Activated Carbon and Diatomite as Filtration Materials for Nutrient Removal from Stormwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology for Testing GAC Properties
2.2. Methodology for Determining Nutrients
2.3. Methodology of Conducting Experiments
- Experiment 1 (exp.1): three rainfall simulations lasting 30 min and with a height of about 32 mm (at 1–2-day intervals)—comparison of the effectiveness of diatomite addition [7].
- Experiment 2 (exp.2): single rainfall simulation (120 min, 43 mm)—testing the effectiveness of the columns after a 3.5-month drought.
- Experiment 3 (exp.3): three rainfall simulations (120 min, 43 mm) (with an interval of 1–2 days)—testing the effectiveness of the columns under less intense rain than in experiment 1.
- Experiment 1 (exp.1)—intense rain for 30 min;
- Experiment 2 (exp.2)—less intense rain for 120 min after a drought period;
- Experiment 3 (exp.3)—less intense rain for 120 min;
- Experiment 4 (exp.4)—less intense rain for 120 min with enrichment of GAC filtration media.
- A height of 31.6 mm, 30 min of rainfall, rainfall intensity of 310 mL min−1, and a total inflow to the column of 9.3 L3;
- A height of 42.9 mm, 120 min of rainfall, a rainfall intensity of 105 mL min−1, and a total inflow to the column of 12.6 L3.
3. Results
3.1. Characteristics of GAC
3.2. Column Experiments Results
3.2.1. Effect of GAC Addition on Changes in Nutrient Concentrations
3.2.2. Nutrient Removal Efficiency—Influence of Sorbents, Drought, and Variable Rainfall Intensity
NH4+ Removal
- In column C1 with only sand, NH4+ was removed after the drought period (exp.2) at a similar level as in exp.3 and almost twice as high as in exp.1, which may suggest that rainfall intensity has a greater impact on NH4+ removal than drying of the filter mixture.
- In the columns with diatomite (C2 and C3), the differences in efficiency were not as significant, but the trends were reversed. After the drought period (exp.2), NH4+ removal was lower than in exp.1, and in subsequent simulations (exp.3), when the filter media were undried, NH4+ removal decreased.
- As mentioned above, in the column without diatomite, less intensive rainfall (exp.3: 105 mL min−1) was better purified (reduction 46%) than in experiments with high-intensity rain (exp.1: 310 mL min−1) (EMC reduction of 27%).
- In the columns with diatomite, such a tendency was not observed in C2; the average reduction for less intensive rainfall (exp.3) was 77%, while during 30 min rainfall (exp.1), it was 93%. In column C3, the decrease in reduction was not as significant, decreasing from 94 to 90%.
NO3− Removal
- In column 1, with only sand after the drought period, the NO3− concentration was higher than the inlet concentration, and comparing exp.2 with exp.1 shows that the effectiveness of NO3− removal decreased after the drought period.
- In the columns with diatomite (C2 and C3), comparing exp.2 to exp.1 showed an increase in NO3− removal efficiency, which may suggest that drying of filter media containing diatomite does not have an adverse effect on NO3− removal.
- In the experiments with high-intensity rain (exp.1: 310 mL min−1), no removal was observed; on the contrary, NO3− concentrations in the effluents were higher than those in synthetic rain. In the column without diatomite, the NO3− concentration increased by approximately 11% on average. The columns with diatomite, i.e., C2 and C3, also did not remove NO3−, and the increase in concentration was even greater: approximately 22% and 15%, on average, for C2 and C3, respectively.
- At lower rain intensity (exp.3: 105 mL min−1), NO3− reductions were observed, and the effluents from all columns had similarly reduced concentrations—6–8% in EMC. The influence of GAC addition on NO3− removal (Table 6, comparison of exp.3 and exp.4) is as follows:
- In the column without diatomite (C1), the addition of carbon (under low rainfall intensity conditions) significantly improved NO3− removal to an average level of about 39%.
- In the columns with diatomite (C2 and C3), no improvement in NO3− removal was observed due to the addition of GAC. NO3− concentrations significantly increased compared to inlet concentrations to 90 and 97%.
N Removal (The Sum of N-NO3− and N-NH4+)
PO43− Removal
- In column C1, GAC addition in exp.4 resulted in a PO43− reduction at around 23%, which was significantly lower than in exp.3 with the same precipitation parameters, where a reduction of 30% was achieved.
- In column C2, PO43− reduction was significantly lower at 61%, while in the other experiments, it was over 90%.
- In column C3, PO43− reduction was complete, but in the other experiments, it was also very high, at 97–98%.
4. Discussion
5. Conclusions
- The GAC additive did not improve phosphate removal in a column without diatomite, as evidenced by the results of experiment 4 for column C1. In the same experiment, also for column C1, it was observed that the presence of GAC caused a 2.4-fold deterioration in ammonium ion removal while increasing the nitrate ion removal efficiency by 6.5-fold.
- The combination of diatomite and GAC did not contribute to the removal of total nitrogen and phosphates. Nitrogen, considered as the sum of NO3− and NH4+ ions, was removed at the same level regardless of the addition of GAC. Phosphate removal using diatomite alone was very high (exp.3 for C2 and C3), while the addition of GAC caused a 1.5-fold deterioration in column performance, which was observed in C2 during exp.4. In the case of column C3 in exp.3, without GAC addition, almost complete phosphorus removal occurred up to 60 min, while the addition of GAC shortened this time to 30 min. Nevertheless, it was primarily diatomite that was responsible for the effective removal of phosphates, achieving an efficiency close to 100%.
- Our experiments confirmed the results of other researchers, suggesting that drought periods can impair nitrogen removal, as demonstrated in C1. Analysis of the impact of various rainfall scenarios showed that short-term, intense rainfall reduced pollutant removal efficiency, primarily due to reduced water contact time with the filter substrate. This effect was most noticeable for nitrogen, while phosphorus removal remained relatively stable due to sorption processes. Variants with sorption materials showed less sensitivity to variable hydraulic conditions.
- The change in rainfall intensity, tested in 30 min and 2 h variants, did not have a significant impact on phosphorus removal efficiency. Columns C2 and C3 were characterized by similarly high efficiency, while slightly worse results were observed in column C1. For nitrogen, an increase in removal efficiency was observed with less intense rainfall, which can be associated with a longer contact time of rainwater with the filtration material in the columns.
- Referring to the criteria described in the table in the introduction (Table 1), the columns studied by the authors meet criterion 2, and the others are as follows:
- Water retention—the focus was on water quality, not flow quantity.
- Pollutant removal:
- −
- Very good (>60%) for phosphorus (C2 and C3, exp.1–4);
- −
- Moderate (from 30 to 60%) for phosphorus (C1, exp.1 and exp.3) and nitrogen (C2 and C3, exp.1–3);
- −
- Poor (< 30%) for phosphorus (C1; exp.2 and exp.4) and nitrogen (C1, exp.1–4; C2, exp.4; and C3, exp.4).
- Safety (leaching) was not measured directly. In the future, the authors will focus on the mechanisms of nutrient removal. Based on their experiments, the authors conclude that using only GAC is safe and does not cause nutrient leaching. Combining both sorbents (GAC and diatomite) increases nitrate concentrations (exp.4, C2 and C3).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| ASAP | Accelerated Surface Area and Porosimetry system |
| BET | Brunauer–Emmett–Teller method |
| BJH | Barrett–Joyner–Halenda method |
| C1, C2, C3 | designations of columns |
| COD | chemical oxygen demand |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EMC | event mean concentration |
| exp.1 | experiment 1 described in this article |
| exp.2 | experiment 2 described in this article |
| exp.3 | experiment 3 described in this article |
| exp.4 | experiment 4 described in this article |
| GAC | granular activated carbon |
| ICDD | International Centre for Diffraction Data |
| ISA | Ionic Strength Adjuster |
| IUPAC | International Union of Pure and Applied Chemistry |
| LFF | Long Fine Focus |
| NBSs | Nature-Based Solutions |
| Powder Diffraction File | |
| SBAC | sludge-based activated carbon |
| SBET | surface areas calculated using the Brunauer–Emmett–Teller BET method |
| SEM | scanning electron microscope |
| TN | total nitrogen |
| TP | total phosphorus |
| XRD | X-ray diffraction |
References
- European Commission Nature-Based Solutions. Available online: https://research-and-innovation.ec.europa.eu/research-area/environment/nature-based-solutions_en (accessed on 7 July 2025).
- Kuller, M.; Bach, P.M.; Ramirez-Lovering, D.; Deletic, A. Framing Water Sensitive Urban Design as Part of the Urban Form: A Critical Review of Tools for Best Planning Practice. Environ. Model. Softw. 2017, 96, 265–282. [Google Scholar] [CrossRef]
- Bak, J.; Barjenbruch, M. Benefits, Inconveniences, and Facilities of the Application of Rain Gardens in Urban Spaces from the Perspective of Climate Change—A Review. Water 2022, 14, 1153. [Google Scholar] [CrossRef]
- Osheen; Singh, K.K. Rain Garden—A Solution to Urban: A Review. In Lecture Notes in Civil Engineering Sustainable Engineering Proceedings of EGRWSE 2018; Agnihotri, A.K., Bansal, A., Reddy, K., Eds.; Springer: Singapore, 2019; pp. 27–36. [Google Scholar]
- Nowogoński, I. Runoff Volume Reduction Using Green Infrastructure. Land 2021, 10, 297. [Google Scholar] [CrossRef]
- Jeon, M.; Guerra, H.B.; Choi, H.; Kwon, D.; Kim, H.; Kim, L.H. Stormwater Runoff Treatment Using Rain Garden: Performance Monitoring and Development of Deep Learning-Based Water Quality Prediction Models. Water 2021, 13, 3488. [Google Scholar] [CrossRef]
- Grela, A.; Łach, M.; Pamuła, J.; Łach, K.; Godyń, I.; Malina, D.; Wzorek, Z.; Setlak, K.; Grela, D. Effect of Diatomite Application on the Removal of Biogenic Pollutants in Rain Gardens. Materials 2024, 17, 6279. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Water Quality Improvement through Bioretention Media: Nitrogen and Phosphorus Removal. Water Environ. Res. 2006, 78, 284–293. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Laboratory Study of Biological Retention for Urban Stormwater Management. Water Environ. Res. 2001, 73, 5–14. [Google Scholar] [CrossRef]
- Ekanayake, D.; Loganathan, P.; Johir, M.A.H.; Kandasamy, J.; Vigneswaran, S. Enhanced Removal of Nutrients, Heavy Metals, and PAH from Synthetic Stormwater by Incorporating Different Adsorbents into a Filter Media. Water Air Soil. Pollut. 2021, 232, 96. [Google Scholar] [CrossRef]
- Hong, E.; Seagren, E.A.; Davis, A.P. Sustainable Oil and Grease Removal from Synthetic Stormwater Runoff Using Bench-Scale Bioretention Studies. Water Environ. Res. 2006, 78, 141–155. [Google Scholar] [CrossRef]
- Nocoń, W.; Moraczewska-Majkut, K.; Wiśniowska, E. Microplastics in Surface Water under Strong Anthropopression. Desalination Water Treat. 2018, 134, 174–181. [Google Scholar] [CrossRef]
- Johansson, G.; Fedje, K.K.; Modin, O.; Haeger-Eugensson, M.; Uhl, W.; Andersson-Sköld, Y.; Strömvall, A.M. Removal and Release of Microplastics and Other Environmental Pollutants during the Start-up of Bioretention Filters Treating Stormwater. J. Hazard. Mater. 2024, 468, 133532. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.E.; Fernandes, J.N.; David, L.M. Key Issues for Sustainable Urban Stormwater Management. Water Res. 2012, 46, 6787–6798. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, Harmful Algae and Biodiversity—Challenging Paradigms in a World of Complex Nutrient Changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Zhang, L.; Wang, W.; Guan, Y. Enhanced Nitrogen Removal and Mitigation of Nitrous Oxide Emission Potential in a Lab-Scale Rain Garden with Internal Water Storage. J. Water Process Eng. 2021, 42, 102147. [Google Scholar] [CrossRef]
- Smol, M.; Włóka, D. Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment. Sustainability 2022, 14, 6432. [Google Scholar] [CrossRef]
- Tian, J.; Jin, J.; Chiu, P.C.; Cha, D.K.; Guo, M.; Imhoff, P.T. A Pilot-Scale, Bi-Layer Bioretention System with Biochar and Zero-Valent Iron for Enhanced Nitrate Removal from Stormwater. Water Res. 2019, 148, 378–387. [Google Scholar] [CrossRef]
- Sharma, R.; Malaviya, P. Management of Stormwater Pollution Using Green Infrastructure: The Role of Rain Gardens. Wiley Interdiscip. Rev. Water 2021, 8, e1507. [Google Scholar] [CrossRef]
- Biswal, B.K.; Vijayaraghavan, K.; Adam, M.G.; Lee Tsen-Tieng, D.; Davis, A.P.; Balasubramanian, R. Biological Nitrogen Removal from Stormwater in Bioretention Cells: A Critical Review. Crit. Rev. Biotechnol. 2022, 42, 713–735. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, F.; Liu, Z.; Ai, L. Removal of Ammonia Nitrogen and Phosphorus by Biochar Prepared from Sludge Residue after Rusty Scrap Iron and Reduced Iron Powder Enhanced Fermentation. J. Environ. Manag. 2021, 282, 111970. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, M.; Huo, L.; Yang, P. Efficient Removal of Phosphorus from Constructed Wetlands Using Solidified Lanthanum/Aluminum Amended Attapulgite/Biochar Composite as a Novel Phosphorus Filter. Sci. Total Environ. 2022, 833, 155233. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, L.; Canga, E.; Hansen, H.C.B.; Kjærgaard, C.; Heckrath, G.J.; Poulsen, T.G. Long-Term Phosphorus Removal by Ca and Fe-Rich Drainage Filter Materials under Variable Flow and Inlet Concentrations. Water Res. 2023, 247, 120792. [Google Scholar] [CrossRef]
- Liu, A.; Jiang, Y.; Dockko, S.; Guan, Y. Characterizing Stormwater Treatment Efficiency at the Laboratory Scale for Effective Rain Garden Design. Desalination Water Treat. 2015, 54, 1334–1343. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Le, W.; You, C.; Liu, Z.; Liu, W.; Zhang, R. Field Performance of Rain Garden in Red Soil Area in Southern China. Water 2023, 15, 267. [Google Scholar] [CrossRef]
- Zhang, W.; Sang, M.; Sun, H.; Che, W.; Li, J. Influence of Rainfall on the Performance of Bioretention Systems Modified with Activated Carbon and Biochar. J. Hydro-Environ. Res. 2021, 38, 63–71. [Google Scholar] [CrossRef]
- Yang, H.; McCoy, E.L.; Grewal, P.S.; Dick, W.A. Dissolved Nutrients and Atrazine Removal by Column-Scale Monophasic and Biphasic Rain Garden Model Systems. Chemosphere 2010, 80, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.E.; Clausen, J.C. A Field Evaluation of Rain Garden Flow and Pollutant Treatment. Water Air Soil Pollut. 2005, 167, 123–138. [Google Scholar] [CrossRef]
- LeFevre, G.H.; Paus, K.H.; Natarajan, P.; Gulliver, J.S.; Novak, P.J.; Hozalski, R.M. Review of Dissolved Pollutants in Urban Storm Water and Their Removal and Fate in Bioretention Cells. J. Environ. Eng. 2015, 141, 04014050. [Google Scholar] [CrossRef]
- Ma, J.; Lenhart, J.H.; Tracy, K. Orthophosphate Adsorption Equilibrium and Breakthrough on Filtration Media for Storm-Water Runoff Treatment. J. Irrig. Drain. Eng. 2011, 137, 244–250. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Z.; Nie, Y.; Liu, H.; Li, P.; Yang, J.; Wu, B. Efficiently Optimized Multi-Fillers for Rain Gardens: Long-Term Pollution Control Performance. Water Cycle 2025, 6, 387–398. [Google Scholar] [CrossRef]
- Gilchrist, S.; Borst, M.; Stander, E.K. Factorial Study of Rain Garden Design for Nitrogen Removal. J. Irrig. Drain. Eng. 2014, 140, 04013016. [Google Scholar] [CrossRef]
- Strong, P.; Hudak, P.F. Nitrogen and Phosphorus Removal in a Rain Garden Flooded with Wastewater and Simulated Stormwater. Environ. Qual. Manag. 2015, 25, 63–69. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Evaluation of Biochar as a Potential Filter Media for the Removal of Mixed Contaminants from Urban Storm Water Runoff. J. Environ. Eng. 2014, 140, 04014043. [Google Scholar] [CrossRef]
- Xiong, J.; Ren, S.; He, Y.; Wang, X.C.; Bai, X.; Wang, J.; Dzakpasu, M. Bioretention Cell Incorporating Fe-Biochar and Saturated Zones for Enhanced Stormwater Runoff Treatment. Chemosphere 2019, 237, 124424. [Google Scholar] [CrossRef] [PubMed]
- Husna, T.; Aminuddin, A.G.; Nor Azazi, Z. The Impact of Stormwater Runoff on Nutrient Removal in Sand Columns. Appl. Mech. Mater. 2014, 567, 155–160. [Google Scholar] [CrossRef]
- Kus, B.; Kandasamy, J. Low-Cost Filtration System to Treat First-Flush Stormwater. Water Air Soil Pollut. Focus. 2009, 9, 347–355. [Google Scholar] [CrossRef]
- Yue, C.; Li, L.Y.; Johnston, C. Exploratory Study on Modification of Sludge-Based Activated Carbon for Nutrient Removal from Stormwater Runoff. J. Environ. Manag. 2018, 226, 37–45. [Google Scholar] [CrossRef]
- Wang, M.; Zhuang, J.; Sun, C.; Wang, L.; Zhang, M.; Fan, C.; Li, J. The Application of Rain Gardens in Urban Environments: A Bibliometric Review. Land 2024, 13, 1702. [Google Scholar] [CrossRef]
- Prochaska, C.A.; Zouboulis, A.I. Removal of Phosphates by Pilot Vertical-Flow Constructed Wetlands Using a Mixture of Sand and Dolomite as Substrate. Ecol. Eng. 2006, 26, 293–303. [Google Scholar] [CrossRef]
- Kang, J.; Davila, M.; Mireles, S.; Ho, J. Nitrate Leaching from Sand and Pumice Geomedia Amended with Pyrogenic Carbon Materials. Environments 2017, 4, 70. [Google Scholar] [CrossRef]
- Marvin, J.T.; Passeport, E.; Drake, J. State-of-the-Art Review of Phosphorus Sorption Amendments in Bioretention Media: A Systematic Literature Review. J. Sustain. Water Built Environ. 2020, 6, 03119001. [Google Scholar] [CrossRef]
- Czerniakowski, Z.W.; Gargała-Polar, M. Ogrody Deszczowe Jako Sposób Retardacji Strat Wody Opadowej w Terenach Zieleni Miejskiej. Pol. J. Sustain. Dev. 2020, 24, 17–24. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Stormwater to Street Trees: Engineering Urban Forests for Stormwater Management. 2013. Available online: https://www.epa.gov/sites/default/files/2015-11/documents/stormwater2streettrees.pdf (accessed on 16 July 2025).
- Jiang, C.; Wang, T.; Wu, X.; Dang, Z.; Li, H. Replacement Depth and Lifespan Prediction of Enhanced Bioretention Media under TSS Impact Conditions. Environ. Technol. 2025, 46, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, M.; Szpakowski, W.; Poznańska, E.; Boogaard, F.C.; Bobkowska, K.; Gajewska, M. Technical Solutions and Benefits of Introducing Rain Gardens—Gdańsk Case Study. Sci. Total Environ. 2022, 835, 155487. [Google Scholar] [CrossRef]
- Kravchenko, M.; Trach, Y.; Trach, R.; Tkachenko, T.; Mileikovskyi, V. Improving the Efficiency and Environmental Friendliness of Urban Stormwater Management by Enhancing the Water Filtration Model in Rain Gardens. Water 2024, 16, 1316. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Dosen, A.; Blanton, T.N. PDF-5+: A comprehensive Powder Diffraction FileTM for materials characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- PN-EN ISO 6878:2006; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. Polish Committee for Standardization (PKN): Warsaw, Poland, 2006.
- International Standards Water Quality-Determination of Phosphorus-Ammonium Molybdate Spectrometric Method. Available online: https://cdn.standards.iteh.ai/samples/36917/7f66bff84db146e398013e961ce7ab4c/ISO-6878-2004.pdf (accessed on 29 July 2025).
- Detektor Elektordy Laboratoryjne. Available online: http://www.detektor.biz/strony/elektrody_rodzaje.php (accessed on 18 July 2025).
- Elmetron Ph/Jonometr CPI-601. Available online: https://elmetron.com.pl/CPI-601.html (accessed on 18 July 2025).
- Elmetron Laboratoryjny Przyrząd Wielofunkcyjny CX-505. Available online: https://elmetron.com.pl/CX-505.html (accessed on 18 July 2025).
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural Bio-Waste Materials as Potential Sustainable Precursors Used for Activated Carbon Production: A Review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Abdullah, M.O.; Lim, L.L.P.; Yeo, T.H.C. Surface Modification and Characterization of Coconut Shell-Based Activated Carbon Subjected to Acidic and Alkaline Treatments. J. Appl. Sci. Process Eng. 2017, 4, 186–194. [Google Scholar]
- Huang, P.H.; Cheng, H.H.; Lin, S.H. Adsorption of Carbon Dioxide onto Activated Carbon Prepared from Coconut Shells. J. Chem. 2015, 2015, 106590. [Google Scholar] [CrossRef]
- Yang, J.; Han, S. Kinetics and Equilibrium Study for the Adsorption of Lysine on Activated Carbon Derived from Coconut Shell. Desalination Water Treat. 2018, 120, 261–271. [Google Scholar] [CrossRef]
- Asada, T.; Oikawa, K.; Kawata, K.; Ishihara, S.; Iyobe, T.; Yamada, A. Study of Removal Effect of Bisphenol A and .BETA.-Estradiol by Porous Carbon. J. Health Sci. 2004, 50, 588–593. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Novel Activation Process for Preparing Highly Microporous and Mesoporous Activated Carbons. Carbon 2001, 39, 877–886. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.Y.; Liang, Y.; Chen, Q.; Li, Z.S.; Li, C.H.; Wang, Z.H.; Li, W. Coconut-Based Activated Carbon Fibers for Efficient Adsorption of Various Organic Dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- ‘Micropore’ in IUPAC Compendium of Chemical Terminology, 5th ed.; International Union of Pure and Applied Chemistry: Durham, NC, USA, 2025; Online version 5.0.0, 2025. [CrossRef]
- Commission Directive 2009/90/EC of 31 July 2009 Laying Down, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, Technical Specifications for Chemical Analysis and Monitoring of Water Status. Available online: https://eur-lex.europa.eu/eli/dir/2009/90/oj/eng (accessed on 5 August 2025).
- Bratieres, K.; Fletcher, T.D.; Deletic, A.; Zinger, Y. Nutrient and Sediment Removal by Stormwater Biofilters: A Large-Scale Design Optimisation Study. Water Res. 2008, 42, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Wystalska, K.; Grosser, A. Sewage Sludge-Derived Biochar and Its Potential for Removal of Ammonium Nitrogen and Phosphorus from Filtrate Generated during Dewatering of Digested Sludge. Energies 2024, 17, 1310. [Google Scholar] [CrossRef]
- Laurenson, G.; Laurenson, S.; Bolan, N.; Beecham, S.; Clark, I. The Role of Bioretention Systems in the Treatment of Stormwater. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 120, pp. 223–274. [Google Scholar]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydraulic and Pollutant Removal Performance of Stormwater Filters under Variable Wetting and Drying Regimes. Water Sci. Technol. 2007, 56, 11–19. [Google Scholar] [CrossRef]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydrologic and Pollutant Removal Performance of Stormwater Biofiltration Systems at the Field Scale. J. Hydrol. 2009, 365, 310–321. [Google Scholar] [CrossRef]
- Kim, H.; Seagren, E.A.; Davis, A.P. Engineered Bioretention for Removal of Nitrate from Stormwater Runoff. Water Environ. Res. 2003, 75, 355–367. [Google Scholar] [CrossRef]
- Søberg, L.C.; Viklander, M.; Blecken, G.T. Nitrogen Removal in Stormwater Bioretention Facilities: Effects of Drying, Temperature and a Submerged Zone. Ecol. Eng. 2021, 169, 106302. [Google Scholar] [CrossRef]
- Yu, S.; He, K.; Xia, C.; Qin, H. Modeling the Effect of the Submerged Zone on Nitrogen Removal Efficiency of a Bioretention System under Dry-Wet Alterations. J. Hydrol. 2023, 623, 129788. [Google Scholar] [CrossRef]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Ergas, S.J.; Mihelcic, J.R. Long-Term Field Performance of a Conventional and Modified Bioretention System for Removing Dissolved Nitrogen Species in Stormwater Runoff. Water Res. 2020, 170, 115336. [Google Scholar] [CrossRef] [PubMed]

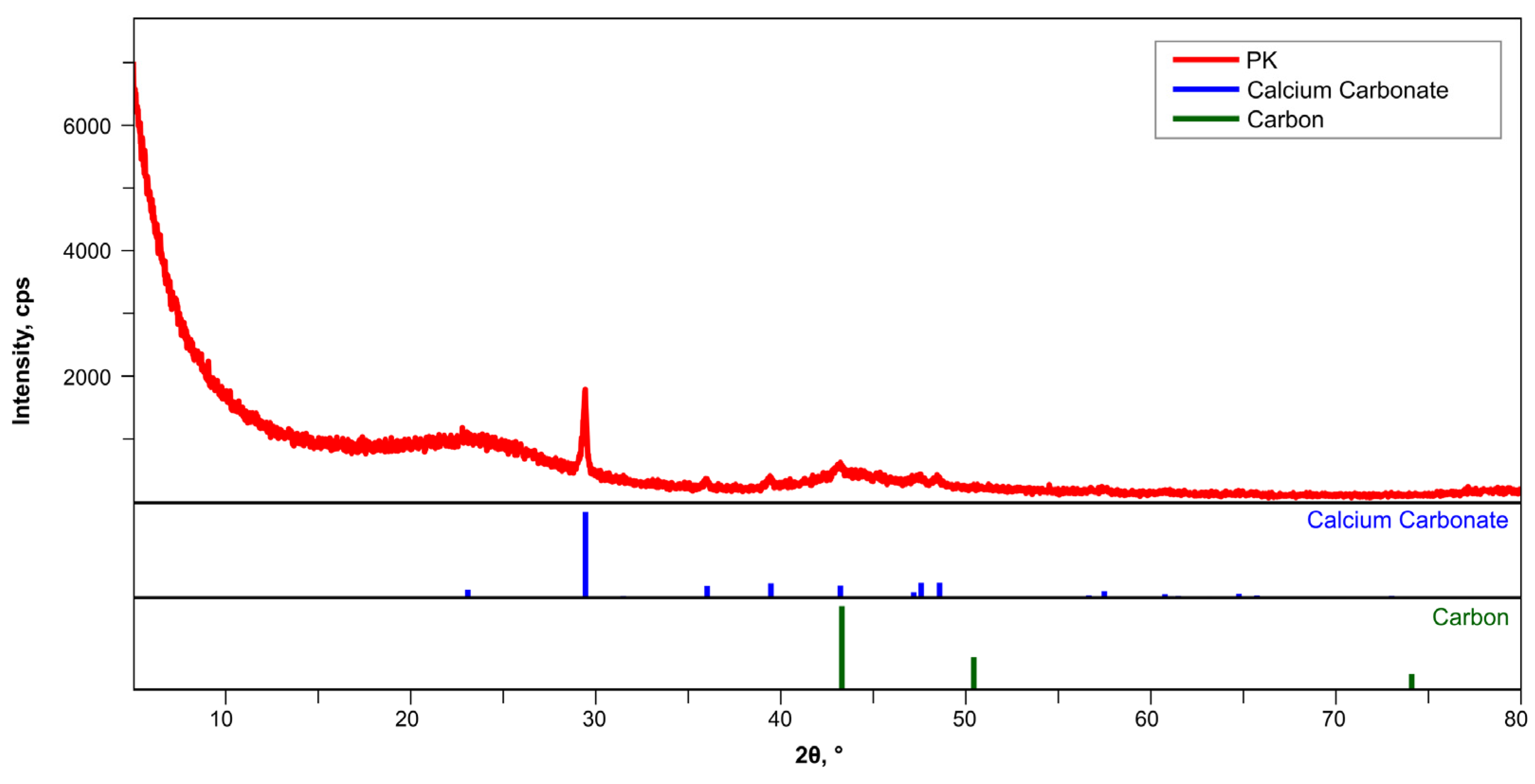

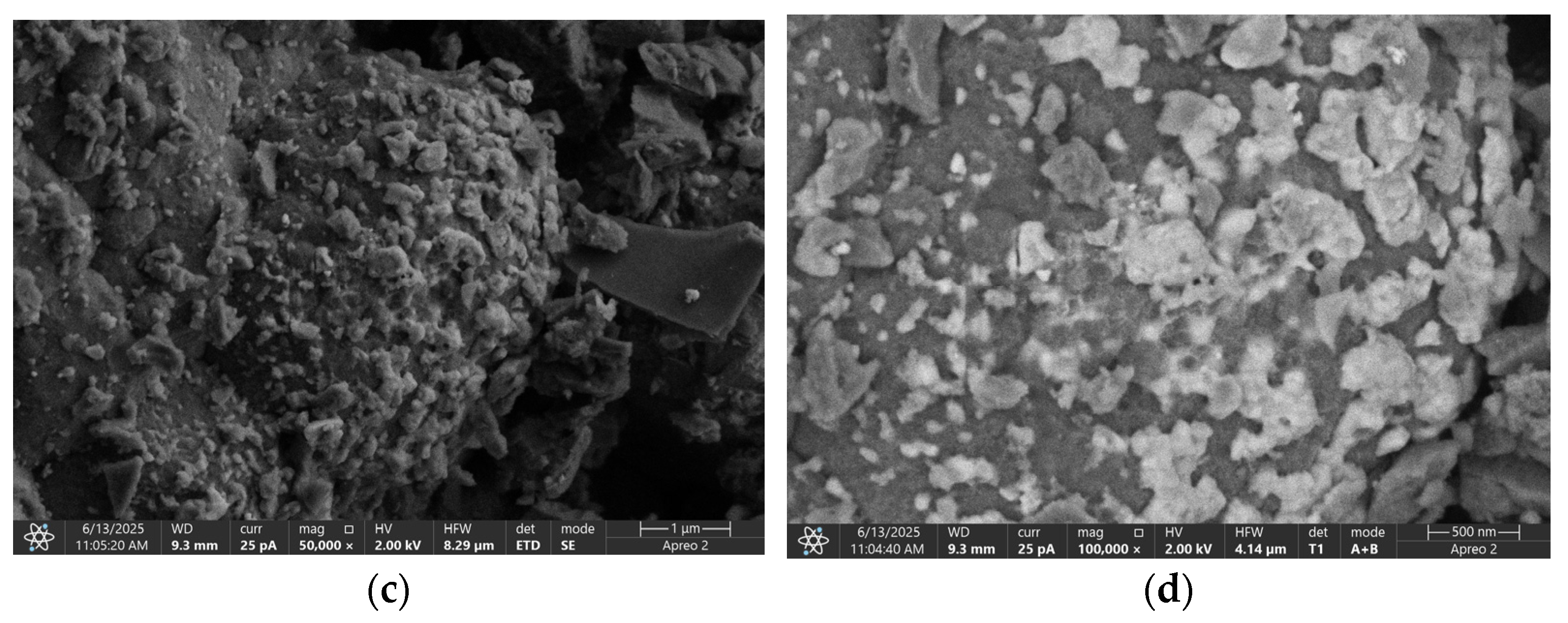
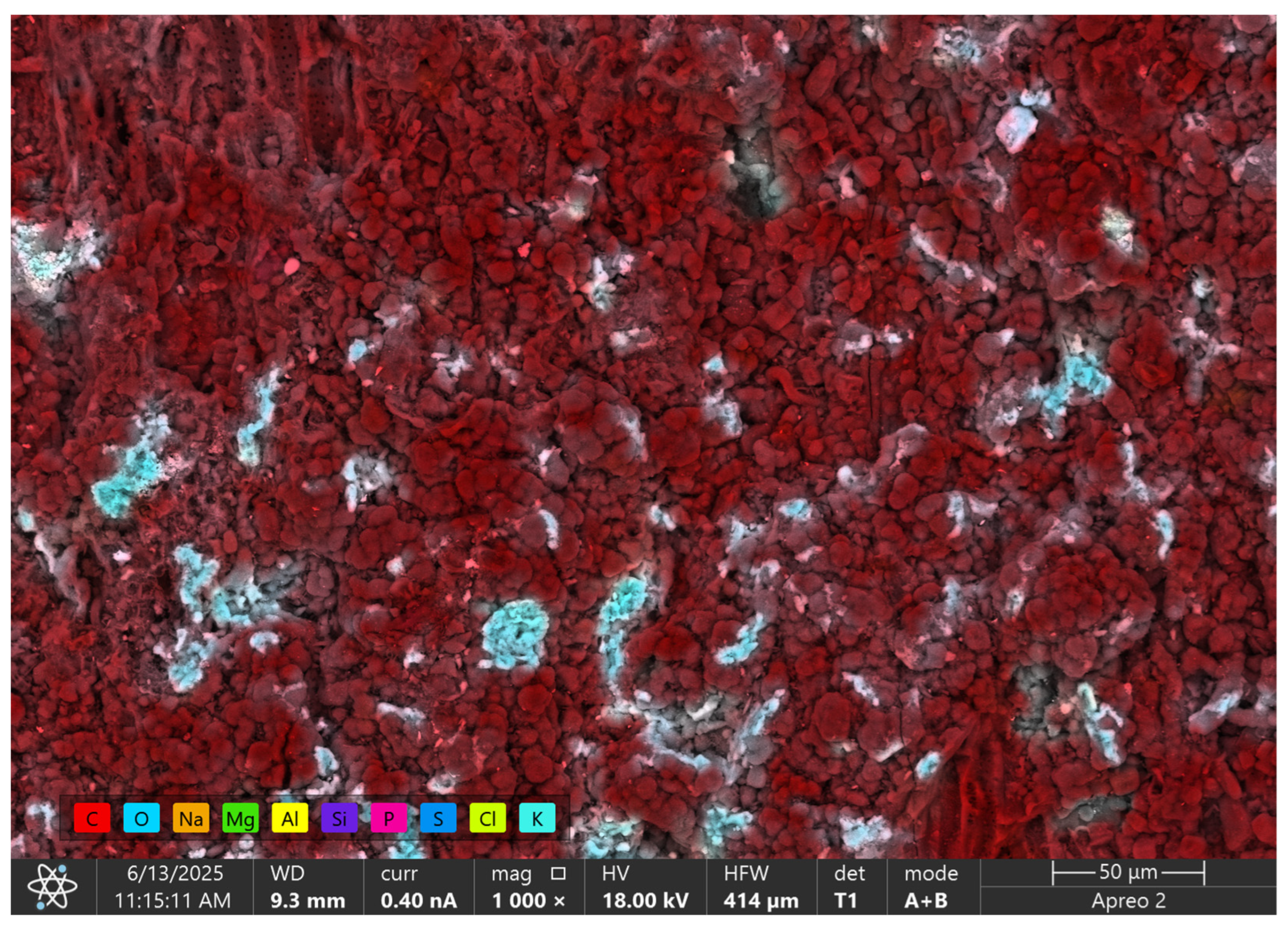



| No. | Material | Criteria | References | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| 1 | gravel (20 cm), river sand (33 cm), soil (33 cm) | yes | yes | yes | [8] |
| 2 | mixture of sand to soil medium consists of 90.6% sand, 6.9% silt, and 2.5% clay with 0.7% organic matter | no | yes | no | [28] |
| 3 | gravel + mixture of river sand and dolomite (10:1, w/w) | N/A | yes | N/A | [41] |
| 4 | dolomite—20 cm, sand and diatomite (2:1)—45 cm, gravel—5 cm | no | yes | no | [7] |
| 5 | soil (40 cm), sand (40 cm), and bottom ash (40 cm) | yes | yes | yes | [6] |
| 6 | sandy loam soil, mulch | no | yes | no | [9] |
| 7 | mulch (bark) 50 mm, soil 250 mm (clay content of red soil was above 40%), sand filter 100 mm, gravel drainage 200 mm (the particle size of the gravel was 20–30 mm) | yes | yes | yes | [26] |
| 8 | 8–27 cm layer of 11 types of fillers, including 5 natural materials, 3 industrial wastes, and 3 artificial materials | yes | yes | yes | [32] |
| 9 | gravel (30 cm), soil with 25% expanded shale aggregate (50 cm), hardwood mulch (10 cm) | yes | yes | no | [34] |
| 10 | 3 cm gravel, 20 cm sand, and 5 cm tea leaf waste or wood chips or coconut husk | yes | yes | N/A | [37] |
| 11 | pea gravel + sandy media (90% sand and 10% Sphagnum peat moss) + wood chips (20 cm) + sandy media | yes | yes | yes | [33] |
| 12 | gravel, fine sand, sorption material: sand, biochar, Sphagnum peat or ash, sandy loam mixed with pumice stone and a sorption material, sandy loam mixed with pumice stone | yes | yes | no | [13] |
| 13 | biochar 22.9 cm + pea gravel 2 × 7.6 cm | N/A | yes | N/A | [35] |
| 14 | mix: gravel + sand + soil + 4% rice husk biochar or iron-coated biochar | N/A | yes | N/A | [36] |
| 15 | gravel (10 cm), sand with 5% woodchips and 5% sugarcane bagasse (V/V) (20 cm), soil with 10% biochar (V/V) (40 cm), 5–10 cm crumb of pine bark | yes | yes | yes | [17] |
| 16 | mix: sand + pumice + activated char or biochar | yes | yes | N/A | [42] |
| 17 | gravel (10 cm), media layer (70 cm): 94% garden soil + 6% activated carbon or biochar in the upper or biochar in the lower | yes | yes | yes | [27] |
| 18 | gravel (5 cm), media layer (2.5 cm): silica sand, gravel, zeolite, activated carbon, or slag, sail layer (22.5 cm), bark (12.5 cm) | N/A | yes | yes | [25] |
| 19 | GAC, zeolite, an engineered media | N/A | yes | N/A | [31] |
| 20 | mix: soil + 0.3% GAC | N/A | yes | N/A | [10] |
| 21 | mix: GAC + sand (1:1 and 1:10) | N/A | yes | N/A | [38] |
| 22 | SBAC—sludge-based activated carbon | no | yes | no | [39] |
| Nitrate Electrode | Ammonium Electrode | |
|---|---|---|
| Detection range | 0.6–6000 mg L−1 | 0.9–9000 mg L−1 |
| Operating temperature range | 0–40 °C | 0–40 °C |
| Required solution pH | 2–12 | 1–8 |
| Half-cell | Ag/AgCl | Ag/AgCl |
| Reference electrode | Chloride-selective with a double junction filled with 1 M L−1 KCl and 1 M L−1 (NH4)2SO4 | Chloride-selective with a double junction filled with 1 M L−1 KCl and 0.1 M L−1 CH3COOLi |
| Required ISA ionic strength buffer | Yes (2 mL/100 mL sample) | Yes (25 mL/25 mL sample) |
| Interfering ions | K+, Rb+, H+, Cs+, Li+, Na+ | ClO4− |
| Sample | BET Surface (SBET, m2 g−1) | Total Pore Volume (Vtotal, cm3 g−1) | Average Pore Diameter (DA, nm) |
|---|---|---|---|
| Activated carbon | 872.77 | 0.415 | 1.903 |
| Element | C | O | Na | Mg | Al | Si | P | S | Cl | K |
|---|---|---|---|---|---|---|---|---|---|---|
| Atomic % | 83.1 | 13.5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 2.5 |
| Experiment | C1 | C2 | C3 |
|---|---|---|---|
| exp.1 | 27% | 93% | 94% |
| exp.2 | 43% | 84% | 92% |
| exp.3 | 46% | 77% | 90% |
| exp.4 | 18% | 44% | 33% |
| Experiment | C1 | C2 | C3 |
|---|---|---|---|
| exp.1 | −11% | −22% | −15% |
| exp.2 | −20% | 4% | 0% |
| exp.3 | 6% | 8% | 7% |
| exp.4 | 39% | −90% | −97% |
| Amount of GAC Additive | N Removal (%) | P Removal (%) | Reference |
|---|---|---|---|
| 1 g L−1 leachate | 72% NO3-N (initial concentration 2 mg L−1), synthetic rain from distilled water enriched with nutrients | 41% PO4-P (initial concentration 1 mg L−1), synthetic rain from distilled water enriched with nutrients | [39] |
| 1 g L−1 leachate | 38% for NO3-N leachate from stormwater | 20% for PO4-P leachate from stormwater | |
| 3 g L−1 leachate | 72% for NO3-N leachate from stormwater | 31% for PO4-P leachate from stormwater | |
| 18 cm horizontal column completely filled with GAC | not examined | 33% | [31] |
| 20 g L−1 in synthetic model solution | 17% removal from synthetic model solution | 86% removal from synthetic model solution | [65] |
| two experiments: GAC and sand (ratio of 1:1), GAC and sand (ratio of 1:10) | 7–14% TN | 11% TP | [38] |
| 2.5 cm GAC layer | 72–85% TN | 60–92% TP | [25] |
| 10.2 cm GAC layer | not examined | 20–30% | [35] |
| 70 cm filtration layer with 6% GAC | 22–52% TN 55–72% NH4+-N 16–44% NO3−-N | 60–82% TP 60–82% PO43−-P | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grela, A.; Pamuła, J.; Łach, K.; Godyń, I.; Malina, D.; Grela, D. Activated Carbon and Diatomite as Filtration Materials for Nutrient Removal from Stormwater. Materials 2025, 18, 4742. https://doi.org/10.3390/ma18204742
Grela A, Pamuła J, Łach K, Godyń I, Malina D, Grela D. Activated Carbon and Diatomite as Filtration Materials for Nutrient Removal from Stormwater. Materials. 2025; 18(20):4742. https://doi.org/10.3390/ma18204742
Chicago/Turabian StyleGrela, Agnieszka, Justyna Pamuła, Karolina Łach, Izabela Godyń, Dagmara Malina, and Damian Grela. 2025. "Activated Carbon and Diatomite as Filtration Materials for Nutrient Removal from Stormwater" Materials 18, no. 20: 4742. https://doi.org/10.3390/ma18204742
APA StyleGrela, A., Pamuła, J., Łach, K., Godyń, I., Malina, D., & Grela, D. (2025). Activated Carbon and Diatomite as Filtration Materials for Nutrient Removal from Stormwater. Materials, 18(20), 4742. https://doi.org/10.3390/ma18204742








