Implant Surface Variability Between Progressive Knife-Edge Thread Design and International Organization for Standardization Thread with and Without Tapping Area: A Model Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIC | Bone-to-implant contact |
| ESA | External surface area |
| ESAAPI | External surface area of apical half |
| ESACOR | External surface area of coronal half |
| ΔESA | Percentage of external surface difference |
| ΔESAAPI | Percentage of external surface difference in apical half |
| ΔESACOR | Percentage of external surface difference in coronal half |
| FEA | Finite element analysis |
| ISQ | Implant stability quotient |
| ISO | International Organization for Standardization |
| IT | Insertion torque |
References
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontology 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Farronato, D.; Manfredini, M.; Stocchero, M.; Caccia, M.; Azzi, L.; Farronato, M. Influence of Bone Quality, Drilling Protocol, Implant Diameter/Length on Primary Stability: An In Vitro Comparative Study on Insertion Torque and Resonance Frequency Analysis. J. Oral Implantol. 2020, 46, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Jacobsson, M. Bone-metal interface in osseointegration. J. Prosthet. Dent. 1987, 57, 597–607. [Google Scholar] [CrossRef] [PubMed]
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part II. Research on implant osseointegration: Material testing, mechanical testing, imaging and histoanalytical methods. Oral Maxillofac. Surg. 2014, 18, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar]
- Davies, J.E. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar]
- Boronat López, A.; Balaguer Martínez, J.; Lamas Pelayo, J.; Carrillo García, C.; Peñarrocha Diago, M. Resonance frequency analysis of dental implant stability during the healing period. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, e244–e247. [Google Scholar]
- do Vale Souza, J.P.; de Moraes Melo Neto, C.L.; Piacenza, L.T.; Freitas da Silva, E.V.; de Melo Moreno, A.L.; Penitente, P.A.; Brunetto, J.L.; Dos Santos, D.M.; Goiato, M.C. Relation Between Insertion Torque and Implant Stability Quotient: A Clinical Study. Eur. J. Dent. 2021, 15, 618–623. [Google Scholar] [CrossRef]
- Huang, H.; Wu, G.; Hunziker, E. The clinical significance of implant stability quotient (ISQ) measurements: A literature review. J. Oral Biol. Craniofac. Res. 2020, 10, 629–638. [Google Scholar] [CrossRef]
- Badalia, I.; Kumar, M.; Bansal, A.; Salil, F.; Ramandeep, F. Measuring Implants Stability—A Review. Dent. J. Adv. Stud. 2017, 5, 105–111. [Google Scholar] [CrossRef]
- Hiranmayi, K.V. Factors influencing implant stability. J. Dent. Implant. 2018, 8, 69–76. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmer, P.W. How does dental implant macrogeometry affect primary implant stability? A narrative review. Int. J. Implant. Dent. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Cahyaningtyas, N.A.; Miranda, A.; Metta, P.; Bawono, C.A. Dental implant macrodesign features in the past 10 years: A systematic review. J. Indian Soc. Periodontol. 2023, 27, 131–139. [Google Scholar] [CrossRef]
- Ryu, H.S.; Namgung, C.; Lee, J.H.; Lim, Y.J. The influence of thread geometry on implant osseointegration under immediate loading: A literature review. J. Adv. Prosthodont. 2014, 6, 547–554. [Google Scholar] [CrossRef]
- Kreve, S.; Ferreira, I.; da Costa Valente, M.L.; Dos Reis, A.C. Relationship between dental implant macro-design and osseointegration: A systematic review. Oral Maxillofac. Surg. 2024, 28, 1–14. [Google Scholar] [CrossRef]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implants Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.R.; Desai, S.R.; Patel, J.R.; Alqhtani, N.R.; Alqahtani, A.S.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M.; Karobari, M.I. Investigating the impact of diameters and thread designs on the Biomechanics of short implants placed in D4 bone: A 3D finite element analysis. BMC Oral Health 2023, 23, 686. [Google Scholar] [CrossRef]
- McCullough, J.J.; Klokkevold, P.R. The effect of implant macro-thread design on implant stability in the early post-operative period: A randomized, controlled pilot study. Clin. Oral Implants Res. 2017, 28, 1218–1226. [Google Scholar] [CrossRef]
- Zita Gomes, R.; de Vasconcelos, M.R.; Lopes Guerra, I.M.; de Almeida, R.A.B.; de Campos Felino, A.C. Implant Stability in the Posterior Maxilla: A Controlled Clinical Trial. Biomed Res. Int. 2017, 2017, 6825213. [Google Scholar] [CrossRef]
- Hoekstra, J.W.M.; van Oirschot, B.A.; Jansen, J.A.; van den Beucken, J.J. Innovative implant design for continuous implant stability: A mechanical and histological experimental study in the iliac crest of goats. J. Mech. Behav. Biomed. Mater. 2021, 122, 104651. [Google Scholar] [CrossRef]
- Mangano, C.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Iezzi, G. Early Bone Formation around Immediately Loaded Transitional Implants Inserted in the Human Posterior Maxilla: The Effects of Fixture Design and Surface. Biomed Res. Int. 2017, 2017, 4152506. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Hart, C.N.; Halbritter, S.A.; Morton, D.; Buser, D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin. Implant Dent. Relat. Res. 2009, 11, 338–347. [Google Scholar] [CrossRef]
- Hicklin, S.P.; Schneebeli, E.; Chappuis, V.; Janner, S.F.; Buser, D.; Brägger, U. Early loading of titanium dental implants with an intra-operatively conditioned hydrophilic implant surface after 21 days of healing. Clin. Oral Implants Res. 2016, 27, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kokovic, V.; Jung, R.; Feloutzis, A.; Todorovic, V.S.; Jurisic, M.; Hämmerle, C.H. Immediate vs. early loading of SLA implants in the posterior mandible: 5-year results of randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, e114–e119. [Google Scholar] [CrossRef]
- Romanos, G.E.; Kuyunov, O.; Sacks, D.; Calvo-Guirado, J.L.; Delgado-Ruiz, R. Apical stability of implants with progressive thread design in vitro, based on clinicians with different levels of experience. J. Periodontol. 2019, 90, 1320–1324. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Ghizzoni, M.; Cusaro, B.; Beretta, M.; Maiorana, C.; Souza, F.Á.; Poli, P.P. High Insertion Torque-Clinical Implications and Drawbacks: A Scoping Review. Medicina 2025, 61, 1187. [Google Scholar] [CrossRef]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.B.; Helms, J.A. Multiscale analyses of the bone-implant interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.B.; Shimano, A.C.; Macedo, A.P.; Valente, M.L.; dos Reis, A.C. Influence of torsional strength on different types of dental implant platforms. Implant Dent. 2015, 24, 281–286. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. Reaction of crestal bone around implants depending on mucosal tissue thickness. A 1-year prospective clinical study. Stomatologija 2009, 11, 83–91. [Google Scholar]
- Berglundh, T.; Mombelli, A.; Schwarz, F.; Derks, J. Etiology, pathogenesis and treatment of peri-implantitis: A European perspective. Periodontology 2000 2024, 00, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4,135 implants. Clin. Implant Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implants 2007, 22, 743–754. [Google Scholar] [PubMed]
- Freitas, A.C., Jr.; Bonfante, E.A.; Giro, G.; Janal, M.N.; Coelho, P.G. The effect of implant design on insertion torque and immediate micromotion. Clin. Oral Implants Res. 2012, 23, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Benalcázar-Jalkh, E.B.; Nayak, V.V.; Gory, C.; Marquez-Guzman, A.; Bergamo, E.T.; Tovar, N.; Coelho, P.G.; Bonfante, E.A.; Witek, L. Impact of implant thread design on insertion torque and osseointegration: A preclinical model. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e48–e55. [Google Scholar] [CrossRef]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between Insertion Torque and Implant Stability Quotient in Tapered Implants with Knife-Edge Thread Design. Biomed Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Schupbach, P.; Fiorellini, J.; Lin, Z.; Kim, D.M. The impact of bone compression on bone-to-implant contact of an osseointegrated implant: A canine study. Int. J. Periodontics Restor. Dent. 2012, 32, 637–645. [Google Scholar]
- Marconcini, S.; Giammarinaro, E.; Toti, P.; Alfonsi, F.; Covani, U.; Barone, A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clin. Implant Dent. Relat. Res. 2018, 20, 322–332. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Pereira, G.M.A.; Gehrke, A.F.; Junior, N.B.; Dedavid, B.A. Effects of insertion torque on the structure of dental implants with different connections: Experimental pilot study in vitro. PLoS ONE 2021, 16, e0251904. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Eliers Treichel, T.L.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Mazón, P.; de Aza, P.N. Impact of Different Titanium Implant Thread Designs on Bone Healing: A Biomechanical and Histometric Study with an Animal Model. J. Clin. Med. 2019, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Tumedei, M.; Aramburú Júnior, J.; Eliers Treichel, T.L.; Kolerman, R.; Lepore, S.; Piattelli, A.; Iezzi, G. Histological and Histomorphometrical Evaluation of a New Implant Macrogeometry. A Sheep Study. Int. J. Environ. Res. Public Health 2020, 17, 3477. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R. Osseointegration of metallic devices: Current trends based on implant hardware design. Arch. Biochem. Biophys. 2014, 561, 99–108. [Google Scholar] [CrossRef]

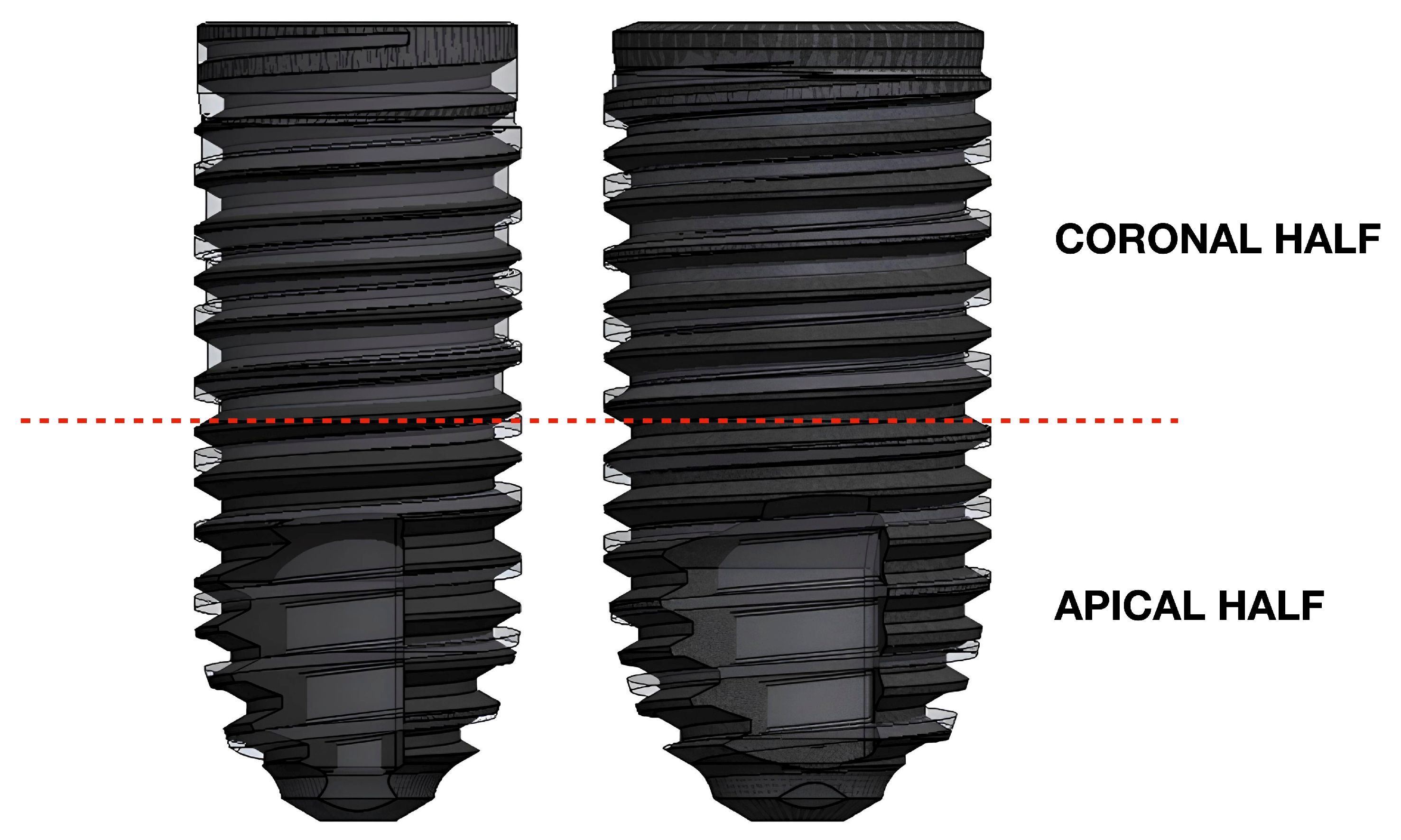
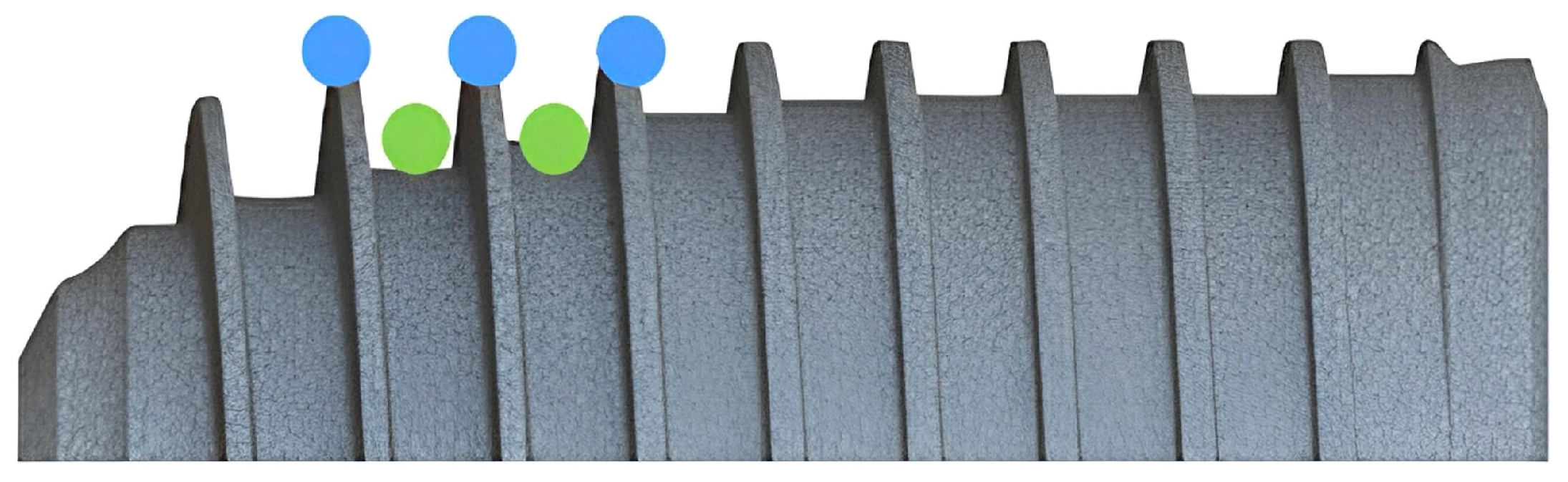
| Implant Model | Diameter (mm) | Length (mm) | Thread Depth (mm) | Thread Angle (°) | Thread Pitch (mm) | Total Surface Area (mm2) |
|---|---|---|---|---|---|---|
| Model A | 3.8 | 9.5 | NA * | NA * | 0.85 | 156.020 |
| Model B | 3.8 | 9.5 | 0.32 | 60° | 0.6 | 164.332 |
| Model C | 3.8 | 9.5 | 0.32 | 60° | 0.6 | 158.268 |
| Model D | 4.6 | 9.5 | NA * | NA * | 0.85 | 205.466 |
| Model E | 4.6 | 9.5 | 0.32 | 60° | 0.6 | 194.425 |
| Model F | 4.6 | 9.5 | 0.32 | 60° | 0.6 | 187.584 |
| Implant Model | ESACOR (mm2) | ΔESACOR | ESAAPI (mm2) | ΔESAAPI | ESA (mm2) | ΔESA |
|---|---|---|---|---|---|---|
| Model A | 66.936 | −18.9% | 85.599 | +9.3% | 152.535 | −5.2% |
| Model B | 82.509 | 78.338 | 160.847 | |||
| Model A | 66.936 | −18.9% | 85.599 | +18.4% | 152.535 | −1.5% |
| Model C | 82.509 | 72.274 | 154.783 | |||
| Model D | 92.961 | −2.2% | 104.982 | +14.3% | 197.943 | +5.9% |
| Model E | 95.034 | 91.868 | 186.902 | |||
| Model D | 92.961 | −2.2% | 104.982 | +23.5% | 197.943 | +9.9% |
| Model F | 95.034 | 85.027 | 180.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farronato, D.; Poncia, L.; Vidotto, M.; Maurino, V.; Romano, L. Implant Surface Variability Between Progressive Knife-Edge Thread Design and International Organization for Standardization Thread with and Without Tapping Area: A Model Analysis. Materials 2025, 18, 5113. https://doi.org/10.3390/ma18225113
Farronato D, Poncia L, Vidotto M, Maurino V, Romano L. Implant Surface Variability Between Progressive Knife-Edge Thread Design and International Organization for Standardization Thread with and Without Tapping Area: A Model Analysis. Materials. 2025; 18(22):5113. https://doi.org/10.3390/ma18225113
Chicago/Turabian StyleFarronato, Davide, Luca Poncia, Marco Vidotto, Vittorio Maurino, and Leonardo Romano. 2025. "Implant Surface Variability Between Progressive Knife-Edge Thread Design and International Organization for Standardization Thread with and Without Tapping Area: A Model Analysis" Materials 18, no. 22: 5113. https://doi.org/10.3390/ma18225113
APA StyleFarronato, D., Poncia, L., Vidotto, M., Maurino, V., & Romano, L. (2025). Implant Surface Variability Between Progressive Knife-Edge Thread Design and International Organization for Standardization Thread with and Without Tapping Area: A Model Analysis. Materials, 18(22), 5113. https://doi.org/10.3390/ma18225113








