Study on Alkali-Activated Slag Mortar Based on Co-Modified Recycled Fine Aggregate with Nano-SiO2 and Sodium Silicate Integrating Waste Liquid Recycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Modification Process of RFA and Waste NMS Recycling Process
2.2.2. Mix Proportion and Sample Preparation
2.3. Test Methods for Mortar Properties
2.3.1. Fluidity Test
2.3.2. XRD Test
2.3.3. Capillary Water Absorption Test
2.3.4. Compressive Strength Test
2.3.5. Drying Shrinkage Test
2.3.6. Microstructure Characterization
3. Results and Discussions
3.1. Fluidity of Mortar
3.2. XRD Analysis
3.3. Capillary Water Absorption
3.4. Compressive Strength
3.5. Drying Shrinkage
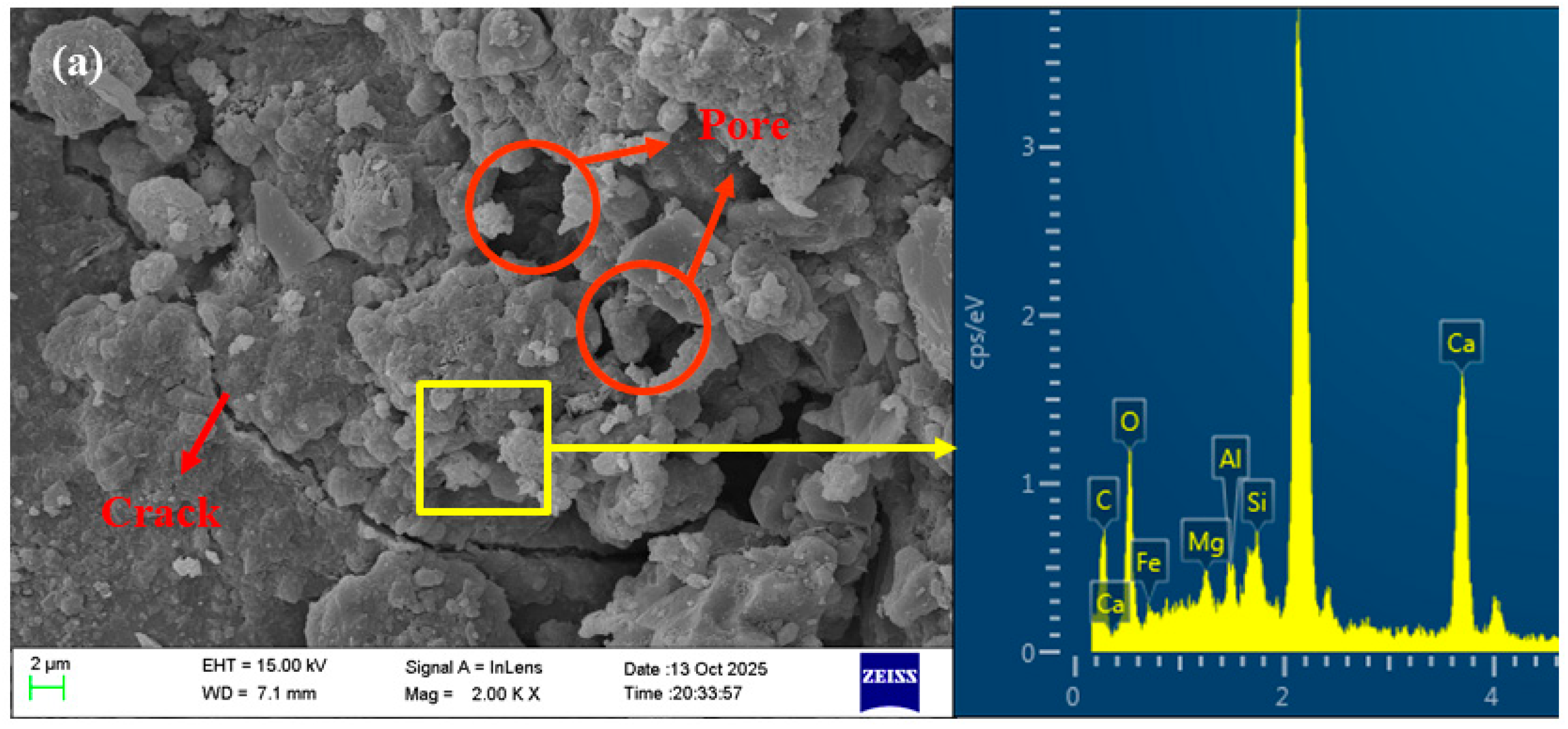
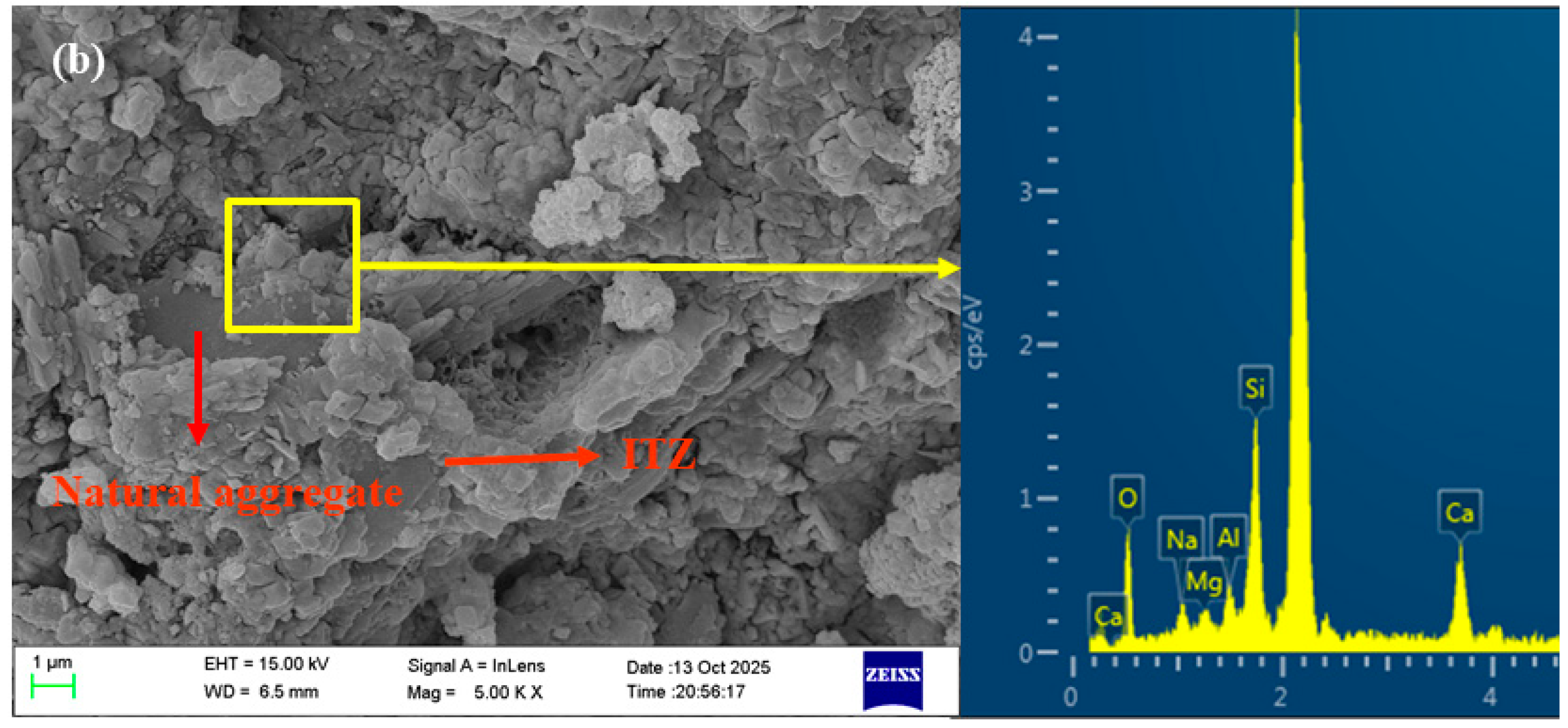
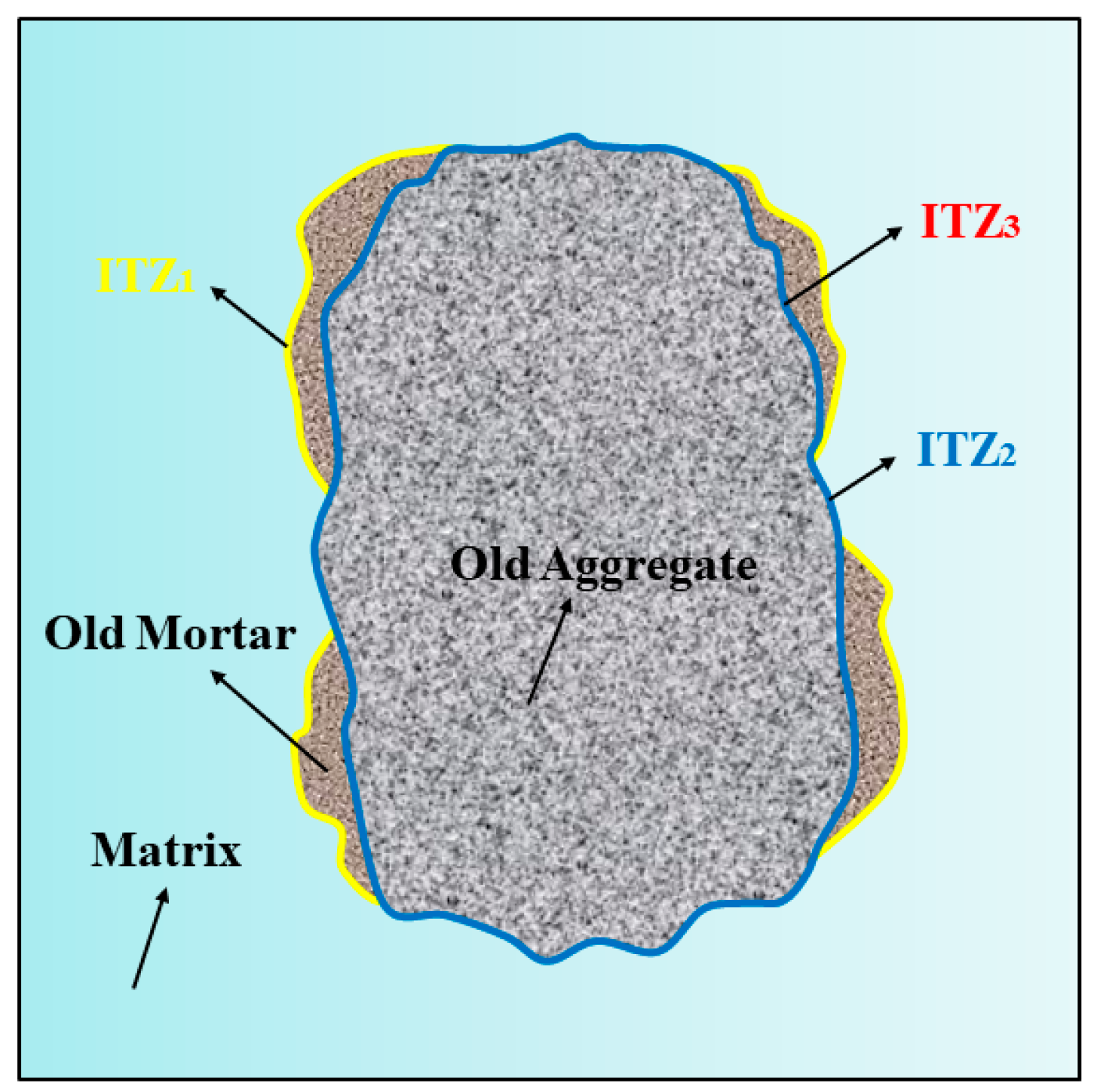
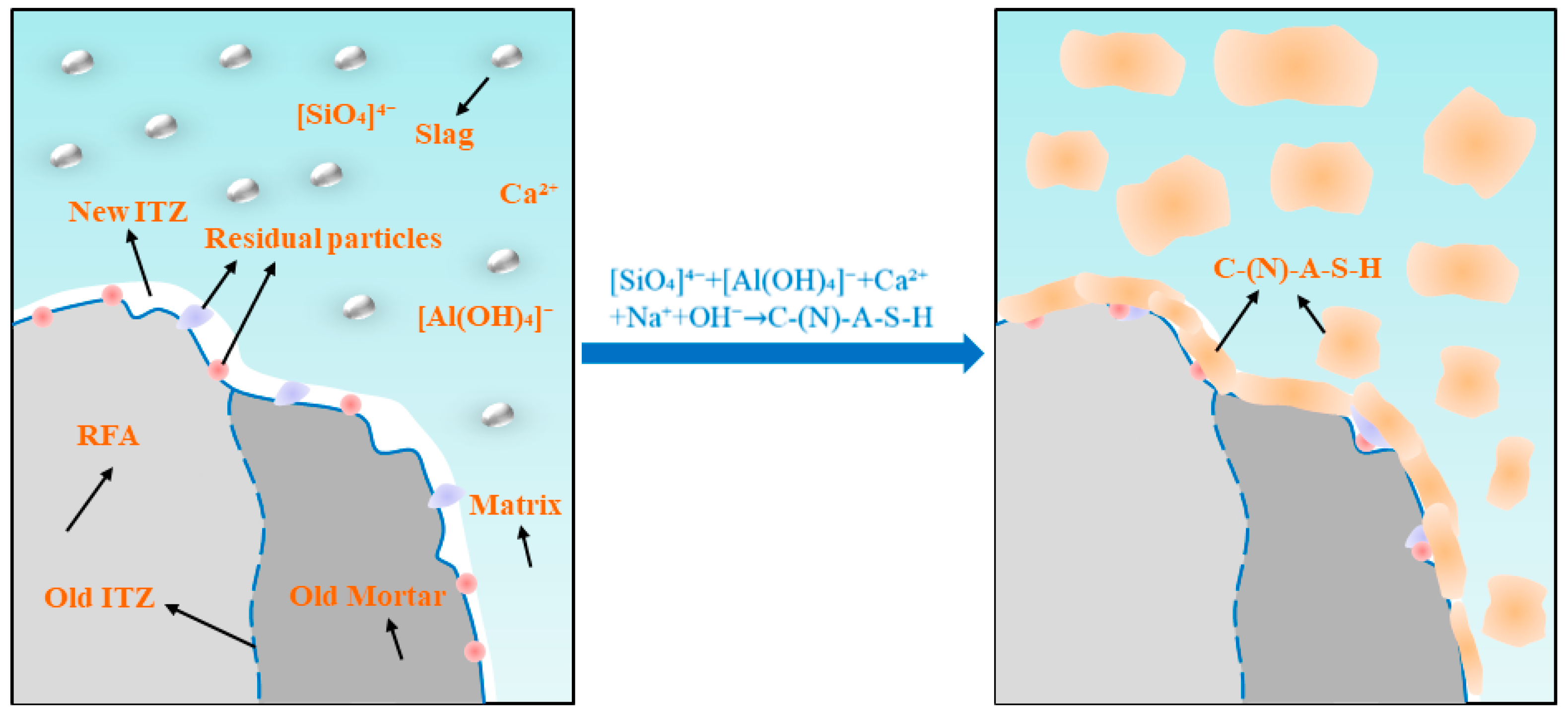
3.6. Microstructure Analysis
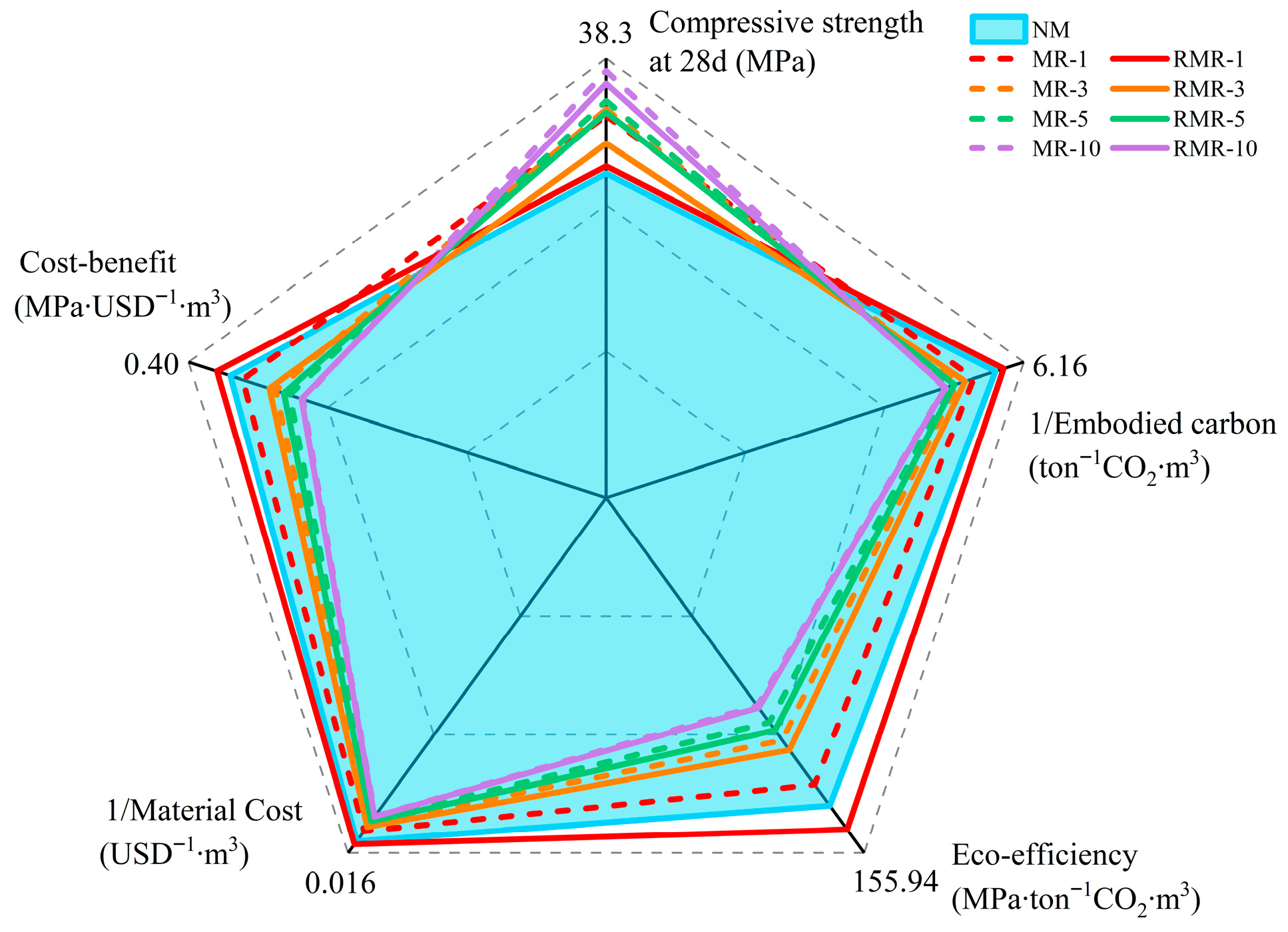
3.7. Economical and Environmental Analysis
4. Conclusions
- (1)
- Immersion modification of RFA using NMS significantly improved the overall performance of AASRMs. As the amount of MRFA added increased, the mortar’s workability improved significantly, while capillary water absorption and drying shrinkage were effectively suppressed, and the compressive strength was significantly enhanced. In particular, MR-10 achieved a 28-day compressive strength as high as 38.3 MPa, with capillary water absorption height and drying shrinkage strain reduced by 49.5% and 40.2%, respectively, compared to the control NM. This indicates that the incorporation of MRFA effectively remedied the defects of RFA, thereby improving the mechanical and durability properties of mortar.
- (2)
- The pores and cracks on the surface of the RFA were repaired through the filling effect of NS and the film-forming action of sodium silicate gel. This reduces the water absorption rate of MRFA and encapsulates loose microparticles to form a dense protective shell, thereby enhancing the stiffness of the aggregate. NS and sodium silicate can be transported into the RFA through microcracks, where they react with Ca2+ and Al3+ to form C-(N)-A-S-H gel, thereby strengthening the microstructure of the ITZ. Simultaneously, residual NS and sodium silicate particles on the surface of MRFA serve as nucleation sites, promoting the uniform growth and densification of C-(N)-A-S-H gel in the new ITZ, thus reinforcing the previously weak ITZ. This microstructure optimization effectively inhibited capillary water absorption and drying shrinkage and significantly improved the mechanical properties of the AASRMs.
- (3)
- Using RNMS as an alkali activator raw material can ensure performance advantages at different MRFA dosages. Although RMRs exhibit slightly slower early strength development and have slightly higher capillary water absorption and drying shrinkage strain than MRs with the same MRFA content, their performance is still far superior to that of the blank NM group, and their fluidity is basically consistent with that of MRs. This indicates that RNMS, as an alkali activator raw material, can effectively promote the alkali activation reaction of the matrix and maintain the excellent performance of AASRMs with MRFA incorporation.
- (4)
- From the perspectives of economics and the environment, although the incorporation of MRFA increases material costs and embodied carbon, the recycling and reuse of NMS significantly improve the eco-efficiency and cost-effectiveness of AASRMs. At low replacement rates, the overall benefits of RMR-1 surpassed those of the blank NM group, with the embodied carbon reduced by 11.9%. With the same amount of MRFA added, RMRs demonstrated better sustainability and higher cost-effectiveness than MRs. This confirms that the simultaneous use of RNMS and MRFA can enhance the performance of AASRMs while balancing economic and environmental benefits.
- (5)
- Based on the findings of this study, several key directions for future research are recommended to advance the field. First, it is essential to conduct a comprehensive investigation of the long-term durability of AASRMs prepared with recycled activators, including their performance under freeze–thaw cycles, sulfate attack, and long-term drying shrinkage. To deepen our understanding of these mechanisms, future studies should incorporate quantitative microstructural analyses. Although this study qualitatively links microstructural improvements to performance, techniques such as quantitative image analysis of backscattered electron (BSE-SEM) micrographs can be used to establish direct numerical correlations between key microstructural indicators (such as porosity or gel fraction from image analysis) and observed macroscopic performance. Second, the applicability of this NMS pretreatment method can be explored in other AAM systems, such as low-calcium fly ash-based geopolymers, in which the interaction mechanisms may differ. Finally, detailed techno-economic analyses and comprehensive life cycle assessments (LCA) will provide valuable data for quantifying the actual economic and environmental benefits of this integrated “modification–recycling” approach, paving the way for its potential industrial application.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RFA | Recycled fine aggregate |
| MRFA | Modified recycled fine aggregate |
| NMS | Nano-SiO2–sodium silicate mixed solution |
| RNMS | Recycled nano-SiO2–sodium silicate mixed solution |
| NS | Nano-silica |
| AASRM | Alkali-activated slag recycled fine aggregate mortar |
| C&DW | Construction and demolition waste |
| ITZ | Interfacial transition zone |
| NA | Natural aggregate |
| RA | Recycled aggregate |
| CH | Calcium hydroxide |
| C-S-H | Calcium–silicate–hydrate |
| C-(N)-A-S-H | Calcium–(sodium)–aluminate–silicate–hydrate |
| OPC | Ordinary Portland Cement |
| GGBS | Ground granulated blast-furnace slag |
| AAM | Alkali-activated material |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| Ms | Modulus |
References
- Lee, S.; Chang, H.; Lee, J. Construction and demolition waste management and its impacts on the environment and human health: Moving forward sustainability enhancement. Sustain. Cities Soc. 2024, 102, 105855. [Google Scholar] [CrossRef]
- Fecko, T.; Kormošová, L.; Ramos, A.P.; Halvonik, J. Punching shear behaviour of full-scale slab-column connections cast from concrete with coarse recycled aggregate. Eng. Struct. 2025, 345, 121460. [Google Scholar] [CrossRef]
- Youssef, O.F. Sustainable concrete with recycled brick and ceramic aggregates: A statistical validation and performance evaluation. Next Mater. 2025, 9, 101319. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Wang, Y.; Zhang, M. Roles of carbonated recycled fines and aggregates in hydration, microstructure and mechanical properties of concrete: A critical review. Cem. Concr. Compos. 2023, 138, 104994. [Google Scholar] [CrossRef]
- Hu, R.; Zhou, Y.; Xing, F. Strategies to Improve the Life Cycle Net CO2 Benefit of Recycled Aggregate Concrete. Engineering 2025, in press. [Google Scholar] [CrossRef]
- Ma, X.; Hu, H.; Luo, Y.; Yao, W.; Wei, Y.; She, A. A carbon footprint assessment for usage of recycled aggregate and supplementary cementitious materials for sustainable concrete: A life-cycle perspective in China. J. Clean. Prod. 2025, 490, 144772. [Google Scholar] [CrossRef]
- GB/T 25176-2010; Recycled fine aggregate for concrete and mortar. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2010.
- GB/T 25177-2010; Recycled coarse aggregate for concrete. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2010.
- Wang, J.; Feng, C.; Wang, Y.; Du, M.; Luo, W.; Zhao, X.; Zhang, W.; Zhu, J. Study on the method of reinforcing recycled fine aggregate by wrapping slag and cement powder. Constr. Build. Mater. 2025, 483, 141771. [Google Scholar] [CrossRef]
- Vahidi, A.; Mostaani, A.; Gebremariam, A.T.; Di Maio, F.; Rem, P. Feasibility of utilizing recycled coarse aggregates in commercial concrete production. J. Clean. Prod. 2024, 474, 143578. [Google Scholar] [CrossRef]
- Panghal, H.; Kumar, A. Enhancing concrete performance: Surface modification of recycled coarse aggregates for sustainable construction. Constr. Build. Mater. 2024, 411, 134432. [Google Scholar] [CrossRef]
- Deng, Q.; Zhuang, Y.; Nasr, A.; Liu, H.; Duan, Z. Assessing the effect of particle characteristics on the rheological properties of mortar with recycled fine aggregate. J. Build. Eng. 2025, 107, 112673. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Zhang, L.; Zeng, L.; Wang, X.; Dang, J. Influence of geometric shape, pore structure and surface modification of recycled fine aggregate on the rheology behaviour and strength development of mortar. J. Build. Eng. 2024, 91, 109604. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Faleschini, F.; Manso, J.M.; Ortega-López, V. Self-compacting concrete manufactured with recycled concrete aggregate: An overview. J. Clean. Prod. 2020, 262, 121362. [Google Scholar] [CrossRef]
- Li, X.; Tian, C.; Li, M.; Zhan, Q.; Wang, X.; Dong, W. Study on the Performance Enhancement of Recycled Fine Aggregate Through Carbonation with Calcium Source Supplied by Industrial Waste Residue. Materials 2025, 18, 1589. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, J.; Shi, C. Autogenous shrinkage and drying shrinkage of recycled aggregate concrete: A review. J. Clean. Prod. 2021, 295, 126435. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Wang, C.; Li, Y.; Fan, Y.; Liu, X.; Ding, Y.; Shen, W. Multi-parameter analysis of nano-SiO2 modified recycled aggregate concrete: Fatigue damage and microscopic mechanisms. Structures 2025, 79, 109414. [Google Scholar] [CrossRef]
- Rezaeicherati, F.; Memarzadeh, A.; Esmailpour, A.; Fallahnejad, H.; Ghorbanzadeh, A.; Nematzadeh, M. Durability evaluation and environmental implications of blended cement with colloidal nano-silica for use in recycled fine aggregate concrete: Experimental and theoretical study. Constr. Build. Mater. 2023, 402, 132926. [Google Scholar] [CrossRef]
- Rezaei, F.; Memarzadeh, A.; Davoodi, M.-R.; Dashab, M.-A.; Nematzadeh, M. Mechanical features and durability of concrete incorporating recycled coarse aggregate and nano-silica: Experimental study, prediction, and optimization. J. Build. Eng. 2023, 73, 106715. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, C.; Chiang, K.; Wang, X.; Zhu, Y.; Zhao, Z.; Luo, H. Influence of Nano-Silicon Dioxide in the Enhancement of Surface Structure of Public Filler and Properties of Recycled Mortar. Buildings 2024, 14, 2093. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, G.; Tu, Y.; Xiao, X.; Cheng, S.; Xu, X. Study on mechanical properties and microstructure of recycled fine aggregate concrete modified by Nano-SiO2. J. Mater. Cycles Waste Manag. 2023, 25, 2135–2145. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, J.; Lin, W.; Xu, W.; Hu, R. Creep and Shrinkage Properties of Nano-SiO2-Modified Recycled Aggregate Concrete. Materials 2024, 17, 1904. [Google Scholar] [CrossRef]
- Shahbazpanahi, S.; Tajara, M.K.; Faraj, R.H.; Mosavi, A. Studying the C–H Crystals and Mechanical Properties of Sustainable Concrete Containing Recycled Coarse Aggregate with Used Nano-Silica. Crystals 2021, 11, 122. [Google Scholar] [CrossRef]
- García-Taengua, E.; Sonebi, M.; Hossain, K.M.A.; Lachemi, M.; Khatib, J. Effects of the addition of nanosilica on the rheology, hydration and development of the compressive strength of cement mortars. Compos. Part B Eng. 2015, 81, 120–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, L.; Xing, F.; Lin, W.; Hu, R.; Guo, M.; Zhu, Z. Effect of nano-SiO2 modification on the seismic performance of recycled aggregate concrete shear walls. Eng. Struct. 2024, 307, 117945. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, W.; Lin, W.; Zhuang, J.; Xing, F.; Hu, R. Cyclic Behavior and Stress–Strain Model of Nano-SiO2-Modified Recycled Aggregate Concrete. Materials 2024, 17, 1180. [Google Scholar] [CrossRef]
- Zhan, P.; Xu, J.; Wang, J.; Zuo, J.; He, Z. A review of recycled aggregate concrete modified by nanosilica and graphene oxide: Materials, performances and mechanism. J. Clean. Prod. 2022, 375, 134116. [Google Scholar] [CrossRef]
- Kim, K.M.; Heo, Y.S.; Kang, S.P.; Lee, J. Effect of sodium silicate- and ethyl silicate-based nano-silica on pore structure of cement composites. Cem. Concr. Compos. 2014, 49, 84–91. [Google Scholar] [CrossRef]
- Li, L.; Xuan, D.; Sojobi, A.O.; Liu, S.; Chu, S.H.; Poon, C.S. Development of nano-silica treatment methods to enhance recycled aggregate concrete. Cem. Concr. Compos. 2021, 118, 103963. [Google Scholar] [CrossRef]
- Chen, W.Z.; Jiao, C.J.; Zhang, X.C.; Yang, Y.; Chen, X.F. Study on the microstructure of recycled aggregate concrete strengthened by the nano-SiO2 soaking method. Structures 2023, 58, 105388. [Google Scholar] [CrossRef]
- Zeng, W.; Zhao, Y.; Zheng, H.; Poon, C.S. Improvement in corrosion resistance of recycled aggregate concrete by nano silica suspension modification on recycled aggregates. Cem. Concr. Compos. 2020, 106, 103476. [Google Scholar] [CrossRef]
- Kumar, R.R. Evaluation of mechanical and carbonation properties of self-compacting treated recycled coarse aggregates with two-stage mixing approaches. Clean. Waste Syst. 2022, 3, 100060. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zuo, J.; Zhao, P.; Xie, X.; Li, S.; Lyu, K.; Wu, C.; Shah, S.P. Evaluation of Various Modification Methods for Enhancing the Performance of Recycled Concrete Aggregate. J. Renew. Mater. 2022, 10, 2685–2698. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Liu, R.; Lyu, K.; Zuo, J.; Li, S.; Liu, L.; Shah, S.P. Research on the durability of nano-SiO2 and sodium silicate co-modified recycled coarse aggregate (RCA) concrete. Constr. Build. Mater. 2023, 378, 131185. [Google Scholar] [CrossRef]
- Raza, M.H.; Khan, M.; Zhong, R.Y. Investigating the impact of alkaline activator on the sustainability potential of geopolymer and alternative hybrid materials. Mater. Today Sustain. 2024, 26, 100742. [Google Scholar] [CrossRef]
- Tejas, S.; Pasla, D. Approach to design sustainable alkali-activated slag recycled aggregate concrete: Mechanical and micro-structural characterization. Case Stud. Constr. Mater. 2024, 21, e03886. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Moranville-Regourd, M.; Kamali-Bernard, S. 10-Cements Made From Blastfurnace Slag. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 469–507. [Google Scholar]
- Mangat, P.; Lambert, P. 18-Sustainability of alkali-activated cementitious materials and geopolymers. In Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing: London, UK, 2016; pp. 459–476. [Google Scholar]
- Liu, C.; Li, Z.; Ye, G. Mitigating the efflorescence of alkali-activated slag mortars by aluminosilicate-based lightweight fine aggregate. Cem. Concr. Res. 2025, 195, 107917. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; de Matos, P.R.; Monteiro, S.N.; Vieira, C.M.F. Rheological and the Fresh State Properties of Alkali-Activated Mortars by Blast Furnace Slag. Materials 2021, 14, 2069. [Google Scholar] [CrossRef]
- Caldas, P.H.C.H.; de Azevedo, A.R.G.; Marvila, M.T. Silica fume activated by NaOH and KOH in cement mortars: Rheological and mechanical study. Constr. Build. Mater. 2023, 400, 132623. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mitigation strategies in alkali-activated slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of alkali dosage and silicate modulus on autogenous shrinkage of alkali-activated slag cement paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Tsai, C.J. Investigate the mechanical behavior of alkali-activated slag mortar using sustainable waste sodium silicate-bonded sand as an alkali activator and carbon free aggregate. Constr. Build. Mater. 2024, 429, 136448. [Google Scholar] [CrossRef]
- Fořt, J.; Mildner, M.; Keppert, M.; Abed, M.; Černý, R. Potential of industrial waste as alternative alkaline activator for development of eco-efficient mortars. Case Stud. Constr. Mater. 2023, 18, e01716. [Google Scholar] [CrossRef]
- JGJ/T 98-2010; Specification for Mix Proportion Design of Masonry Mortar. Ministry of Housing and Urban-Rural Develop-ment of China: Beijing, China, 2010.
- Shi, Z.; Shi, C.; Wan, S.; Zhang, Z. Effects of alkali dosage and silicate modulus on alkali-silica reaction in alkali-activated slag mortars. Cem. Concr. Res. 2018, 111, 104–115. [Google Scholar] [CrossRef]
- Xi, X.; Zheng, Y.; Zhuo, J.; Zhang, P.; Golewski, G.L.; Du, C. Influence of water glass modulus and alkali content on the properties of alkali-activated thermally activated recycled cement. Constr. Build. Mater. 2024, 452, 138867. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Durón-Sifuentes, M.; Díaz-Guillén, J.A.; Escalante-García, J.I. Effect of waste glass incorporation on the properties of geopolymers formulated with low purity metakaolin. Cem. Concr. Compos. 2020, 107, 103492. [Google Scholar] [CrossRef]
- Liu, M.; Hu, R.; Zhang, Y.; Wang, C.; Ma, Z. Effect of ground concrete waste as green binder on the micro-macro properties of eco-friendly metakaolin-based geopolymer mortar. J. Build. Eng. 2023, 68, 106191. [Google Scholar] [CrossRef]
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. General Administration of Quality Supervision, Inspection and Quarantine of PR China: Beijing, China, 2005.
- ASTM C1585; Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2020.
- JGJ/T 70-2009; Standard for Test Method of Performance on Building Mortar. Ministry of Construction of the People’s Republic of China: Beijing, China, 2009.
- Li, B.; Zhang, W.; Fang, X.; Kong, X.; Liu, F.; Li, G. Coupling effects of sewage sludge and recycled fine aggregate on the properties of geopolymer recycled mortars. Case Stud. Constr. Mater. 2024, 20, e03067. [Google Scholar] [CrossRef]
- Kumar, G.S. Influence of fluidity on mechanical and permeation performances of recycled aggregate mortar. Constr. Build. Mater. 2019, 213, 404–412. [Google Scholar] [CrossRef]
- Lu, D.; Qu, F.; Su, Y.; Cui, K. Nano-engineered the interfacial transition zone between recycled fine aggregates and paste with graphene oxide for sustainable cement composites. Cem. Concr. Compos. 2024, 154, 105762. [Google Scholar] [CrossRef]
- Luan, C.; Liu, J.; Zhao, S.; Li, Y.; Yang, Y. Enhancing the properties of UHPC with recycled concrete powder and iron ore tailings sand, and evaluating the environmental impact. Constr. Build. Mater. 2024, 452, 138769. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D. Effect of silica modulus on the geopolymerization activity of natural pozzolans. Constr. Build. Mater. 2019, 219, 31–43. [Google Scholar] [CrossRef]
- Gao, G.; Xu, H.; Bao, J.; Wang, Y.; Cui, Y.; Song, Q.; Sun, J. Durability-related properties of alkali-activated recycled aggregate concrete: Effect of alkali equivalent, recycled coarse aggregate replacement rate and slag content. Constr. Build. Mater. 2025, 486, 142018. [Google Scholar] [CrossRef]
- Yi, J.; Fu, S.; Huang, J.; Chen, Y. Synergistic modification of recycled aggregate using carbonation and hydrophobic Nano-Silica: Effect on interfacial performance of recycled aggregate concrete. J. Build. Eng. 2025, 107, 112740. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B. Study on the influence of nano-silica substitution of reactive Si on the properties and hydration process of alkali-activated cementitious materials paste. Constr. Build. Mater. 2024, 443, 137759. [Google Scholar] [CrossRef]
- Lyu, K.; Sun, B.; Liu, X.; Xie, X.; Liu, R. Evaluation of the ITZ modification efficiency via aggregate surface coating with nano SiO2 (NS) and its influence on properties. Case Stud. Constr. Mater. 2022, 17, e01488. [Google Scholar] [CrossRef]
- Yang, T.; Gong, L.; Jin, C.; Qin, J.; Dang, D.; Cui, X. Study on the pore characteristics and ITZ properties of recycled aggregate concrete by desert sand subjecting to salt freeze-thaw environments. J. Build. Eng. 2025, 108, 112918. [Google Scholar] [CrossRef]
- Lyu, K.; Garboczi, E.J.; She, W.; Miao, C. The effect of rough vs. smooth aggregate surfaces on the characteristics of the interfacial transition zone. Cem. Concr. Compos. 2019, 99, 49–61. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Zhang, B.; Chen, W.; Zhong, H.; Yang, J.; Yu, T.; Feng, Y. Effects of pretreated recycled fine aggregates on the mechanical properties and microstructure of alkali-activated mortar. Case Stud. Constr. Mater. 2024, 20, e02819. [Google Scholar] [CrossRef]
- ThéRéné, F.; Keita, E.; Naël-Redolfi, J.; Boustingorry, P.; Bonafous, L.; Roussel, N. Water absorption of recycled aggregates: Measurements, influence of temperature and practical consequences. Cem. Concr. Res. 2020, 137, 106196. [Google Scholar] [CrossRef]
- Adessina, A.; Fraj, A.B.; Barthélémy, J.-F.; Chateau, C.; Garnier, D. Experimental and micromechanical investigation on the mechanical and durability properties of recycled aggregates concrete. Cem. Concr. Res. 2019, 126, 105900. [Google Scholar] [CrossRef]
- Gomes, H.C.; da Silva Bezerra, A.C.; de Souza Rodrigues, C.; Poggiali, F.S.J.; Kim, B. Carbonation for enhancement of fine recycled aggregate applied to mortar. Clean. Waste Syst. 2025, 12, 100413. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Tam, V.W.Y.; Tan, J.; Shen, A.; Zhang, C.; Zhang, J. Feasibility of recycled aggregates modified with a compound method involving sodium silicate and silane as permeable concrete aggregates. Constr. Build. Mater. 2022, 361, 129747. [Google Scholar] [CrossRef]
- Luo, L.; Yao, W.; Liang, G.W.; Luo, Y. Workability, autogenous shrinkage and microstructure of alkali-activated slag/fly ash slurries: Effect of precursor composition and sodium silicate modulus. J. Build. Eng. 2023, 73, 106712. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, D.; Yan, Y.; Ren, G.; Liu, M. Synergistic effects of alkali content and silicate modulus optimization for enhanced properties in alkali-activated ultra-high performance concrete at ambient and high temperature. Case Stud. Constr. Mater. 2025, 22, e04606. [Google Scholar] [CrossRef]
- Xu, R.; Kong, F.; Yang, R.; Wang, H.; Hong, T. Influences of silicate modulus and alkali content on macroscopic properties and microstructure of alkali-activated blast furnace slag-copper slag. Constr. Build. Mater. 2024, 442, 137622. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Q.; Li, D.; Yu, Y.; Jiang, X.; Xu, J. Autogenous and drying shrinkage properties of precast recycled aggregate concrete. Case Stud. Constr. Mater. 2025, 22, e04355. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Huang, J.; Wang, Z.; Ma, S.; Liu, Y. Relationship between internal humidity and drying shrinkage of recycled aggregate thermal insulation concrete considering recycled aggregate content. Constr. Build. Mater. 2022, 355, 129224. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Zhang, P.; Lin, Q.; Chen, Z.; Qiu, R.; Zheng, X. Nano-SiO2 coating and silane modified bamboo cellulose nanofibers for alkali-activated slag mortar with recycled aggregate: Performance enhancement and mechanism. Constr. Build. Mater. 2025, 458, 139703. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y. Fracture properties and acoustic emission characteristics of manufactured sand recycled fine aggregate concrete. Theor. Appl. Fract. Mech. 2024, 133, 104633. [Google Scholar] [CrossRef]
- Pei, C.; Chen, P.; Tan, W.; Zhou, T.; Li, J. Effect of wet copper tailings on the performance of high-performance concrete. J. Build. Eng. 2023, 74, 106931. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Qian, X.; Wang, L.; Chen, P.; Qiao, P. A renewable admixture to enhance the performance of cement mortars through a pre-hydration method. J. Clean. Prod. 2022, 332, 130095. [Google Scholar] [CrossRef]
- Li, Q.-H.; Zhao, S.-Y.; Huang, B.-T.; Xu, L.-Y.; Xu, S.-L. Simultaneous enhancement of ductility and sustainability of high-strength Strain-Hardening Cementitious Composites (SHCC) using recycled fine aggregates. J. Clean. Prod. 2024, 470, 143357. [Google Scholar] [CrossRef]
- Xie, J.K.; Chen, P.Y.; Li, J.; Xu, Y.; Fang, Y.; Wang, A.G.; Wang, J.L. Directly upcycling copper mining wastewater into a source of mixing water for the preparation of alkali-activated slag materials. Process Saf. Environ. Prot. 2022, 168, 362–371. [Google Scholar] [CrossRef]
- Shi, J.Y.; Tan, J.X.; Liu, B.J.; Chen, J.Z.; Dai, J.D.; He, Z.H. Experimental study on full-volume slag alkali-activated mortars: Air-cooled blast furnace slag versus machine-made sand as fine aggregates. J. Hazard. Mater. 2021, 403, 123983. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, X.; Liu, R.; Lyu, K.; Zhu, Y.; Zuo, J.; Zhang, P.; Wu, C.; Shah, S.P. Optimization formulation of low carbon MSWIFA cement-based composites modified by nano SiO2. J. Build. Eng. 2023, 80, 108043. [Google Scholar] [CrossRef]

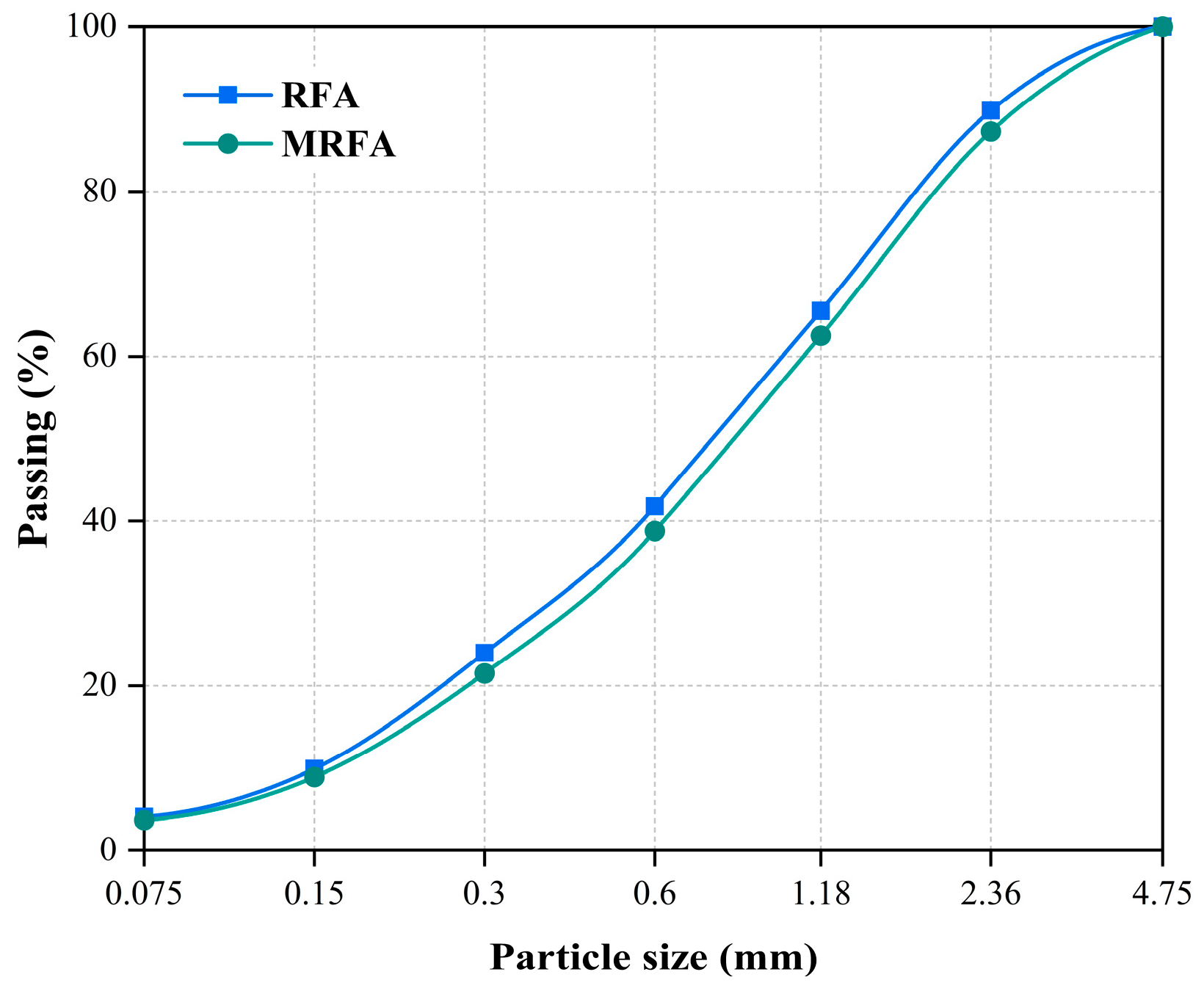
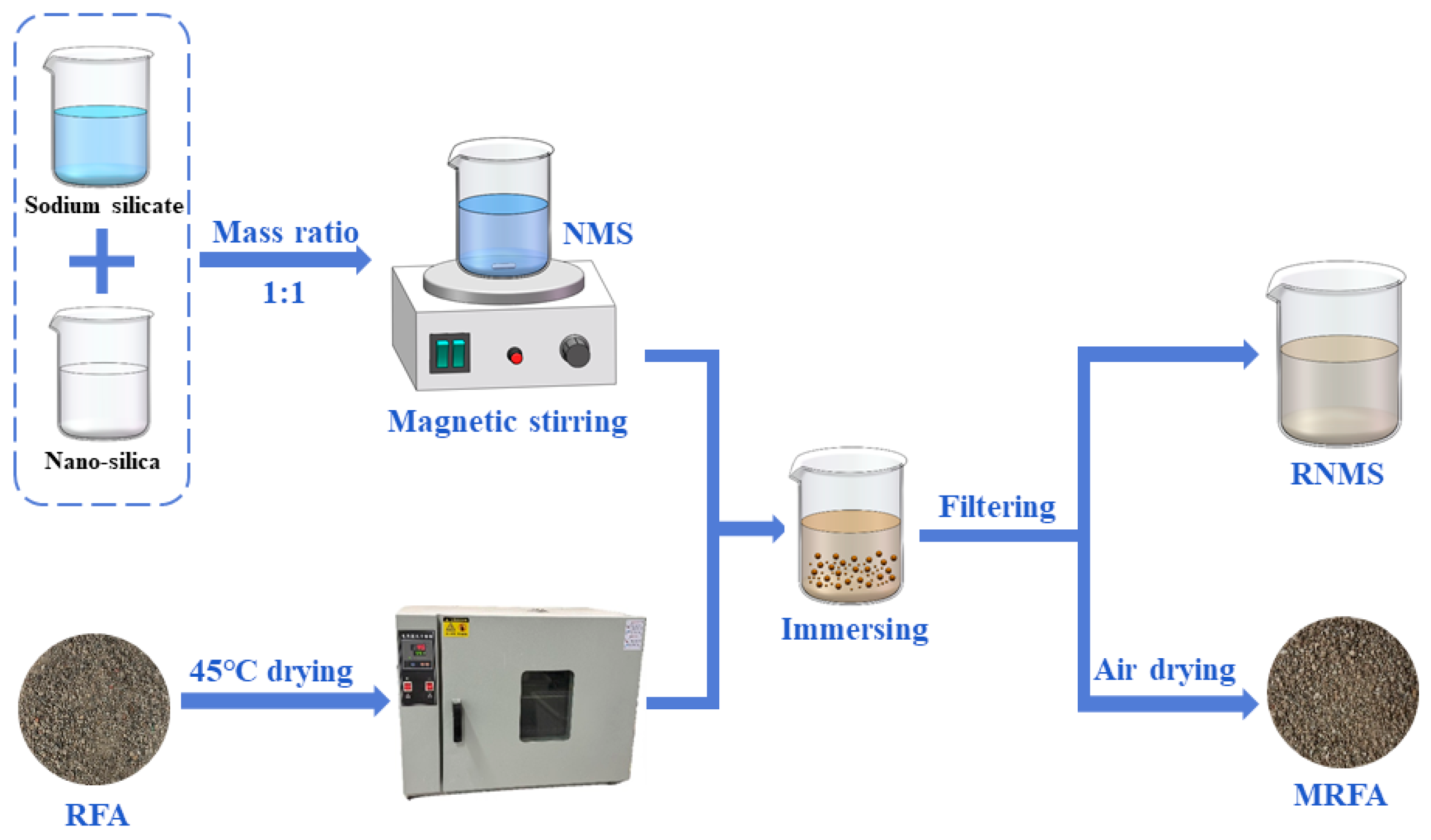

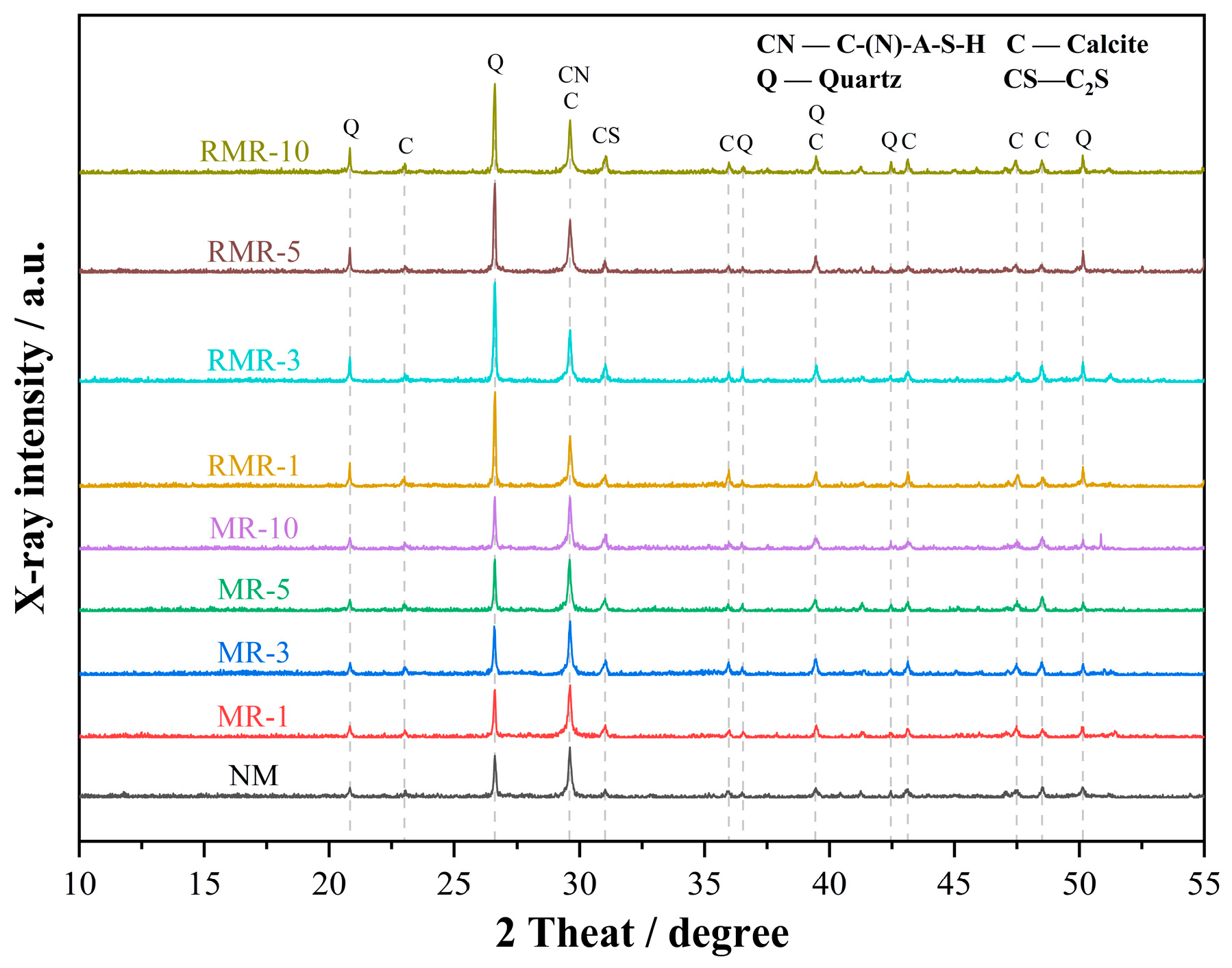





| CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Other |
|---|---|---|---|---|---|---|---|
| 45.06 | 26.71 | 16.34 | 7.80 | 0.52 | 2.32 | 0.36 | 0.89 |
| Type | Bulk Density (kg/m3) | Apparent Density (kg/m3) | Fineness Modulus | Maximum Particle Size (mm) | Water Absorption (%) |
|---|---|---|---|---|---|
| RFA | 1320 | 2385 | 2.69 | 2.5 | 8.86 |
| MRFA | 1297 | 2463 | 2.81 | 2.5 | 5.75 |
| Group | GGBS | Aggregate | Waterglass Solution | RNMS | NaOH | Additional Water | |
|---|---|---|---|---|---|---|---|
| RFA | MRFA | ||||||
| NM | 312 | 1320 | — | 69.2 | — | 17.5 | 234.9 |
| MR-1 | 312 | 1188 | 132 | 69.2 | — | 17.5 | 234.9 |
| MR-3 | 312 | 924 | 396 | 69.2 | — | 17.5 | 234.9 |
| MR-5 | 312 | 660 | 660 | 69.2 | — | 17.5 | 234.9 |
| MR-10 | 312 | — | 1320 | 69.2 | — | 17.5 | 234.9 |
| RMR-1 | 312 | 1188 | 132 | — | 138.3 | 17.5 | 165.8 |
| RMR-3 | 312 | 924 | 396 | — | 138.3 | 17.5 | 165.8 |
| RMR-5 | 312 | 660 | 660 | — | 138.3 | 17.5 | 165.8 |
| RMR-10 | 312 | — | 1320 | — | 138.3 | 17.5 | 165.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Wang, C.; Liu, J.; Liu, Q. Study on Alkali-Activated Slag Mortar Based on Co-Modified Recycled Fine Aggregate with Nano-SiO2 and Sodium Silicate Integrating Waste Liquid Recycling. Materials 2025, 18, 4889. https://doi.org/10.3390/ma18214889
Su Q, Wang C, Liu J, Liu Q. Study on Alkali-Activated Slag Mortar Based on Co-Modified Recycled Fine Aggregate with Nano-SiO2 and Sodium Silicate Integrating Waste Liquid Recycling. Materials. 2025; 18(21):4889. https://doi.org/10.3390/ma18214889
Chicago/Turabian StyleSu, Qiushi, Changbai Wang, Jimin Liu, and Qinghua Liu. 2025. "Study on Alkali-Activated Slag Mortar Based on Co-Modified Recycled Fine Aggregate with Nano-SiO2 and Sodium Silicate Integrating Waste Liquid Recycling" Materials 18, no. 21: 4889. https://doi.org/10.3390/ma18214889
APA StyleSu, Q., Wang, C., Liu, J., & Liu, Q. (2025). Study on Alkali-Activated Slag Mortar Based on Co-Modified Recycled Fine Aggregate with Nano-SiO2 and Sodium Silicate Integrating Waste Liquid Recycling. Materials, 18(21), 4889. https://doi.org/10.3390/ma18214889







