Sustainable Fire-Resistant Materials: Thermal, Physical, Mechanical, and Environmental Behavior of Walls with Waste from the Aquaculture Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Sample Fabrication Method
2.2. Methods
2.2.1. Chemical and Physical Properties
2.2.2. Mechanical Properties
2.2.3. Thermal Properties
2.2.4. Fire Insulating Capacity
2.2.5. Environmental Risk Assessment
- –
- Leaching test
- –
- Radionuclide test
3. Results
3.1. Chemical and Physical Properties
3.2. Mechanical Properties
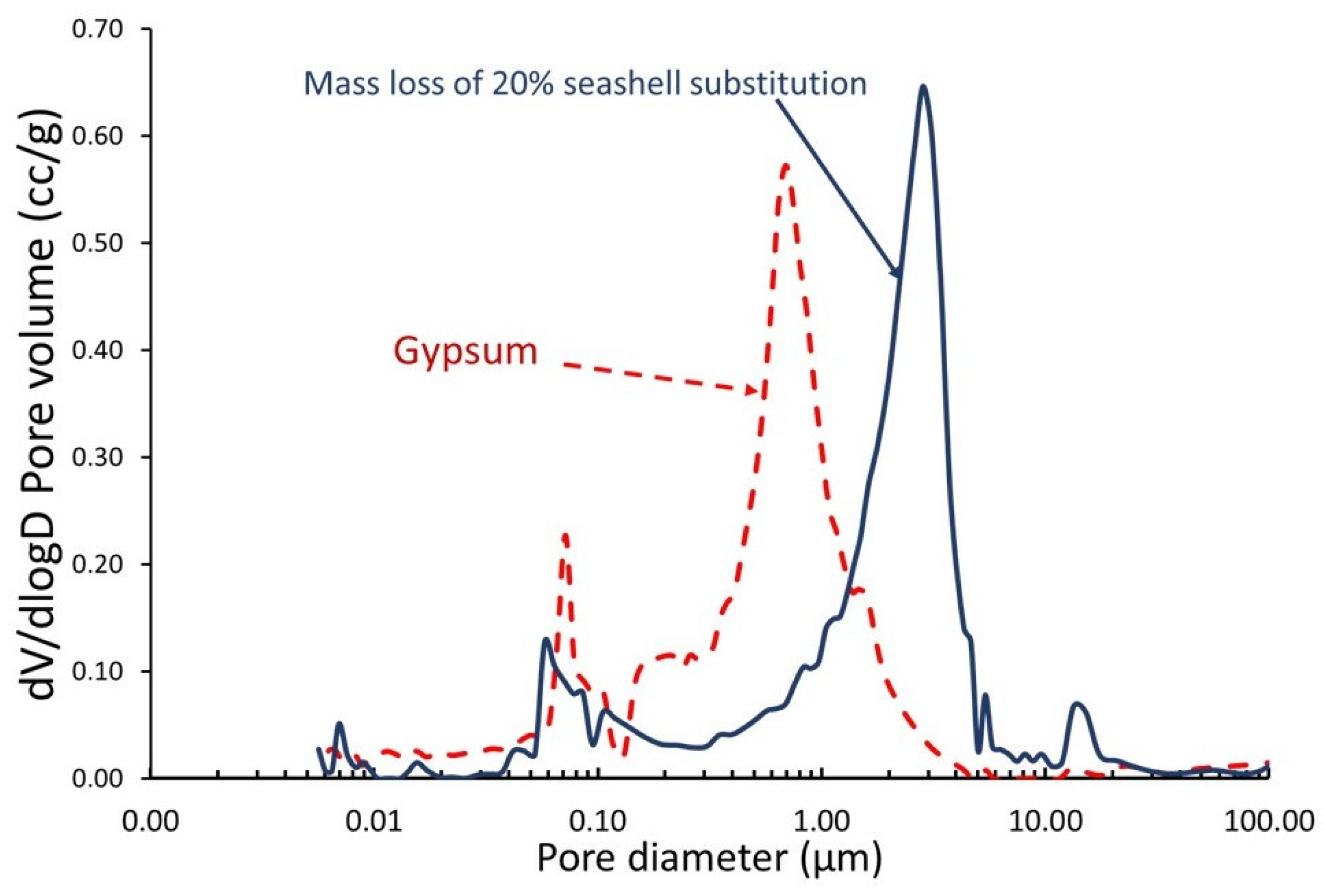
3.3. Thermal Properties
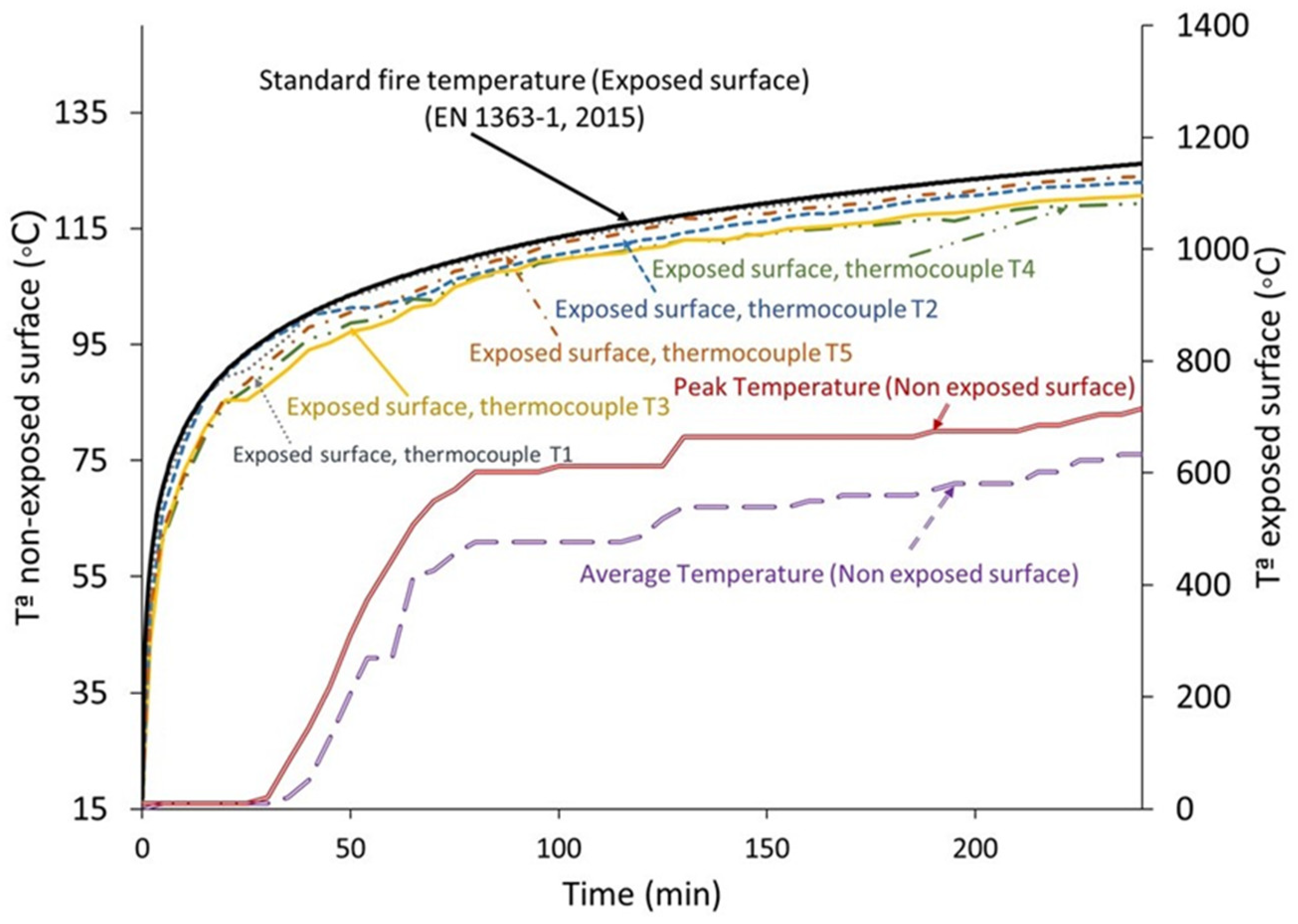
3.4. Fire Resistance
3.5. Environmental Risk Assessment
3.5.1. Leaching Test
3.5.2. Radionuclide Test
4. Conclusions
- –
- Substituting gypsum with seashell waste does not cause a large change in the bulk density of the material. The bulk density of the new material is 1.8% less than that of gypsum.
- –
- The use of seashell waste in place of gypsum has no mechanical disadvantages. When compared to traditional materials, the material made with seashell waste exhibits comparable mechanical properties.
- –
- A 20% substitution of seashell waste for gypsum has a negligible effect on the material’s thermal properties. At 500 °C, the new material’s thermal conductivity decreased to 0.23 W/mK compared to 0.25 W/mK for gypsum. Similarly, at room temperature (20 °C), both materials have comparable thermal conductivities.
- –
- In terms of fire resistance, because of a reduction in water retention capacity, replacing 20% of the gypsum with seashell waste shortens the evaporation plateau’s duration. In contrast to the conventional material, the material containing 20% seashell waste has a lower slope after the evaporation plateau. Therefore, the addition of 20% seashell waste does not affect the fire resistance properties of the material. Additionally, the tested material’s mechanical qualities are satisfactory; during the fire resistance test, it exhibited no discernible crumbling, cracking, or deformation.
- –
- Regarding the environmental properties, the incorporation of 20% shell waste in gypsum-based materials does not affect the material’s potential for leaching nor its radiological behavior.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tayeh, B.A.; Hasaniyah, M.W.; Zeyad, A.M.; Yusuf, M.O. Properties of concrete containing recycled seashells as cement partial replacement: A review. J. Clean. Prod. 2019, 237, 117723. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Don’t waste seafood waste. Nature 2025, 524, 155–157. [Google Scholar] [CrossRef]
- Chiou, I.J.; Chen, C.H.; Li, Y.H. Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Constr. Build. Mater. 2014, 64, 480–487. [Google Scholar] [CrossRef]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Eziefula, U.G.; Ezeh, J.C.; Eziefula, B.I. Properties of seashell aggregate concrete: A review. Constr. Build. Mater. 2018, 192, 480–487. [Google Scholar] [CrossRef]
- Kwon, H.B.; Lee, C.W.; Jun, B.S.; Do Yun, J.; Weon, S.Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Felipe-Sesé, M.; Eliche-Quesada, D.; Corpas-Iglesias, F.A. The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceram. Int. 2011, 37, 3019–3028. [Google Scholar] [CrossRef]
- La Voz de Galicia. Available online: https://www.lavozdegalicia.es/noticia/arousa/2000/08/25/conserveros-plantean-posibilidad-verter-concha-mejillon-mar/0003_169074.htm (accessed on 2 July 2023).
- La Voz de Galicia. Available online: https://www.lavozdegalicia.es/noticia/maritima/2015/10/19/interrogantes-conchaconcha-mejillon/0003_201510G19P22993.htm (accessed on 21 January 2023).
- Diario de Arousa. Available online: https://www.diariodearousa.com/texto-diario/mostrar/2515303/seprona-denunciara-ante-xunta-vertido-concha-mejillon-almeja-terreno-muino-da-correa (accessed on 20 July 2023).
- La Voz de Galicia. Available online: https://www.lavozdegalicia.es/noticia/arousa/2020/04/26/eliminan-vertido-ria-causado-deposito-concha-mejillon/0003_202004A26C10993.htm (accessed on 2 July 2023).
- Faro de Vigo. Available online: https://www.farodevigo.es/arousa/2010/01/21/garavilla-afronta-sellado-vertedero-concha-17871519.html (accessed on 2 July 2023).
- Ndoke, P. Performance of Palm Kernel Shells as a Partial Replacement for Coarse Aggregate in Asphalt Concrete. Leonardo Electron. J. Pract. Technol. 2006, 9, 145–152. [Google Scholar]
- Richardson, A.E.; Fuller, T. Sea shells used as partial aggregate replacement in concrete. Struct. Surv. 2013, 31, 347–354. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar. Materials 2019, 12, 1322. [Google Scholar] [CrossRef]
- Zaid, S.T.; Ghorpade, V.G.; Pradesh Anantapur, A.; Pradesh, A. Experimental Investigation of Snail Shell Ash (SSA) as Partial Replacement of Ordinary Portland Cement in Concrete. Int. J. Eng. Res. Technol. 2014, 3, 1049–1053. [Google Scholar]
- Umoh, A.A.; Ujene, A.O. Improving the strength performance of high volume periwinkle shell ash blended cement concrete with sodium nitrate as accelerator. J. Civ. Eng. Sci. Technol. 2015, 6, 18–22. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Seco, N.; Núñez, A. Use of mussel shells as a soil amendment: Effects on bulk and rhizosphere soil and pasture production. Pedosphere 2012, 22, 152–164. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, D.K.; Ali, M.A.; Kim, P.J. Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming material. Waste Manag. 2008, 28, 2702–2708. [Google Scholar] [CrossRef]
- Asaoka, S.; Yamamoto, T.; Kondo, S.; Hayakawa, S. Removal of hydrogen sulfide using crushed oyster shell from pore water to remediate organically enriched coastal marine sediments. Bioresour. Technol. 2009, 100, 4127–4132. [Google Scholar] [CrossRef]
- Peña-Rodríguez, S.; Bermúdez-Couso, A.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mercury removal using ground and calcined mussel shell. J. Environ. Sci. 2013, 25, 2476–2486. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Li, H.; Chen, T.; Ye, Y.; Zheng, H. Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2502–2530. [Google Scholar] [CrossRef]
- Chang, F.; Li, G.; Haws, M.; Niu, T. Element concentrations in shell of Pinctada margaritifera from French Polynesia and evaluation for using as a food supplement. Food Chem. 2007, 104, 1171–1176. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Martínez-Abella, F.; Carro-López, D. Performance of mussel shell as aggregate in plain concrete. Constr. Build. Mater. 2017, 139, 570–583. [Google Scholar] [CrossRef]
- Bamigboye, G.O.; Okara, O.; Bassey, D.E.; Jolayemi, K.J.; Ajimalofin, D. The use of Senilia senilis seashells as a substitute for coarse aggregate in eco-friendly concrete. J. Build. Eng. 2020, 32, 101811. [Google Scholar] [CrossRef]
- Bamigboye, G.O.; Nworgu, A.T.; Odetoyan, A.O.; Kareem, M.; Enabulele, D.O.; Bassey, D.E. Sustainable Use of Seashells as Binder in Concrete Production: Prospect and Challenges. J. Build. Eng. 2021, 33, 101864. [Google Scholar] [CrossRef]
- Peceño, B.; Arenas, C.; Alonso-Fariñas, B.; Leiva, C. Substitution of coarse aggregates with mollusk-shell waste in acoustic-absorbing concrete. J. Mater. Civ. Eng. 2019, 31, 04019077. [Google Scholar] [CrossRef]
- Peceño, B.; Leiva, C.; Alonso-Fariñas, B.; Gallego-Schmid, A. Is recycling always the best option? Environmental assessment of recycling of seashell as aggregates in noise barriers. Processes 2020, 8, 776. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Liang, Y.; Qu, K.; Lu, K.; Chen, S.; Kong, M. Study on engineering properties of foam concrete containing waste seashell. Constr. Build. Mater. 2020, 260, 119896. [Google Scholar] [CrossRef]
- Varhen, C.; Carrillo, S.; Ruiz, G. Experimental investigation of Peruvian scallop used as fine aggregate in concrete. Constr. Build. Mater. 2017, 136, 533–540. [Google Scholar] [CrossRef]
- Soltanzadeh, F.; Emam-Jomeh, M.; Edalat-Behbahani, A.; Soltan-Zadeh, Z. Development and characterization of blended cements containing seashell powder. Constr. Build. Mater. 2018, 161, 292–304. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Hasaniyah, M.W.; Zeyad, A.M.; Awad, M.M.; Alaskar, A.; Mohamed, A.M.; Alyousef, R. Durability and mechanical properties of seashell partially-replaced cement. J. Build. Eng. 2020, 31, 101328. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Pérez-Ordóñez, J.L. Assessment of mussel shells building solutions: A real-scale application. J. Build. Eng. 2021, 44, 102635. [Google Scholar] [CrossRef]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Implementing by-product management into the life cycle assessment of the mussel sector. Resour. Conserv. Recycl. 2010, 54, 1219–1230. [Google Scholar] [CrossRef]
- Lee, M.; Tsai, W.S.; Chen, S.T. Reusing shell waste as a soil conditioner alternative? A comparative study of eggshell and oyster shell using a life cycle assessment approach. J. Clean. Prod. 2020, 265, 121845. [Google Scholar] [CrossRef]
- Féjean, J.; Lanos, C.; Mélinge, Y.; Baux, C. Behaviour of fire-proofing materials containing gypsum, modifications induced by incorporation of inert filler. Chem. Eng. Res. Des. 2003, 81, 1230–1236. [Google Scholar] [CrossRef]
- Leiva, C.; García-Arenas, C.; Vilches, L.F.; Vale, J.; Gimenez, A.; Ballesteros, J.C.; Fernández-Pereira, C. Use of FGD gypsum in fire resistant panels. Waste Manag. 2010, 30, 1123–1129. [Google Scholar] [CrossRef]
- Thomas, G. Thermal properties of gypsum plasterboard at high temperatures. Fire Mater. 2002, 26, 37–45. [Google Scholar] [CrossRef]
- Alvarenga, R.A.F.; Galindro, B.M.; Helpa, C.F.; Soares, S.R. The recycling of oyster shells: An environmental analysis using life cycle assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Recycled mollusc shells. In New Trends in Eco-efficient and Recycled Concrete; Pacheco-Torgal, F., Tam, V.W.Y., Labrincha, J.A., Zhuang, X.Y., Ding, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–205. [Google Scholar]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, M.; Liu, T.; Yang, G.; Jiang, G.; Gu, H. Research on Compressive Strength and Thermal Conductivity of Lightweight Phosphogypsum-Based Composite Cementitious Materials. Constr. Build. Mater. 2024, 436, 136955. [Google Scholar] [CrossRef]
- Du, J.; Tian, L.; Qi, M.; Zhang, C.; Di, H.; Zhi, X.; Zhu, J. Revealing Maleic Acid Role in the Preparation of α-Hemihydrate Gypsum from Titanium Gypsum through Experiments and DFT Calculations. Sci. Total Environ. 2023, 897, 166405. [Google Scholar] [CrossRef]
- Huang, X.; Shi, Z.; Wang, Z.; Dong, J.; Wang, X.; Zhao, X. Microstructure and Performances of Sludge Soil Stabilized by Fluorogypsum-Based Cementitious Binder. Constr. Build. Mater. 2022, 325, 126702. [Google Scholar] [CrossRef]
- Ma, D.X.; Yin, G.Z.; Ye, W.; Jiang, Y.; Wang, N.; Wang, D.Y. Exploiting Waste towards More Sustainable Flame-Retardant Solutions for Polymers: A Review. Materials 2024, 17, 2266. [Google Scholar] [CrossRef] [PubMed]
- Guirado, E.; Ruiz Martínez, J.D.; Campoy, M.; Leiva, C. Properties and optimization process using machine learning for recycling of fly and bottom ashes in fire-resistant materials. Processes 2025, 13, 933. [Google Scholar] [CrossRef]
- Leiva, C.; Vilches, L.F.; Vale, J.; Fernández-Pereira, C. Fire resistance of biomass ash panels used for internal partitions in buildings. Fire Saf. J. 2009, 44, 622–628. [Google Scholar] [CrossRef]
- Sakkas, K.; Nomikos, P.; Sofianos, A.; Panias, D. Utilization of FeNi-slag for the production of inorganic polymeric materials for construction or for passive fire protection. Waste Biomass Valorization 2014, 5, 403–410. [Google Scholar] [CrossRef]
- Albero, V.; Reig, L.; Hernández-Figueirido, D.; Roig-Flores, M.; Melchor-Eixea, A.; Piquer, A.; Pitarch, A.M. Fire and postfire compressive strength of recycled aggregate concrete made with ceramic stoneware. J. Build. Eng. 2024, 89, 109363. [Google Scholar] [CrossRef]
- Salazar, P.A.; Leiva-Fernández, C.; Luna-Galiano, Y.; Sánchez, R.V.; Fernández-Pereira, C. Physical, mechanical and radiological characteristics of a fly ash geopolymer incorporating titanium dioxide waste as passive fire insulating material in steel structures. Materials 2022, 15, 8493. [Google Scholar] [CrossRef]
- Valanides, M.; Aivaliotis, K.; Oikonomopoulou, K.; Fikardos, A.; Savva, P.; Sakkas, K.; Nicolaides, D. Geopolymerization of recycled glass waste: A sustainable solution for a lightweight and fire-resistant material. Recycling 2024, 9, 16. [Google Scholar] [CrossRef]
- Peceño, B.; Alonso-Fariñas, B.; Vilches, L.F.; Leiva, C. Study of seashell waste recycling in fireproofing material: Technical, environmental, and economic assessment. Sci. Total Environ. 2021, 790, 148102. [Google Scholar] [CrossRef]
- Vilches, L.F.; Fernández-Pereira, C.; Olivares del Valle, J.; Vale, J. Recycling potential of coal fly ash and titanium waste as new fireproof products. Chem. Eng. J. 2003, 95, 155–161. [Google Scholar] [CrossRef]
- Lo, K.-W.; Lin, Y.-W.; Cheng, T.-W.; Lin, K.-L.; Lin, W.-T. Recycling of Silicon Carbide Sludge on the Preparation and Characterization of Lightweight Foamed Geopolymer Materials. Polymers 2021, 13, 4029. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Abu Bakar, B.H.; Mamat, H.; Gong, L. A New, Green, Recyclable Fireproof Insulation Board for Use in Integrated Composite Structure Fire Protection Systems. Fire 2022, 5, 203. [Google Scholar] [CrossRef]
- Peceño, B.; Alonso-Fariñas, B.; Arenas, C.; Leiva, C. Influence of particle size of mussel shells in physical, mechanical and insulating properties of fireproof materials. Procedia Environ. Sci. Eng. Manag. 2021, 8, 323–333. [Google Scholar]
- Peceño, B.; Bakit, J.; Cortes, N.; Alonso-Fariñas, B.; Bonilla, E.; Leiva, C. Assessing durability properties and economic potential of shellfish aquaculture waste in the construction industry: A circular economy perspective. Sustainability 2022, 14, 8383. [Google Scholar] [CrossRef]
- EN 13279-1:2009; Gypsum Binders and Gypsum Plasters—Part 1: Definitions and Requirements. European Committee for Standardization (CEN): Brussels, Belgium, 2009.
- ASTM D3682-13:2013; Standard Test Method for Major and Minor Elements in Combustion Residues from Coal Utilization Processes. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D854:2014; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- Peceño, B.; Leiva, C.; Alonso-Fariñas, B. Aquaculture Waste Ecopanel Having Passive Fire Protection Properties and Compressive and Flexural Strength. Patent No. WO2023092246A1, 21 November 2022. [Google Scholar]
- Li, J.; Zhuang, X.; Leiva, C.; Cornejo, A.; Font, O.; Querol, X.; Moreno, N.; Arenas, C.; Fernández-Pereira, C. Potential utilization of FGD gypsum and fly ash from a Chinese power plant for manufacturing fire-resistant panels. Constr. Build. Mater. 2015, 95, 910–921. [Google Scholar] [CrossRef]
- ASTM E605/E605M-19:2023; Standard Test Methods for Thickness and Density of Sprayed Fire-Resistive Material (SFRM) Applied to Structural Members. ASTM International: West Conshohocken, PA, USA, 2023.
- EN 12859:2012; Gypsum Blocks—Definitions, Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN 13279-2:2014; Gypsum Binders and Gypsum Plasters—Part 2: Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2014.
- Moreno, V.; González-Arias, J.; Ruiz-Martínez, J.D.; Balart-Gimeno, R.; Baena-Moreno, F.M.; Leiva, C. FGD-Gypsum Waste to Capture CO2 and to Recycle in Building Materials: Optimal Reaction Yield and Preliminary Mechanical Properties. Materials 2024, 17, 3774. [Google Scholar] [CrossRef]
- EN 520:2005+A1:2010; Gypsum Plasterboards—Definitions, Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2010.
- ASTM E1461-13:2022; Standard Test Method for Thermal Diffusivity by the Flash Method. ASTM International: West Conshohocken, PA, USA, 2022.
- EN 1363-1:2015; Fire Resistance Tests—Part 1: General Requirements. European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- NEN 7375:2004; Leaching Characteristics of Moulded or Monolithic Building and Waste Materials—Determination of Leaching of Inorganic Components with the Diffusion Test (“The Tank Test”). Netherlands Standards (NEN): Amsterdam, The Netherlands, 2004.
- Peceño, B.; Hurtado-Bermudez, S.; Alonso-Fariñas, B.; Villa-Alfageme, M.; Más, J.L.; Leiva, C. Recycling bio-based wastes into road-base binder: Mechanical, leaching, and radiological implications. Appl. Sci. 2023, 13, 1644. [Google Scholar] [CrossRef]
- Nuccetelli, C.; Pontikes, Y.; Leonardi, F.; Trevisi, R. New perspectives and issues arising from the introduction of (NORM) residues in building materials: A critical assessment on the radiological behaviour. Constr. Build. Mater. 2015, 82, 323–331. [Google Scholar] [CrossRef]
- Lanzón, M.; Castellón, F.J.; Ayala, M. Effect of the expanded perlite dose on the fire performance of gypsum plasters. Constr. Build. Mater. 2022, 346, 128494. [Google Scholar] [CrossRef]
- Nahas, L.; Dahdah, E.; Aouad, S.; El Khoury, B.; Gennequin, C.; Abi Aad, E.; Estephane, J. Highly efficient scallop seashell-derived catalyst for biodiesel production from sunflower and waste cooking oils: Reaction kinetics and effect of calcination temperature studies. Renew. Energy 2023, 202, 1086–1095. [Google Scholar] [CrossRef]
- Freire, M.T.; Veiga, M.R.; Santos Silva, A.; de Brito, J. Studies in ancient gypsum based plasters towards their repair: Physical and mechanical properties. Constr. Build. Mater. 2019, 202, 319–331. [Google Scholar] [CrossRef]
- Sophia, M.; Sakthieswaran, N. Synergistic effect of mineral admixture and bio-carbonate fillers on the physico-mechanical properties of gypsum plaster. Constr. Build. Mater. 2019, 204, 419–439. [Google Scholar] [CrossRef]
- Asadi Ardebili, A.; Villoria Sáez, P.; González Cortina, M.; Tasán Cruz, D.M.; Rodríguez Saíz, Á.; Atanes-Sánchez, E. Mechanical characterization of gypsum mortars with waste from the automotive sector. Constr. Build. Mater. 2023, 370, 130675. [Google Scholar] [CrossRef]
- Zehfuß, J.; Sander, L. Gypsum plasterboards under natural fire—Experimental investigations of thermal properties. Civ. Eng. Des. 2021, 3, 62–72. [Google Scholar] [CrossRef]
- Peceño, B.; Pérez-Soriano, E.M.; Luna-Galiano, Y.; Leiva, C. The Incorporation of Ladle Furnace Slag in Fire Insulating Gypsum-Based Materials. Fire 2023, 6, 416. [Google Scholar] [CrossRef]
- van der Heijden, G.H.A.; Pel, L.; Kopinga, K. Moisture transport and dehydration in heated gypsum. In Proceedings of the 2nd International RILEM Workshop on Concrete Spalling due to Fire Exposure, Delft, The Netherlands, 5–7 October 2011; Available online: https://www.rilem.net/images/publis/90e35dcd871a60711cfc3ab291740b95.pdf (accessed on 31 October 2025).
- Panel Sandwich Group. Panel Sandwich de Fachada Ignífuga. Available online: https://panelsandwich.cl (accessed on 31 October 2025).
- Villalba. Fireproof Wall—Panel Resistente al Fuego de Lana de Roca. Available online: https://www.villalba.cl/producto/fireproof-wall (accessed on 31 October 2025).
- Cintac. Paneles con Aislación—Lana de Roca y Poliuretano. Available online: https://www.cintac.cl/paneles-con-aislacion (accessed on 31 October 2025).
- MultiGips—VG-ORTH. Solid Drywalls for Domestic Construction: Simply Gypsum Blocks (Product Brochure). Available online: https://www.multigips.de/wp-content/uploads/2018/07/2018-08_P004_ImBro_Platte_EN.pdf (accessed on 31 October 2025).
- Chen, W.; Ye, J.; Bai, Y.; Zhao, X.L. Full-scale fire experiments on load-bearing cold-formed steel walls lined with different panels. J. Constr. Steel Res. 2012, 79, 242–254. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, P.; Man, M.; Chen, Z.; Jiang, L.; Wei, B. Experimental and numerical studies on the fire-resistance behaviors of critical walls and columns in modular steel buildings. J. Build. Eng. 2021, 44, 102964. [Google Scholar] [CrossRef]
- Ministry of Infrastructure and Water Management, Rijkswaterstaat Environment. Decree on Soil Quality (Soil Quality Decree) 469; Official Gazette of the Netherlands: The Hague, The Netherlands, 2007. [Google Scholar]
- Council of the European Union. Directive 2013/59/Euratom: Laying Down Basic Safety Standards for Protection Against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, and 97/43/Euratom. Off. J. Eur. Union 2013, 13, 1–73. [Google Scholar]

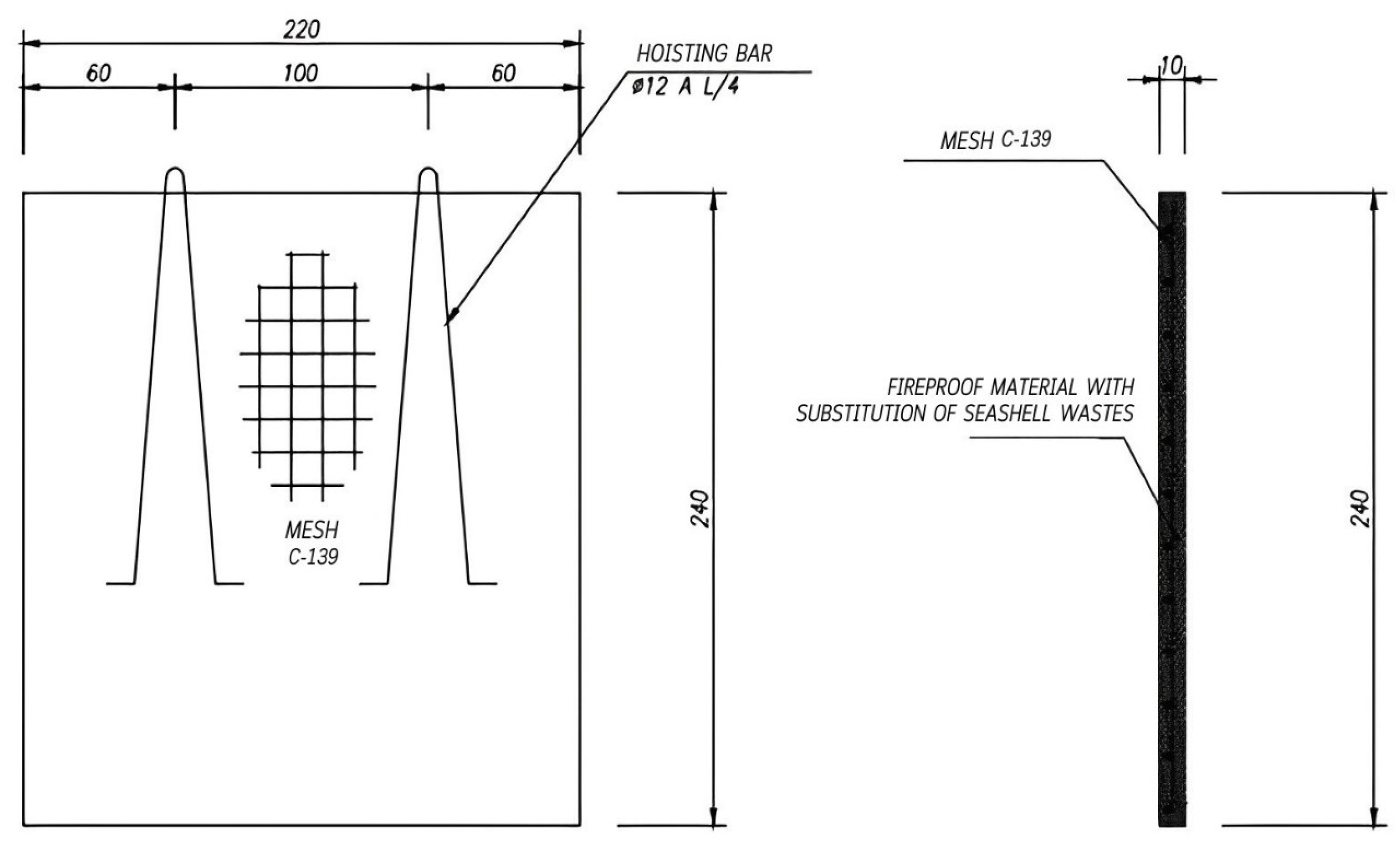





| SW 2 | G 3 | |
|---|---|---|
| SiO2 (wt%) | 0.3 | 0.9 |
| SO3 (wt%) | 0.2 | 45.7 |
| Al2O3 (wt%) | N.D. 4 | 0.2 |
| CaO (wt%) | 54.6 | 40.6 |
| Na2O (wt%) | 0.9 | 0.1 |
| MnO (wt%) | 0.0 | 0.0 |
| LOI 1 (wt%) | 44.0 | 12.5 |
| Median size (μm) | 130.0 | 6.6 |
| Specific gravity (g/cm3) | 2.6 | 2.9 |
| W 1 (%) | D 2 (kg/m3) | |
|---|---|---|
| 100G | 17 ± 1 | 1520 ± 2 |
| 20SW | 15 ± 1 | 1492 ± 17 |
| SH 1 (Shore C) | IR 2 (cm) | Sc 3 (MPa) | Sf 4 (MPa) | |
|---|---|---|---|---|
| 100G | 94 ± 1 | 2.1 ± 0.1 | 14.8 ± 1.4 | 4.0 ± 0.8 |
| 20SW | 94 ± 2 | 1.5 ± 0.1 | 14.7 ± 2.3 | 4.0 ± 0.6 |
| 100G (mg/m2) | 20SW (mg/m2) | DSQD Limits (mg/m2) | |
|---|---|---|---|
| Se | 4 | 3.5 | 4.8 |
| Hg | 0.7 | 0.7 | 1.4 |
| Sn | 2.5 | 2.4 | 50 |
| Ba | 3.7 | 2.3 | 1500 |
| Pb | 3.5 | 1.2 | 400 |
| Sb | 2.6 | 2.2 | 8.7 |
| Cd | 0.7 | 0.7 | 3.8 |
| Co | 0.2 | 0.2 | 60 |
| V | 3.8 | 3.8 | 320 |
| Cr | 0.2 | 0.2 | 120 |
| As | 5.2 | 5 | 260 |
| Mo | 2.4 | 1.6 | 144 |
| Ni | 2.8 | 1.4 | 81 |
| Zn | 9 | 6 | 800 |
| Cu | 1.2 | 1.2 | 98 |
| 100G (mg/m2) | 20SW (mg/m2) | |
|---|---|---|
| K-40 (Bq/kg) | MNDA (1) | MNDA (1) |
| Th-232 (Bq/kg) | MNDA (1) | MNDA (1) |
| Ra-226 (Bq/kg) | 3.6 ± 1.1 | 1.8 ± 0.7 |
| ACI | 0.012 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peceño, B.; Alonso-Fariñas, B.; Vega, G.; Carrizo, D.; Leiva, C. Sustainable Fire-Resistant Materials: Thermal, Physical, Mechanical, and Environmental Behavior of Walls with Waste from the Aquaculture Industry. Materials 2025, 18, 5086. https://doi.org/10.3390/ma18225086
Peceño B, Alonso-Fariñas B, Vega G, Carrizo D, Leiva C. Sustainable Fire-Resistant Materials: Thermal, Physical, Mechanical, and Environmental Behavior of Walls with Waste from the Aquaculture Industry. Materials. 2025; 18(22):5086. https://doi.org/10.3390/ma18225086
Chicago/Turabian StylePeceño, Begoña, Bernabé Alonso-Fariñas, Giovanna Vega, Daniel Carrizo, and Carlos Leiva. 2025. "Sustainable Fire-Resistant Materials: Thermal, Physical, Mechanical, and Environmental Behavior of Walls with Waste from the Aquaculture Industry" Materials 18, no. 22: 5086. https://doi.org/10.3390/ma18225086
APA StylePeceño, B., Alonso-Fariñas, B., Vega, G., Carrizo, D., & Leiva, C. (2025). Sustainable Fire-Resistant Materials: Thermal, Physical, Mechanical, and Environmental Behavior of Walls with Waste from the Aquaculture Industry. Materials, 18(22), 5086. https://doi.org/10.3390/ma18225086








