Nitriding of Commercially Pure (CP) Titanium Grade 2 by the Active Screen Method
Abstract
1. Introduction
1.1. Ion Nitriding of Titanium
1.2. Ion Nitriding Using an Active Screen
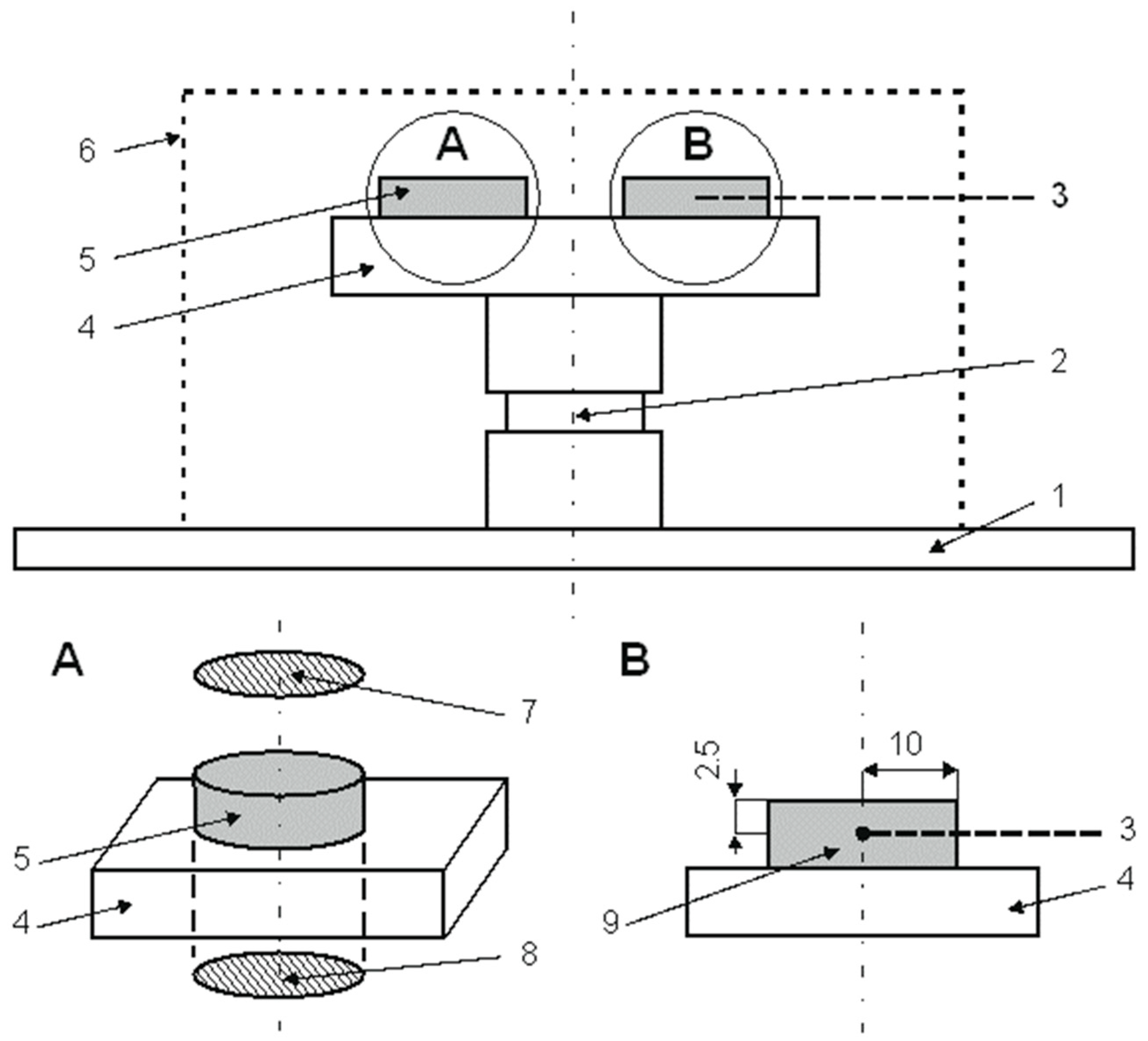
2. Materials and Methods
- Observations using a Carl Zeiss Axiovert 25 light microscope (Carl Zeiss, Oberkochen, Deutschland) and a Jeol 6610Lv scanning electron microscope (JEOL Ltd, Tokio, Japan);
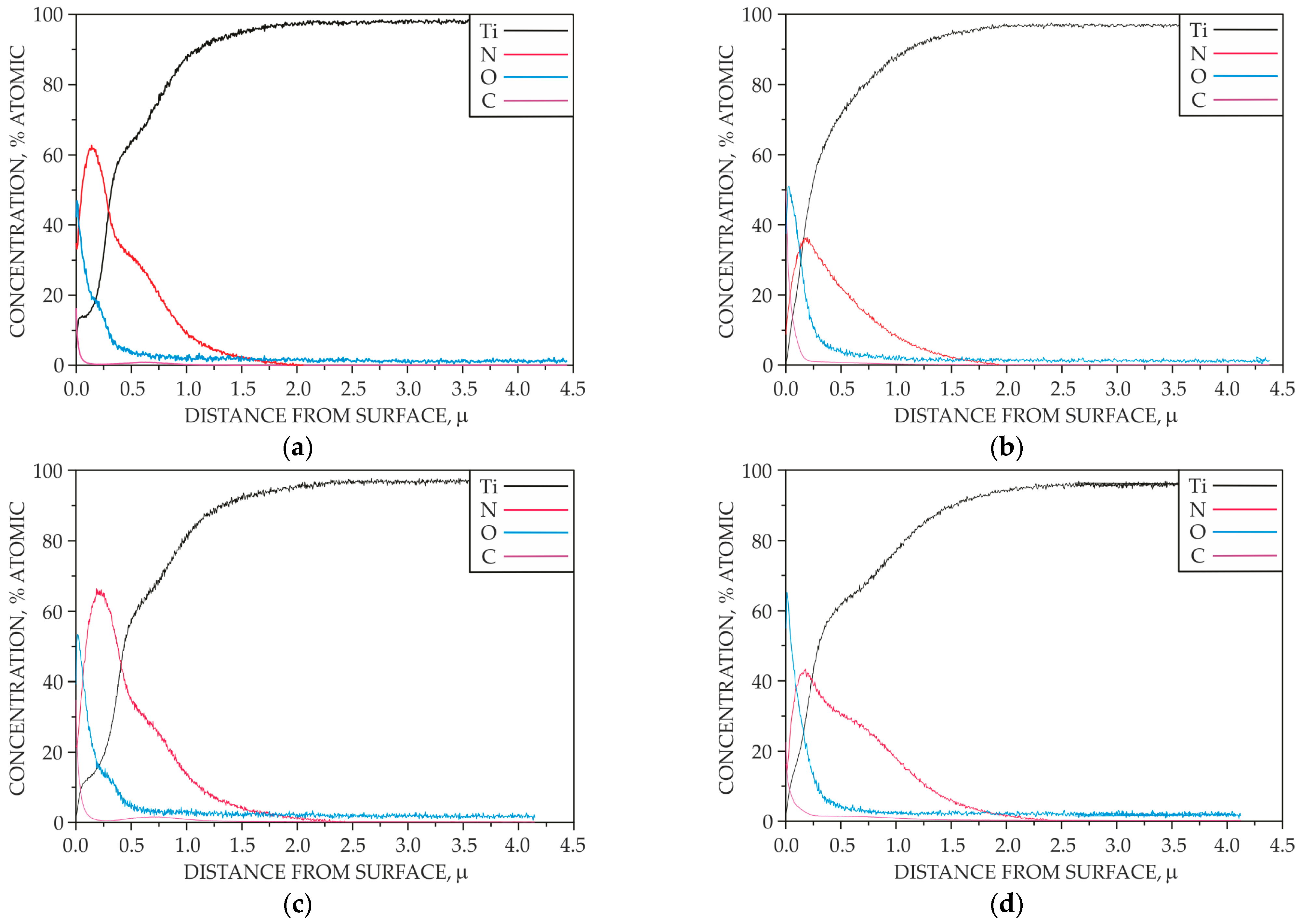
- Examination of the elemental distribution profile using a LECO’s GDS 850A spectrometer (GDOES—Glow Discharge Optical Emission Spectroscopy) (LECO Corporation, St. Joseph, MI, USA) equipped with a Grimm discharge lamp (LECO Corporation, St. Joseph, MI, USA) with a cathode diameter of Ø 4 mm. The spectrometer utilizes the technique of glow discharge optical emission spectrometry in a vacuum. During analysis, the sample surface was bombarded with a stream of ionized argon, resulting in the uniform sputtering of successive layers of atoms from the sample surface. This process was conducted at reduced pressure and without additional thermal effects on the sample surface;
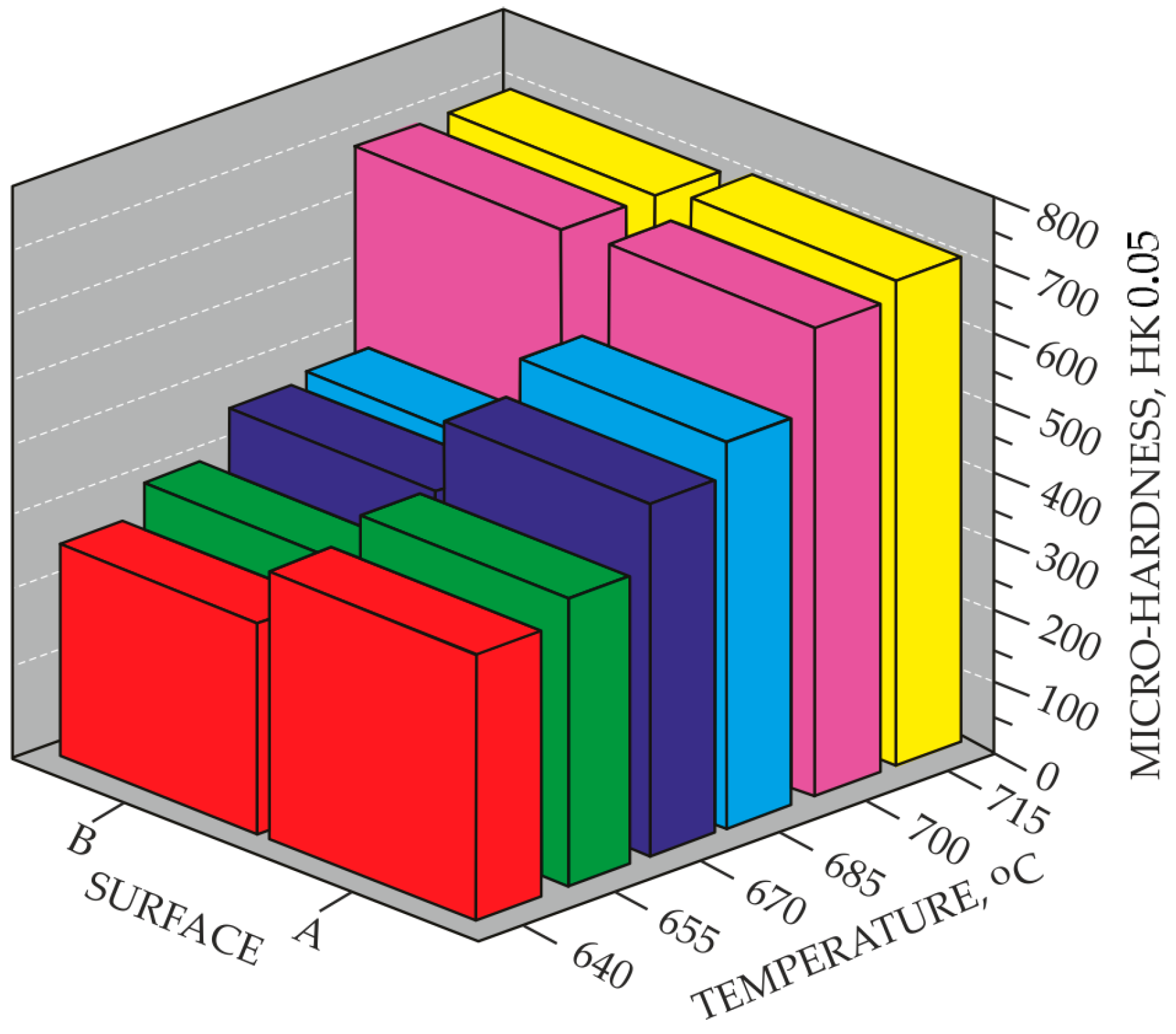
- Examination of surface microhardness of the material in the as-delivered condition and after ion nitriding processes using FutureTech FM-7 microhardness tester (Future-Tech Corporation, Kawaguchi, Japan) Knoop method (providing higher measurement accuracy compared to the Vickers method due to the longer diagonal of the indentation, recommended for measuring the microhardness of thin surface layers of hard and brittle materials without risk of indenter damage).
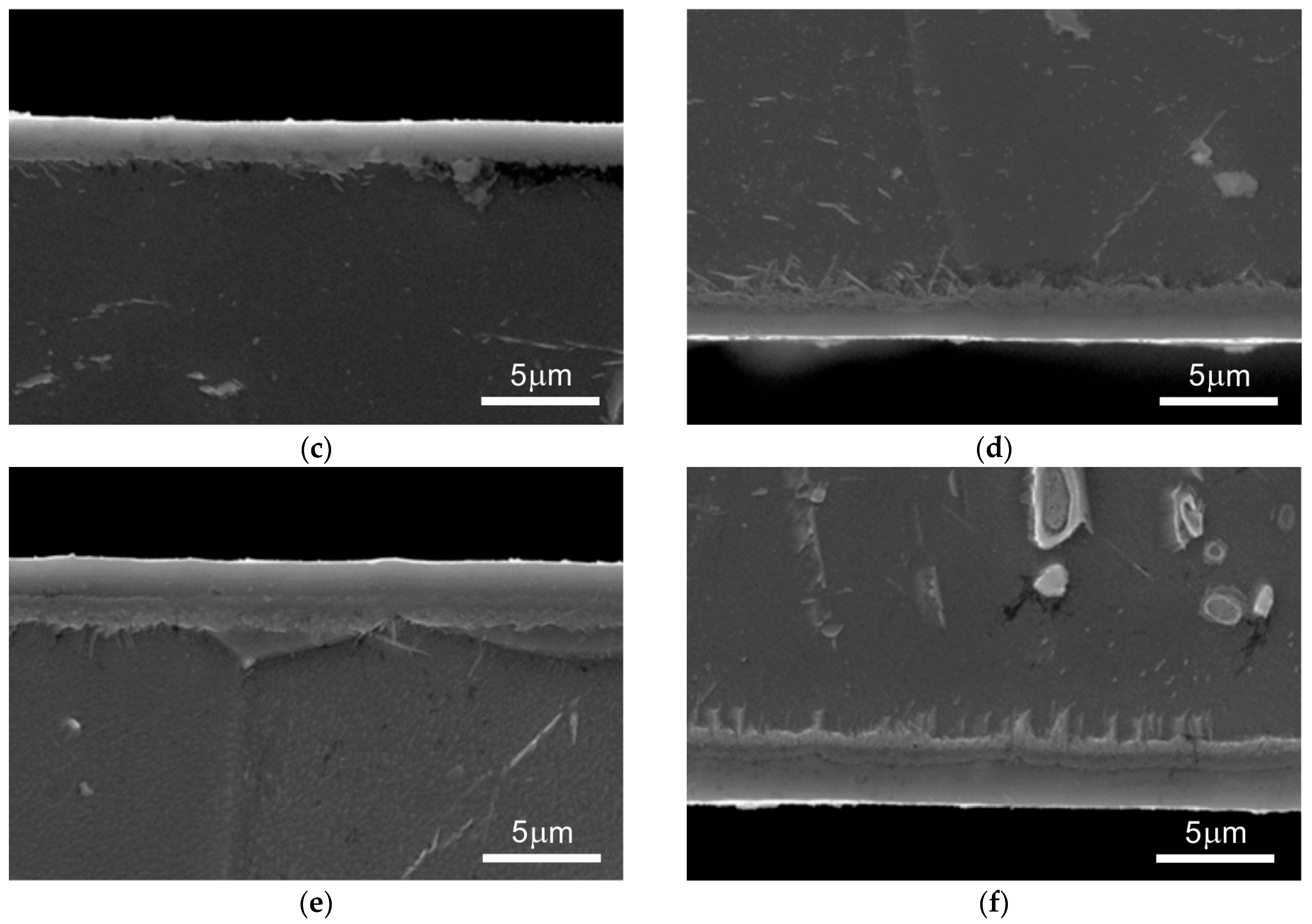
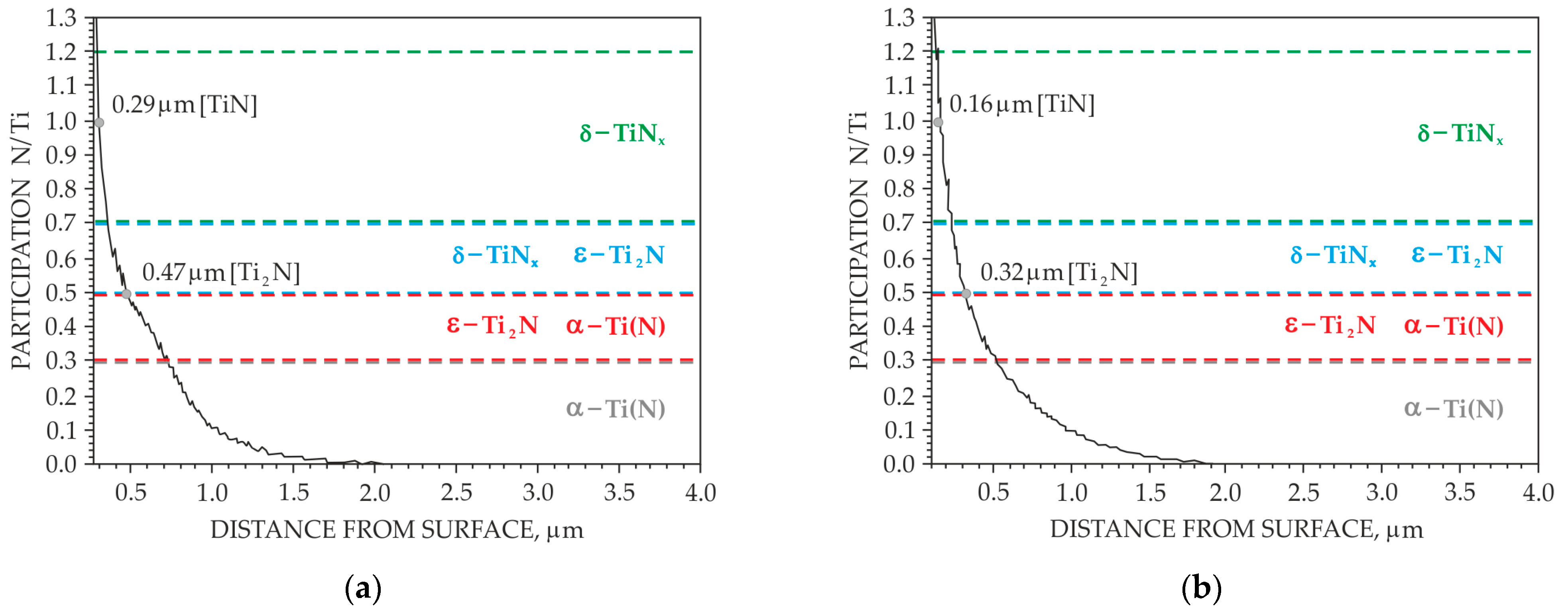
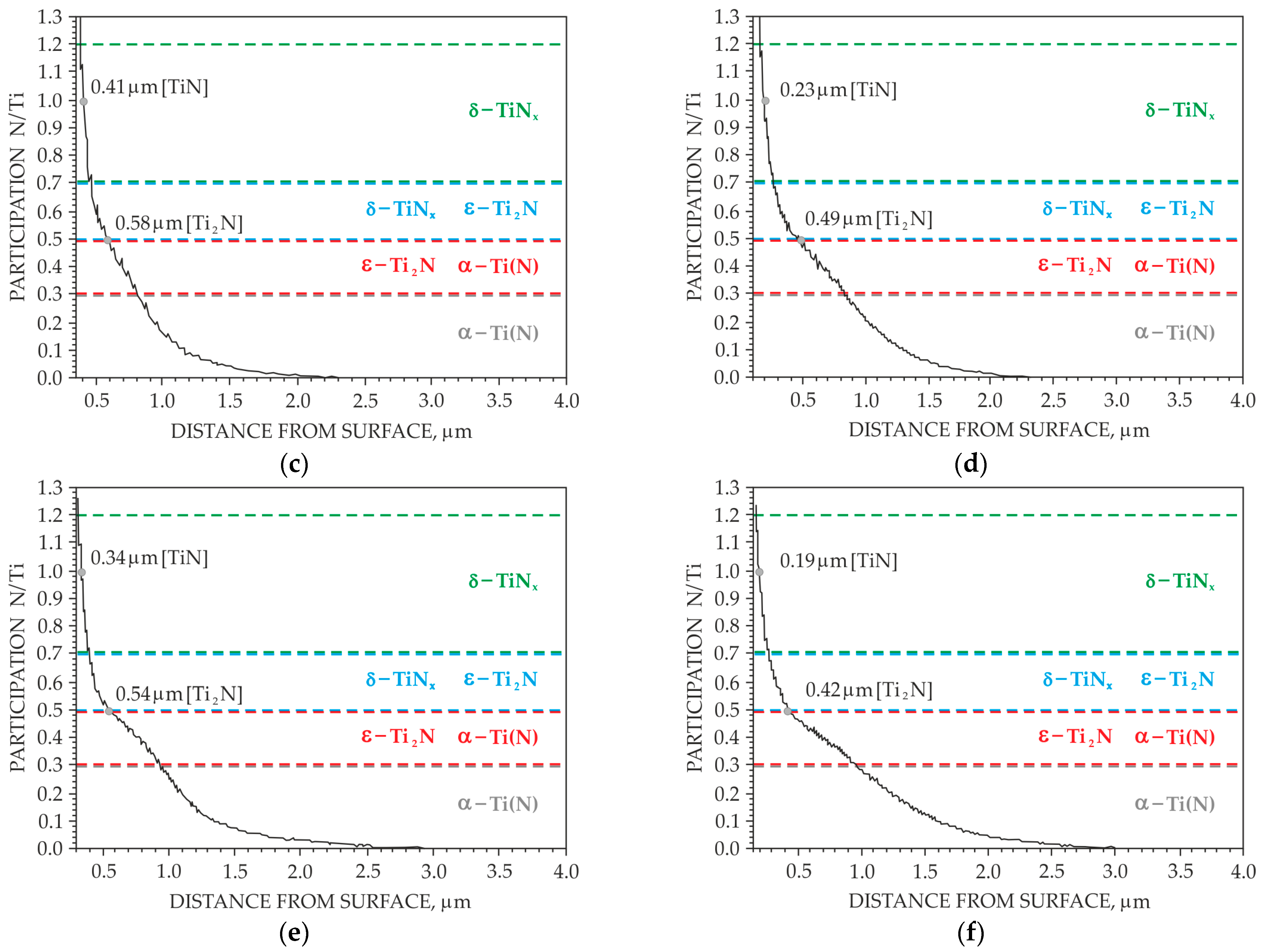
3. Results
3.1. Preliminary Research Results
3.2. Main Research
4. Discussion
5. Conclusions
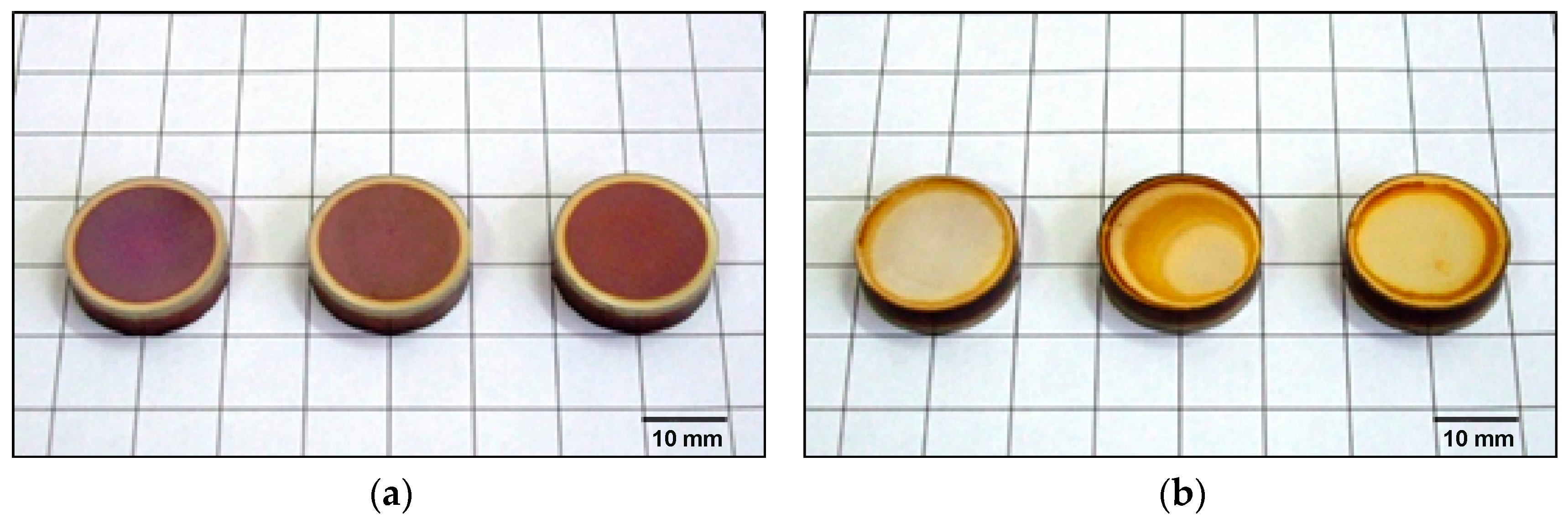
- In order to eliminate the brown coloring of the surface, the adopted parameters of the ion nitriding treatment ensure the formation of homogeneous surface layers of a diffusive nature, over the entire surface of the nitrided sample, at a limit temperature of 700 °C. However, it was only for the treatment carried out at 715 °C that the same depth of nitrogen diffusion was found on both surface A and surface B.
- It was found that homogeneous surface layers on the direct cathode contact surface (surface B) are formed at the ion nitriding process limit temperature of 700 °C by adsorption processes involving atomic nitrogen.
- In order to eliminate the brown coloring of the surface of the nitrided samples, which is indicative of the presence of oxygen during nitriding, the flushing time of the furnace atmosphere must be increased before the main nitriding process.
- The phenomenon of ion bombardment, as well as other active plasma components, contributes to the reduction of carbon and oxygen content in the produced surface layers on surface A of the nitrided sample.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walkowicz, J. Fizykochemiczna Struktura Plazmy a Skład Chemiczny i Fazowy Warstw Wytwarzanych Technikami Plazmowej Inżynierii Powierzchni; Wydawnictwo i Zakład Poligrafii Instytutu Technologii Eksploatacji: Radom, Poland, 2003. [Google Scholar]
- Lachtin, J.M.; Kogan, J.D. Azotirovanie Stali; Masinostrojenie: Moscow, Russia, 1976. [Google Scholar]
- Michel, H.; Gantois, M. Etude de La Nitruration Par Bombardment Ionique Du Titane et Des Alliages de Titane. Mém. Sci. Rev. Métall. 1972, 69, 739–749. [Google Scholar]
- Bollinger, H.; Bucken, B.; Wilberg, R. Untersuchungen Zum Glimmnitrieren von Titan. Metalloberflache 1977, 31, 329–333. [Google Scholar]
- Keller, K. Method of Hardening the Surface of Workpieces Made of Iron and Stee. U.S. Patent No. 3761370, 25 September 1973. [Google Scholar]
- Konuma, M.; Matsumoto, O. Nitriding of Titanium in a Radio Frequency Discharge. J. Less Common Met. 1977, 52, 145–152. [Google Scholar] [CrossRef]
- Matsumoto, O.; Konuma, M.; Kanzaki, Y. Nitriding of Titanium in an r.f. Discharge II: Effect of the Addition of Hydrogen to Nitrogen on Nitriding. J. Less Common Met. 1982, 84, 157–163. [Google Scholar] [CrossRef]
- Liu, M.B.; Gruen, D.M.; Krauss, A.R.; Reis, A.H.; Peterson, S.W. Ion Nitriding of Titanium and Zirconium by a Dc-Glow Discharge Method. High Temp. Sci. 1978, 10, 53–65. [Google Scholar]
- Hu, H.; Murray, P.T.; Fukuda, Y.; Rabalais, J.W. Absolute Cross Sections for Beam-surface Reactions: N2+ on Ti from 0.25 to 3.0 KeV Kinetic Energy. J. Chem. Phys. 1981, 74, 2247–2255. [Google Scholar] [CrossRef]
- Hu, H.; Fukuda, Y.; Baldwin, D.A.; Murray, P.T.; Rabalais, J.W. Interactions of Ion Beams with Surfaces: Dynamics of the Reaction of N2+ with Rhenium. J. Chem. Phys. 1980, 72, 6158–6163. [Google Scholar] [CrossRef]
- Roliński, E. Azotowanie Jonowe Tytanu i Jego Stopów; Prace Naukowe Politechniki Warszawskiej: Warsaw, Poland, 1988. [Google Scholar]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the Microstructure and Properties of Titanium Alloys through Nitriding and Other Surface Engineering Methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Jagielska-Wiaderek, K.; Bala, H.; Wierzchon, T. Corrosion Depth Profiles of Nitrided Titanium Alloy in Acidified Sulphate Solution. Cent. Eur. J. Chem. 2013, 11, 2005–2011. [Google Scholar] [CrossRef]
- Malinov, S.; Zhecheva, A.; Sha, W. Modelling the Nitriding in Titanium Alloys. In Proceedings of the International Surface Engineering Congress-Proceedings of the 1st Congress, Colombus, OH, USA, 7–10 October 2002; ASM International: Almere, The Netherlands, 2003. [Google Scholar]
- Frączek, T. Niekonwencjonalne Niskotemperaturowe Azotowanie Jonowe Materiałów Metalicznych; Wydawnictwo WiPMiFS Politechniki Częstochowskiej: Czestochowa, Poland, 2011. [Google Scholar]
- Frączek, T.; Jeziorski, L. Model Azotowania Jarzeniowego Tytanu w Atmosferze Azotu. Hut. Wiadomości Hut. 2008, 75, 366–369. [Google Scholar]
- Georges, J. Nitriding Process and Nitriding Furnace Therefore. U.S. Patent No. 5989363, 23 November 1999. [Google Scholar]
- Ossowski, M.; Borowski, T.; Tarnowski, M.; Wierzchoń, T. Cathodic Cage Plasma Nitriding of Ti6Al4V Alloy. Mater. Sci. 2016, 22, 25–30. [Google Scholar] [CrossRef][Green Version]
- Tarnowski, M.; Borowski, T.; Skrzypek, S.; Kulikowski, K.; Wierzchoń, T. Shaping the Structure and Properties of Titanium and Ti6Al7Nb Titanium Alloy in Low-Temperature Plasma Nitriding Processes. J. Alloys Compd. 2021, 864, 158896. [Google Scholar] [CrossRef]
- Morgiel, J.; Maj, Ł.; Szymkiewicz, K.; Pomorska, M.; Ozga, P.; Toboła, D.; Tarnowski, M.; Wierzchoń, T. Surface Roughening of Ti-6Al-7Nb Alloy Plasma Nitrided at Cathode Potential. Appl. Surf. Sci. 2022, 574, 151639. [Google Scholar] [CrossRef]
- Sitek, R.; Kamiński, J.; Adamczyk-Cieślak, B.; Molak, R.; Spychalski, M.; Cowell, B.; McCann, J.; Roliński, E. Effect of Plasma Nitriding on Structure and Properties of Titanium Grade 2 Produced by Direct Metal Laser Sintering. Metallogr. Microstruct. Anal. 2022, 11, 852–863. [Google Scholar] [CrossRef]
- Ossowski, M.; Tarnowski, M.; Borowski, T.; Brojanowska, A.; Wierzchoń, T. Azotowanie z Ekranem Aktywnym Jako Alternatywa Dla Azotowania Jarzeniowego Tytanu i Jego Stopów. Inżynieria Mater. 2012, 4, 236–239. [Google Scholar]
- Zhao, C.; Li, C.X.; Dong, H.; Bell, T. Study on the Active Screen Plasma Nitriding and Its Nitriding Mechanism. Surf. Coat. Technol. 2006, 201, 2320–2325. [Google Scholar] [CrossRef]
- Taherkhani, F.; Taherkhani, A. Surface Characterization of Through Cage Plasma Nitriding on the Surface Properties of Low Alloy Steel. Trans. B Mech. Eng. 2010, 17, 253–263. [Google Scholar]
- Betiuk, M.; Kowalski, S. Azotowanie Jarzeniowe Na Potencjale Uzupełniającym. Inżynieria Mater. 2010, 4, 869–873. [Google Scholar]
- de Sousa, R.R.M.; de Araújo, F.O.; Ribeiro, K.J.B.; Mendes, M.W.D.; da Costa, J.A.P.; Alves, C. Cathodic Cage Nitriding of Samples with Different Dimensions. Mater. Sci. Eng. A 2007, 465, 223–227. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Dong, H.; Guo, P.; Gu, D. Nitrogen Mass Transfer and Surface Layer Formation during the Active Screen Plasma Nitriding of Austenitic Stainless Steels. Vacuum 2018, 148, 224–229. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Dong, H. New Insights into the Mechanism of Low-Temperature Active-Screen Plasma Nitriding of Austenitic Stainless Steel. Scr. Mater. 2012, 67, 89–91. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Dong, H. On the Fundamental Mechanisms of Active Screen Plasma Nitriding. Vacuum 2009, 84, 321–325. [Google Scholar] [CrossRef]
- ASTM Standard. Available online: https://luminatitan.com/astm-grade-2-titanium-specs/#:~:text=ASTM%20Grade%202%20Titanium%20%28UNS%20R50400%29%20is%20an,to%20its%20versatility%2C%20wide%20availability%2C%20and%20balanced%20properties (accessed on 11 October 2025).
- Domínguez-Meister, S.; Ibáñez, I.; Dianova, A.; Brizuela, M.; Braceras, I. Nitriding of Titanium by Hollow Cathode Assisted Active Screen Plasma and Its Electro-Tribological Properties. Surf. Coat. Technol. 2021, 411, 126998. [Google Scholar] [CrossRef]
- Sun, W.; Song, Z.; Liu, H.; Wang, J.; Liu, C.; Si, C. Effect of Surface Nanocrystallization Pretreatment on the Microstructure and Fretting Wear of Ti-6Al-4V Titanium Alloy Plasma Nitrided Layer. Mater. Charact. 2025, 223, 114884. [Google Scholar] [CrossRef]
- Lai, T.; Gao, H.; Gao, Y. High-Efficiency Low-Temperature Active Screen Plasma Nitriding by Bias Voltage Optimization of Ti-6Al-4V Titanium Alloy. Thin Solid Films 2025, 825, 140717. [Google Scholar] [CrossRef]
- Rossi, M.C.; Bazaglia Kuroda, P.A.; Solano de Almeida, L.; Rossino, L.S.; Moreira Afonso, C.R. A Detailed Analysis of the Structural, Morphological Characteristics and Micro-Abrasive Wear Behavior of Nitrided Layer Produced in α (CP–Ti), A+β (Ti–6Al–4V), and β (TNZ33) Type Ti Alloys. J. Mater. Res. Technol. 2023, 27, 2399–2412. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, K.; Gao, Y. Influence of Screen Height and Bias Voltage on the Active Screen Plasma Nitriding of Shot-Peened Ti-6Al-4V Titanium Alloy. Surf. Coat. Technol. 2024, 477, 130381. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Hu, Y.; Luo, L.; Jiang, C.; Liu, F.; Jin, W.; Zhu, K.; Long, Z.; Liu, K. Effect of Hydrogen on Microstructure and Mechanical Properties of Plasma-Nitrided Pure Titanium by Cathodic Cage Plasma Nitriding. Surf. Coat. Technol. 2023, 456, 129231. [Google Scholar] [CrossRef]




| Process 1 | Temperature °C | Time h | Pressure Pa | Atomizing Atmosphere mL/min | Nitriding Atmosphere mL/min |
|---|---|---|---|---|---|
| 1 | 640 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| 2 | 655 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| 3 | 670 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| 4 | 685 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| 5 | 700 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| 6 | 715 | 5 | 150 | 360 H2, 160 Ar | 500 N2 |
| Element | C | Fe | O | N | H | Ti |
|---|---|---|---|---|---|---|
| Mass percentage | 0.01 | 0.08 | 0.12 | 0.01 | 0.001 | rest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frączek, T.; Pilarska, M.; Skuza, Z.; Prusak, R. Nitriding of Commercially Pure (CP) Titanium Grade 2 by the Active Screen Method. Materials 2025, 18, 4735. https://doi.org/10.3390/ma18204735
Frączek T, Pilarska M, Skuza Z, Prusak R. Nitriding of Commercially Pure (CP) Titanium Grade 2 by the Active Screen Method. Materials. 2025; 18(20):4735. https://doi.org/10.3390/ma18204735
Chicago/Turabian StyleFrączek, Tadeusz, Milena Pilarska, Zbigniew Skuza, and Rafał Prusak. 2025. "Nitriding of Commercially Pure (CP) Titanium Grade 2 by the Active Screen Method" Materials 18, no. 20: 4735. https://doi.org/10.3390/ma18204735
APA StyleFrączek, T., Pilarska, M., Skuza, Z., & Prusak, R. (2025). Nitriding of Commercially Pure (CP) Titanium Grade 2 by the Active Screen Method. Materials, 18(20), 4735. https://doi.org/10.3390/ma18204735





