Influence of Limestone Powder as Activator on the Enhancement of Early Mechanical Strength and Durability of High Blending Fly Ash Mortar Cured Under Different Temperatures
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Specimen Preparation
2.3. Test Methods
2.3.1. Compressive Strength
2.3.2. Chloride Diffusion Coefficient
2.3.3. Freeze–Thaw Resistance
2.3.4. XRD, TG, and SEM
2.3.5. Total Porosity
3. Results and Discussion
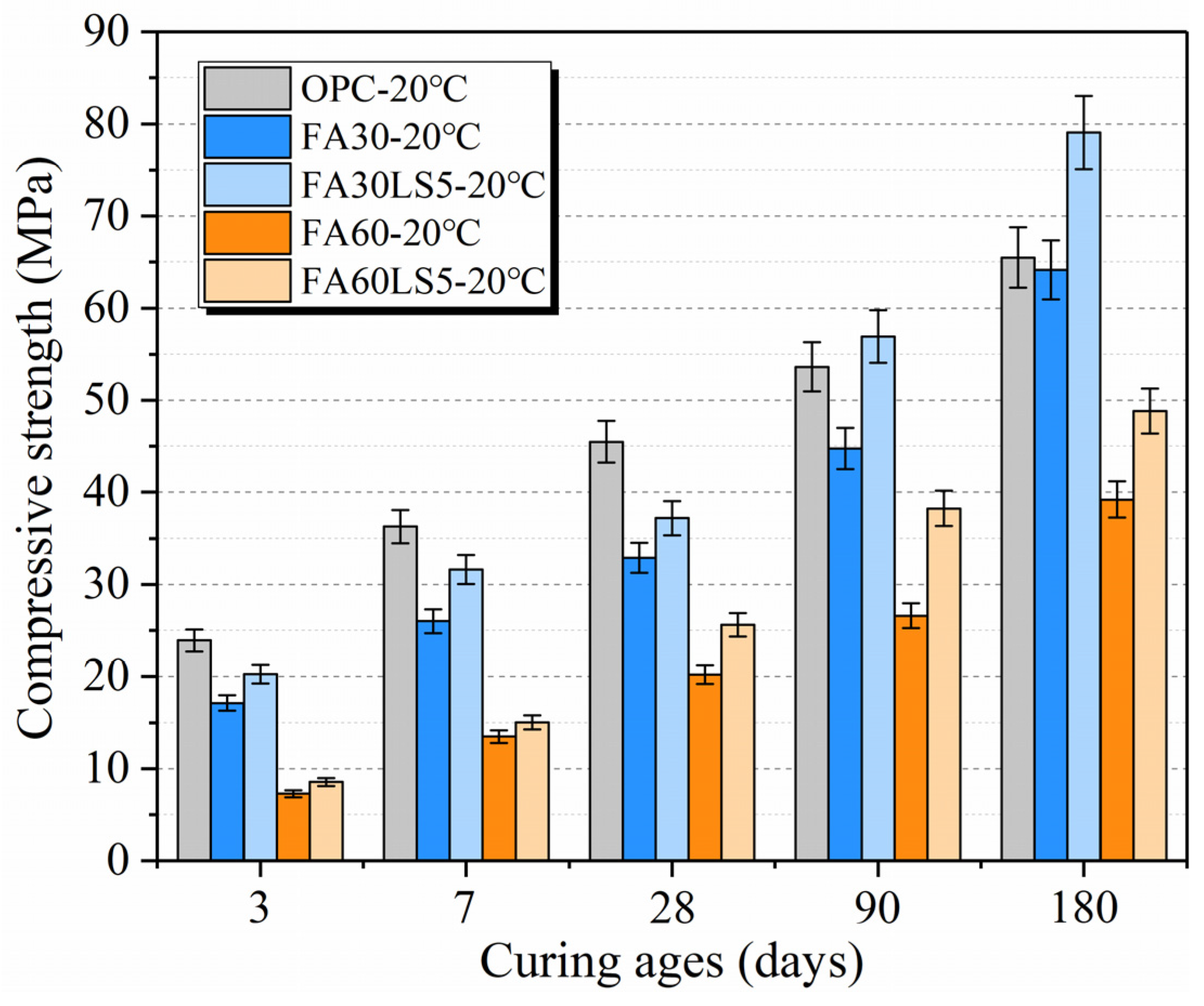
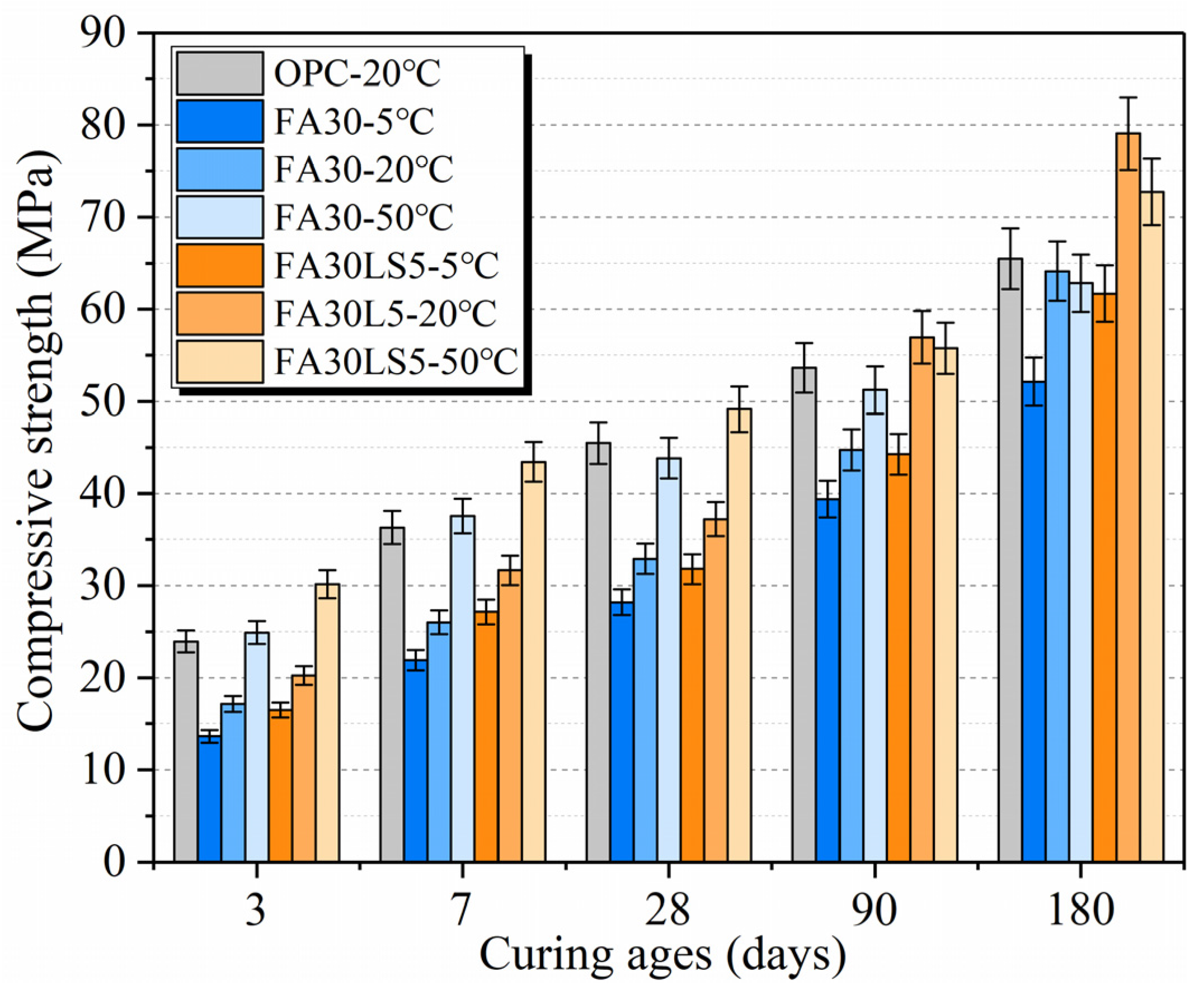
3.1. Compressive Strength
3.2. Compressive Strength After Freeze–Thaw Cycles
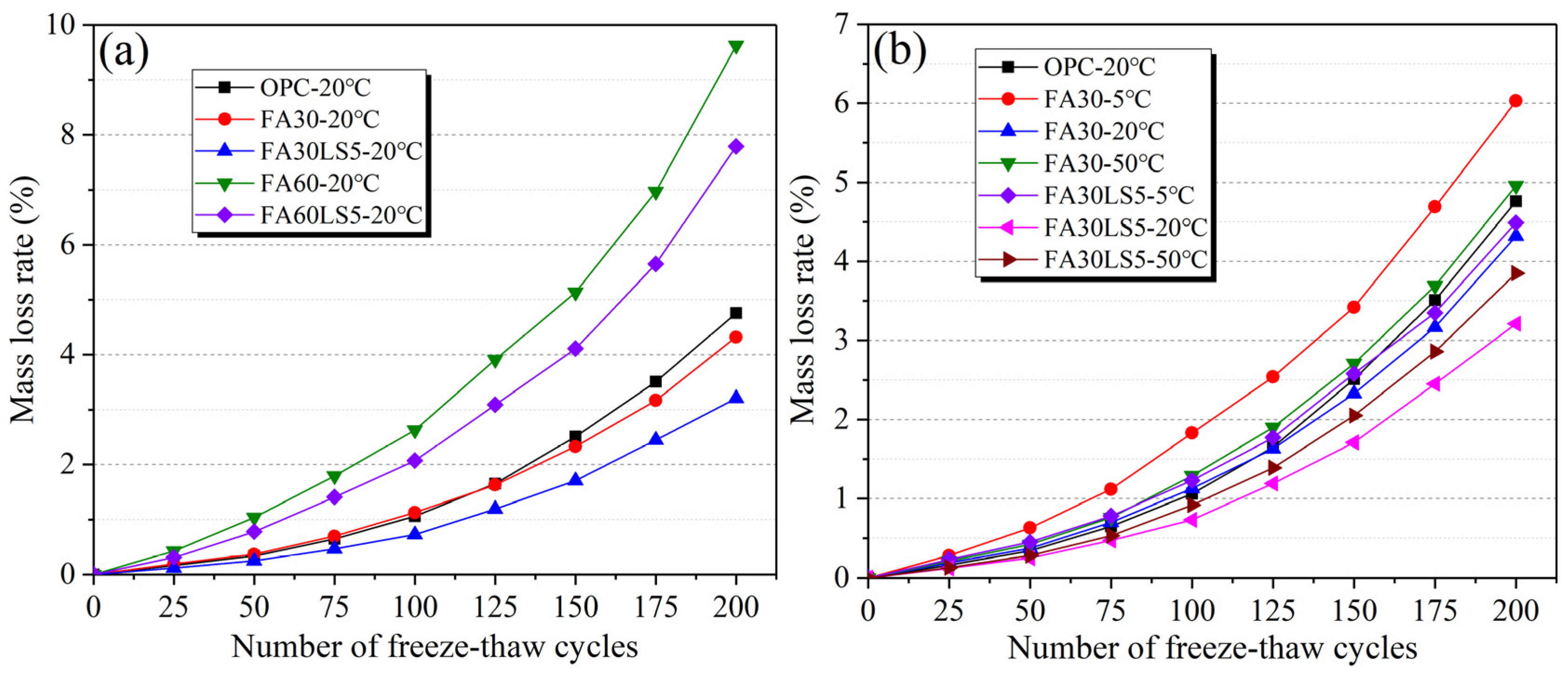
3.3. Mass Loss Rate After Freeze–Thaw Cycles
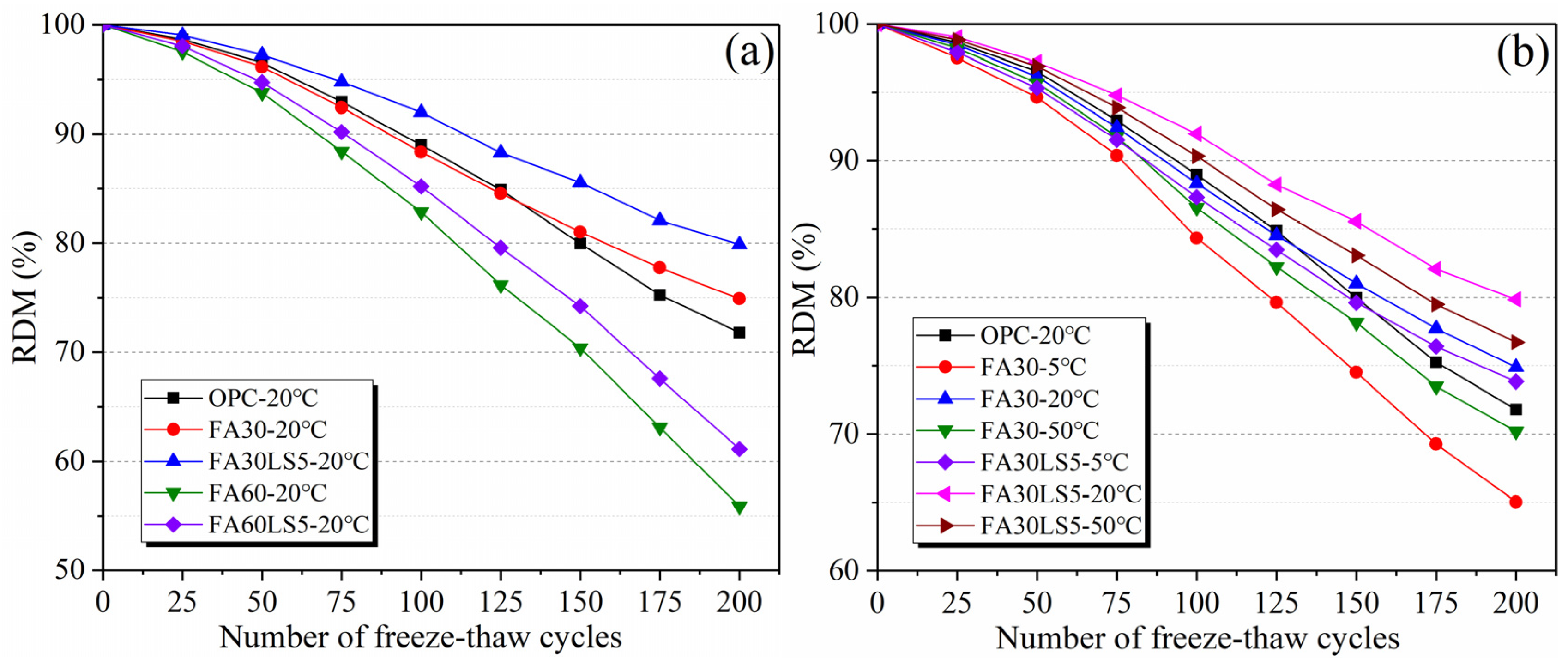
3.4. RDM After Freeze–Thaw Cycles
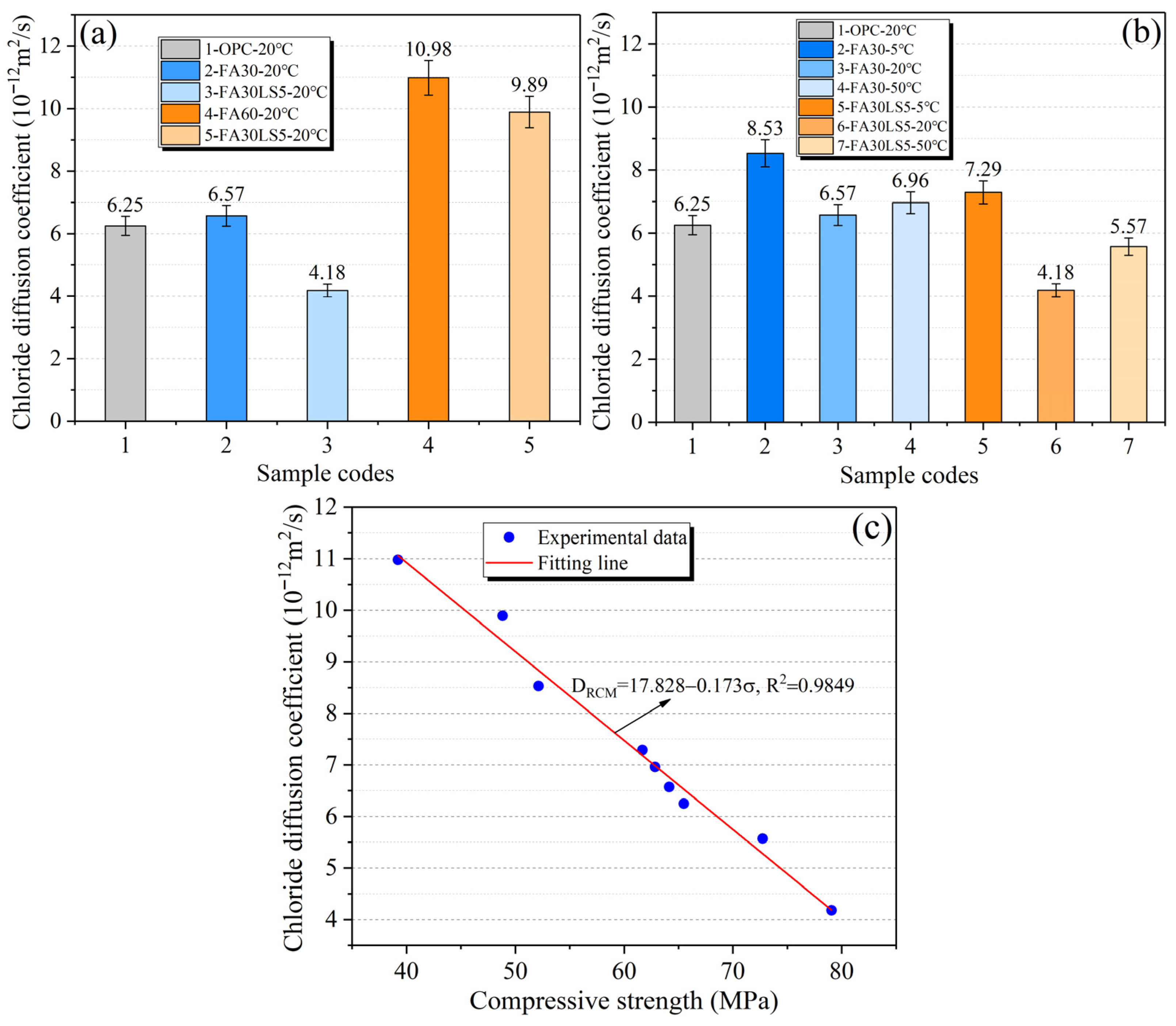
3.5. Chloride Diffusion Coefficient
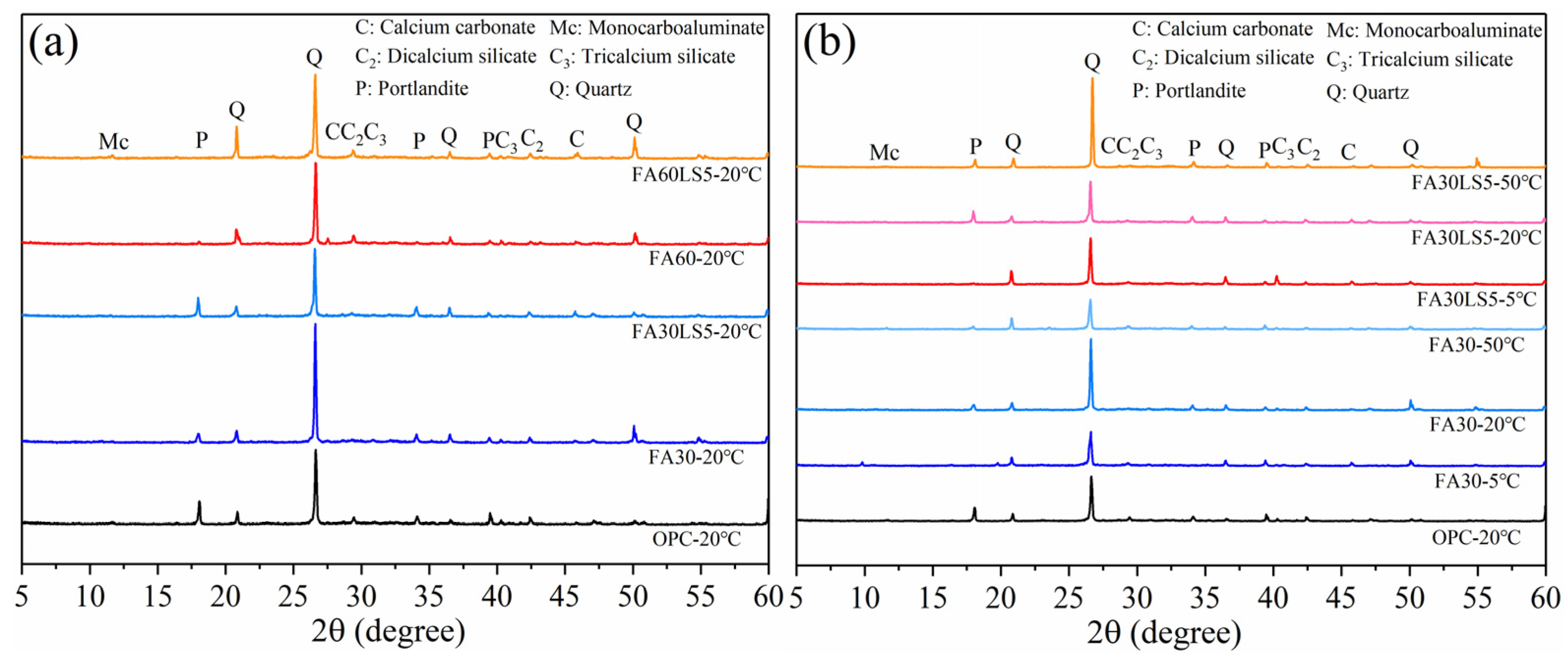
3.6. XRD
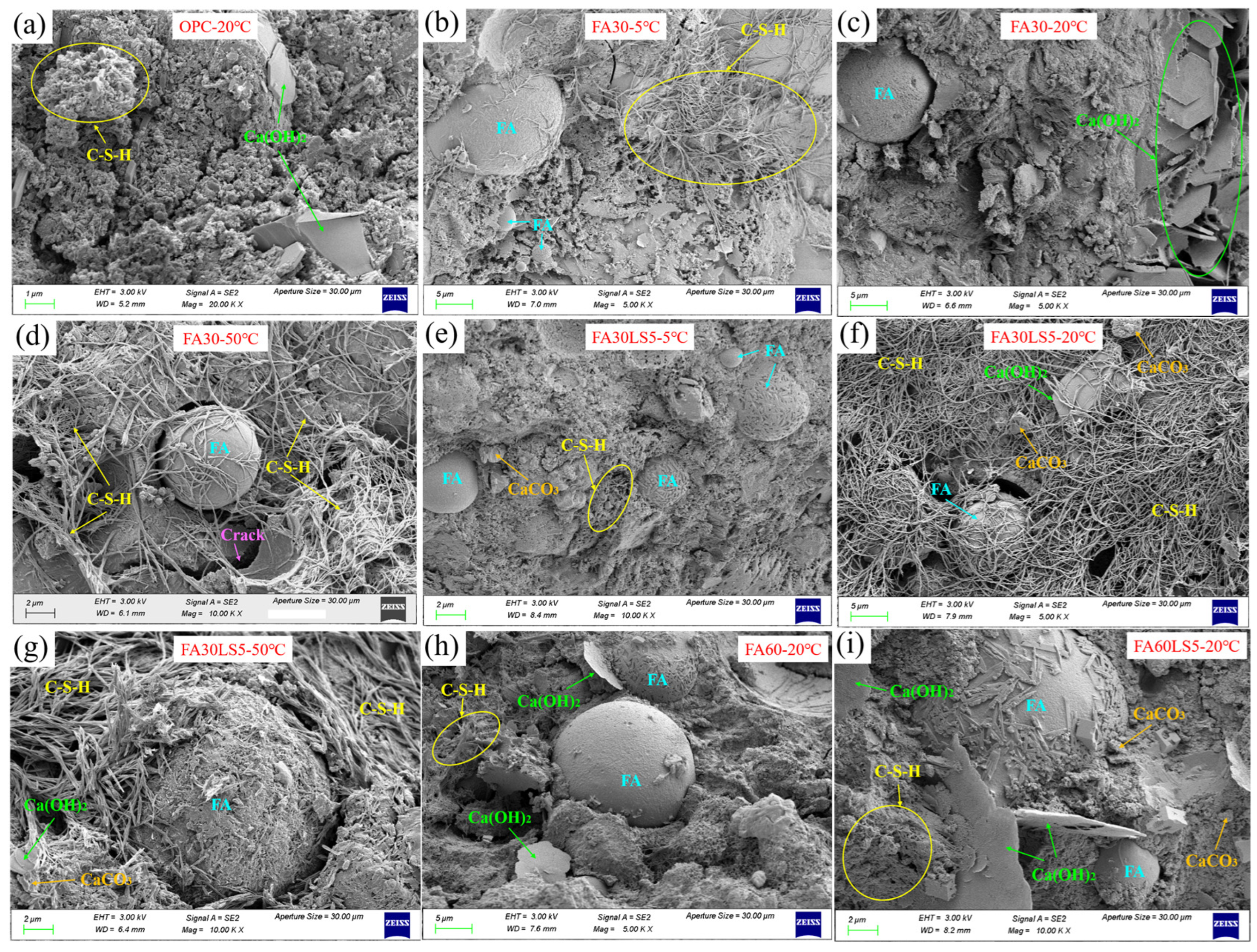
3.7. SEM
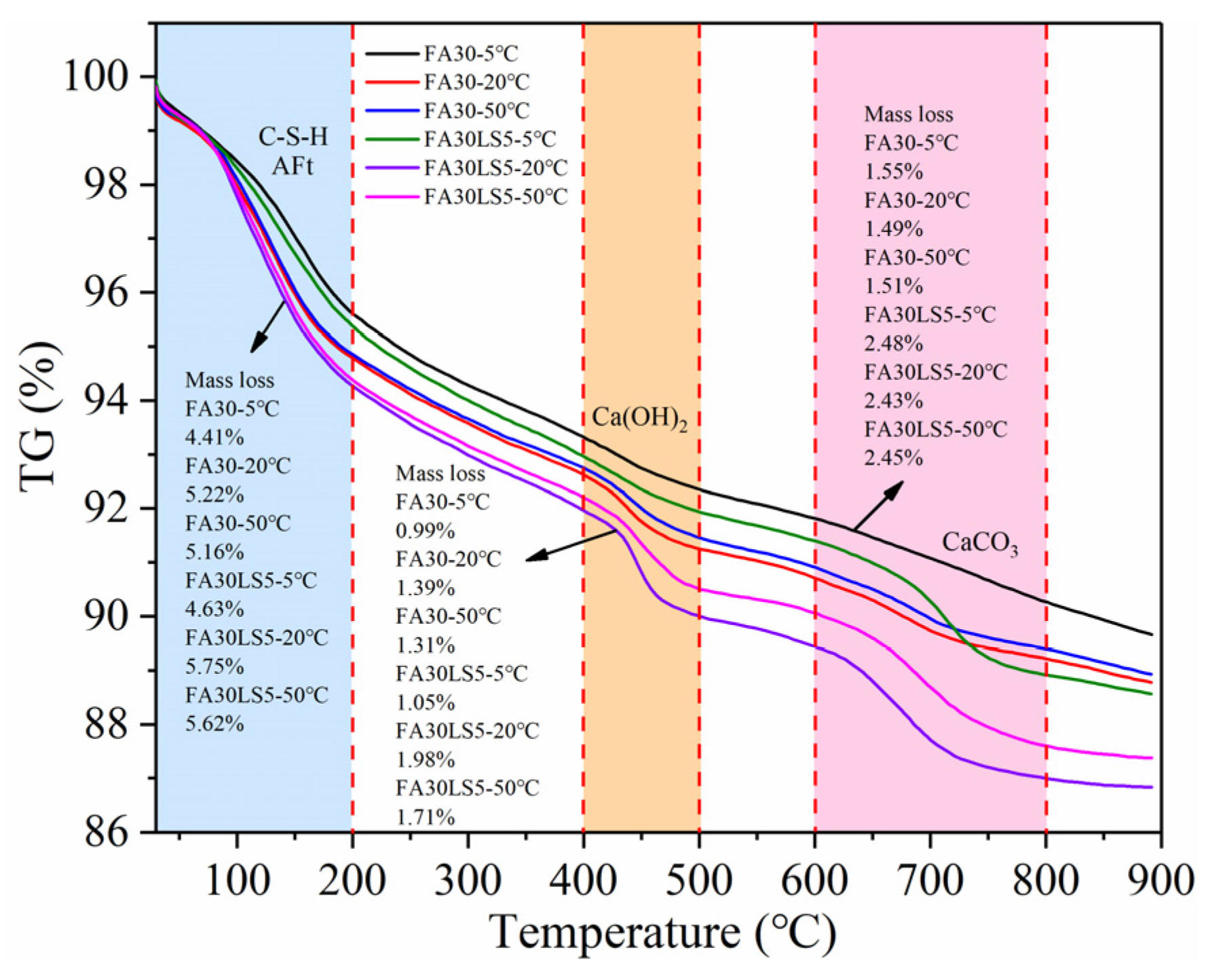
3.8. TG
3.9. Total Porosity
4. Conclusions
- (1)
- LS as an activator can effectively improve the compressive strength of mortars with large amount of FA cured under 20 °C at the early ages. LS can increase the early compressive strength of mortars with 30% and 60% FA by up to 18.2% and 17.7% at 3 d, respectively. Meanwhile, LS as an activator can effectively improve the compressive strength of mortar with a large amount of FA cured under different temperatures at early ages. LS can increase the early compressive strength of mortars with 30% FA cured under 5 °C, 20 °C, and 50 °C by up to 21.0%, 18.2%, and 21.2% at 3 d, respectively.
- (2)
- LS as an activator can significantly enhance the freeze–thaw cycle and chloride ion corrosion resistance of FA mortar. Among them, the FA mortar with LS added under 20 °C curing condition can achieve the maximum resistance to freeze–thaw cycles and chloride ion corrosion, followed by 50 °C curing condition, and the resistance is the smallest at 5 °C curing condition. Meanwhile, the chloride diffusion coefficient of mortars has a negative linear correlation with its compressive strength.
- (3)
- LS as an activator can promote more hydration products of Ca(OH)2 and C-S-H in FA mortars. The addition of LS can promote the pozzolanic reaction of FA, allowing the mortar to produce more hydration products. Meanwhile, the nucleation effect and chemical activity effect of LS can be fully exerted under high temperature of 50 °C and 20 °C, thereby generating more hydration products and enhancing the mechanical strength and resistance to freeze–thaw cycles and chloride ion erosion of mortar matrix. In addition, a low temperature of 5 °C will slow down the hydration rate, and the nucleation effect and chemical activity effect of LS cannot be fully exerted.
- (4)
- LS as an activator can be filled into FA mortars, and making the inner structure denser and the Mc produced by the reaction between LS and Ca(OH)2 can result in a significant reduction in the total porosity of FA mortars. Meanwhile, the addition of LS can greatly reduce the total porosity of FA mortar cured under 20 °C and 50 °C but has little effect on the total porosity of FA mortar cured under 5 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al Khaffaf, I.; Hawileh, R.A.; Sahoo, S.; Abdalla, J.A.; Kim, J.H. Toward carbon-neutral construction: A review of zero-carbon concrete. J. Build. Eng. 2025, 99, 111578. [Google Scholar] [CrossRef]
- Terán-Cuadrado, G.; Tahir, F.; Nurdiawati, A.; Almarshoud, M.A.; Al-Ghamdi, S.G. Current and potential materials for the low-carbon cement production: Life cycle assessment perspective. J. Build. Eng. 2024, 96, 110528. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, Q.L.; Feng, L.; Luo, W.J.; Wen, X.D.; Chu, S.H. Ultra-green cement: Limestone calcined clay cement (LC3) with recycled cement. Cem. Concr. Compos. 2025, 163, 106210. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, compatibility, environmental benefits and future directions of limestone calcined clay cement (LC3) concrete: A review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Singh, P.; Kapoor, K. Evaluation of fly ash-based geopolymer concrete incorporating coarse and fine recycled aggregates. Adv. Cem. Res. 2025, 37, 158–171. [Google Scholar] [CrossRef]
- Yan, R.; Ke, G.; Han, D. Expansion properties of concrete containing circulating fluidised bed fly ash for use in concrete-filled steel tubes. Adv. Cem. Res. 2023, 36, 306–319. [Google Scholar] [CrossRef]
- Papp, V.; Ardelean, I.; Bulátkó, A.; László, K.; Csík, A.; Janovics, R.; Kéri, M. Effect of metakaolin and fly ash on the early hydration and pore structure of Portland cement. Cem. Concr. Res. 2025, 196, 107928. [Google Scholar] [CrossRef]
- Kochchapong, K.; Wattanachai, P.; Chaiwithee, S.; Keereemasthong, T.; Rachtanapun, P.; Jantanasakulwong, K.; Suhr, J.; Sawangrat, C. Accelerating strength development through pozzolanic activity in hydrated fly-ash cement using plasma-activated water. J. Mater. Res. Technol. 2025, 36, 5332–5340. [Google Scholar] [CrossRef]
- Gao, M.; Jing, H.; Dai, J.; Ye, W. Effect of magnesium slag and fly ash on strength and hydration mechanism of binary and ternary blended cement mortar. Case Stud. Constr. Mater. 2025, 22, e04531. [Google Scholar] [CrossRef]
- Bhat, R.; Han, T.; Sant, G.; Neithalath, N.; Kumar, A. A comprehensive analysis of hydration kinetics and compressive strength development of fly ash-Portland cement binders. J. Build. Eng. 2024, 88, 109191. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, C.; Wang, B.; Han, J.; Guo, L.; Tang, N. Study on the micro-rheological properties of fly ash-based cement mortar. Constr. Build. Mater. 2024, 442, 137664. [Google Scholar] [CrossRef]
- Saingam, P.; Chatveera, B.; Promsawat, P.; Hussain, Q.; Nawaz, A.; Makul, N.; Sua-iam, G. Synergizing Portland Cement, high-volume fly ash and calcined calcium carbonate in producing self-compacting concrete: A comprehensive investigation of rheological, mechanical, and microstructural properties. Case Stud. Constr. Mater. 2024, 21, e03832. [Google Scholar] [CrossRef]
- Shamseldeen Fakhri, R.; Thanon Dawood, E. Limestone powder, calcined clay and slag as quaternary blended cement used for green concrete production. J. Build. Eng. 2023, 79, 107644. [Google Scholar] [CrossRef]
- Rashad, A.M.; Abdu, M.F.A. Accelerated aging resistance, abrasion resistance and other properties of alkali-activated slag mortars containing limestone powder. Sustain. Chem. Pharm. 2024, 37, 101406. [Google Scholar] [CrossRef]
- Hamdadou, M.N.-e.; Bignonnet, F.; Deboucha, W.; Ranaivomanana, H.; Leklou, N.; Arroudj, K. Hydration, mechanical and transfer properties of blended cement pastes and mortars prepared with recycled powder or limestone filler. J. Build. Eng. 2023, 78, 107541. [Google Scholar] [CrossRef]
- Rong, H.-l.; Zhou, Z.-h.; Peng, S.-l.; Huang, Y.; Liang, J.-l.; Yang, X.-l.; Shi, J.-c.; Feng, Y.-m. Effect of ultra-fine limestone powder on leaching resistance of cement mortar. Constr. Build. Mater. 2023, 368, 130422. [Google Scholar] [CrossRef]
- Wu, C.; Meng, L.; Wang, D.; Zhang, H.; Ke, L.; Ma, Z. Performance of sulfate species on limestone powder concrete under low temperature pulse current. Phys. Chem. Earth Parts A/B/C 2025, 138, 103850. [Google Scholar] [CrossRef]
- Jia, Z.; Kong, L.; Jia, L.; Ma, L.; Chen, Y.; Zhang, Y. Printability and mechanical properties of 3D printing ultra-high performance concrete incorporating limestone powder. Constr. Build. Mater. 2024, 426, 136195. [Google Scholar] [CrossRef]
- Chang, X.; He, T.; Niu, M.; Zhao, L.; Wang, L.; Wang, Y. Influence of limestone powder mixing method on properties of manufactured sand concrete. Case Stud. Constr. Mater. 2024, 20, e02996. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Shang, H.; Wang, J.; Song, N. Development of sustainable ultra-high-performance concrete (UHPC) by synergistic utilization of red mud and limestone powder. J. Build. Eng. 2024, 90, 109372. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Y.; Luo, L.; Dong, X.; Wang, Q. Effects of graphene oxide on hydration of cement-based materials at different curing temperatures. J. Build. Eng. 2025, 111, 113268. [Google Scholar] [CrossRef]
- Fang, S.; Ma, H.; Shen, Y.; Rong, H.; Ba, M. Synergistic effects of CaCO3 and styrene acrylic emulsion (SAE) on hydration of high-strength cement-based composites at various curing temperatures. J. Build. Eng. 2025, 111, 113195. [Google Scholar] [CrossRef]
- Deschner, F.; Lothenbach, B.; Winnefeld, F.; Neubauer, J. Effect of temperature on the hydration of Portland cement blended with siliceous fly ash. Cem. Concr. Res. 2013, 52, 169–181. [Google Scholar] [CrossRef]
- Bach, T.T.H.; Coumes, C.C.D.; Pochard, I.; Mercier, C.; Revel, B.; Nonat, A. Influence of temperature on the hydration products of low pH cements. Cem. Concr. Res. 2012, 42, 805–817. [Google Scholar] [CrossRef]
- Ogawa, Y.; Uji, K.; Ueno, A.; Kawai, K. Contribution of fly ash to the strength development of mortars cured at different temperatures. Constr. Build. Mater. 2021, 276, 122191. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Han, L.; Chen, L.; Yan, X.; Chen, C. Influence of curing temperature on the mechanical properties and microstructure of limestone powder mass concrete. Struct. Concr. 2020, 22, E745–E755. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Sharp, J.H. Effect of temperature on the hydration of the main clinker phases in portland cements: Part ii, blended cements. Cem. Concr. Res. 1998, 28, 1259–1274. [Google Scholar] [CrossRef]
- Feng, H.; Xin, X.; Guo, A.; Yu, Z.; Shao, Q.; Sheikh, M.N.; Sun, Z. Effect of mix proportion parameters on chloride erosion resistance of fly ash/slag-based engineered geopolymer composites. J. Clean. Prod. 2024, 438, 140785. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Han, L.; Huang, H.; Zhi, F.; Yang, G.; Niu, Y.; Chen, L.; Wang, L.; Chen, Z. Influence of curing temperature on freeze-thaw resistance of limestone powder hydraulic concrete. Case Stud. Constr. Mater. 2022, 17, e01322. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength. Architecture & Building Press: Beijing, China, 2021.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Architecture & Building Press: Beijing, China, 2009.
- Chen, F.; Gao, J.; Qi, B.; Shen, D.; Li, L. Degradation progress of concrete subject to combined sulfate-chloride attack under drying-wetting cycles and flexural loading. Constr. Build. Mater. 2017, 151, 164–171. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Jiang, L.; Jin, W.; Guo, M.-Z.; Xia, K.; Yang, G. Hydrated Lime–Enriched CO2 Sequestration Binders Reinforced by Polyvinyl Alcohol. J. Mater. Civ. Eng. 2023, 35, 04023184. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Hu, C. Towards greener ultra-high performance concrete based on highly-efficient utilization of calcined clay and limestone powder. J. Build. Eng. 2023, 66, 105836. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Gu, X.; Han, D.; Wang, Q. Improving the performance of ultra-high performance concrete containing lithium slag by incorporating limestone powder. J. Build. Eng. 2023, 72, 106610. [Google Scholar] [CrossRef]
- Jin, W.Z.; Tang, X.D.; Bai, Z.P.; Yang, H.; Chen, Z.Y.; Wang, L.; Zhang, L.; Jiang, L.H. Effect of Curing Temperature on Mechanical Strength and Thermal Properties of Hydraulic Limestone Powder Concrete. J. Mater. Eng. Perform. 2024, 33, 11214–11230. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem. Concr. Res. 2011, 41, 579–589. [Google Scholar] [CrossRef]
- Mohammed, T.; Torres, A.; Aguayo, F.; Okechi, I.K. Evaluating carbonation resistance and microstructural behaviors of calcium sulfoaluminate cement concrete incorporating fly ash and limestone powder. Constr. Build. Mater. 2024, 442, 137551. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, J.; Yang, Q.; Li, Q.; Lu, X.; Ye, Z. Hydration of Portland cement in the presence of triethanolamine and limestone powder: Mechanical properties and synergistic mechanism. Constr. Build. Mater. 2024, 438, 137323. [Google Scholar] [CrossRef]
- Liu, K.; Wang, H.; Wang, Y.; Tang, J.; Wang, A.; Liu, T. Sulfate resistance of mortars under semi-immersion condition: Impact of environmental humidity and fly ash content. J. Build. Eng. 2024, 97, 110819. [Google Scholar] [CrossRef]
- Lv, X.; Yang, L.; Wang, F.; Li, J.; Xie, Z. Hydration and microstructure of ternary high-ferrite Portland cement blends incorporating a large amount of limestone powder and fly ash. Constr. Build. Mater. 2023, 372, 130684. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, L.H.; Han, L.; Gu, Y.; Guo, M.Z.; Gao, S.; Zhang, L.; Liu, M.W. Influence of Calcium Leaching on Mechanical and Physical Properties of Limestone Powder-Cement Pastes Cured under Different Temperatures. J. Mater. Civ. Eng. 2022, 34, 04022214. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, Y.; Song, C.; Jin, W.; Xiang, T.; Qiao, M.; Dang, J.; Bai, W.; Yang, Z.; Zhao, J. Effect of calcium stearate hydrophobic agent on the performance of mortar and reinforcement corrosion in mortar with cracks. Constr. Build. Mater. 2024, 450, 138684. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, W.; Lv, Y.; Li, S.; Zhang, X.; Xiang, T.; Gu, C.; Bai, W.; Song, C.; Zhao, J. Study on mechanical, hydrophobic and corrosion resistant properties of methylsiloxan-based mortar modified by micro-nano supplementary cementitious materials. J. Mater. Res. Technol. 2025, 34, 1654–1670. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F.; Alder, C.; Wieland, E.; Lunk, P. Effect of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cem. Concr. Res. 2007, 37, 483–491. [Google Scholar] [CrossRef]
- Zhuang, W.; Li, S.; Wang, Z.; Zhang, Z.; Yu, Q. Impact of micromechanics on dynamic compressive behavior of ultra-high performance concrete containing limestone powder. Compos. Part B Eng. 2022, 243, 110160. [Google Scholar] [CrossRef]


| Materials | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | LOI |
|---|---|---|---|---|---|---|---|---|
| OPC | 62.58 | 20.39 | 5.43 | 3.76 | 1.73 | 2.28 | 0.78 | 3.05 |
| FA | 2.47 | 48.92 | 36.86 | 6.79 | 0.73 | 0.58 | 1.07 | 2.58 |
| LS | 56.34 | 0.37 | 0.19 | 0.18 | 1.69 | - | - | 41.23 |
| Samples | w/b | Water | Cement | FA | LS | Sand | SP |
|---|---|---|---|---|---|---|---|
| OPC | 0.35 | 194 | 554 | 0 | 0 | 1662 | 3.32 |
| FA30 | 0.35 | 194 | 387.8 | 166.2 | 0 | 1662 | 3.32 |
| FA30LS5 | 0.35 | 194 | 387.8 | 166.2 | 27.7 | 1662 | 3.32 |
| FA60 | 0.35 | 194 | 221.6 | 332.4 | 0 | 1662 | 3.32 |
| FA60LS5 | 0.35 | 194 | 221.6 | 332.4 | 27.7 | 1662 | 3.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Jin, W.; Li, J.; Huang, M.; Fang, P. Influence of Limestone Powder as Activator on the Enhancement of Early Mechanical Strength and Durability of High Blending Fly Ash Mortar Cured Under Different Temperatures. Materials 2025, 18, 5087. https://doi.org/10.3390/ma18225087
Chen Q, Jin W, Li J, Huang M, Fang P. Influence of Limestone Powder as Activator on the Enhancement of Early Mechanical Strength and Durability of High Blending Fly Ash Mortar Cured Under Different Temperatures. Materials. 2025; 18(22):5087. https://doi.org/10.3390/ma18225087
Chicago/Turabian StyleChen, Qingfeng, Weizhun Jin, Jingjing Li, Min Huang, and Pengfei Fang. 2025. "Influence of Limestone Powder as Activator on the Enhancement of Early Mechanical Strength and Durability of High Blending Fly Ash Mortar Cured Under Different Temperatures" Materials 18, no. 22: 5087. https://doi.org/10.3390/ma18225087
APA StyleChen, Q., Jin, W., Li, J., Huang, M., & Fang, P. (2025). Influence of Limestone Powder as Activator on the Enhancement of Early Mechanical Strength and Durability of High Blending Fly Ash Mortar Cured Under Different Temperatures. Materials, 18(22), 5087. https://doi.org/10.3390/ma18225087






