Preparation, Properties and Chemical Modification Methods of the Fire-Fighting Foam for Coal Spontaneous Combustion
Abstract
1. Introduction
2. Three-Phase Foam
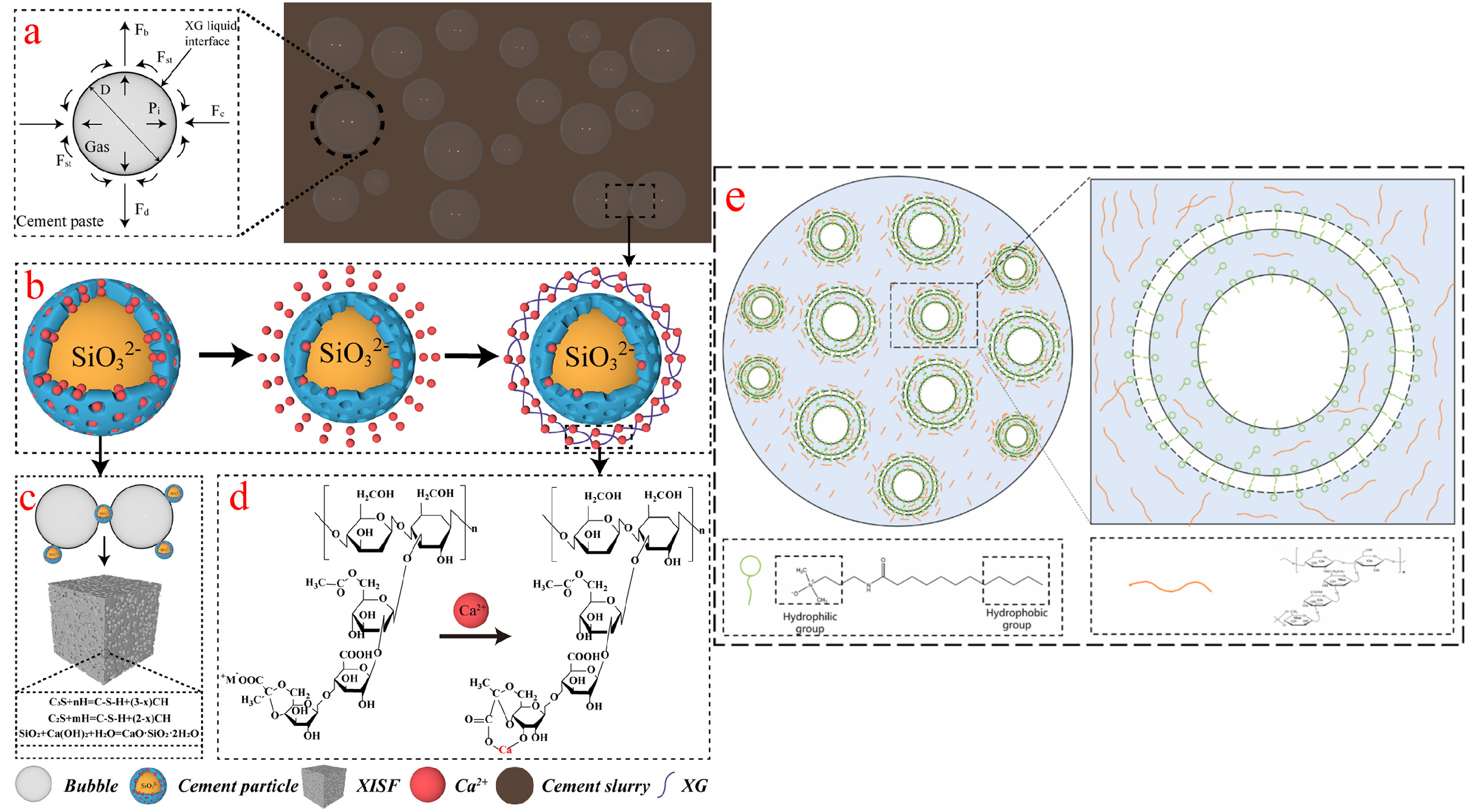
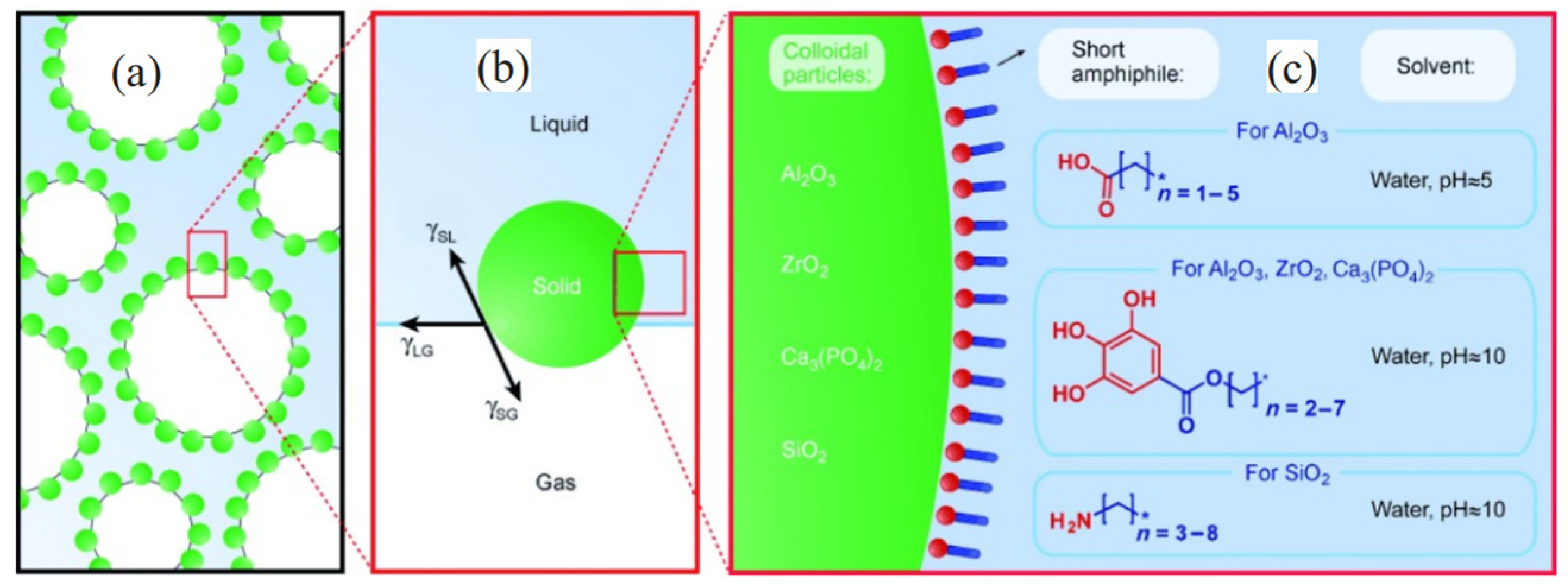
2.1. Formation Mechanism and Microstructure
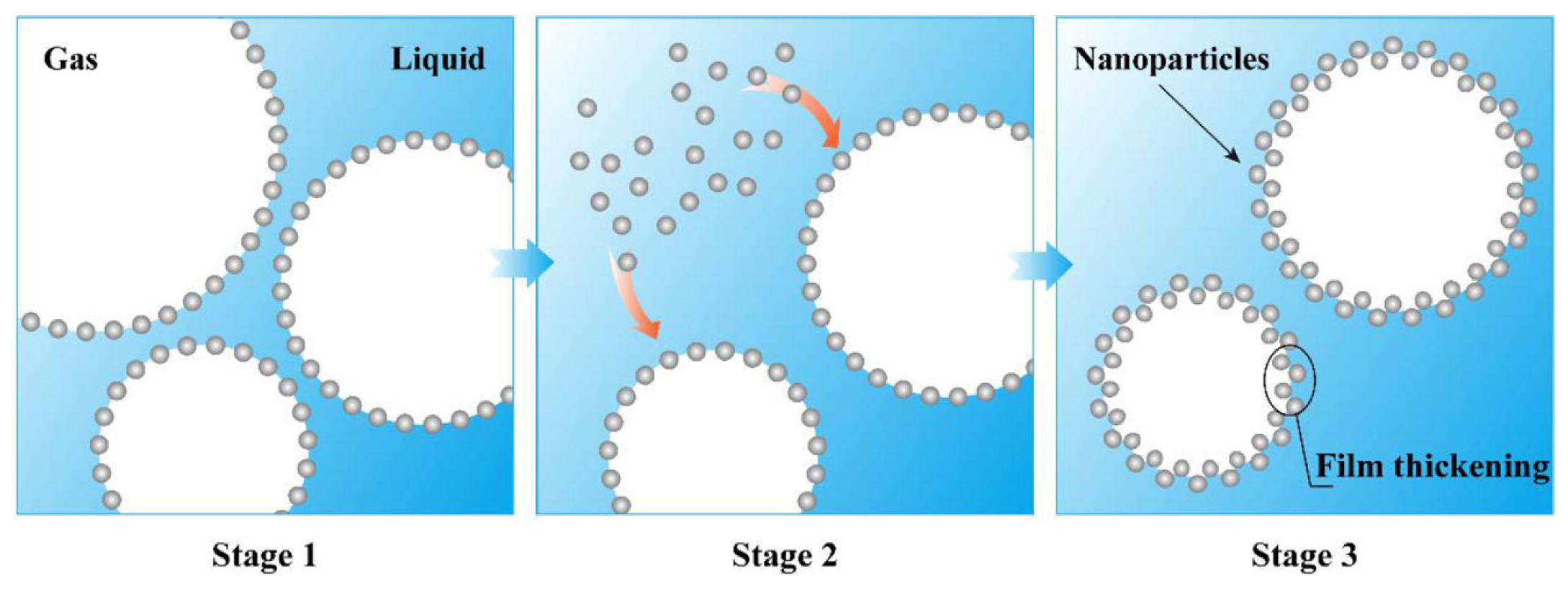
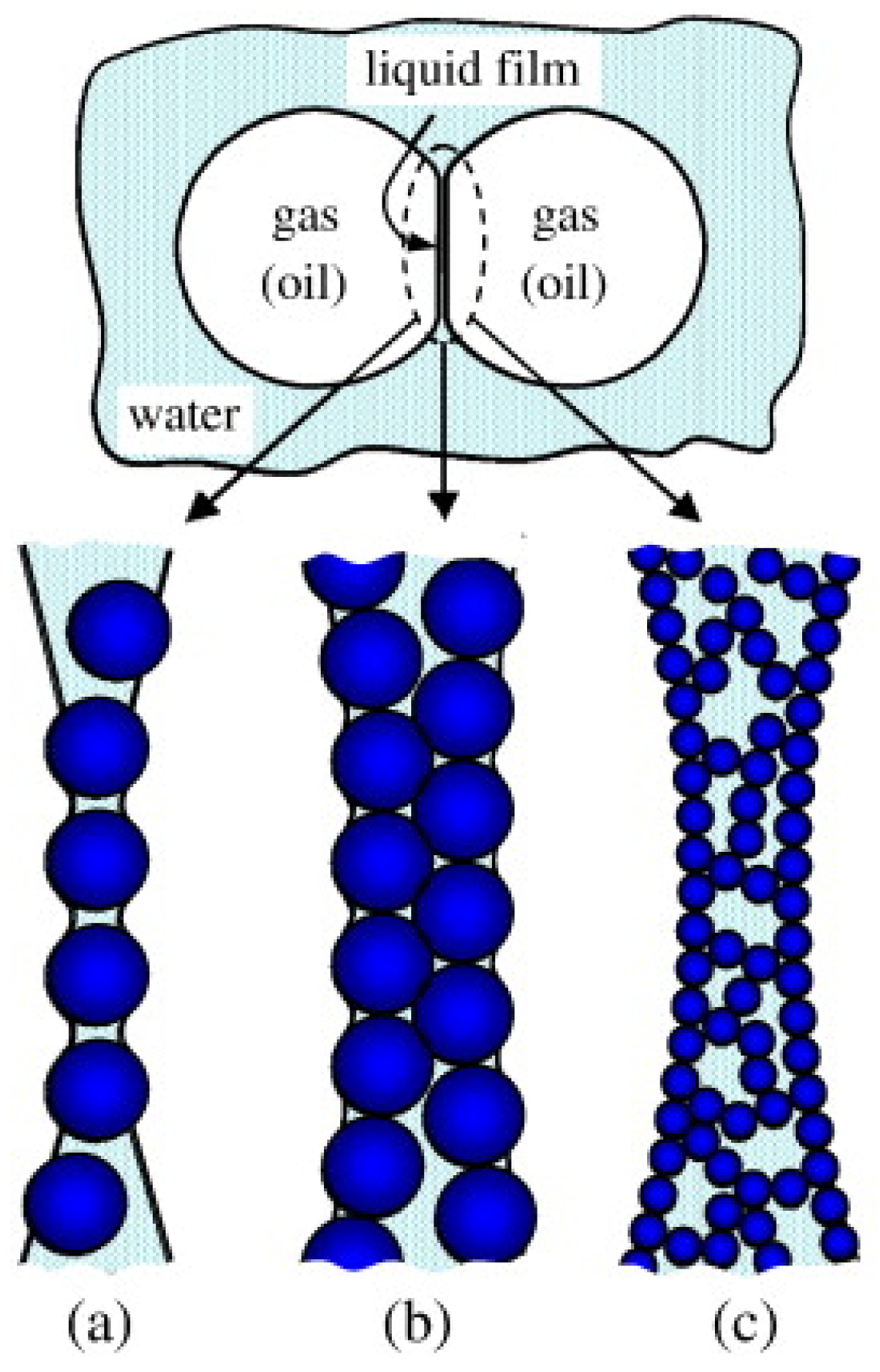
2.2. Influencing Factors for the Properties of Three-Phase Foam
3. Gel Foam
3.1. Inhibition Mechanism
3.2. Components of Gel Foam
3.3. Diffusion Characteristics in Porous Media
4. Curing Foam
4.1. Inhibition Mechanism
4.2. Inorganic Curing Foam
| Ref. | Admixture | Properties |
|---|---|---|
| [165] | Expanded vermiculite powder and silica powder | Silica powder enhances the compressive strength of materials. Foam concretes incorporating expanded vermiculite powder exhibit promising prospects in thermal conductivity. |
| [166] | Rice husk | Rice husk ash exhibits higher pozzolanic activity than fly ash, enhancing the degree of hydration. Under various preset pressures and airflow velocity conditions, the average blocking efficiency of the novel inorganic curing foam was 8.1–18.1% higher than that of traditional inorganic curing foam. |
| [153] | Fly ash (FA), blast furnace slag (BFS) and silica fume (SF) | When the blast furnace slag content is 20%, the foam content is twice that of the cementitious slurry, and the water–cement ratio is 0.5, the overall performance of cement-based foam material (CBFM) can be optimized. CBFM with BFS as a mineral admixture has a more uniform closed-cell structure than FA and SF. |
| [120] | Blast furnace slag | The activator dissolves the glassy structure of slag, generating more hydration products. This enhances the compressive strength of cement-based foam materials and further improves structural density. |
| [167] | Fly ash, granulated blast furnace slag (GBS) | The foam concrete with 100% GBS at a water-to-binder ratio of 0.68 exhibits better performance than the reference foam concrete. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szurgacz, D.; Tutak, M.; Brodny, J.; Sobik, L.; Zhironkina, O. The Method of Combating Coal Spontaneous Combustion Hazard in Goafs—A Case Study. Energies 2020, 13, 4538. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Deng, J.; Shu, C.-M.; Zeng, Q.; Guo, T.; Zhang, Y. Microcharacteristic analysis of CH4 emissions under different conditions during coal spontaneous combustion with high-temperature oxidation and in situ FTIR. Energy 2020, 209, 118494. [Google Scholar] [CrossRef]
- Tutak, M. Application of Model-Based Tests for Analysing the Consequences of Mine Fires. Multidiscip. Asp. Prod. Eng. 2018, 1, 767–774. [Google Scholar] [CrossRef]
- Xian, X.; Jiang, S.; Yin, C.-H.; Wu, Z. Experimental investigation on cement-based foam developed to prevent spontaneous combustion of coal by plugging air leakage. Fuel 2021, 301, 121091. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Liang, H.; Gao, Y.; Bi, Q.; Qu, B. Effects of igneous intrusions on the structure and spontaneous combustion propensity of coal: A case study of bituminous coal in Daxing Mine, China. Fuel 2018, 216, 181–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Y.; Shi, X.; Che, B. Determination of ignition temperature and kinetics and thermodynamics analysis of high-volatile coal based on differential derivative thermogravimetry. Energy 2022, 240, 122493. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tang, G.; Yang, Y.; Shao, S.; Ding, Y. Research and Prevention of Upper Remaining Coal Spontaneous Combustion Induced by Air Leakage in Multi-Inclination Regeneration Roof: A Case Study in the Luwa Coal Mine, China. Energy 2023, 275, 127484. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Zhang, J.; Sun, J.; Huang, J.; Taherdangkoo, R. New Insights of Grouting in Coal Mass: From Small-Scale Experiments to Microstructures. Sustainability 2021, 13, 9315. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Wen, H. Design and performance of a novel foaming device for plugging air leakage in underground coal mines. Powder Technol. 2019, 344, 842–848. [Google Scholar] [CrossRef]
- Shi, G.-Q.; Ding, P.-X.; Guo, Z.; Wang, Y.-M. Modeling temperature distribution upon liquid-nitrogen injection into a self heating coal mine goaf. Process Saf. Environ. Prot. 2019, 126, 278–286. [Google Scholar] [CrossRef]
- Wang, D.; Dou, G.; Zhong, X.; Xin, H.; Qin, B. An experimental approach to selecting chemical inhibitors to retard the spontaneous combustion of coal. Fuel 2014, 117, 218–223. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Jing, Z.; Feng, C. Effect of polydispersity on the structural characteristics of two-phase foam. Int. J. Multiph. Flow 2023, 164, 104465. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Du, W.; Dong, H.; Wang, B.; Wang, Y.; Cao, X. Preparation and characteristic study of the hydrogel of coal spontaneous combustion environmental protection. Fuel 2024, 360, 130505. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Hao, Y.; Li, H. Experimental investigation of the flow and extinguishment characteristics of gel-stabilized foam used to control coal fire. Energy 2022, 247, 123484. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Shi, S.; Wang, G.G.X.; Li, H.; Wang, T. Micro-particles stabilized aqueous foam for coal spontaneous combustion control and its flow characteristics. Process Saf. Environ. Prot. 2020, 139, 262–272. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Q.; Bu, Y.; Yang, Y.; Sun, S.; Li, S.; Tao, M.; Mao, Z. Review and prospects of research on materials to prevent and extinguish mine fires. Fire Mater. 2023, 47, 739–757. [Google Scholar] [CrossRef]
- Ge, T.; Cai, C.; Zhang, M. Microwave absorption properties of organic sulfur compounds in coal: Application of desulfurization. J. Sulfur Chem. 2021, 42, 322–334. [Google Scholar] [CrossRef]
- Wang, T.; Fan, H.; Yang, W.; Meng, Z. Stabilization mechanism of fly ash three-phase foam and its sealing capacity on fractured reservoirs. Fuel 2020, 264, 116832. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903. [Google Scholar] [CrossRef]
- Xi, X.; Shi, Q. Study of the preparation and extinguishment characteristic of the novel high-water-retaining foam for controlling spontaneous combustion of coal. Fuel 2021, 288, 119354. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Hu, Z.; Kang, R.; Zhang, J. Development of an environmentally friendly gel foam and assessment of its thermal stability and fire suppression properties in liquid pool fires. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133990. [Google Scholar] [CrossRef]
- Si, Y.; Li, T.; Clegg, P. Mixed Aqueous-and-Oil Foams via the Spinning Together of Separate Particle-Stabilized Aqueous and Oil Foams. Langmuir 2022, 38, 4243–4249. [Google Scholar] [CrossRef] [PubMed]
- Vavra, E.; Bai, C.; Puerto, M.; Ma, K.; Mateen, K.; Hirasaki, G.J.; Biswal, S.L. Effects of velocity on N2 and CO2 foam flow with in-situ capillary pressure measurements in a high-permeability homogeneous sandpack. Sci. Rep. 2023, 13, 10029. [Google Scholar] [CrossRef]
- Du, D.; Zhang, X.; Li, Y.; Zhao, D.; Wang, F.; Sun, Z. Experimental study on rheological properties of nanoparticle-stabilized carbon dioxide foam. J. Nat. Gas Sci. Eng. 2020, 75, 103140. [Google Scholar] [CrossRef]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755. [Google Scholar] [CrossRef]
- Zhao, J.; Torabi, F.; Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 2021, 287, 119443. [Google Scholar] [CrossRef]
- Rafati, R.; Haddad, A.S.; Hamidi, H. Experimental study on stability and rheological properties of aqueous foam in the presence of reservoir natural solid particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 19–31. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z.; Miao, Q.; Deng, Z.; Zhu, Y. Foams Stabilized by In Situ-Modified Nanoparticles and Anionic Surfactants for Enhanced Oil Recovery. Energy Fuels 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Xi, X.; Jiang, S.; Shi, Q.; Yin, C. Experimental investigation on the leakage plugging and fire extinguishment characteristics of industrial solid waste-based composite foam slurry materials. Energy 2023, 269, 126780. [Google Scholar] [CrossRef]
- Zhu, J.; Da, C.; Chen, J.; Johnston, K.P. Ultrastable N2/Water Foams Stabilized by Dilute Nanoparticles and a Surfactant at High Salinity and High Pressure. Langmuir 2022, 38, 5392–5403. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Lu, Y.; Li, Y.; Wang, D. Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv. Powder Technol. 2014, 25, 1527–1533. [Google Scholar] [CrossRef]
- Michaylov, M. Preventing and fighting spontaneous combustion by foam pulp in Bobov dol coal field. In Proceedings of the 7th US Mine Ventilation Symposium, Lexington, KY, USA, 5–7 June 1995; Wala, A.M., Ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 1995; pp. 185–190. [Google Scholar]
- Zhou, F.; Ren, W.; Wang, D.; Song, T.; Li, X.; Zhang, Y. Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int. J. Coal Geol. 2006, 67, 95–100. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Li, S.; Jiang, L.; Wang, J.; Wang, P. Utilization of Surfactant-Stabilized Foam for Enhanced Oil Recovery by Adding Nanoparticles. Energy Fuels 2014, 28, 2384–2394. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- AlYousef, Z.A.; Almobarky, M.A.; Schechter, D.S. The effect of nanoparticle aggregation on surfactant foam stability. J. Colloid Interface Sci. 2018, 511, 365–373. [Google Scholar] [CrossRef]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Emrani, A.S.; Nasr-El-Din, H.A. Stabilizing CO2-Foam using Nanoparticles. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar]
- Fu, C.; Yu, J.; Liu, N. Nanoparticle-stabilized CO2 foam for waterflooded residual oil recovery. Fuel 2018, 234, 809–813. [Google Scholar] [CrossRef]
- Hurtado, Y.; Beltrán, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Effects of Surface Acidity and Polarity of SiO2 Nanoparticles on the Foam Stabilization Applied to Natural Gas Flooding in Tight Gas-Condensate Reservoirs. Energy Fuels 2018, 32, 5824–5833. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergy between Nanoparticles and Surfactants in Stabilizing Foams for Oil Recovery. Energy Fuels 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M.; Risal, A.R. Experimental investigation of minimization in surfactant adsorption and improvement in surfactant-foam stability in presence of silicon dioxide and aluminum oxide nanoparticles. J. Pet. Sci. Eng. 2017, 159, 115–134. [Google Scholar] [CrossRef]
- AlYousef, Z.; Almobarky, M.; Schechter, D. Enhancing the Stability of Foam by the Use of Nanoparticles. Energy Fuels 2017, 31, 10620–10627. [Google Scholar] [CrossRef]
- Aroonsri, A.; Worthen, A.; Hariz, T.; Johnston, K.; Huh, C.; Bryant, S. Conditions for Generating Nanoparticle-Stabilized CO2 Foams in Fracture and Matrix Flow. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Farhadi, H.; Riahi, S.; Ayatollahi, S.; Ahmadi, H. Experimental study of nanoparticle-surfactant-stabilized CO2 foam: Stability and mobility control. Chem. Eng. Res. Des. 2016, 111, 449–460. [Google Scholar] [CrossRef]
- Wang, G.; Wang, K.L.; Lu, C.J. Advances of Researches on Improving the Stability of Foams by Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 242, 012020. [Google Scholar] [CrossRef]
- Fameau, A.-L.; Saint-Jalmes, A.; Cousin, F.; Houinsou Houssou, B.; Novales, B.; Navailles, L.; Nallet, F.; Gaillard, C.; Boué, F.; Douliez, J.-P. Smart Foams: Switching Reversibly between Ultrastable and Unstable Foams. Angew. Chem. Int. Ed. 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Velikov, K.P.; Durst, F.; Velev, O.D. Direct Observation of the Dynamics of Latex Particles Confined inside Thinning Water−Air Films. Langmuir 1998, 14, 1148–1155. [Google Scholar] [CrossRef]
- Ju, S.; Huang, Q.; Wang, G.; Qiao, J.; Wang, S.; Qin, C. Synergistic Stabilization Mechanism of SiO2 Nanoparticles and Anionic Surfactants during Foam Fracturing. Energy Fuels 2022, 36, 5327–5336. [Google Scholar] [CrossRef]
- Majeed, T.; Kamal, M.S.; Zhou, X.; Solling, T. A Review on Foam Stabilizers for Enhanced Oil Recovery. Energy Fuels 2021, 35, 5594–5612. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Panahpoori, D.; Dehdari, B.; Riazi, M.; Parsaei, R. Visualization experiments on the impact of surfactant and nanoparticle on EOR potential of foam injection. In Proceedings of the Saint Petersburg 2018, Moscow, Russia, 16–18 October 2018. [Google Scholar]
- Façanha, J.M.F.; Lopes, L.F.; Fritis, G.; Godoy, P.; Weber dos Santos, R.; Chapiro, G.; Perez-Gramatges, A. Bubble-growth regime for confined foams: Comparison between N2–CO2/foam and CO2/foam stabilized by silica nanoparticles. J. Pet. Sci. Eng. 2022, 218, 111006. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable Particle-Stabilized Foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef]
- Jing, Z.; Feng, C.; Ma, X.; Xu, D.; Wang, S. Mechanical evolution of bubble structure and interactive migration behaviors of two particles in flowing wet foam. J. Rheol. 2022, 66, 349–364. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous Foams Stabilized Solely by Silica Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef]
- Li, S.; Qiao, C.; Li, Z.; Wanambwa, S. Properties of Carbon Dioxide Foam Stabilized by Hydrophilic Nanoparticles and Hexadecyltrimethylammonium Bromide. Energy Fuels 2017, 31, 1478–1488. [Google Scholar] [CrossRef]
- Jing, Z.; Feng, C. Influence mechanisms of several parameters on the interaction between flowing wet foam and settling particle. Int. J. Multiph. Flow 2022, 150, 104015. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Ye, H.; Yang, L.; Luo, D.; Peng, B. Nanoparticles as foam stabilizer: Mechanism, control parameters and application in foam flooding for enhanced oil recovery. J. Pet. Sci. Eng. 2021, 202, 108561. [Google Scholar] [CrossRef]
- Jing, Z.; Feng, C. Alternating advancing behavior of two-phase wet foam fluid in heterogeneous porous media. Chem. Eng. Sci. 2025, 308, 121405. [Google Scholar] [CrossRef]
- Dedovets, D.; Li, Q.; Leclercq, L.; Nardello-Rataj, V.; Leng, J.; Zhao, S.; Pera-Titus, M. Multiphase Microreactors Based on Liquid–Liquid and Gas–Liquid Dispersions Stabilized by Colloidal Catalytic Particles. Angew. Chem. Int. Ed. 2022, 61, e202107537. [Google Scholar] [CrossRef]
- Lanza, F.; Sinha, S.; Hansen, A.; Rosso, A.; Talon, L. Transition from viscous fingers to foam during drainage in heterogeneous porous media. Phys. Fluids 2023, 35, 103119. [Google Scholar] [CrossRef]
- Benali, B.; Sæle, A.; Liu, N.; Fernø, M.A.; Alcorn, Z.P. Pore-level Ostwald ripening of CO2 foams at reservoir pressure. Transp. Porous Media 2023, 150, 427–445. [Google Scholar] [CrossRef]
- Yu, W.; Kanj, M.Y. Review of foam stability in porous media: The effect of coarsening. J. Pet. Sci. Eng. 2022, 208, 109698. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, X.; Kanj, M.Y. Microfluidic Investigation of Foam Coarsening Dynamics in Porous Media at High-Pressure and High-Temperature Conditions. Langmuir 2022, 38, 2895–2905. [Google Scholar] [CrossRef]
- Su, X.; Liu, Z.; Li, Y.; Du, D. Effect of foam quality on foam three phase displacement characteristics in porous media-A mechanistic numerical study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131624. [Google Scholar] [CrossRef]
- Lozano, L.F.; Cedro, J.B.; Zavala, R.V.Q.; Chapiro, G. How simplifying capillary effects can affect the traveling wave solution profiles of the foam flow in porous media. Int. J. Non-Linear Mech. 2022, 139, 103867. [Google Scholar] [CrossRef]
- Jones, S.; Getrouw, N.; Vincent-Bonnieu, S. Foam flow in a model porous medium: I. The effect of foam coarsening. Soft Matter 2018, 14, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Hu, X.; Cheng, W.; Yu, X.; Wu, M.; Zhao, Y.; Lu, Y.; Pan, R.; Niu, H.; Hu, S. Development of a novel composite inhibitor modified with proanthocyanidins and mixed with ammonium polyphosphate. Energy 2020, 213, 118901. [Google Scholar] [CrossRef]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of new gel foam and evaluation of its fire extinguishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Zhao, Y.-Y.; Hu, X.-M.; Wu, M.-Y.; Xue, D. A novel fire prevention and control plastogel to inhibit spontaneous combustion of coal: Its characteristics and engineering applications. Fuel 2020, 263, 116693. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Yuan, Y.; Qi, G.; Hu, X.; Li, J.; Liang, Y.; Guo, B. Study on the characteristics and mechanism of a new type of antioxidant gel foam for coal spontaneous combustion prevention. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127254. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wu, M.; Shao, Z.; Li, Y.; Zhao, Y.; Zhang, K. Carbon dioxide sealing-based inhibition of coal spontaneous combustion: A temperature-sensitive micro-encapsulated fire-retardant foamed gel. Fuel 2020, 266, 117036. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, N.; Miao, X.; Li, F.; Wang, J.; Zong, R.; Lu, S. Comparative studies on foam stability, oil-film interaction and fire extinguishing performance for fluorine-free and fluorinated foams. Process Saf. Environ. Prot. 2020, 133, 201–215. [Google Scholar] [CrossRef]
- Zhou, R.; Lang, X.; Zhang, X.; Tao, B.; He, L. Thermal stability and insulation characteristics of three-phase fire-fighting foam exposed to radiant heating. Process Saf. Environ. Prot. 2021, 146, 360–368. [Google Scholar] [CrossRef]
- Qing, G.; Wanxing, R.; Wei, L. Study on the stability of foamed gel and its structural evolution characteristics. Combust. Sci. Technol. 2023, 195, 2877–2888. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Z.; Ye, Q.; Lu, Y. Research Progress of Gel Foam Extinguishing Agent in Coal Mines. Fire 2023, 6, 470. [Google Scholar] [CrossRef]
- Chen, J.; Jia, B.; Fu, S.; Wen, Y.; Liang, Y.; Tian, F. Novel PFA-Based Inorganic Three-Phase Foam for Inhibiting Coal Spontaneous Combustion. ACS Omega 2023, 8, 24615–24623. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhang, L.; Lu, Y. Preparation and Inhibition Characteristic of Multi-Phase Foamed Gel for Preventing Spontaneous Combustion of Coal. Adv. Mater. Res. 2013, 634–638, 3678–3682. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Liu, S.; Zhang, H.; Li, J.; Zhang, H.; Wang, Z. A novel biomass sodium alginate gel foam to inhibit the spontaneous combustion of coal. Fuel 2022, 314, 122779. [Google Scholar] [CrossRef]
- Fan, X.-L.; Ma, L.; Sheng, Y.-J.; Liu, X.-X.; Wei, G.-M.; Liu, S.-M. Experimental investigation on the characteristics of XG/GG/HPAM gel foam and prevention of coal spontaneous combustion. Energy 2023, 284, 128710. [Google Scholar] [CrossRef]
- Ma, L.; Fan, X.; Wei, G.; Sheng, Y.; Liu, S.; Liu, X. Preparation and characterization of antioxidant gel foam for preventing coal spontaneous combustion. Fuel 2023, 338, 127270. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Li, F.; Guo, Y.; Chen, C.; Chen, C.; Li, R.; Yang, Z. A novel H-TiO2/gel co-stabilized three-dimensional network synergistic fire-retardant foam gel for coal-pile. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129642. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Yang, J.; Zhang, H.; Zhang, H.; Li, J.; Wang, Z. A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion. Energies 2022, 15, 5207. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.-B.; Liu, Z.; Yang, J.-N.; Zhang, H.L.; Li, J.; Zhang, H. Study on the Performance of a Novel Highly Stable Nano-Hydroxyapatite Gel Foam to Inhibit Coal Spontaneous Combustion. Combust. Sci. Technol. 2022, 196, 3120–3134. [Google Scholar] [CrossRef]
- Nie, S.-B.; Zhang, H.; Han, C.; Li, J.; Yang, J.-N.; Zhang, H.L.; Dai, G.; Su, H. Preparation of New Eco-Friendly Gel Foam Based on Biomass Pectin Material for Fire Prevention of Coal. Combust. Sci. Technol. 2022, 196, 2863–2877. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.; Zhu, J.; Shi, J. Study on the composition and structure of foamed gel for fire prevention and extinguishing in coal mines. Process Saf. Environ. Prot. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Dong, H.; Cheng, W.; Wang, W.; Liang, Y. Examination of characteristics of anti-oxidation compound inhibitor for preventing the spontaneous combustion of coal. Fuel 2022, 310, 122160. [Google Scholar] [CrossRef]
- Qiao, J.; Hu, X.-M.; Liang, Y.-T.; Zhang, Q.; Wang, W.; Zhao, Y.-Y.; Ju, S.; Tian, F.-C. Preparation and characterization of PVA-H18 gel foam for preventing spontaneous combustion of coal based on interfacial self-assembly. Fuel 2022, 327, 125081. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Li, F.; Chen, C.; Chen, C.; Li, R.; Zou, R.; Zhang, M. Response Surface Analysis (RSA) optimization of temperature-resistant gel foam fabrication and performance evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130260. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Wang, Z.; Wang, F.; Zhang, L.; Ma, H.; Wang, W. Preparation and characterization of highly stable double-crosslinked gel foam for inhibiting coal spontaneous combustion. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133179. [Google Scholar] [CrossRef]
- Huang, Z.; Quan, S.; Hu, X.; Zhang, Y.; Gao, Y.; Ji, Y.; Qi, X.; Yin, Y.H. An Environmentally Friendly Antioxidant Foamed Gel for Inhibiting Spontaneous Combustion of Coal. Combust. Sci. Technol. 2022, 195, 4144–4165. [Google Scholar] [CrossRef]
- Nie, S.-B.; Cai, Z.; Hu, D.; Han, C. Preparation and Characteristics of Biomass Gel Foam with High Foaming and Film-Forming Properties for Preventing Coal Spontaneous Combustion. Combust. Sci. Technol. 2023, 196, 5167–5184. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B. Film-Forming Property and Oxygen Barrier Characteristic of Gel-Stabilized Foam Used for Controlling Spontaneous Combustion of Coal. Energy Fuels 2021, 35, 12083–12090. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Zhang, H.; Chen, C. Study on Highly Stable Biomass Gel Foam with Ultra-Strong Film-Forming Performance Based on the Double Network Structure to Inhibit Coal Spontaneous Combustion. Combust. Sci. Technol. 2023, 197, 1426–1442. [Google Scholar] [CrossRef]
- Yang, F.; Lu, Y.; Yan, Z.; Wang, G.G.X.; Hu, X.; Gu, W. Colloidal Particle-Stabilized Foam To Control the Coal Spontaneous Combustion: Stability Mechanism Analysis and Extinguishing Properties. Energy Fuels 2020, 34, 14822–14831. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wen, H.; Zhao, J.; Liu, S. Study on the Inhibition Characteristics of Coal Spontaneous Combustion by Silica Gel Foam. ACS Omega 2024, 9, 14033–14042. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Liu, Z.; Wang, F.; Yang, N.; Lou, R.; Qin, C.; Yu, M.; Yu, Y. Permeation-diffusion characteristics and air-leakage blocking mechanism for the fire-extinguishing inorganic gel flows in loose broken coal particles. Fuel 2022, 328, 125245. [Google Scholar] [CrossRef]
- Li, S.; Zhou, G.; Wang, Y.; Jing, B.; Qu, Y. Synthesis and characteristics of fire extinguishing gel with high water absorption for coal mines. Process Saf. Environ. Prot. 2019, 125, 207–218. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Wang, D.-M.; Wang, L.-Y.; Zhong, X.-X.; Chu, T.-X. Experimental research on inhibition performances of the sand-suspended colloid for coal spontaneous combustion. Saf. Sci. 2012, 50, 822–827. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Wei, J.; Bian, Y.; Luo, H. Preparation of foamed gel for preventing spontaneous combustion of coal. Fuel 2021, 300, 121024. [Google Scholar] [CrossRef]
- Guo, J.; Cai, G.; Jin, Y.; Zheng, X.; Liu, Y. An Improved Composite Fly Ash Gel to Extinguish Underground Coal Fire in Close Distance Coal Seams: A Case Study. Adv. Mater. Sci. Eng. 2020, 2020, 5695471. [Google Scholar] [CrossRef]
- Lei, B.; He, B.; Xiao, B.; Du, P.; Wu, B. Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 2020, 265, 116976. [Google Scholar] [CrossRef]
- Ren, W.; Guo, Q.; Wang, Z. Application of foam–gel technology for suppressing coal spontaneous combustion in coal mines. Nat. Hazards 2016, 84, 1207–1218. [Google Scholar] [CrossRef]
- Rao, W.-H.; Liao, W.; Wang, H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retardant and smoke-suppressant flexible polyurethane foams based on reactive phosphorus-containing polyol and expandable graphite. J. Hazard. Mater. 2018, 360, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, D.-M. Enhanced fire behavior of rigid polyurethane foam by intumescent flame retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Sun, G.; Wang, K.; Liu, T.; Wang, J.; Wang, F. A study on a Janus-type composite solidified foam and its characteristics for preventing and controlling spontaneous combustion of coal. Energy 2023, 275, 127433. [Google Scholar] [CrossRef]
- Hu, X.-M.; Cheng, W.-M.; Wang, D.-M. Properties and applications of novel composite foam for blocking air leakage in coal mine. Russ. J. Appl. Chem. 2014, 87, 1099–1108. [Google Scholar] [CrossRef]
- Wu, J.; Yan, H.; Wang, J.; Wu, Y.; Zhou, C. Flame retardant polyurethane elastomer nanocomposite applied to coal mines as air-leak sealant. J. Appl. Polym. Sci. 2013, 129, 3390–3395. [Google Scholar] [CrossRef]
- Zhao, B.; Song, F.; Tan, L.; Yang, R.; Pan, Z.; Zhang, M.; Zhou, Y. Production, thermal recycling, and application of cardanol-based polyurethane foam with phenol-carbamate bonds. Chem. Eng. J. 2024, 494, 152941. [Google Scholar] [CrossRef]
- Nguyen-Ha, T.M.; Nguyen, T.B.; Nguyen, T.A.; Pham, L.H.; Nguyen, D.H.; Nguyen, D.M.; Hoang, D.; Oh, E.; Suhr, J. Novel high-performance sustainable polyurethane nanocomposite foams: Fire resistance, thermal stability, thermal conductivity, and mechanical properties. Chem. Eng. J. 2023, 474, 145585. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Cheng, W.; Wang, D.; Nie, W. Synthesis and Characterization of Phenol-Urea-Formaldehyde Foaming Resin Used to Block Air Leakage in Mining. Polym. Compos. 2014, 35, 2056–2066. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Ibrahim, T. Comparison of Toughening Effects of Various Additives on Phenolic Foam. ACS Omega 2024, 9, 4695–4704. [Google Scholar] [CrossRef]
- DSouza, G.C.; Ng, H.; Charpentier, P.; Xu, C.C. Recent Developments in Biobased Foams and Foam Composites for Construction Applications. ChemBioEng Rev. 2024, 11, 7–38. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Ye, L. A Novel Elastic Urea–Melamine–Formaldehyde Foam: Structure and Properties. Ind. Eng. Chem. Res. 2016, 55, 8743–8750. [Google Scholar] [CrossRef]
- Yuan, W.; Li, D.; Shen, Y.; Jiang, Y.; Zhang, Y.; Gu, J.; Tan, H. Preparation, characterization and thermal analysis of urea-formaldehyde foam. RSC Adv. 2017, 7, 36223–36230. [Google Scholar] [CrossRef]
- Liu, X.; Dai, C.; Ye, L.; Zhang, Y.; He, Z.; Zhao, X. Co-Condensation of Phloroglucinol-Nonylphenol-Urea-Formaldehyde Resin Enables High Toughness and Enhanced Warpage Resistance. Ind. Eng. Chem. Res. 2024, 63, 3127–3139. [Google Scholar] [CrossRef]
- Xi, X.; Tao, Y.; Jiang, S.; Yin, C. Study on the formation mechanism and mechanical properties of composite foam slurry material for mine plugging. Energy 2023, 281, 128295. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Wang, W.; Qi, G.; Sun, Y.; Zhao, Z.; Cui, X. Experimental study on the effect of preinhibition pressure on the inhibition of coal spontaneous combustion. Fire Saf. J. 2024, 149, 104235. [Google Scholar] [CrossRef]
- Sonnenschein, M. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green flame-retardant flexible polyurethane foam based on polyphenol-iron-phytic acid network to improve the fire safety. Compos. Part B Eng. 2022, 239, 109958. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Bo, C.; Zhang, M.; Hu, L.; Zhou, Y. Phosphorus-containing tung oil-based siloxane toughened phenolic foam with good mechanical properties, fire performance and low thermal conductivity. Mater. Des. 2020, 192, 108668. [Google Scholar] [CrossRef]
- Chen, X.; Yu, W.; Ma, L.; Zhou, S.; Liu, X. Mechanical properties and thermal characteristics of different-density phenolic foams. J. Therm. Anal. Calorim. 2021, 144, 393–401. [Google Scholar] [CrossRef]
- Ma, Z. Thermal Conductivity of Phenolic Foams. In Phenolic Based Foams: Preparation, Characterization, and Applications; Sandhya, P.K., Sreekala, M.S., Thomas, S., Eds.; Springer Nature: Singapore, 2022; pp. 155–174. [Google Scholar]
- Soloveva, O.; Solovev, S.; Vankov, Y.; Shakurova, R. Experimental Studies of the Effective Thermal Conductivity of Polyurethane Foams with Different Morphologies. Processes 2022, 10, 2257. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, J.; Tan, H.; Lv, S.; Zhang, Y. Preparation and properties of a polyvinyl alcohol toughened urea-formaldehyde foam for thermal insulation applications. Constr. Build. Mater. 2016, 120, 104–111. [Google Scholar] [CrossRef]
- Wan, M.; Shi, C.; Qian, X.; Qin, Y.; Jing, J.; Che, H.; Ren, F.; Li, J.; Yu, B.; Zhou, K. Design of novel double-layer coated ammonium polyphosphate and its application in flame retardant thermoplastic polyurethanes. Chem. Eng. J. 2023, 459, 141448. [Google Scholar] [CrossRef]
- Yin, S.; Ren, X.; Zheng, R.; Li, Y.; Zhao, J.; Xie, D.; Mei, Y. Improving fire safety and mechanical properties of waterborne polyurethane by montmorillonite-passivated black phosphorus. Chem. Eng. J. 2023, 464, 142683. [Google Scholar] [CrossRef]
- Saffar, T.; Bouafif, H.; Braghiroli, F.L.; Magdouli, S.; Langlois, A.; Koubaa, A. Production of Bio-based Polyol from Oxypropylated Pyrolytic Lignin for Rigid Polyurethane Foam Application. Waste Biomass Valorization 2020, 11, 6411–6427. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Chen, Y.-C. Synthesis of bio-based polyurethane foam modified with rosin using an environmentally-friendly process. J. Clean. Prod. 2020, 276, 124203. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, S.; Hu, L.; Zhang, M.; Huang, Q.; Sha, Y.; Jia, P.; Zhou, Y. Molecularly engineered cardanol derived epoxy vitrimers based on dynamic disulfide and dynamic ester exchanges with desirable dynamic response, degradability, and recyclability. Chem. Eng. J. 2023, 477, 147284. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ha, T.M.N.; Nguyen, B.T.; Ha, D.; Vu Vo, T.; Nguyen, D.M.; Vo, D.K.; Nguyen, N.T.; Nguyen, T.V.; Hoang, D. Microwave-assisted polyol liquefication from bamboo for bio-polyurethane foams fabrication. J. Environ. Chem. Eng. 2023, 11, 109605. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Kurańska, M.; Benes, H.; Kockova, O.; Kucała, M.; Malewska, E.; Schmidt, B.; Michałowski, S.; Zemła, M.; Prociak, A. Rebiopolyols—New components for the synthesis of polyurethane biofoams in line with the circular economy concept. Chem. Eng. J. 2024, 490, 151504. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Karaaslan, M.A.; Wan, X.; Chen, S.; Hua, Q.; Renneckar, S. Bio-based non-flammable foams with a circular end-of-life based on the self-foaming process. Chem. Eng. J. 2023, 470, 143957. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Formulation optimization of unreinforced and lignin nanoparticle-reinforced phenolic foams using an analysis of variance approach. Compos. Sci. Technol. 2012, 72, 667–674. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High bio-content polyurethane (PU) foam made from bio-polyol and cellulose nanocrystals (CNCs) via microwave liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Tao, J.; Yang, F.; Wu, T.; Shi, J.; Zhao, H.-B.; Rao, W. Thermal insulation, flame retardancy, smoke suppression, and reinforcement of rigid polyurethane foam enabled by incorporating a P/Cu-hybrid silica aerogel. Chem. Eng. J. 2023, 461, 142061. [Google Scholar] [CrossRef]
- Burgaz, E.; Kendirlioglu, C. Thermomechanical behavior and thermal stability of polyurethane rigid nanocomposite foams containing binary nanoparticle mixtures. Polym. Test. 2019, 77, 105930. [Google Scholar] [CrossRef]
- Mougel, C.; Garnier, T.; Cassagnau, P.; Sintes-Zydowicz, N. Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer 2019, 164, 86–117. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Progress in Bio-Based Phenolic Foams: Synthesis, Properties, and Applications. ChemBioEng Rev. 2021, 8, 612–632. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, P. Experimental investigation on spontaneous combustion of coal affected by exothermic reaction of polyurethane in underground coal mines. J. Therm. Anal. Calorim. 2022, 147, 337–346. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T.; Zhou, J.; Ma, L.; Liu, Q.; Xu, X. Study on the flame retardant property of reactive halogen-free organic grouting reinforcement materials for coal mines. Min. Saf. Environ. Prot. 2023, 50, 40–45. [Google Scholar]
- Yang, R.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, mechanical properties and fire behaviors of rigid polyurethane foam with a reactive flame retardant containing phosphazene and phosphate. Polym. Degrad. Stab. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- Shao, N.; Dong, C.; Wei, X.; Su, Y.; Dong, Z.; Zhang, Z. Quantitative characterization and control mechanism of pore structure in geopolymer foams with addition of various surfactants. Cem. Concr. Compos. 2024, 149, 105522. [Google Scholar] [CrossRef]
- Feneuil, B.; Roussel, N.; Pitois, O. Optimal cement paste yield stress for the production of stable cement foams. Cem. Concr. Res. 2019, 120, 142–151. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, B.; Shi, Q.; Hao, M.; Shao, X.; Jiang, Z.; Ma, Z. Study on the adsorption of cement particles on surfactant and its effect on the characteristics of inorganic curing foam for prevention of coal spontaneous combustion in a goaf. Fuel 2023, 333, 126407. [Google Scholar] [CrossRef]
- Yuanliang, X.; Zhongshuai, H.; Chao, L.; Chao, Z.; Yamei, Z. Unveiling the role of Portland cement and fly ash in pore formation and its influence on properties of hybrid alkali-activated foamed concrete. Constr. Build. Mater. 2024, 411, 134336. [Google Scholar] [CrossRef]
- Dang, J.; Tang, X.; Xiao, J.; Duan, Z.; Han, A. Role of recycled brick powder and alkaline solution on the properties of eco-friendly alkali-activated foam concrete. J. Clean. Prod. 2024, 436, 140381. [Google Scholar] [CrossRef]
- Xi, X.; Sun, L.; Shi, Q.; Tian, F.; Guo, B. Effects of mineral admixture on properties of cement-based foam material developed for preventing coal spontaneous combustion. Fuel 2023, 342, 127785. [Google Scholar] [CrossRef]
- Rooholamini, H.; Bayat, A.; Kazemian, F. Mechanical and fracture properties of alkali activated concrete containing different pozzolanic materials. Road Mater. Pavement Des. 2020, 23, 802–821. [Google Scholar] [CrossRef]
- Amin, M.; Tayeh, B.A.; Agwa, I.S. Effect of using mineral admixtures and ceramic wastes as coarse aggregates on properties of ultrahigh-performance concrete. J. Clean. Prod. 2020, 273, 123073. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Wang, B.; Zhao, Y.; Kang, M.; Wang, P. Properties of pervious concrete with steel slag as aggregates and different mineral admixtures as binders. Constr. Build. Mater. 2020, 257, 119543. [Google Scholar] [CrossRef]
- Ali, B.; Ahmed, H.; Kurda, R.; Qureshi, L.; Hafez, H.; Cadosch, H.; Raza, A. Enhancing the Hardened Properties of Recycled Concrete (RC) through Synergistic Incorporation of Fiber Reinforcement and Silica Fume. Materials 2020, 13, 4112. [Google Scholar] [CrossRef]
- Gu, X.; Wang, S.; Liu, J.; Wang, H.; Xu, X.; Wang, Q.; Zhu, Z. Effect of hydroxypropyl methyl cellulose (HPMC) as foam stabilizer on the workability and pore structure of iron tailings sand autoclaved aerated concrete. Constr. Build. Mater. 2023, 376, 130979. [Google Scholar] [CrossRef]
- Gong, S.; Gao, N.; Han, L.; Luo, H.A. A theoretical model for bubble coalescence by coupling film drainage with approach processes. Chem. Eng. Sci. 2020, 213, 115387. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Z.; Li, J.; Xu, Y.; Xin, H. Effect of Interface Structure and Behavior on the Fluid Flow Characteristics and Phase Interaction in the Petroleum Industry: State of the Art Review and Outlook. Energy Fuels 2023, 37, 9914–9937. [Google Scholar] [CrossRef]
- Zawala, J.; Miguet, J.; Rastogi, P.; Atasi, O.; Borkowski, M.; Scheid, B.; Fuller, G.G. Coalescence of surface bubbles: The crucial role of motion-induced dynamic adsorption layer. Adv. Colloid Interface Sci. 2023, 317, 102916. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xing, Y.; Zhu, C.; Liu, Q.; Yang, Z.; Zhang, R.; Zhang, Y.; Xia, Y.; Gui, X. Effect of roughness on wettability and floatability: Based on wetting film drainage between bubbles and solid surfaces. Int. J. Min. Sci. Technol. 2022, 32, 1389–1396. [Google Scholar] [CrossRef]
- Li, G.; Tan, H.; He, X.; Zhang, J.; Deng, X.; Zheng, Z. Research on the properties of wet-ground waste limestone powder as foam stabilizer in foamed concrete. Constr. Build. Mater. 2022, 329, 127203. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. Enhancing the strength of pre-made foams for foam concrete applications. Cem. Concr. Compos. 2018, 87, 164–171. [Google Scholar] [CrossRef]
- Koksal, F.; Sahin, Y.; Gencel, O. Influence of expanded vermiculite powder and silica fume on properties of foam concretes. Constr. Build. Mater. 2020, 257, 119547. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Liang, Y.; Sun, G.; Tang, H.; Wang, W. A study on the characteristics of a novel inorganic solidified foam for the prevention and control of the spontaneous combustion of coal. Constr. Build. Mater. 2022, 347, 128516. [Google Scholar] [CrossRef]
- Oren, O.H.; Gholampour, A.; Gencel, O.; Ozbakkaloglu, T. Physical and mechanical properties of foam concretes containing granulated blast furnace slag as fine aggregate. Constr. Build. Mater. 2020, 238, 117774. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- She, W.; Du, Y.; Miao, C.; Liu, J.; Zhao, G.; Jiang, J.; Zhang, Y. Application of organic- and nanoparticle-modified foams in foamed concrete: Reinforcement and stabilization mechanisms. Cem. Concr. Res. 2018, 106, 12–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, G.; Hu, X.; Xue, D.; Wang, K. Study on the Preparation of Inorganic Solidified Foam with High Stability and Its Prevention and Treatment of Coal Spontaneous Combustion. Energy Fuels 2023, 37, 14268–14279. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, B.; Shi, Q.; Hao, M.; Shao, X.; Jiang, Z.; Ma, Z. Study on the preparation and properties of colloidal gas foam concrete to prevent spontaneous combustion of coal. Energy 2023, 283, 128551. [Google Scholar] [CrossRef]
- Nakum, A.V.; Arora, N.K. Fresh and mechanical characterization of fly ash/slag by incorporating steel fiber in self-compacted geopolymer concrete. Constr. Build. Mater. 2023, 368, 130481. [Google Scholar] [CrossRef]
- Chen, Y. Experimental study on application performance of foamed concrete prepared based on a colloidal NanoSiO2-stabilized foam. Constr. Build. Mater. 2023, 409, 134012. [Google Scholar] [CrossRef]
- Xiao, M.; Li, F.; Yang, P.; Li, B.; Wei, J.; Yu, Q. Influence of slurry characteristics on the bubble stability in foamed concrete. J. Build. Eng. 2023, 71, 106500. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Ma, M.; Zhang, Z.; Ji, X. Slurry rheological behaviors and effects on the pore evolution of fly ash/metakaolin-based geopolymer foams in chemical foaming system with high foam content. Constr. Build. Mater. 2023, 379, 131259. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Z.; Jia, Z.; Liu, C.; Ma, L.; Liu, Z. Effect of formic acid as an accelerator on foam-stability, compressive strength, and pore size distribution of foam concrete. J. Build. Eng. 2023, 66, 105923. [Google Scholar] [CrossRef]
- Yuanliang, X.; Chao, Z.; Chun, C.; Yamei, Z. Effect of superabsorbent polymer on the foam-stability of foamed concrete. Cem. Concr. Compos. 2022, 127, 104398. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Li, P.; Chen, D.; Xu, Y.; Feng, Y.; Yu, Y.; Zhang, H. Effect of characteristics of chemical combined of graphene oxide-nanosilica nanocomposite fillers on properties of cement-based materials. Constr. Build. Mater. 2019, 225, 745–753. [Google Scholar] [CrossRef]
- Xiong, Y.; Pang, B.; Liu, Z.; Liu, C.; Hu, Z.; Ma, L. Effect of foam temperature on foam stability of foamed concrete and stabilization mechanisms. J. Build. Eng. 2023, 77, 107492. [Google Scholar] [CrossRef]
- Tran, N.; Nguyen, T.; Ngo, T.; Le, P.; Le, A.-T. Strategic progress in foam stabilisation towards high-performance foam concrete for building sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 375, 133939. [Google Scholar] [CrossRef]
- Cho, S.; van Rooyen, A.; Kearsley, E.; van Zijl, G. Foam stability of 3D printable foamed concrete. J. Build. Eng. 2022, 47, 103884. [Google Scholar] [CrossRef]
- Briceño-Ahumada, Z.; Mikhailovskaya, A.; Staton, J.A. The role of continuous phase rheology on the stabilization of edible foams: A review. Phys. Fluids 2022, 34, 031302. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Ramakrishnan, S.; Pasupathy, K.; Sanjayan, J. Collapse of fresh foam concrete: Mechanisms and influencing parameters. Cem. Concr. Compos. 2021, 122, 104151. [Google Scholar] [CrossRef]
- Hu, X.; Song, Y.; Zhang, Q.; Wu, M.; Lu, W.; Du, H.; Yang, Z. Study on the influence of dry ice phase change behavior on the micropore structure and hydration properties of mining grouting materials based on experiments and molecular simulations. Constr. Build. Mater. 2024, 425, 136035. [Google Scholar] [CrossRef]
- Ganguly, S.; Tang, X.S. 3D Printing of High Strength Thermally Stable Sustainable Lightweight Corrosion-Resistant Nanocomposite by Solvent Exchange Postprocessing. ACS Sustain. Chem. Eng. 2025, 13, 423–435. [Google Scholar] [CrossRef]
- Huo, S.; Guo, Y.; Yang, Q.; Wang, H.; Song, P. Two-dimensional nanomaterials for flame-retardant polymer composites: A mini review. Adv. Nanocompos. 2024, 1, 240–247. [Google Scholar] [CrossRef]


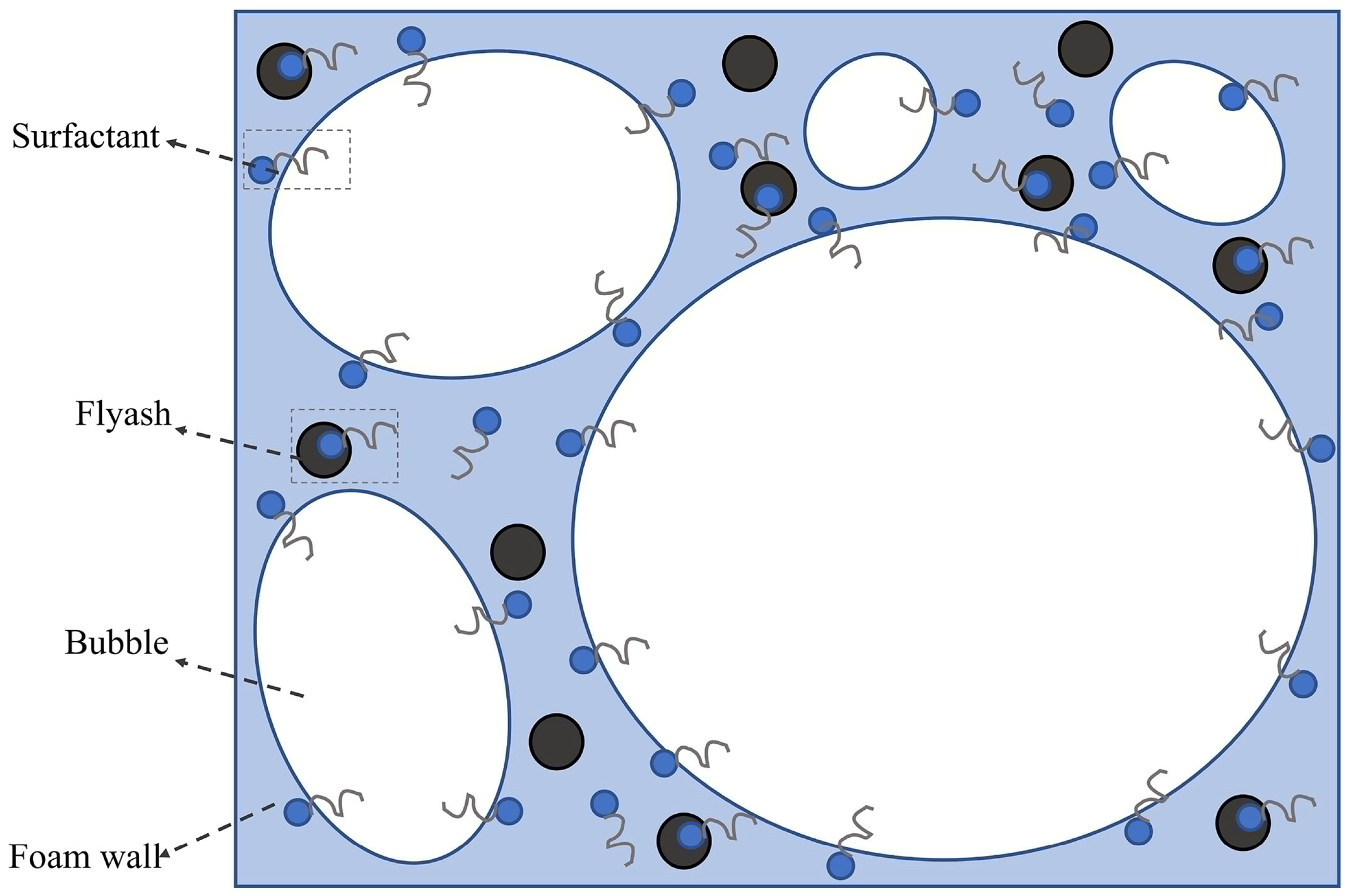
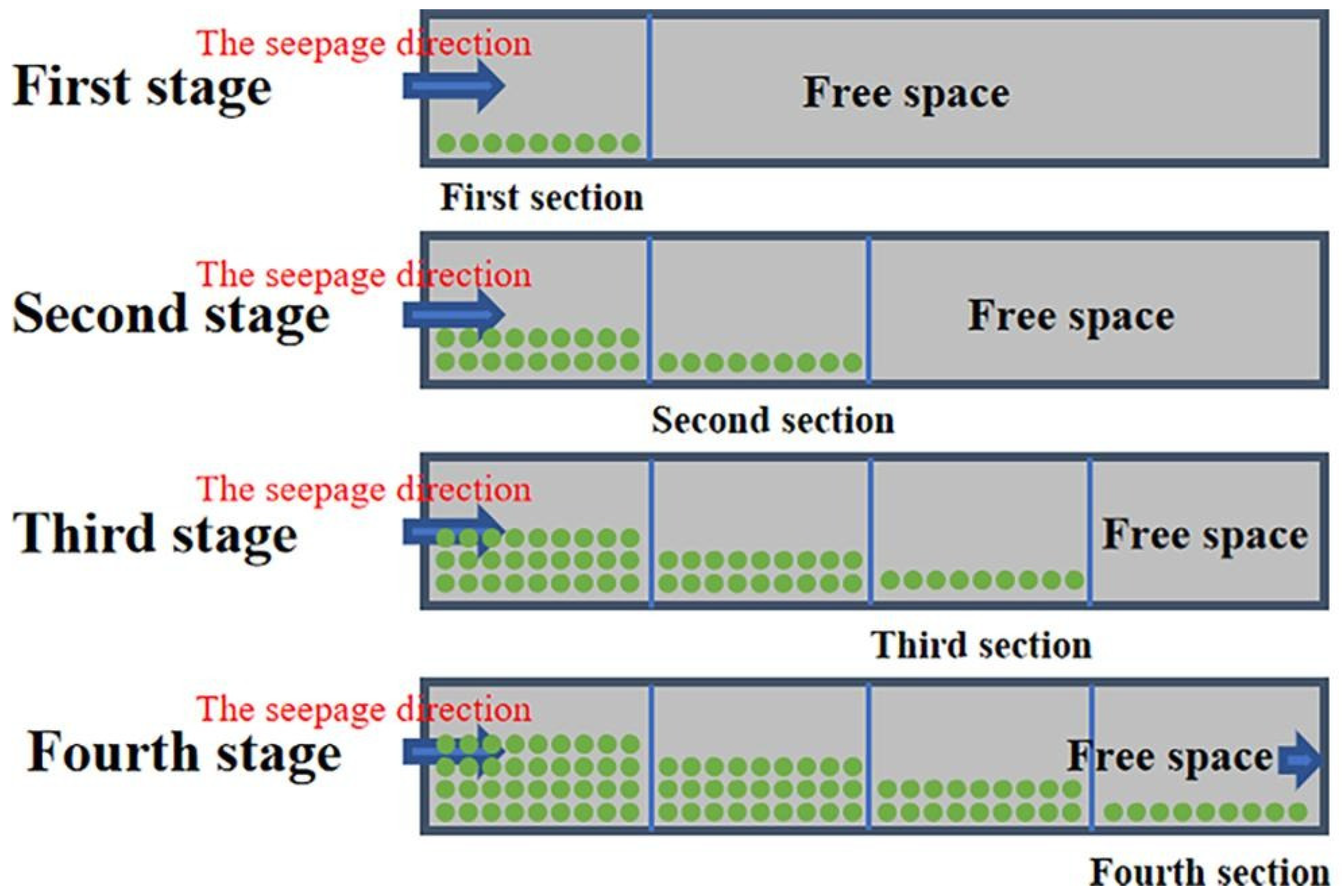



| Name | Components | Configuration Procedure | Thermal Stability | Inhibition Rate | Fire Extinguishing Performance | Ref. |
|---|---|---|---|---|---|---|
| New gel foam | (a) Compound foaming agent (sodium α-alkenyl sulfonate: fatty alcohol polyoxyethylene ether sodium sulfate = 8:4); (b) gelling agent; (c) organic crosslinking agent. | (1) Foaming solution + gelling agent (stirring) → Liquid A; (2) inorganic aluminum salt solution + ligand (stirring) → Liquid B; (3) Liquid A + Liquid B → Gel foam. | Not mentioned | The inhibition rate increased by 61.72% compared to two-phase foam. | No significant reignition of the coal body was observed. | [71] |
| Novel high-water-retaining foam | (a) Polymer composite (PC), a mixture of the microbial polysaccharide and galactomannan biopolymer; (b) organic boron complex; (c) foaming agent prepared from anionic surfactant and non-ionic surfactant. | (1) Foaming agent + water → base fluid (The concentration is 3 g/L); (2) polymer composite + base fluid (stirring about 15 min) → Dispersion liquid; (3) dispersion liquid + organic boron complex → uniform foaming solution; (4) mixed solutions + high-pressure air → high-water-retaining foam. | Not mentioned | Not mentioned | Within 30 min, the temperature of the burning coal dropped from approximately 700 °C to 34.7 °C. | [21] |
| Novel biomass sodium alginate gel foam | (a) Sodium alginate (SA); (b) calcium L-lactate (CL); (c) alkyl glycoside (APG); (d) tea saponin (TS). | (1) SA solution + 0.2 g APG + 0.1 g TS (Stir well) → Solution A; (2) dissolve CL + water → Solution B; (3) Solution A + Solution B (mechanical stirring) → biomass sodium alginate gel foam. | Not mentioned | The CO inhibition rate is 60.5% at 200 °C. | During the first 60 min, the coal sample temperature rapidly decreased from 965 °C to 90 °C, and after 200 min, it dropped to 30 °C. | [81] |
| PVA-H18 gel foam (PGF) | (a) Nanoparticles (hydrophobic); (b) sodium bicarbonate; (c) sodium tetraborate; (d) PVA. | (1) 3 g PVA + 100 mL deionized water (stirring) → PVA solution; (2) PVA solution + sodium bicarbonate (stirring) → PVA solution with PH 8.5; (3) 0.7 g nanosilica + foam + 0.4 g sodium tetraborate (stirring) → PGF. | Retain water for over 15 h at 100 °C. | The temperature of the coal sample dropped from 865 °C to 100 °C within 30 s. | Effectively preventing the recurrence of a coal fire after extinguishing. | [90] |
| XG/GG/HPAM gel foam | (a) Surfactants; (b) anionic polysaccharide xanthan gum (XG); (c) galactomannan guar gum (GG); (d) metal crosslinker; (e) gelling agent polyacrylamide (HPAM); (f) lab-made inhibitor. | Surfactant + XG + GG + HPAM + inhibitor (high-speed stirring) → XG/GG/HPAM gel foam. | The rate of water loss was 8.50% after heating at 100 °C for 1 h. | The inhibition rate at 100 °C is 74.48%. | Not mentioned | [82] |
| A novel foam gel | (a) Sodium metaborate tetrahydrate; (b) tetrabutyl titanate; (c) glucose monomer; (d) sodium bicarbonate (NaHCO3); (e) acrylic acid (AA); (f) acrylamide (AM); (g) potassium persulfate (KPS); (h) sodium hydroxide (NaOH); (i) commercially available N,N′-methylene bisacrylamide (MBA); (j) ethanol. | (1) Sodium acrylate + AM + crosslinking agent MBA + sodium bicarbonate (stirring)→Solution A; (2) sodium borate + initiator KPS + deionized water (stirring) → Solution B; (3) foaming agent + Solution B + prepared H-TiO2 (ultrasonication) → new solution B; (4) new Solution B + Solution A → Foam gel. | Not mentioned | The inhibition rate is 52.63%. | Has a marked inhibitory effect on smoke. | [84] |
| Temperature-resistant gel foam | (a) Acrylic acid (AA); (b) acrylamide (AM); (c) 2-Acryloylamino-2-methyl-1-propanesulfonic acid (AMPS); (d) N,N′-Methylenebisacrylamide (MBA); (e) ammonium persulfate (APS); (f) sodium hydroxide(NaOH); (g) calcium lignosulfonate (CLS). | (1) Acrylic acid + sodium hydroxide solution → Sodium acrylate solution; (2) AMPS + MBA+ CLS + sodium acrylate solution (stirring) → mixed solution; (3) APS+ mixed solution (stirring) → polymer solution. | Not mentioned | Not mentioned | Generated an enhanced mechanism with greater temperature resistance, stability and considerable potential application areas. | [91] |
| Highly stable double-crosslinked gel foam | (a) Fatty alcohol polyethylene ether sodium sulfate (AES); (b) polyether modified tri-siloxane (GT-248); (c) sodium alginate (SA); (d) carboxymethyl cellulose sodium (CMC); (e) ethylenediaminetetraacetic acid disodium (EDTA); (f) gluconate-δ-lactone (GDL). | (1) AES + GT-248 → foam agent (AG); (2) EDTA + CaCl2 + water (stirring) → EDTA-Ca solution; (3) 0.5 g SA + 0.05 g CMC + AG → SA/CMC thickener solution (left for 12 h) → SC solution; (4) SC solution+ EDTA-Ca+ GDL (thoroughly foamed) → double-crosslinked gel foam. | Not mentioned | The CO inhibition rate is 44.37% at 100 °C. | Extinguished the heat source within 470 s and reduced the temperature to 87 °C within 1300 s. | [92] |
| Environmentally friendly gel foam | (a) Alphaolefin sulfonate (AOS); (b) alkyl ethoxy polyglycosides (AEG); (c) sodium silicate; (d) sodium bicarbonate. | (1) Foaming agents: AOS and AEG; (2) gelling agents: sodium silicate; (3) crosslinking agents: sodium bicarbonate. | Thermal stability depends on the formulation. | Not mentioned | As the concentration of NaHCO3 increases, the fire extinguishing performance improves. | [22] |
| An environmentally friendly antioxidant foamed gel | (a) Modified sodium polyacrylate (MSP); (b) konjac glucomannan; (c) sodium dodecyl sulfate; (d) sodium alpha-olefin sulfonate; (e) modified silicone polyether microemulsion; (f) montmorillonite; (g) tert-butyl hydroquinone (TBHQ). | (1) Crosslinking agent + foaming agent + foam stabilizer + deionized water → mixed solution; (2) MSP + montmorillonite/TBHQ → slowly mixed; (3) stirred for 5 min. | Suppressed the thermal decomposition stage after 300 °C. | Inhibit the oxidation reaction of coal. | Prevents coal from contacting oxygen. Antioxidant components mitigate chemisorption and chemical reactions. | [93] |
| SA-Ca2+@TA-GF | (a) Tannic acid (TA) and calcium L-lactate (CL); (b) sodium alginate (SA); (c) composite foaming agent (CFA). | (1) TA + CFA + SA solution → mixed solution; (2) CL+ mixed solution (mechanical stirring) → gel foam. | Not mentioned | At 200 °C, the inhibition rate is 79.6%. | Within 20 min, the temperature of the coal decreased rapidly from 965 °C to 98.8 °C. | [85] |
| Biomass gel foam | (a) Carboxymethyl chitosan (CMCS); (b) composite crosslinking agent (CCA); (c) composite foaming agent (CFA) was a combination of anionic and non-ionic surfactants with a 1:1 ratio; (d) foam stabilizer agent (FSA). | (1) CMCS + water → CMCS solution; (2) CMCS solution + CFA + FSA + CCA (high-speed stirring) → biomass gel foam. | The water-holding rate was 49.34% after heating for 10 h at 80 °C. | The CO inhibition rate is 67.43% at 100 °C. | Excellent flame-retardant properties. | [94] |
| New eco-friendly gel foam based on biomass pectin material (LMP-Ca) | (a) Low methoxyl pectin (LMP); (b) calcium L-lactate (Ca-L); (c) Biomass compound foaming agent (BF) was composed of tea saponin (TS) and alkyl glycoside (APG). | (1) BF solution + LMP solution (mechanically stirred) → foaming solution; (2) Ca-L solution + foaming solution (fully stirred) → LMP-Ca. | Not mentioned | The CO inhibition rate is 72.1% at 180 °C. | The temperature of the coal decreased from 960 °C to 60 °C within 20 min, with no reignition occurring. | [87] |
| Gel-stabilized foam | (a) Thickening agent (TA); (b) crosslinking agent (CA); (c) foaming agent (FA) composed of the surfactant compound. | (1) TA + CA + FA + water → uniform foaming solution; (2) gel-stabilized foam. | Not mentioned | The foam can form a dense covering film with an excellent oxygen barrier | Not mentioned | [95] |
| PE/SA-Ca | (a) Pectin (PE); (b) sodium alginate (SA); (c) calcium L-lactate (Ca-L); (d) biomass foaming systems (BS). | (1) 0.2 wt.% APG + 0.1 wt.% TS (stirring) → biomass foaming system BS; (2) Ca-L + water → Ca-L crosslinking agent solution; (3) PE solution + SA solution → mixed solution of SA/PE; (4) BS + SA/PE solution + Ca-L crosslinking agent solution (stirring fully) → PE/SA-Ca. | Maintains membrane integrity under high temperature. | The CO inhibition rate is 78.06% at 180 °C. | Within 20 min, the coal fire temperature was reduced from 960 °C to 68.9 °C, effectively preventing coal dust reignition. | [96] |
| CPSF | (a) Fly ash (FA); (b) sodium alginate (SA); (c) sodium dodecyl sulfate (SDS). | (1) FA particles+ SA solution→stable gel suspensions (SGS); (2) 15 parts of SDS+ 100 parts of water (stirring) → foaming solution; (3) SGS + foaming solution (stirred fully) → CPSF. | Not mentioned | Not mentioned | It adheres well to the coal particle surface, significantly delays the onset time of CO, and shows good inhibition performance. | [97] |
| Silica gel foam | (a) Foaming agent: sodium dodecyl sulfate (SDS); (b) gel agent: sodium polyacrylate; (c) crosslinking agent: konjac gum; (d) foam stabilizer: xanthan gum; (e) nanosilica; (f) antioxidant: tert-butyl hydroquinone (TBHQ); (g) modification reagent: montmorillonite. | (1) Foaming agent + foam stabilizer + crosslinking agent + gel agent → gel foam state; (2) acrylic acid + potassium persulfate → modified montmorillonite (O-MMT); (3) modified montmorillonite (cause free radical reaction) → antioxidant system; (4) gel foam state + antioxidant system (fully mixed) + nanoscale particles → silica gel foam. | Not mentioned | Within the range of 60 to 100 °C, the concentration of free radicals shows a marked downward trend. | Modified nanosilica particles and antioxidants can enhance the suppression efficiency of foam liquid films while improving their mechanical strength and stability. | [98] |
| Species | Raw Materials and Forms | Advantage | Disadvantage | Refs. |
|---|---|---|---|---|
| Polyurethane foam | Polyether polyols, isocyanates, diffusion crosslinkers, foaming agents, catalysts and flame retardants are expanded and cured according to a certain proportion. | Good viscoelasticity and stability, good sealing effect. | Polyurethane is a flammable substance, producing a lot of toxic smoke in case of fire. At the same time, the foaming reaction releases more heat, resulting in higher production costs. | [110,111,112]. |
| Phenolic foam | Using phenolic resin as a substrate, then adding curing agent and foaming agent, closed-cell foam material is formed after a chemical reaction, which is mainly used in filling and sealing wall construction in high-volume areas of coal mines. | It overcomes the disadvantages of large heat release and inflammability, low thermal conductivity, while having a short reaction time, good adiabatic performance and high expansion rate, and it can be used continuously at 140 °C–160 °C. | It is prone to breaking, and phenolic substances are toxic and can form carcinogens. The foam is brittle and powdery. Compared with polyurethane foam, the bonding force is weaker and the cost is higher. | [113,114,115,116]. |
| Urea–formaldehyde foam | It is a polymer foam material formed by chemical or physical foaming under the action of a foaming agent and hardener, with urea–formaldehyde resin as the base material. | Lightweight, high expansion ratio, no heat transfer, non-combustible and low reaction heat release temperature, low production cost, only half of the cost of phenolic foam. | Low strength, weak bearing capacity. Irritant gas is released during the reaction and pollutes the working environment. | [117,118,119]. |
| Cured polymer composite foam | Synthetic with various types of raw materials. | Depending on the material used and the preparation method, different composite foams have different characteristics. | The mechanical properties are different from those of conventional filling materials, and each material needs to be studied and analyzed. | [120,121]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Li, Y.; Li, H.; Bai, M.; Jing, Z. Preparation, Properties and Chemical Modification Methods of the Fire-Fighting Foam for Coal Spontaneous Combustion. Materials 2025, 18, 4888. https://doi.org/10.3390/ma18214888
Feng C, Li Y, Li H, Bai M, Jing Z. Preparation, Properties and Chemical Modification Methods of the Fire-Fighting Foam for Coal Spontaneous Combustion. Materials. 2025; 18(21):4888. https://doi.org/10.3390/ma18214888
Chicago/Turabian StyleFeng, Chenchen, Ying Li, Hua Li, Mengmeng Bai, and Zefeng Jing. 2025. "Preparation, Properties and Chemical Modification Methods of the Fire-Fighting Foam for Coal Spontaneous Combustion" Materials 18, no. 21: 4888. https://doi.org/10.3390/ma18214888
APA StyleFeng, C., Li, Y., Li, H., Bai, M., & Jing, Z. (2025). Preparation, Properties and Chemical Modification Methods of the Fire-Fighting Foam for Coal Spontaneous Combustion. Materials, 18(21), 4888. https://doi.org/10.3390/ma18214888