Influence of Initiator Content and Polymerization Conditions on the Properties of Polyacrylate Mortar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportion and Mixing Procedure
2.3. Experimental Program and Testing Methods
2.3.1. Polymerization Time Experiment
2.3.2. Low-Temperature Polymerization Test
2.3.3. Mechanical Tests
2.3.4. SEM Analysis
3. Results and Discussion
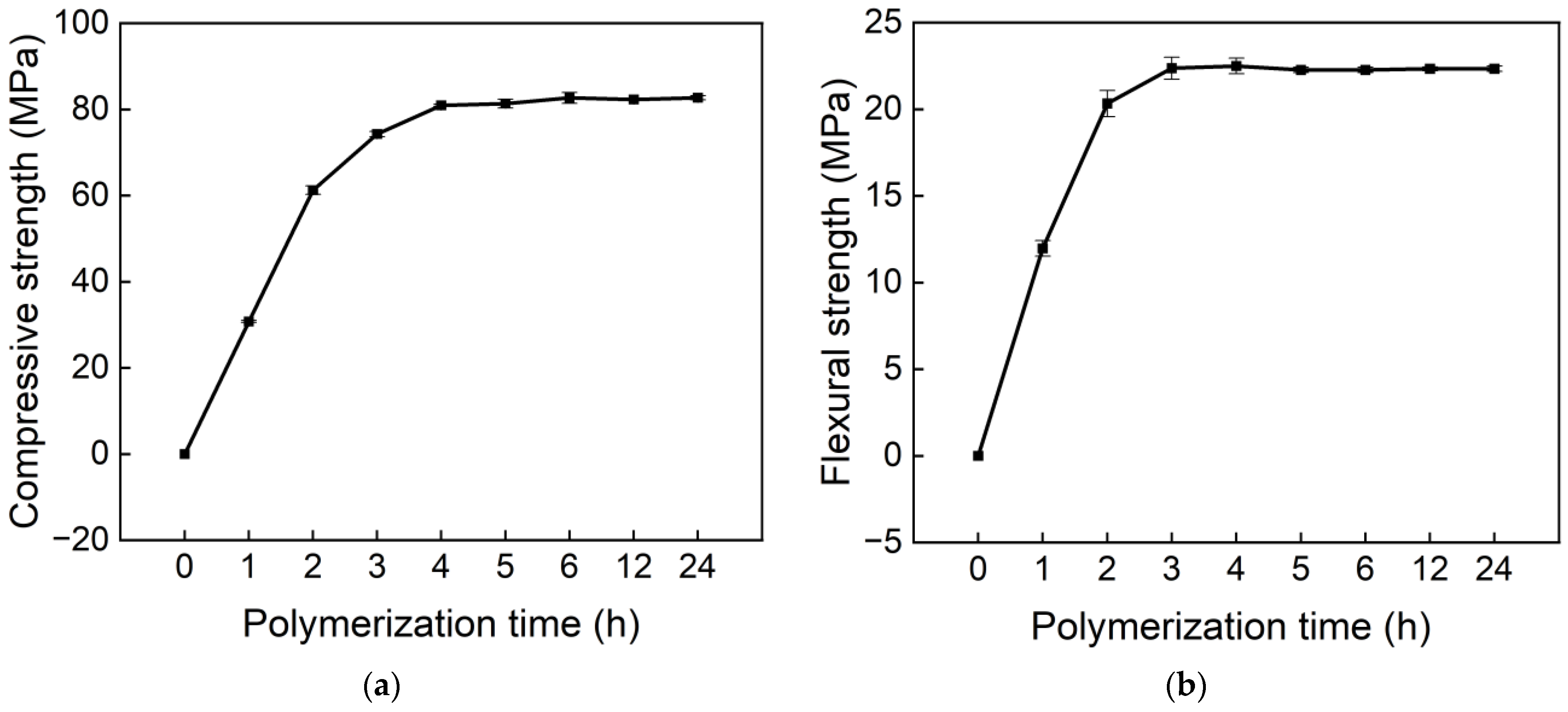
3.1. Polymerization Time
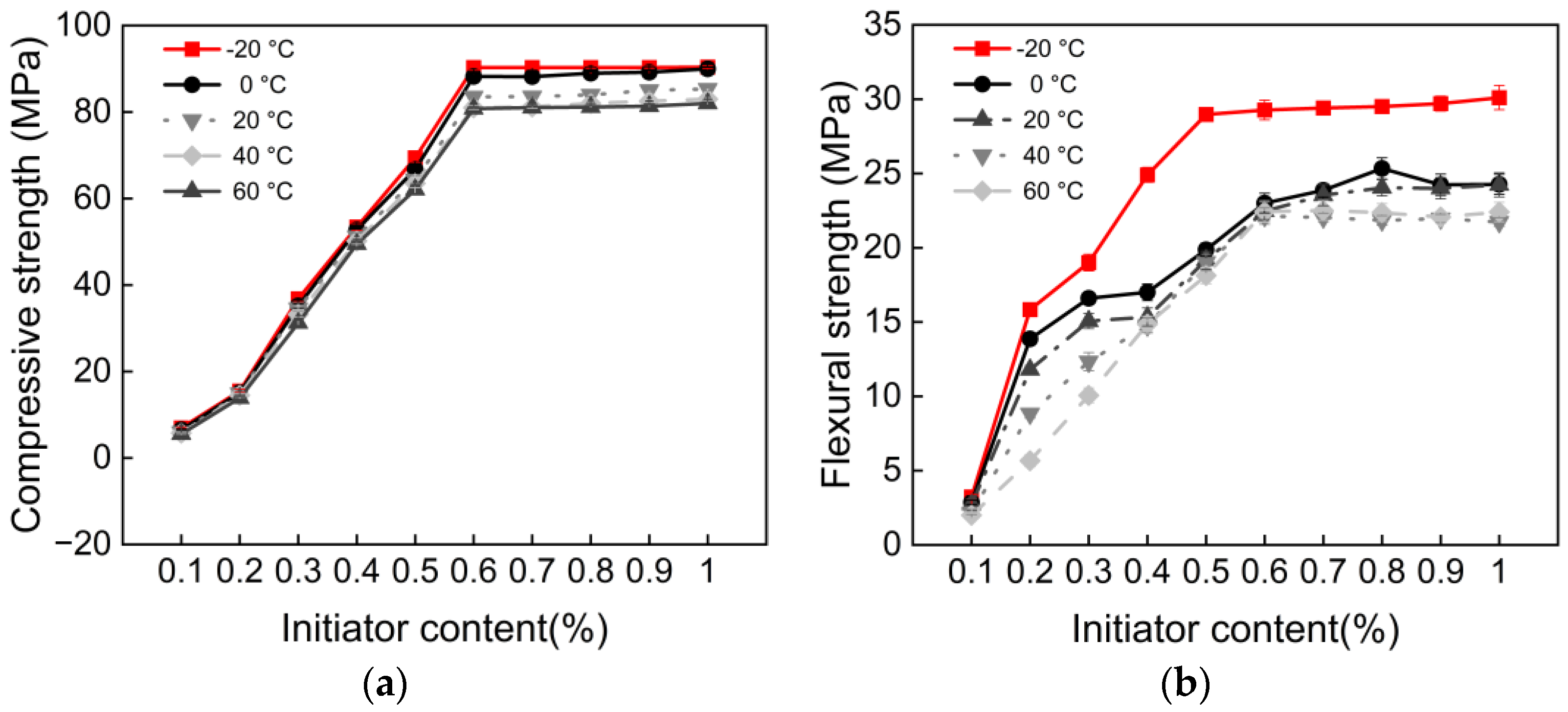
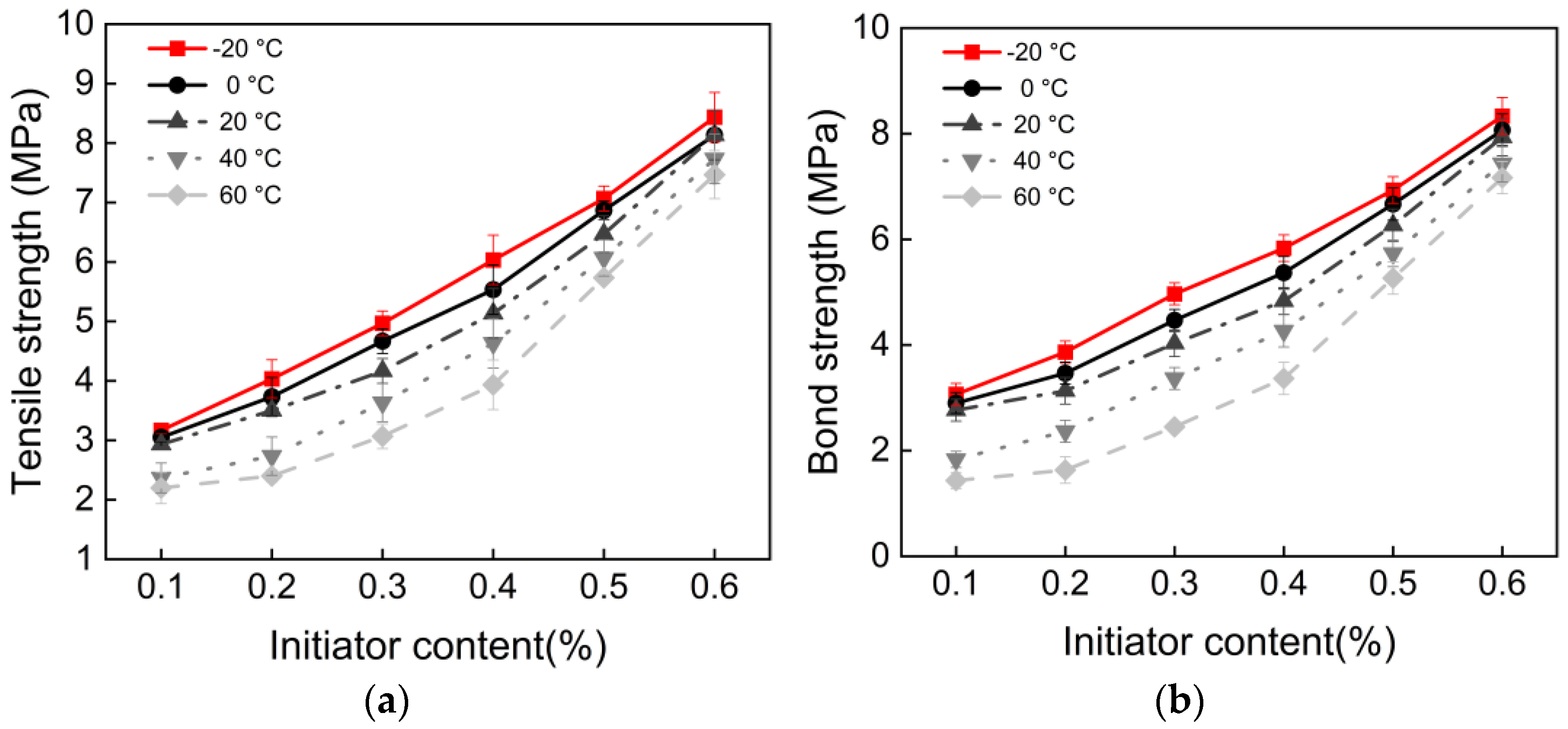
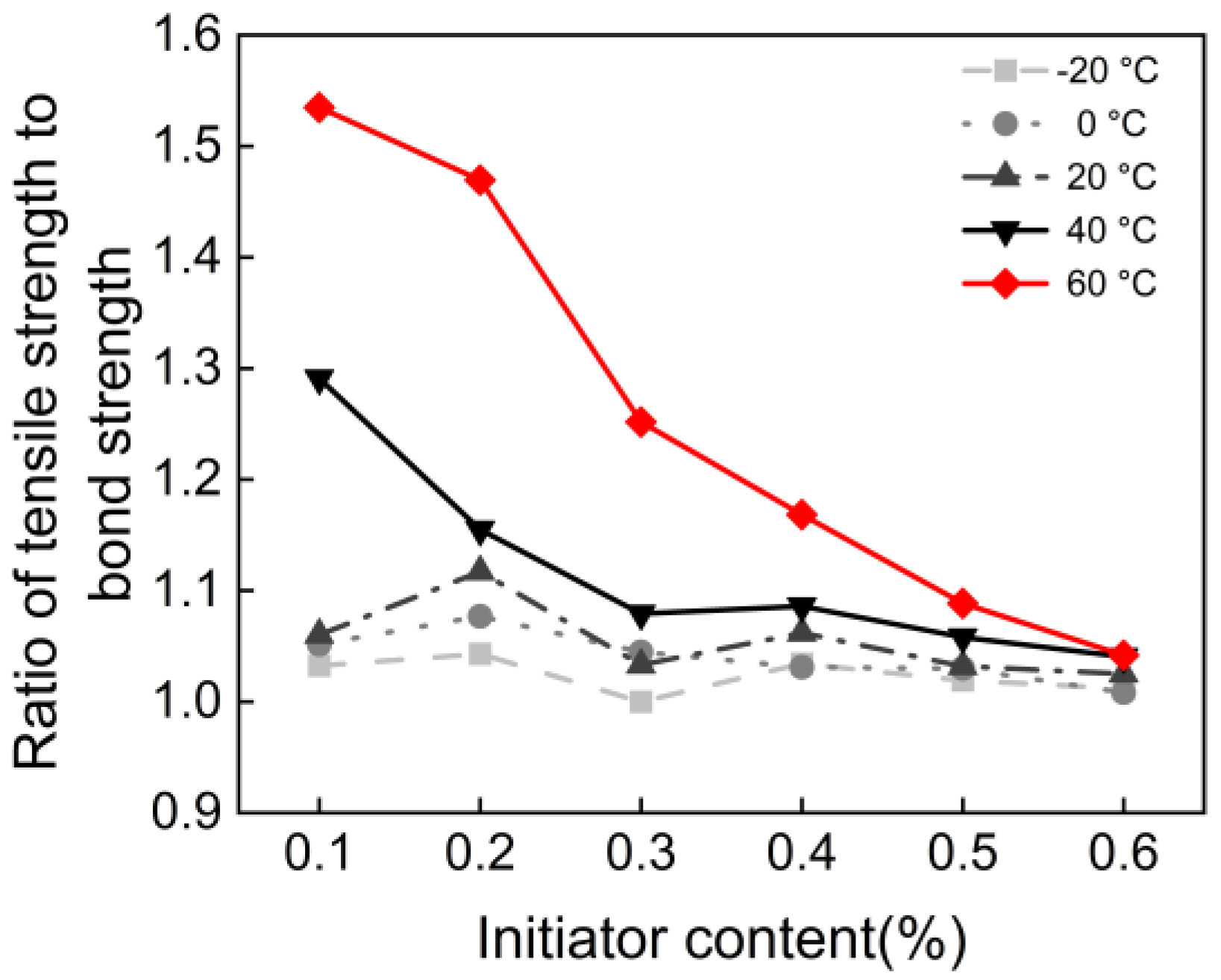
3.2. Initiator Content and Polymerization Temperature
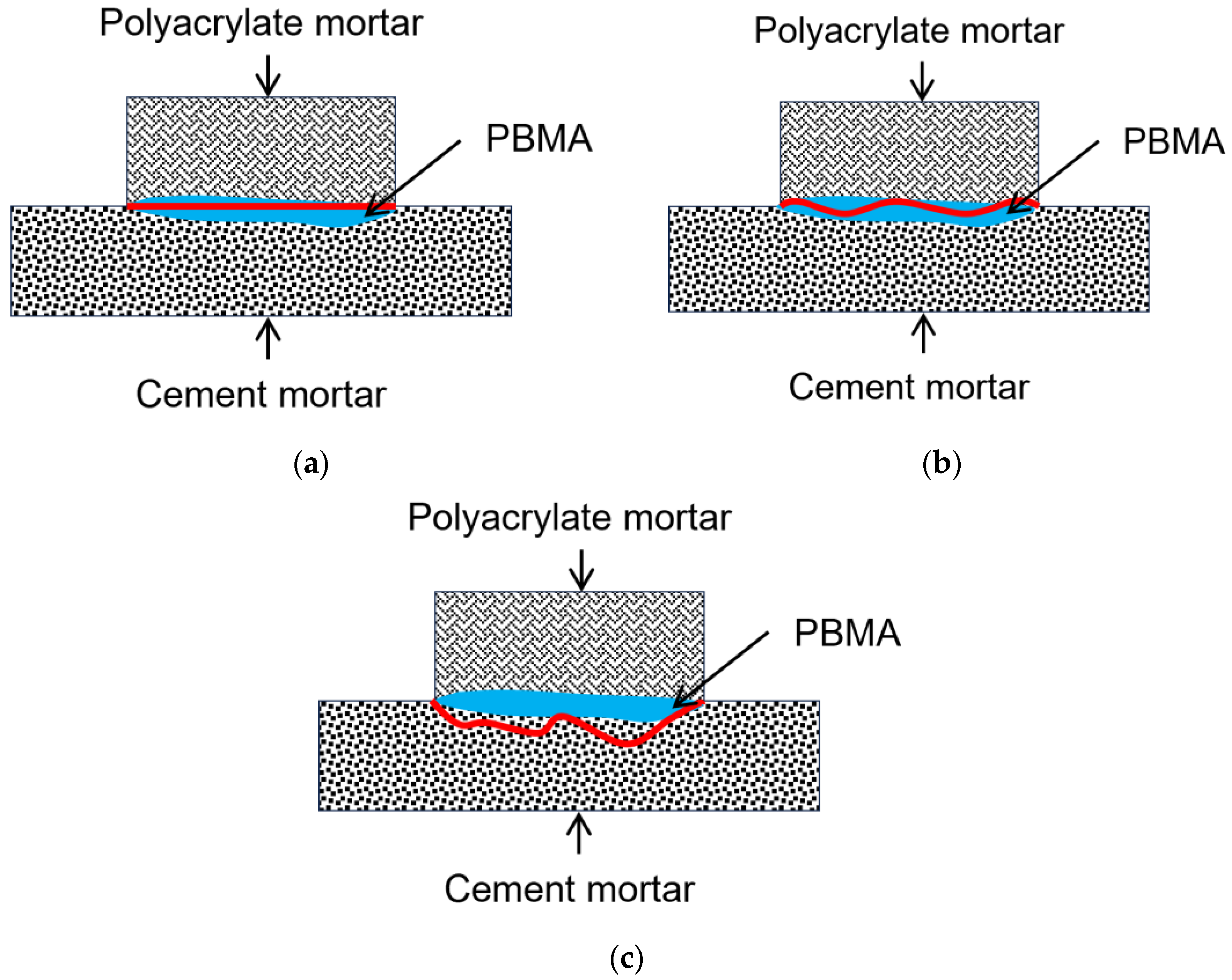
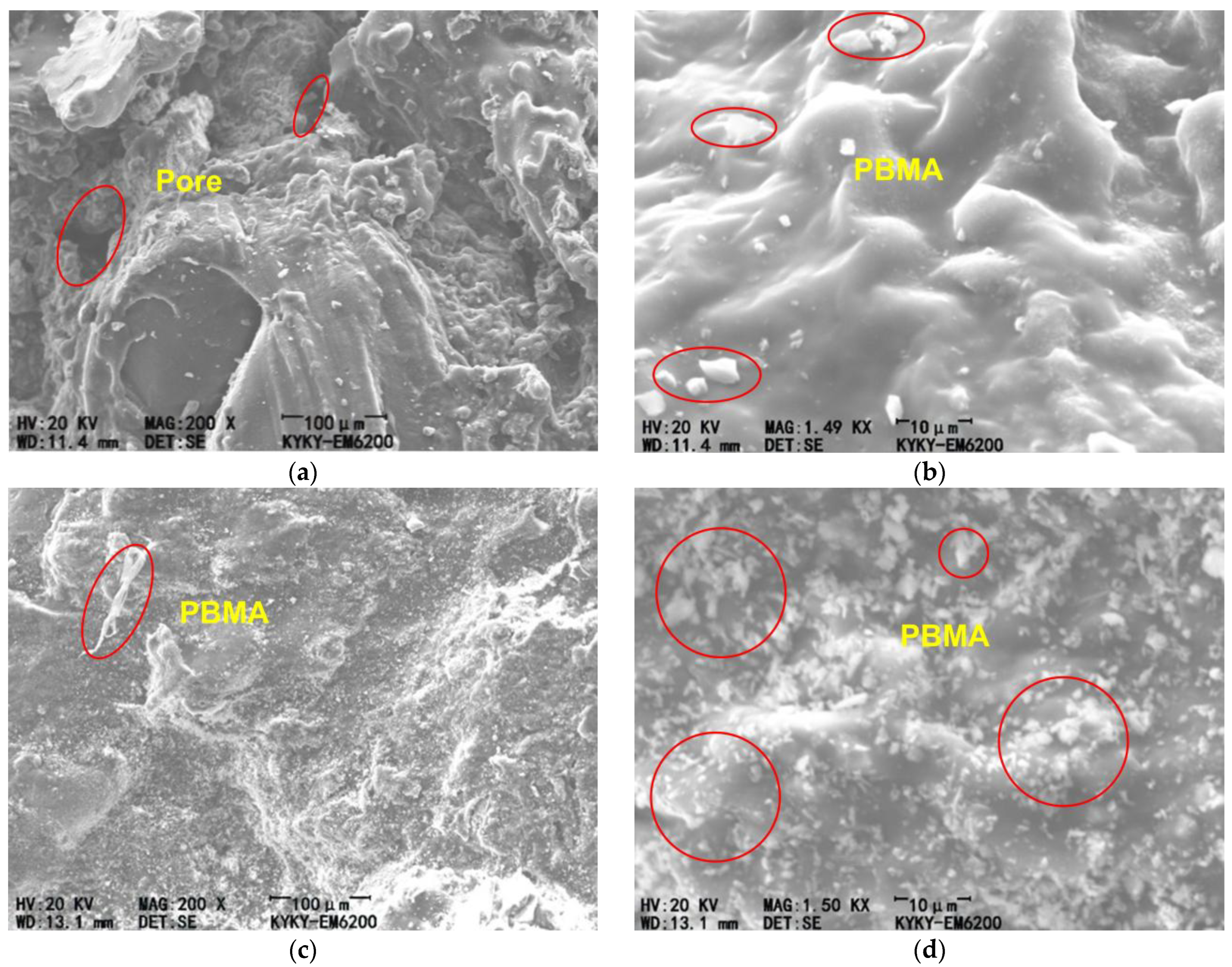
3.3. SEM Observations
4. Conclusions
- (1)
- At 60 °C, the mechanical strength of polyacrylate mortar increases rapidly within 3–5 h and then stabilizes, indicating that the polymerization reaction is essentially complete. To ensure sufficient reaction under all temperature conditions, 12 h was determined as the standard curing time in this study.
- (2)
- There exists an optimal initiator content of 0.6%. When the content is below this value, compressive, flexural, tensile, and bonding strengths increase significantly with higher content. Beyond this threshold, performance growth enters a plateau stage. This indicates that 0.6% is the optimal dosage for achieving efficient monomer conversion and the formation of a complete polymer network in this system.
- (3)
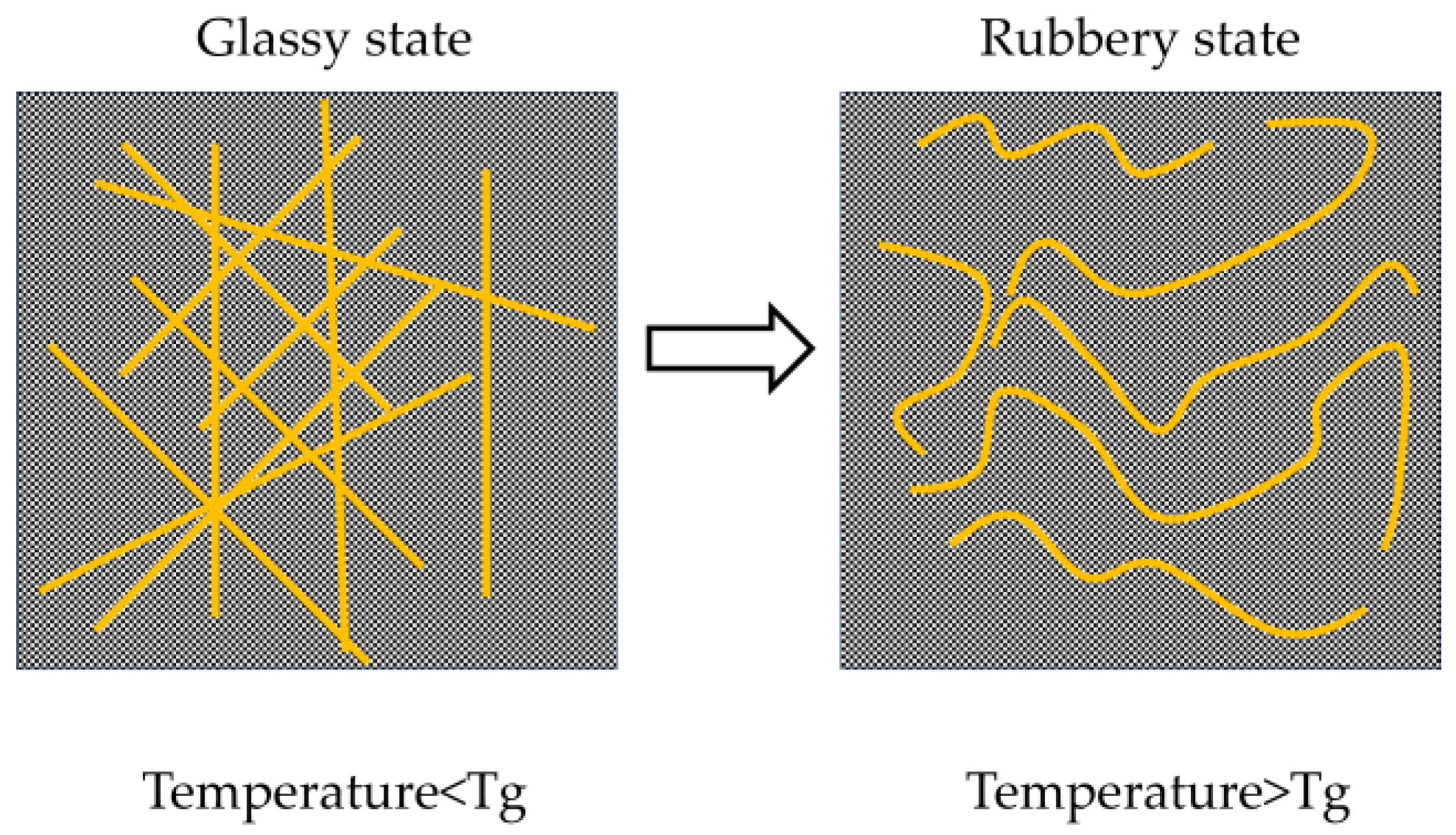
- All mechanical properties show a negative correlation with temperature. From a pure performance perspective, curing at −20 °C results in the highest mechanical strength. This is attributed to the stable redox initiation reaction of BPO/DMA at low temperatures, which favors the formation of polymers with higher molecular weight and more regular structures. Additionally, the polymer chains remain in a glassy state, exhibiting extremely high modulus.
- (4)
- SEM analysis reveals that at an initiator content of 0.6%, the polymer phase is more continuous and compact and adheres more firmly to the aggregates, which microscopically explains the superiority of its macroscopic performance.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tripathi, B. Effects of polymers on cement hydration and properties of concrete: A review. ACS Omega 2024, 9, 2014–2021. [Google Scholar] [CrossRef]
- Yang, S.; He, S.; Liu, S. Effect of different curing temperatures on the hydration of polyacrylate emulsion polymer-modified cement. J. Build. Eng. 2024, 94, 109938. [Google Scholar] [CrossRef]
- Chaudhury, R.; Sharma, U.; Thapliyal, P.; Singh, L. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Olsson, J.A.; Miller, S.A.; Alexander, M.G. Near-term pathways for decarbonizing global concrete production. Nat. Commun. 2023, 14, 4574. [Google Scholar] [CrossRef]
- Moini, M.; Olek, J.; Youngblood, J.P.; Magee, B.; Zavattieri, P.D. Additive manufacturing and performance of architectured cement-based materials. Adv. Mater. 2018, 30, 1802123. [Google Scholar] [CrossRef]
- Zhang, X.; Du, M.; Fang, H.; Shi, M.; Zhang, C.; Wang, F. Polymer-modified cement mortars: Their enhanced properties, applications, prospects, and challenges. Constr. Build. Mater. 2021, 299, 124290. [Google Scholar] [CrossRef]
- Lou, C.; Xu, J.; Wang, T.; Ren, W. Microstructure and pore structure of polymer-cement composite joint sealants. Sci. Rep. 2021, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Feng, P.; Liu, Q.; Zhang, Y.; Yu, Z.; Yan, S. In-situ polymerization-modified cement composites: A critical review. Constr. Build. Mater. 2024, 449, 138294. [Google Scholar] [CrossRef]
- Salami, B.A.; Bahraq, A.A.; ul Haq, M.M.; Ojelade, O.A.; Taiwo, R.; Wahab, S.; Adewumi, A.A.; Ibrahim, M. Polymer-enhanced concrete: A comprehensive review of innovations and pathways for resilient and sustainable materials. Next Mater. 2024, 4, 100225. [Google Scholar] [CrossRef]
- Nodehi, M. Epoxy, polyester and vinyl ester based polymer concrete: A review. Innov. Infrastruct. Solut. 2022, 7, 64. [Google Scholar] [CrossRef]
- Ataabadi, H.S.; Zare, A.; Rahmani, H.; Sedaghatdoost, A.; Mirzaei, E. Lightweight dense polymer concrete exposed to chemical condition and various temperatures: An experimental investigation. J. Build. Eng. 2021, 34, 101878. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Wong, H.S.; Abousnina, R.; Alajarmeh, O.S.; Zhuge, Y.; Schubel, P. Optimal design for epoxy polymer concrete based on mechanical properties and durability aspects. Constr. Build. Mater. 2020, 232, 117229. [Google Scholar] [CrossRef]
- Ayse, K.; Filiz, K. Properties of concrete containing waste expanded polystyrene and natural resin. Constr. Build. Mater. 2016, 105, 572–578. [Google Scholar] [CrossRef]
- Kępniak, M.; Chyliński, F.; Łukowski, P.; Woyciechowski, P. Recycled aggregate integration for enhanced performance of polymer concrete. Materials 2024, 17, 4007. [Google Scholar] [CrossRef]
- Yin, P.; Huang, L.; Yan, L.; Zhu, D. Compressive behavior of concrete confined by CFRP and transverse spiral reinforcement. Part A Exp. Study. Mater. Struct. 2016, 49, 1001–1011. [Google Scholar] [CrossRef]
- Heidarnezhad, F.; Jafari, K.; Ozbakkaloglu, T. Effect of polymer content and temperature on mechanical properties of lightweight polymer concrete. Constr. Build. Mater. 2020, 260, 119853. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Martinez-Barrera, G.; Vigueras-Santiago, E.; Martinez-Lopez, M.; Gencel, O. Mechanical improvement of polymer concrete by using aged polyester resin, nanosilica and gamma rays. J. Build. Eng. 2022, 58, 105083. [Google Scholar] [CrossRef]
- Zeldin, A.; Kukacka, L.; Carciello, N. Influence of the ratio of styrene and acrylonitrile in crosslinked polymeric binders for use in high-temperature polymer concrete composites. J. Appl. Polym. Sci. 1979, 24, 1759–1765. [Google Scholar] [CrossRef]
- Murcia, D.H.; Al Shanti, S.; Hamidi, F.; Rimsza, J.; Yoon, H.; Gunawan, B.; Abdellatef, M.; Taha, M.R. Development and characterization of a sustainable bio-polymer concrete with a low carbon footprint. Polymers 2023, 15, 628. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Li, X. Investigation on the working performance of a non-dispersible grouting material for the crack repairment of underwater structures. Constr. Build. Mater. 2023, 407, 133558. [Google Scholar] [CrossRef]
- Li, C.; Bai, J.; Jiang, Y.; Xiao, H.; Wang, W.; Xu, F. Investigating the seepage control and plugging capabilities of polyurethane-cement composites: A comprehensive study on material properties. Constr. Build. Mater. 2024, 416, 135191. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, R.; Wu, J.; Chen, C.-H. Influence of soaking and polymerization conditions on the properties of polymer concrete. Constr. Build. Mater. 2006, 20, 706–712. [Google Scholar] [CrossRef]
- Giustozzi, F. Polymer-modified pervious concrete for durable and sustainable transportation infrastructures. Constr. Build. Mater. 2016, 111, 502–512. [Google Scholar] [CrossRef]
- Jiang, C.; Huang, S.; Gao, P.; Chen, D. Experimental study on the bond and durability properties of mortar incorporating polyacrylic ester and silica fume. Adv. Cem. Res. 2018, 30, 56–65. [Google Scholar] [CrossRef]
- Medina, N.F.; Barluenga, G.; Hernández-Olivares, F. Combined effect of Polypropylene fibers and Silica Fume to improve the durability of concrete with natural Pozzolans blended cement. Constr. Build. Mater. 2015, 96, 556–566. [Google Scholar] [CrossRef]
- Çakır, Ö.; Sofyanlı, Ö.Ö. Influence of silica fume on mechanical and physical properties of recycled aggregate concrete. HBRC J. 2015, 11, 157–166. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarnia, K. The effect of silica fume on durability of alkali activated slag concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]
- Almeida, A.E.d.S.; Sichieri, E.P. Mineralogical study of polymer modified mortar with silica fume. Constr. Build. Mater. 2006, 20, 882–887. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Xie, X.; Zhang, L.; Guo, X.; Tu, R. Heat transfer blockage effect of small-scale PMMA flame under exterior heat radiation. Int. J. Therm. Sci. 2017, 122, 26–32. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and mechanical properties of PMMA/reduced graphene oxide nanocomposites prepared via in situ polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- Soroush, M.; Grady, M.C.; Kalfas, G.A. Free-radical polymerization at higher temperatures: Systems impacts of secondary reactions. Comput. Chem. Eng. 2008, 32, 2155–2167. [Google Scholar] [CrossRef]
- Grady, M.C.; Simonsick, W.J.; Hutchinson, R.A. Studies of higher temperature polymerization of n-butyl methacrylate and n-butyl acrylate. In Macromolecular Symposia; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2002; Volume 182, pp. 149–168. [Google Scholar]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021.
- JTG E50-2006; Test Method of Geosynthetics for Highway Engineering. China Communications Press: Beijing, China, 2006.
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. China Architecture & Building Press: Beijing, China, 2009.
- Awsiuk, K.; Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Dąbczyński, P.; Kostruba, A.; Ohar, H.; Shymborska, Y.; Nastyshyn, S.; Budkowski, A. Temperature-controlled orientation of proteins on temperature-responsive grafted polymer brushes: Poly (butyl methacrylate) vs. poly (butyl acrylate): Morphology, wetting, and protein adsorption. Biomacromolecules 2019, 20, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Fleischhaker, F.; Haehnel, A.P.; Misske, A.M.; Blanchot, M.; Haremza, S.; Barner-Kowollik, C. Glass-Transition-, Melting-, and Decomposition Temperatures of Tailored Polyacrylates and Polymethacrylates: General Trends and Structure–Property Relationships. Macromol. Chem. Phys. 2014, 215, 1192–1200. [Google Scholar] [CrossRef]
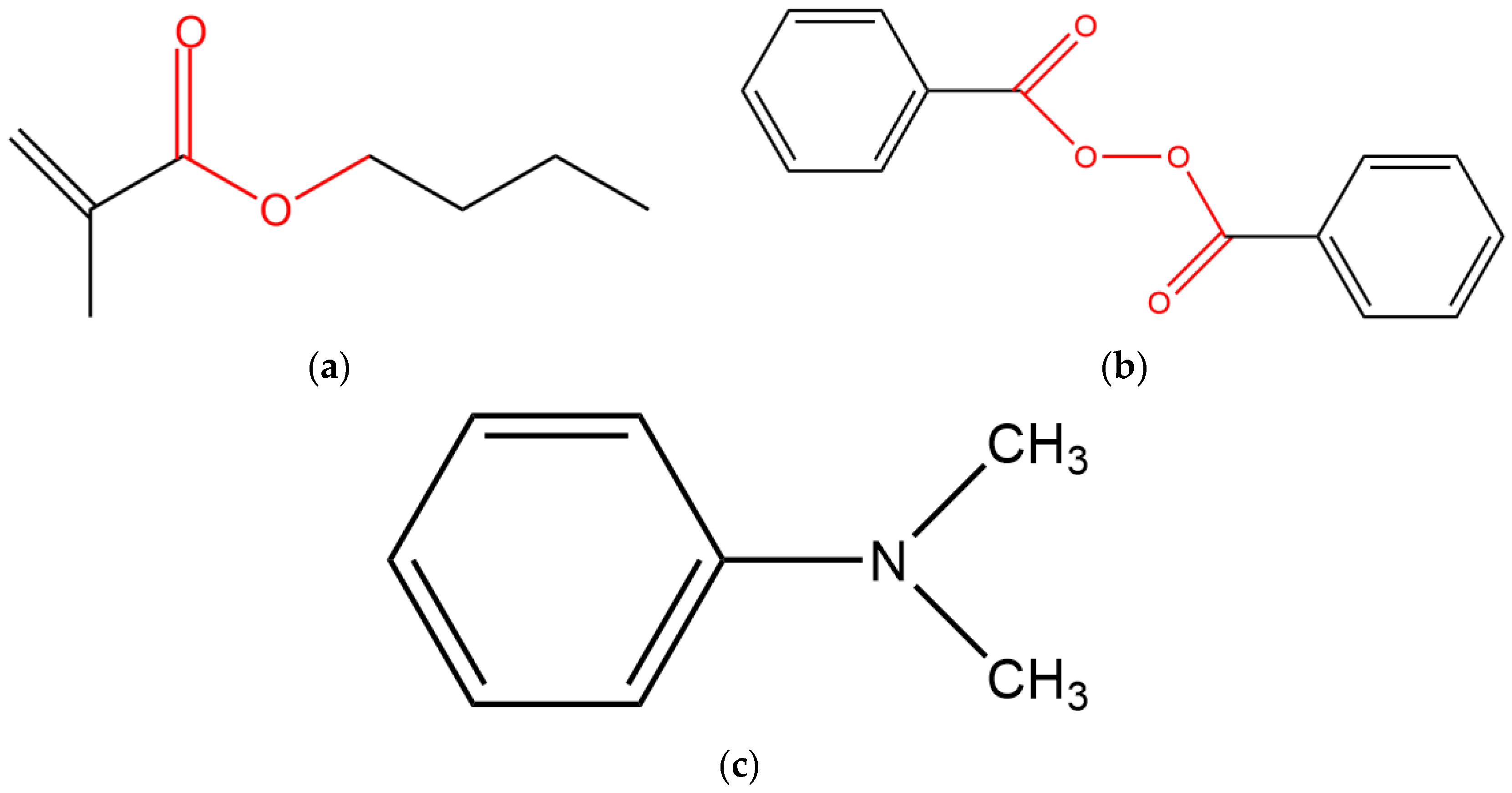
| Chemical Composition: % | BMA | SF | BPO | DMA |
|---|---|---|---|---|
| Silicon dioxide (SiO2) | - | 87.29 | - | - |
| Aluminum oxide (Al2O3) | - | 0.47 | - | - |
| Iron (III) oxide (Fe2O3) | - | 0.63 | - | - |
| Calcium oxide (CaO) | - | 0.81 | - | - |
| Magnesium oxide (MgO) | - | 4.47 | - | - |
| Titanium dioxide (TiO2) | - | - | - | - |
| Sulfur trioxide (SO3) | - | 0.22 | - | - |
| Sodium oxide (Na2O) | - | 1.25 | - | - |
| Potassium oxide (K2O) | - | 1.28 | -- | - |
| Loss on ignition | - | 2.70 | - | - |
| Molecular formula | C9H14O2 | - | C14H10O4 | C8H11N |
| Melting Point (°C) | <−75 | - | 105 | 2.5 |
| Boiling point (°C) | 160 | - | 349.7 | 193.1 |
| Relative density (g/cm3) | 0.889 | 1.334 | 0.96 | |
| Purity | >99.5% | - | - | - |
| Constituent Components | Particle Size (mm) | Mass Percentage (%) |
|---|---|---|
| Quartz sand A | 2.36–4.75 | 54.3 |
| Quartz sand B | 0.25–0.3 | 32.5 |
| SF | <0.25 | 13.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Han, C.; Zhang, T.; Guo, D.; Dai, Y.; Ding, W. Influence of Initiator Content and Polymerization Conditions on the Properties of Polyacrylate Mortar. Materials 2025, 18, 4737. https://doi.org/10.3390/ma18204737
Huang Z, Han C, Zhang T, Guo D, Dai Y, Ding W. Influence of Initiator Content and Polymerization Conditions on the Properties of Polyacrylate Mortar. Materials. 2025; 18(20):4737. https://doi.org/10.3390/ma18204737
Chicago/Turabian StyleHuang, Zhengqiang, Chong Han, Tianhang Zhang, Dongyang Guo, Yonggui Dai, and Wencheng Ding. 2025. "Influence of Initiator Content and Polymerization Conditions on the Properties of Polyacrylate Mortar" Materials 18, no. 20: 4737. https://doi.org/10.3390/ma18204737
APA StyleHuang, Z., Han, C., Zhang, T., Guo, D., Dai, Y., & Ding, W. (2025). Influence of Initiator Content and Polymerization Conditions on the Properties of Polyacrylate Mortar. Materials, 18(20), 4737. https://doi.org/10.3390/ma18204737







