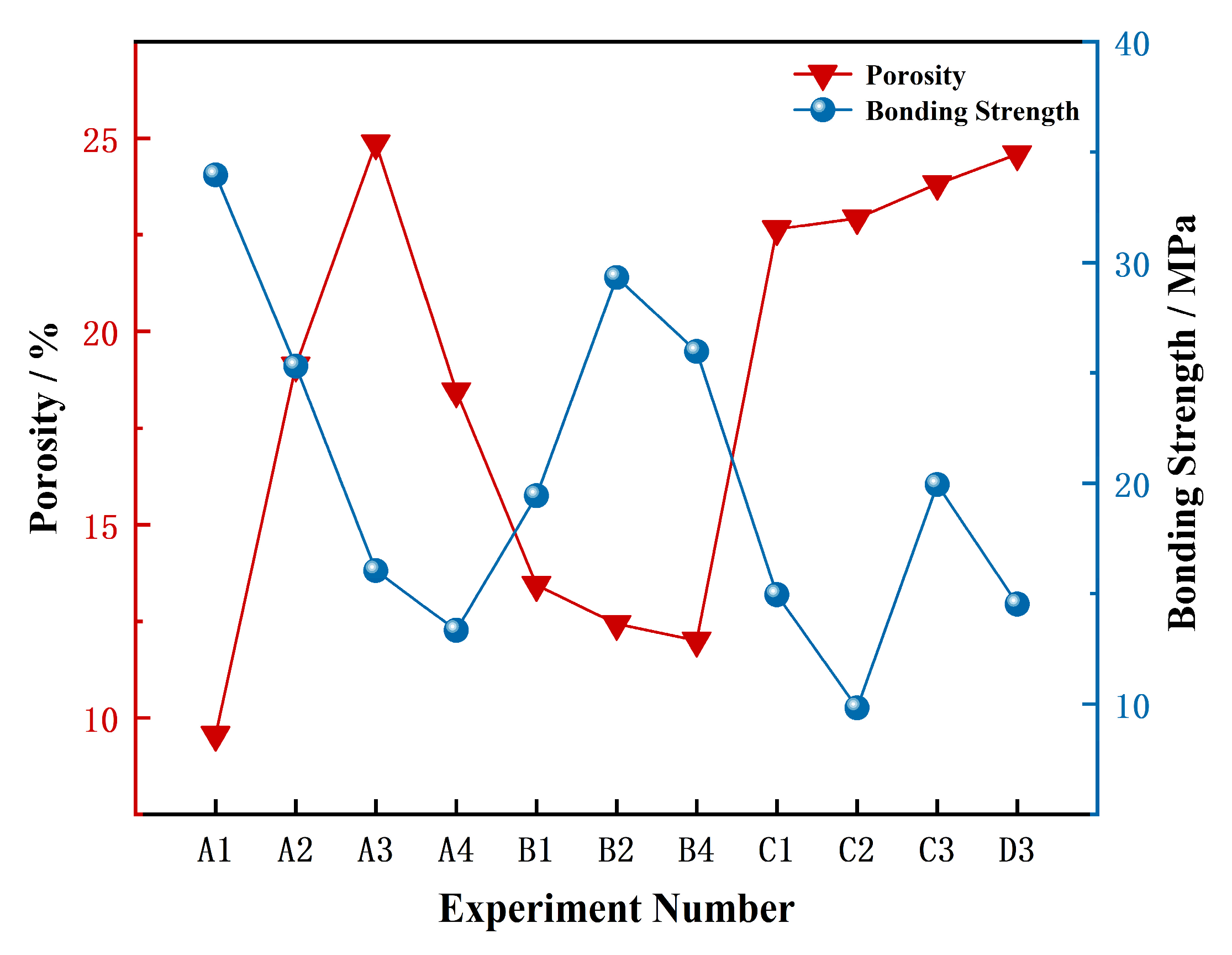
3.2. Effect of Process Parameters on Coating Bonding Strength
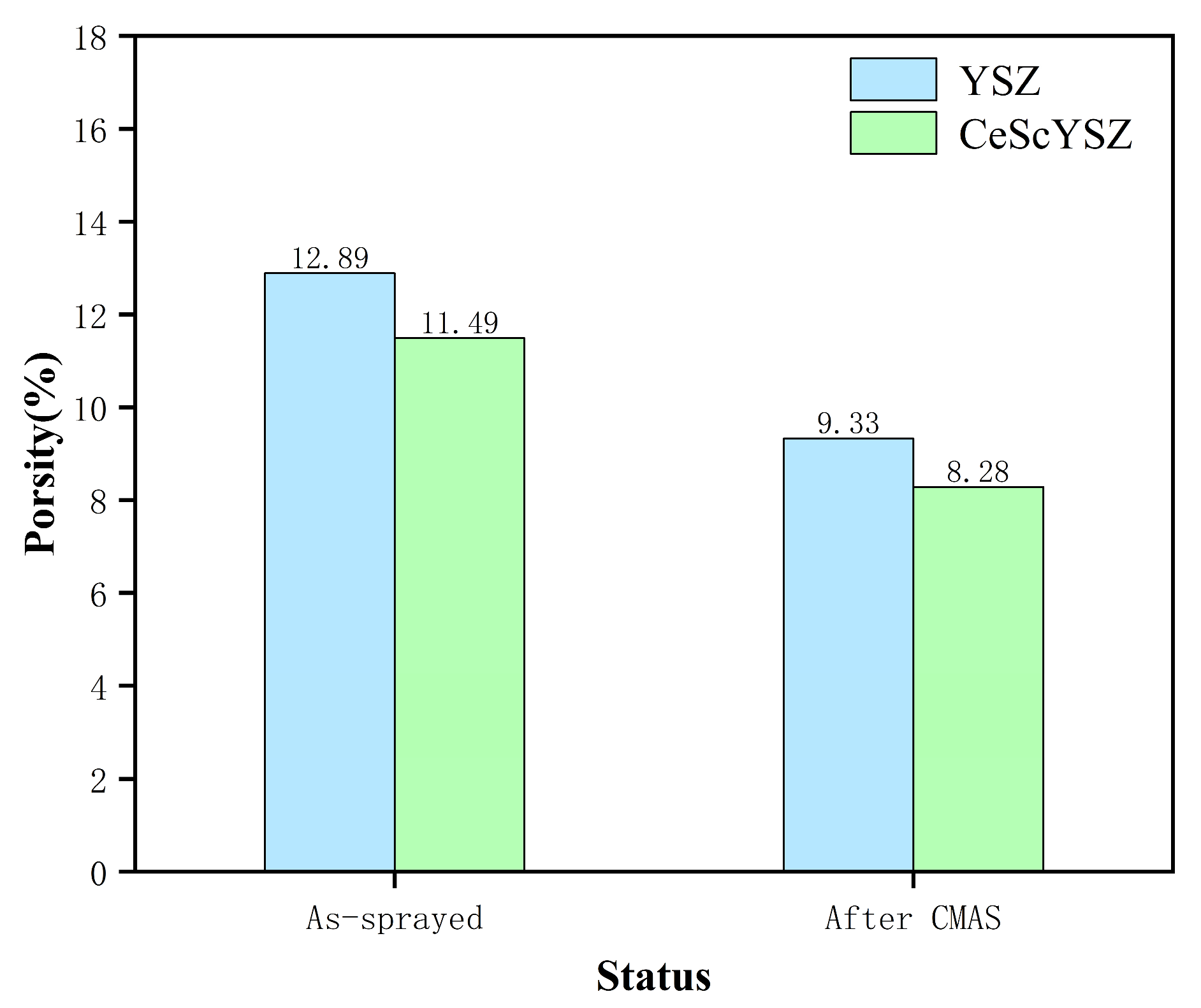
3.3. Research on the Thermal Insulation Capacity and CMAS Corrosion Resistance of CeScYSZ Coatings with Optimized Process
4. Conclusions
- (1)
- An orthogonal experimental design with five factors and four levels was implemented to optimize the process parameters for depositing the CeScYSZ coating, using porosity and bonding strength as the key response criteria. The final optimized parameters were determined as follows: spray distance, 100 mm; current, 500 A; primary gas (Ar) flow rate, 30 L/min; hydrogen (H2) flow rate, 6 L/min; and powder feed rate, 45 g/min.
- (2)
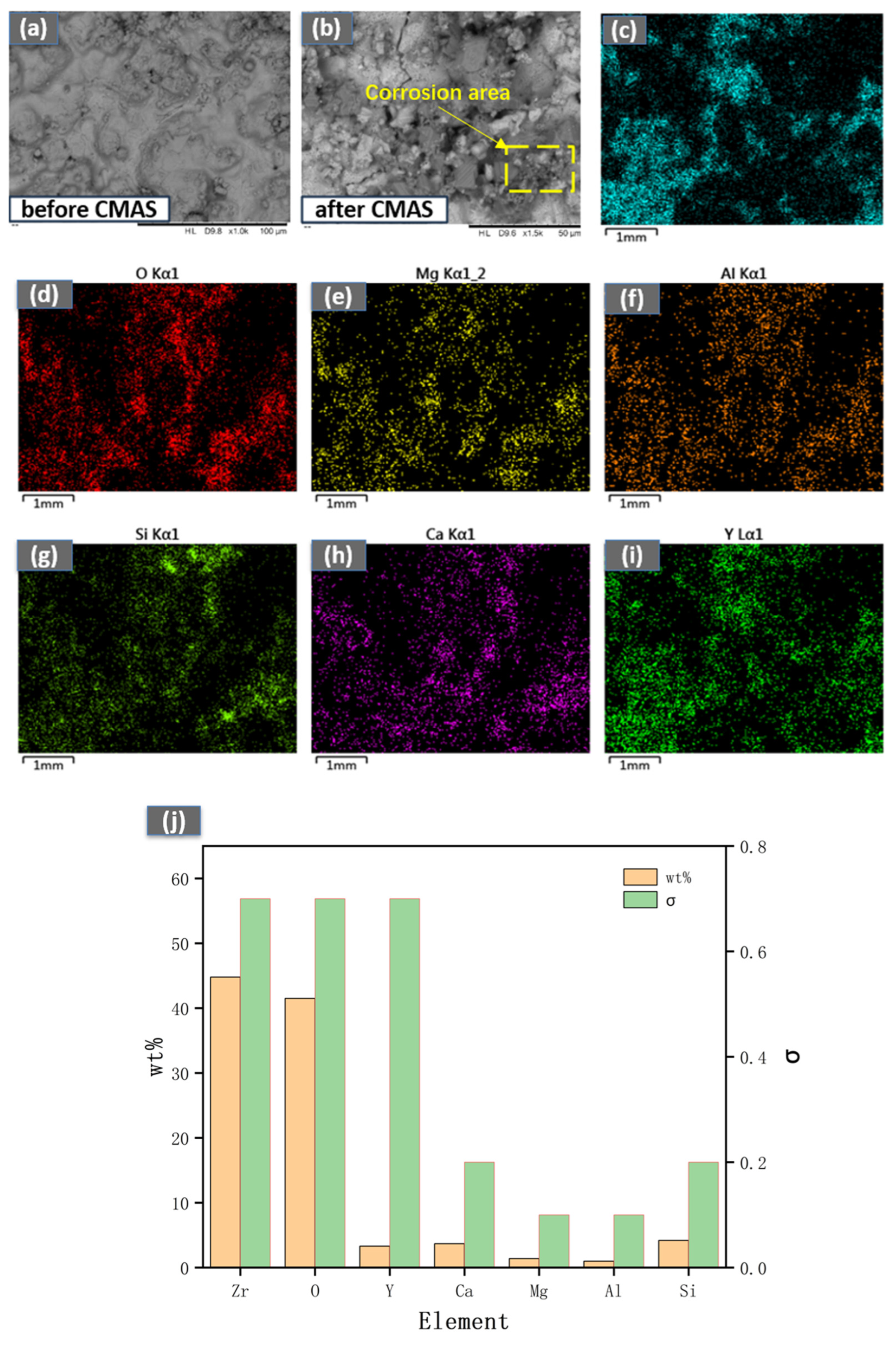
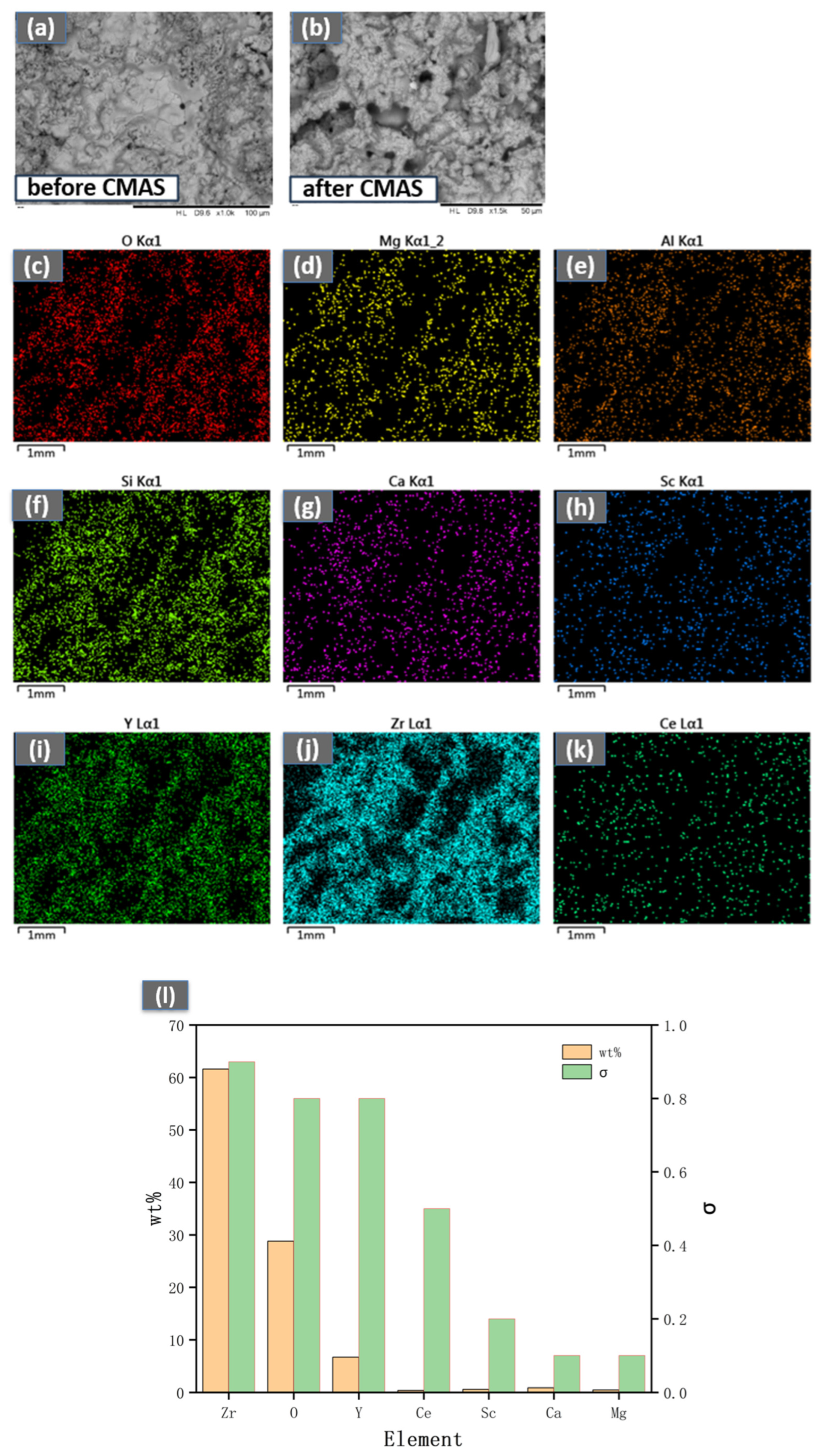
- The CeScYSZ coating demonstrates enhanced thermal insulation capability relative to the conventional YSZ coating. During CMAS attack, the melt dissolves Y from YSZ, inducing a t→m transformation that creates a porous m-phase layer. Concurrently, MgO infiltrates through cracks, enhancing CMAS penetration. Thermal stress causes significant cracking. In contrast, CeScYSZ forms a barrier layer of CaZrO3 that inhibits melt infiltration. Ce4+ consumes alkaline ions, aiding protective layer formation, while Sc3+ effectively stabilizes the t-ZrO2 lattice even in depleted zones. Crucially, the Ce–Sc synergy inhibits Y3+ dissolution, entirely blocking the transformation pathway. Thus, CeScYSZ exhibits superior CMAS resistance and phase stability over YSZ.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ito, K.; Kuriki, H.; Araki, H.; Kuroda, S.; Enoki, M. Detection of segmentation cracks in top coat of thermal barrier coatings during plasma spraying by non-contact acoustic emission method. Sci. Technol. Adv. Mater. 2014, 15, 035007. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, J.; Liu, Y.; Wang, Y.; Ajayi, A.; Wang, Z. Research on crack propagation behaviour of EB-PVD TBCs based on TGO evolution. Sci. Rep. 2023, 13, 17550. [Google Scholar] [CrossRef]
- Vaßen, R.; Kagawa, Y.; Subramanian, R.; Zombo, P.; Zhu, D. Testing and evaluation of thermal-barrier coatings. Mrs Bull. 2012, 37, 911–916. [Google Scholar] [CrossRef]
- Wu, Y.; Hong, D.; Zhong, X.; Niu, Y.; Zheng, X. Research progress on hafnium-based thermal barrier coatings materials. Ceram. Int. 2023, 49, 21133–21141. [Google Scholar] [CrossRef]
- Krishnamurthy, K.N.; Akash Deep, B.N.; Birur Jayanna, V.; Sumalatha, C.P.; Khan, A.; Kamangar, S.; Islam, S.; Razak, A.; Jelila, Y.D. Influence of ceramic thermal barrier coating on diesel engine performance using scum oil biodiesel at different compression ratios. Sci. Rep. 2025, 15, 31094. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Pan, W.; Xu, Q.; Qi, L.H.; Wang, J.D.; Miao, H.Z.; Mori, K.; Torigoe, T. Novel Low Thermal Conductivity Ceramic Materials for Thermal Barrier Coatings. Key Eng. Mater. 2004, 509, 1497–1500. [Google Scholar]
- Peng, H.; Yu, Y.; Shi, T.; Bai, B.; Yan, Z.; Yuan, K. Effects of Induction Plasma Spheroidization on Properties of Yttria-Stabilized Zirconia Powders for Thermal Barrier Coating Applications. Materials 2024, 17, 1518. [Google Scholar] [CrossRef] [PubMed]
- Parchovianský, M.; Parchovianská, I.; Hanzel, O.; Netriová, Z.; Pakseresht, A. Phase evaluation, mechanical properties and thermal behavior of hot-pressed LC-YSZ composites for TBC applications. Materials 2022, 15, 2839. [Google Scholar] [CrossRef]
- Cao, Z.; An, S.; Song, X. Effect of thermal treatment at high temperature on phase stability and transformation of Yb2O3 and Y2O3 co-doped ZrO2 ceramics. Sci. Rep. 2022, 12, 9955. [Google Scholar] [CrossRef]
- Chen, L.B. Yttria-stabilized zirconia thermal barrier coatings—A review. Surf. Rev. Lett. 2006, 13, 535–544. [Google Scholar] [CrossRef]
- Lughi, V.; Clarke, D.R. High temperature aging of YSZ coatings and subsequent transformation at low temperature. Surf. Coat. Technol. 2005, 200, 1287–1291. [Google Scholar] [CrossRef]
- Lipkin, D.M.; Krogstad, J.A.; Yan, G.; Johnson, C.A.; Nelson, W.A.; Levi, C.G. Phase Evolution upon Aging of Air-Plasma Sprayed t’-Zirconia Coatings: I—Synchrotron X-Ray Diffraction. J. Am. Ceram. Soc. 2013, 96, 290–298. [Google Scholar] [CrossRef]
- Hou, Z.; Yang, W.; Zhan, Y.; Zhang, X.; Zhang, J. Effect of Calcination Temperature on the Microstructure, Composition and Properties of Agglomerated Nanometer CeO2-Y2O3-ZrO2 Powders for Plasma Spray–Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Nanomaterials 2024, 14, 995. [Google Scholar] [CrossRef]
- Wei, X.; Hou, G.; An, Y.; Yang, P.; Zhao, X.; Zhou, H.; Chen, J. Effect of doping CeO2 and Sc2O3 on structure, thermal properties and sintering resistance of YSZ. Ceram. Int. 2021, 47, 6875–6883. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Li, W.; Chen, H.; Liu, H.; Cheng, X. Shakedown analysis and experimental study of thermal barrier coatings. Materials 2022, 15, 6446. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, W.; Huang, J.; Ye, D. Investigation of CMAS resistance of sacrificial plasma-sprayed mullite-YSZ protective layer on 8YSZ thermal barrier coating. Corros. Sci. 2020, 173, 108764. [Google Scholar] [CrossRef]
- Yuan, P.L. Effect of co-doping of CeO2 and Sc2O3 on mechanical properties and CMAS corrosion resistance of YSZ ceramics. IOP Conf. Ser. Earth Environ. Science. IOP Publ. 2024, 1393, 012010. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Cai, C.; Wang, Y.; Zhou, Y.; Yang, L.; Zhou, G. Comparison of CMAS corrosion and sintering induced microstructural characteristics of APS thermal barrier coatings. J. Mater. Sci. Technol. 2019, 35, 440–447. [Google Scholar] [CrossRef]
- GB/T 8642-2002; Thermal Spraying—Determination of Tensile Adhesive Strength. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2002.
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Vassen, R.; Cao, X.Q.; Tietz, F.; Basu, D.; Stöver, D. Influence of porosity on thermal conductivity and sintering in thermal barrier coatings. J. Am. Ceram. Soc. 2000, 83, 2023–2028. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Zhu, C.; Li, P.; Javed, A.; Liang, G.Y.; Xiao, P. An investigation on the microstructure and oxidation behavior of laser remelted air plasma sprayed thermal barrier coatings. Surf. Coat. Technol. 2012, 206, 3739–3746. [Google Scholar] [CrossRef]
- Ma, X.; He, Y.; Wang, D.; Zhang, J. Enhanced high-temperature corrosion resistance of (Al2O3–Y2O3)/Pt micro-laminated coatings on 316L stainless steel alloy. Corros. Sci. 2012, 54, 183–192. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, L.; Huang, Y.; Lei, H.; Zhang, W. Impact of interfacial texture on failure behaviour of 8YSZ thermal barrier coatings under thermal cyclic loading. J. Eur. Ceram. Soc. 2024, 44, 7265–7276. [Google Scholar] [CrossRef]
- Xu, P.; Chen, C.; Chen, S.; Lu, W.; Qian, Q.; Zeng, Y. Machine learning-assisted design of yttria-stabilized zirconia thermal barrier coatings with high bonding strength. ACS Omega 2022, 7, 21052–21061. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Liu, Y.F.; Kang, Y.; Wang, Y.; Wang, Z.; Tao, W. Corrosion behavior of Sc2O3–Y2O3 co-stabilized ZrO2 thermal barrier coatings with CMAS attack. Ceram. Int. 2019, 45, 15763–15767. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Li, Q.; Li, Y. Investigation on the phase stability, sintering and thermal conductivity of Sc2O3–Y2O3–ZrO2 for thermal barrier coating application. Mater. Des. 2010, 31, 2972–2977. [Google Scholar] [CrossRef]
- Dong, Y.S.; Yan, C.L.; Li, J.; Jiang, Z.C.; Mo, W.L.; Zhang, Q.J. Study on thermal shock resistance of Sc2O3 and Y2O3 co-stabilized ZrO2 thermal barrier coatings. Ceram. Int. 2023, 49, 20034–20040. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Ye, F. Phase stability and thermal conductivity of RE2O3 (RE = La, Nd, Gd, Yb) and Yb2O3 co-doped Y2O3 stabilized ZrO2 ceramics. Ceram. Int. 2016, 42, 7360–7365. [Google Scholar] [CrossRef]
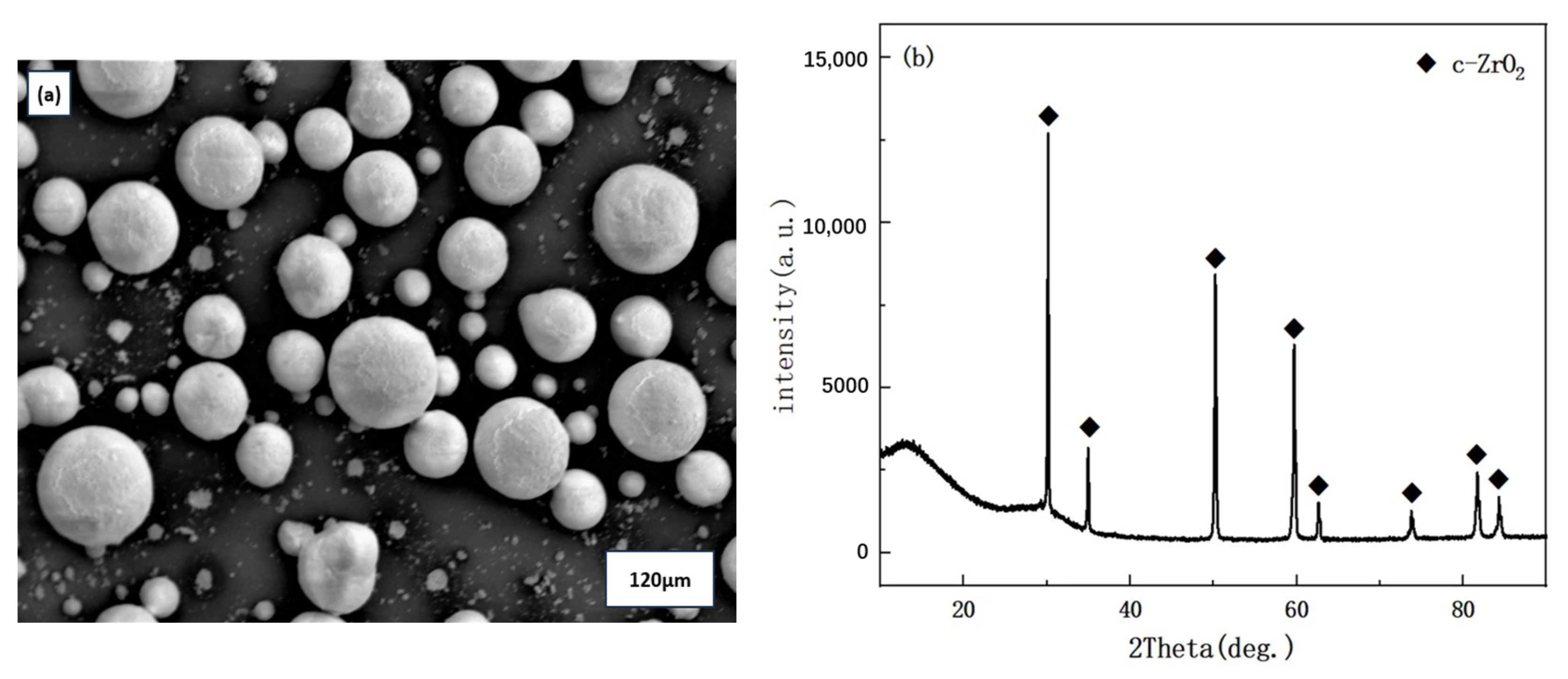

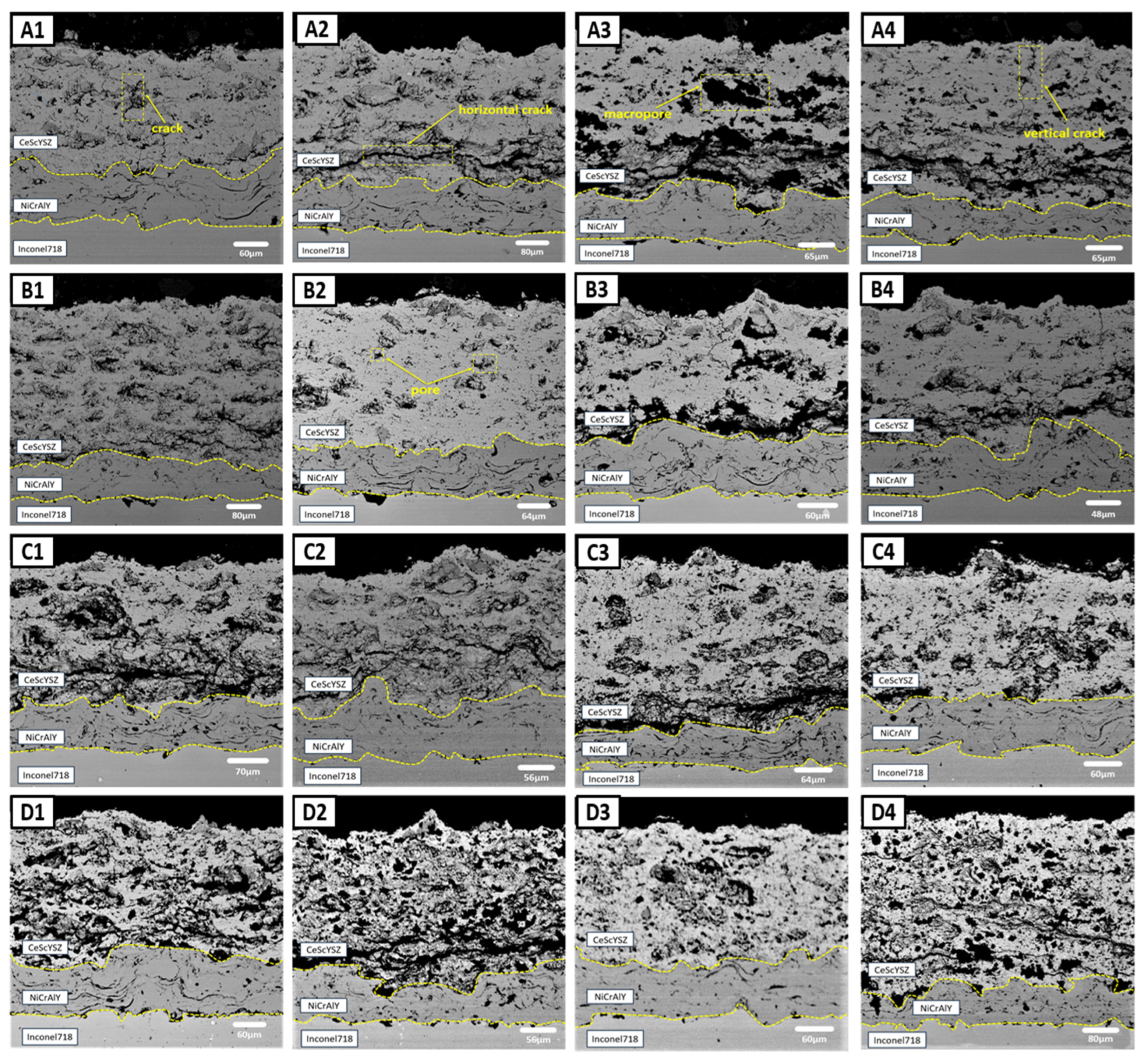


| Exp. | Spray Distance | Current | Ar | H2 | Powder Feed Rate |
|---|---|---|---|---|---|
| (mm) | (A) | (L/min) | (L/min) | (g/min) | |
| A1 | 90 | 500 | 30 | 6 | 35 |
| A2 | 90 | 550 | 35 | 8 | 40 |
| A3 | 90 | 600 | 40 | 10 | 45 |
| A4 | 90 | 650 | 45 | 12 | 50 |
| B1 | 100 | 500 | 35 | 10 | 50 |
| B2 | 100 | 550 | 30 | 12 | 45 |
| B3 | 100 | 600 | 45 | 6 | 40 |
| B4 | 100 | 650 | 40 | 8 | 35 |
| C1 | 110 | 500 | 40 | 12 | 40 |
| C2 | 110 | 550 | 45 | 10 | 35 |
| C3 | 110 | 600 | 30 | 8 | 50 |
| C4 | 110 | 650 | 35 | 6 | 45 |
| D1 | 120 | 500 | 45 | 8 | 45 |
| D2 | 120 | 550 | 40 | 6 | 50 |
| D3 | 120 | 600 | 35 | 12 | 35 |
| D4 | 120 | 650 | 30 | 10 | 40 |
| Stride (mm) | N2 (L/min) | Number of Coating Layers | Spray Distance (mm) | Current (A) | Ar (L/min) | H2 (L/min) | Powder Feed Rate (g/min) |
|---|---|---|---|---|---|---|---|
| 4 | 3.5 | 3 | 90 | 600 | 46 | 6 | 29 |
| Exp. | Spray Distance | Current | Ar | H2 | Powder Feed Rate | Porosity |
|---|---|---|---|---|---|---|
| (mm) | (A) | (L/min) | (L/min) | (g/min) | (%) | |
| A1 | 90 | 500 | 30 | 6 | 35 | 9.65 |
| A2 | 90 | 550 | 35 | 8 | 40 | 19.12 |
| A3 | 90 | 600 | 40 | 10 | 45 | 24.87 |
| A4 | 90 | 650 | 45 | 12 | 50 | 18.45 |
| B1 | 100 | 500 | 35 | 10 | 50 | 13.44 |
| B2 | 100 | 550 | 30 | 12 | 45 | 12.43 |
| B3 | 100 | 600 | 45 | 6 | 40 | 35.08 |
| B4 | 100 | 650 | 40 | 8 | 35 | 12 |
| C1 | 110 | 500 | 40 | 12 | 40 | 22.65 |
| C2 | 110 | 550 | 45 | 10 | 35 | 22.93 |
| C3 | 110 | 600 | 30 | 8 | 50 | 23.82 |
| C4 | 110 | 650 | 35 | 6 | 45 | 30.82 |
| D1 | 120 | 500 | 45 | 8 | 45 | 32.27 |
| D2 | 120 | 550 | 40 | 6 | 50 | 32.85 |
| D3 | 120 | 600 | 35 | 12 | 35 | 24.59 |
| D4 | 120 | 650 | 30 | 10 | 40 | 30.48 |
| Range (R) | −12.03 | −7.59 | −8.08 | −5.57 | −9.54 | |
| Optimal Level | 90 | 500 | 30 | 12 | 35 |
| Exp. | A1 | A2 | A3 | A4 | B1 | B2 | B4 | C1 | C2 | C3 | D3 |
| Number | |||||||||||
| Bonding | 33.96 | 25.31 | 16.04 | 13.34 | 19.44 | 29.32 | 25.97 | 14.95 | 9.83 | 19.94 | 14.53 |
| Strength (MPa) | |||||||||||
| Spray Distance (mm) | Current (A) | Ar (L/min) | H2 (L/min) | Powder Feed Rate (g/min) | |||||||
| Range (R) | 10.38 | 5.94 | 16.15 | 18.86 | 5.11 | ||||||
| Optimal Level | 100 | 500 | 30 | 6 | 45 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, K.; Li, Z. Optimization of Plasma-Sprayed CeScYSZ Thermal Barrier Coating Parameters and Investigation of Their CMAS Corrosion Resistance. Materials 2025, 18, 5114. https://doi.org/10.3390/ma18225114
Li R, Wang K, Li Z. Optimization of Plasma-Sprayed CeScYSZ Thermal Barrier Coating Parameters and Investigation of Their CMAS Corrosion Resistance. Materials. 2025; 18(22):5114. https://doi.org/10.3390/ma18225114
Chicago/Turabian StyleLi, Rongbin, Keyu Wang, and Ziyan Li. 2025. "Optimization of Plasma-Sprayed CeScYSZ Thermal Barrier Coating Parameters and Investigation of Their CMAS Corrosion Resistance" Materials 18, no. 22: 5114. https://doi.org/10.3390/ma18225114
APA StyleLi, R., Wang, K., & Li, Z. (2025). Optimization of Plasma-Sprayed CeScYSZ Thermal Barrier Coating Parameters and Investigation of Their CMAS Corrosion Resistance. Materials, 18(22), 5114. https://doi.org/10.3390/ma18225114





