The Evaluation of Thermal Stability, Electric Conductivity and Carbide Morphology of Austenitic Ductile Iron Castings
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation
2.2. Microstructure Analysis
2.3. Carbides Identification
2.4. Thermal Stability
2.5. Electric Conductivity
3. Results and Discussion
3.1. Chemical Composition
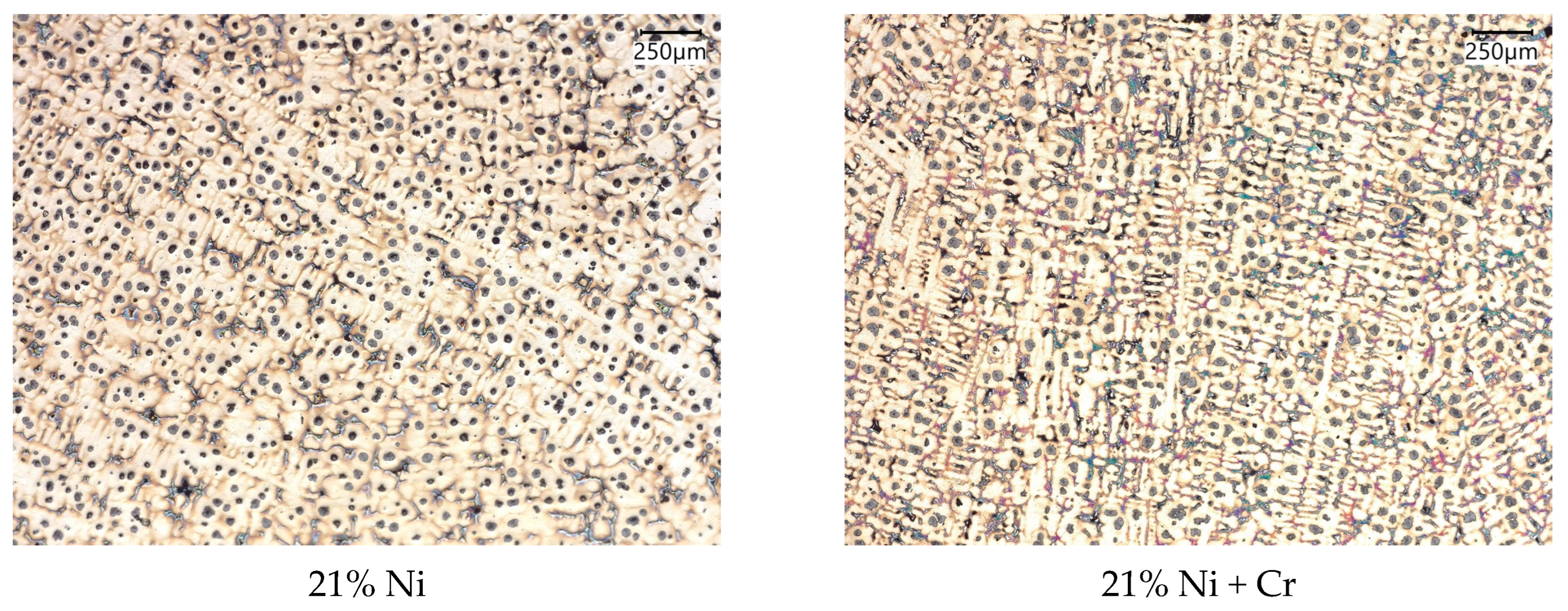
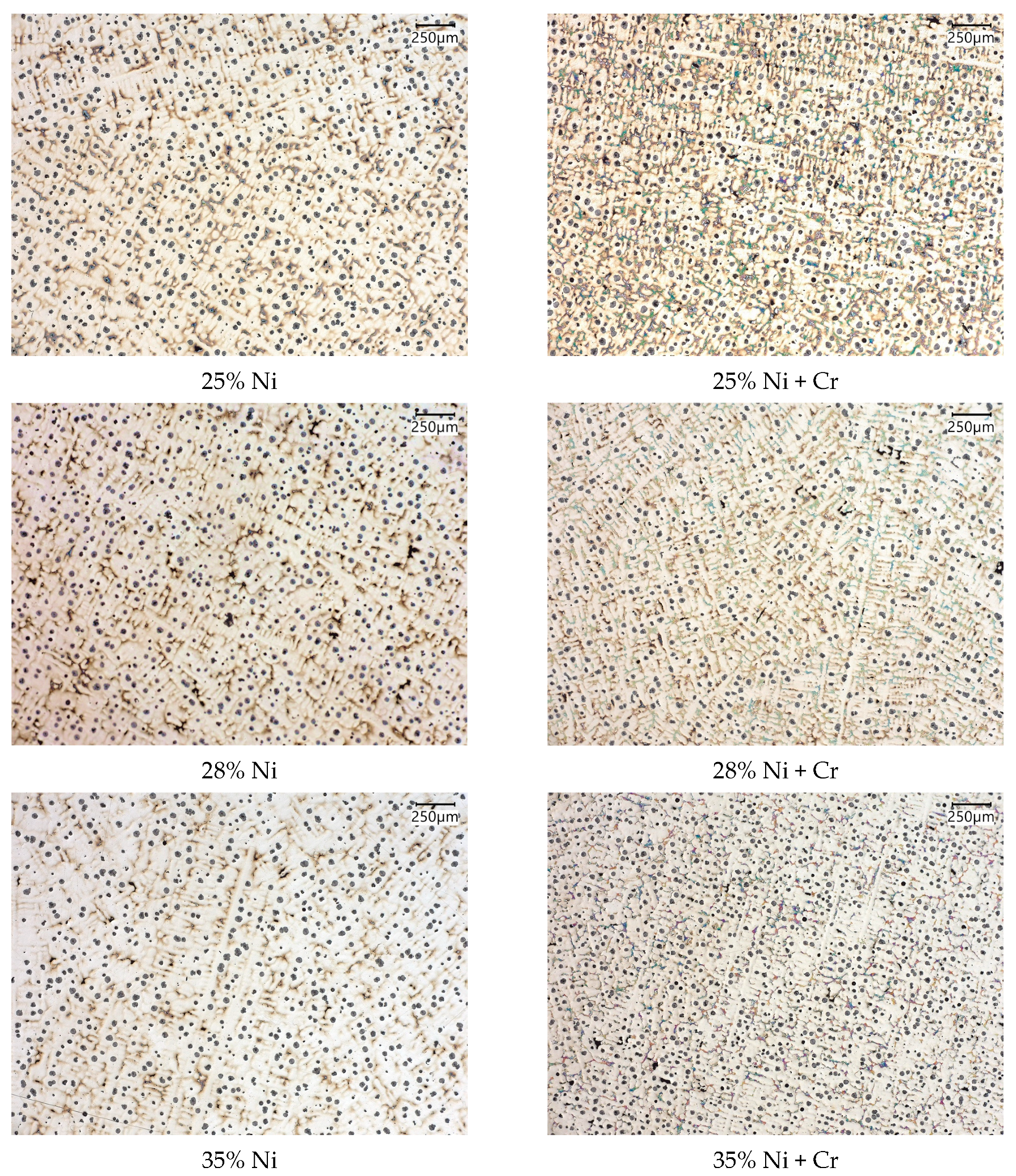
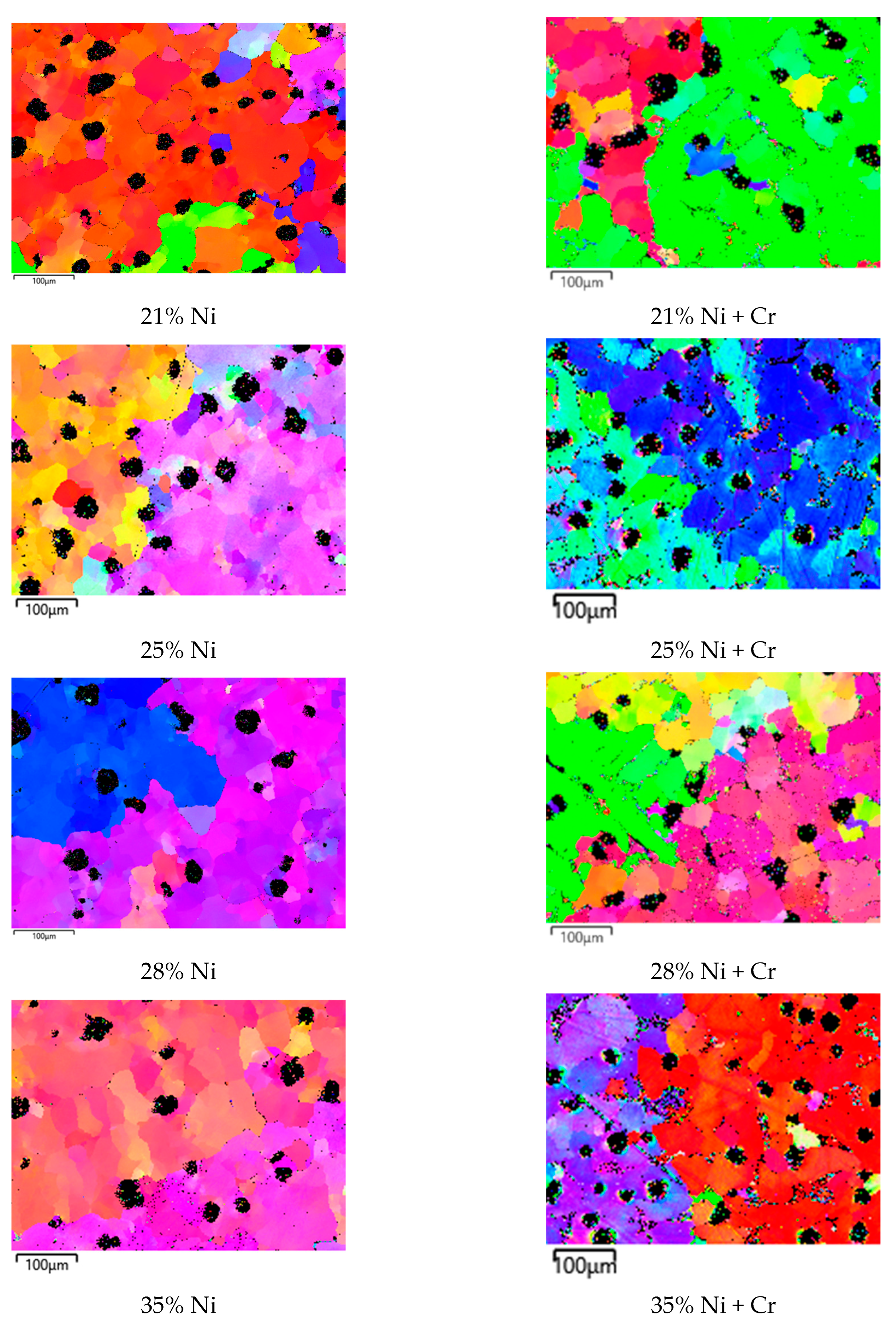
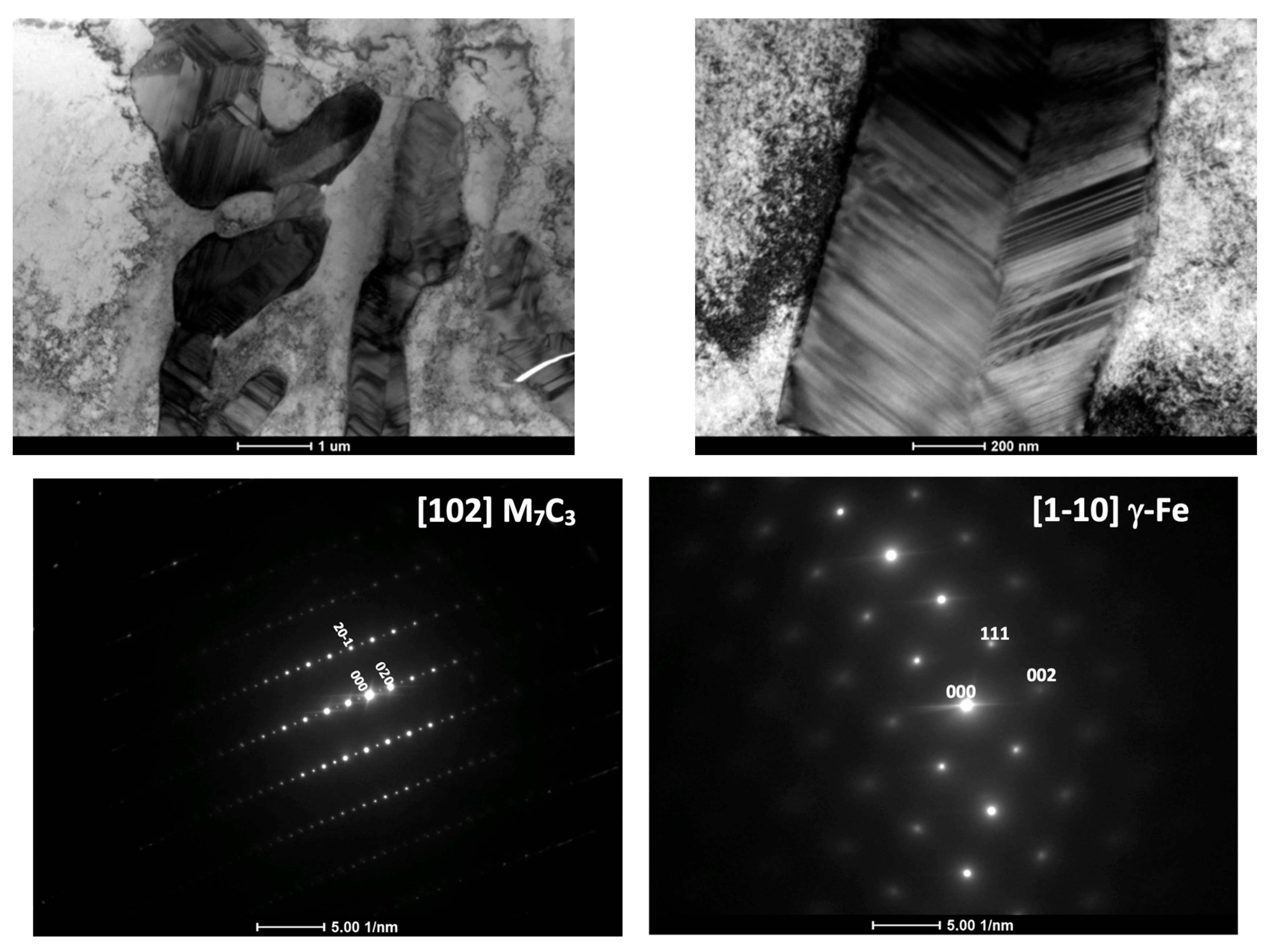
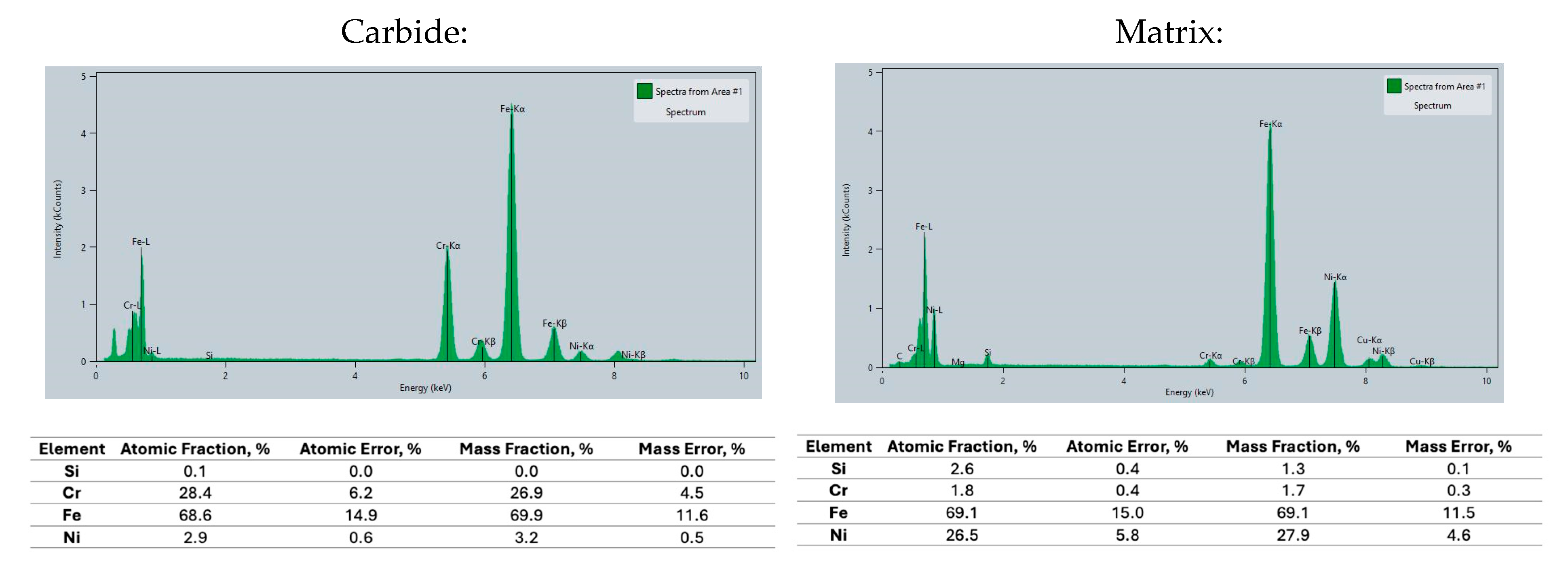
3.2. Microstructure and Carbides Identification
3.3. Resistance Towards Oxidation at High Temperatures
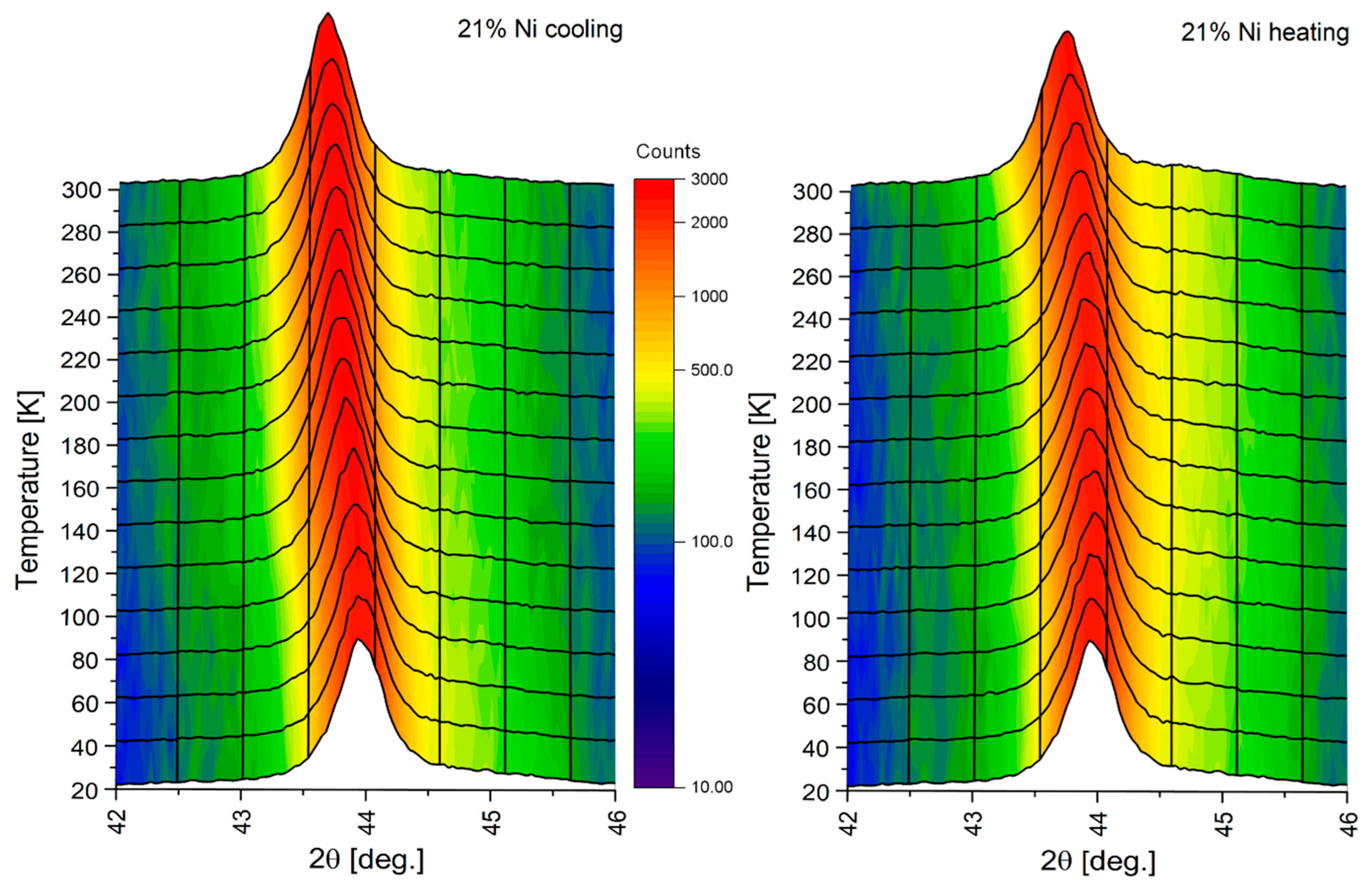
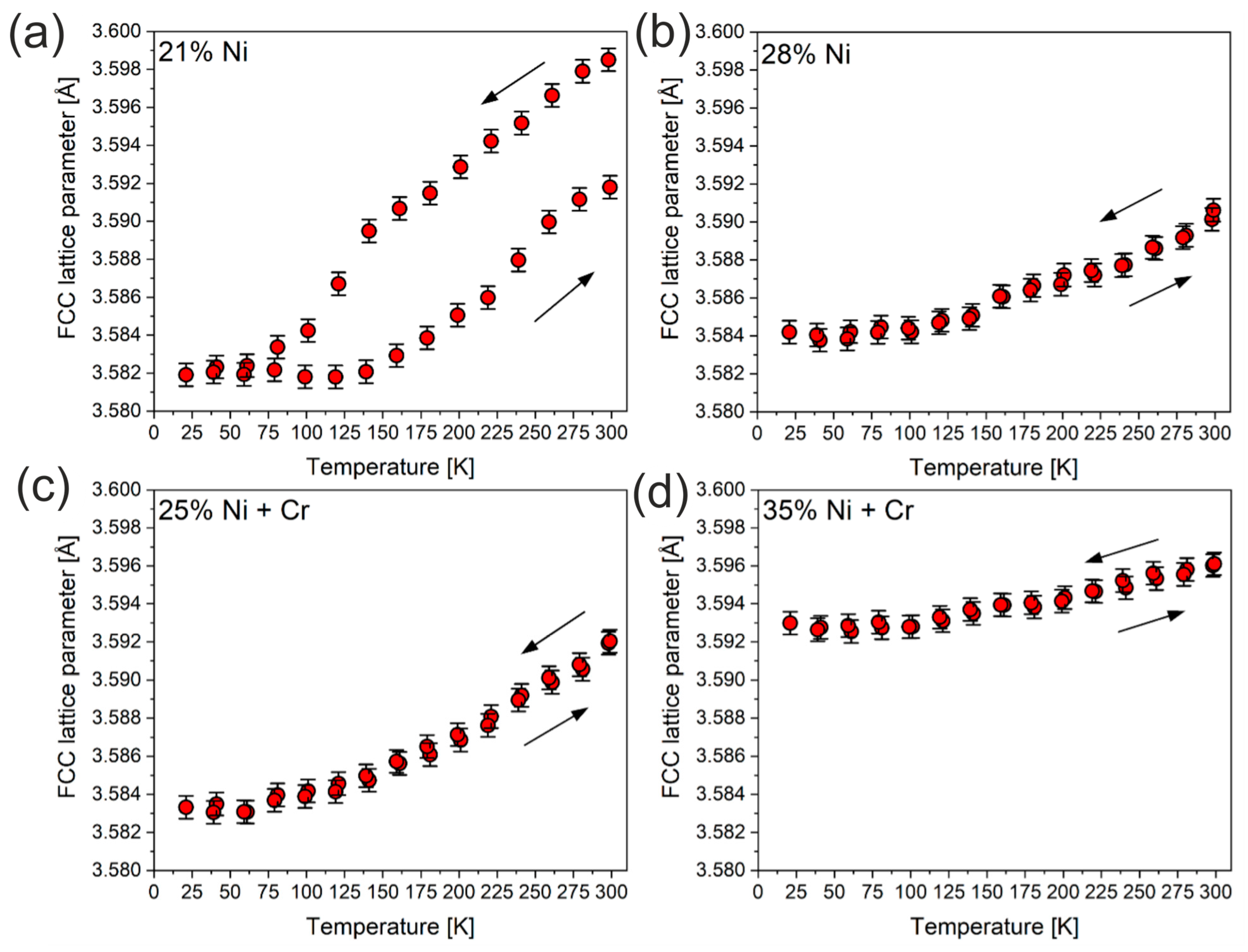
3.4. Thermal Stability at Cryogenic Temperatures
3.5. Electrical Conductivity
4. Conclusions
- In investigated slightly hypo-eutectic ductile iron with a nickel range of 21–35 wt.%, the highly branched dendritic microstructure is predominantly present with graphite nodules located in the interdendritic regions and on the austenite grain boundaries. The highly branched austenitic dendrites determine the uniform distribution of graphite nodules which results in a high-quality engineering material.
- TEM microanalysis revealed that austenitic metallic matrix in chromium-alloyed ductile iron contains Cr = 1.7 ± 0.3 wt.% next to eutectic carbide M7C3 type.
- XRD analysis shows the high thermal stability of high-nickel ductile iron. In particular, it has been proved that the nickel content plays a key role in thermal stability under cryogenic conditions. The minimum required nickel content of 25 wt.% was satisfactory to provide thermal stability at cryogenic temperatures down to 25 K.
- The electrical conductivity characteristic for investigated austenitic high-nickel cast iron was provided, and the attained values are at the level of Ni-based superalloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Foundrymen’s Society. Ductile Iron Handbook, Des Plaines, 2nd ed.; American Foundrymen’s Society: Schaumburg, IL, USA, 1993. [Google Scholar]
- Yang, Y.L.; Cao, Z.Y.; Lian, Z.S.; Yu, H.X. A study on microstructure of ductile Ni-resist cast iron for exhaust manifolds and mechanical property at the condition of alternative thermal cycles. Adv. Mater. Res. 2011, 194–196, 95–99. [Google Scholar] [CrossRef]
- Janus, A.; Granat, K. Heat treatment of Ni–Mn–Cu cast iron. Arch. Civ. Mech. Eng. 2014, 14, 602–607. [Google Scholar] [CrossRef]
- Kühn, H.-J. Thermomechanical fatigue of heat-resistant austenitic cast iron EN-GJSA-XNiSiCr35-5-2 (Ni-Resist D-5S). Int. J. Fatigue 2017, 99, 295–302. [Google Scholar] [CrossRef]
- Alabbasian, F.; Boutorabi, S.M.A.; Kheirandish, S. Effect of inoculation and casting modulus on the microstructure and mechanical properties of ductile Ni-resist cast iron. Mater. Sci. Eng. A 2016, 651, 467–473. [Google Scholar] [CrossRef]
- Moe, G. Properties and Applications of Ni-Resist and Ductile Ni-Resist Alloys, 2nd ed.; Nickel Institute: Durham, NC, USA, 2022. [Google Scholar]
- Fragassa, C.; Radović, N.; Pavlović, A.; Minak, G. Comparison of mechanical properties in compacted and spheroidal graphite irons. Tribol. Ind. 2016, 38, 45–56. [Google Scholar]
- Rashidi, M.M.; Hasbullah, I.M. The effects of solidification on the microstructure and mechanical properties of modified ductile Ni-resist iron with a high manganese content. Mater. Sci. Eng. A 2014, 597, 395–407. [Google Scholar] [CrossRef]
- Rashidi, M.M.; Idris, M.H. Microstructure and mechanical properties of modified ductile Ni-resist with higher manganese content. Mater. Sci. Eng. A 2013, 574, 226–234. [Google Scholar] [CrossRef]
- Bork, M.; Chulist, R.; Górny, M.; Kowalczyk, M.; Marosz, J. The influence of nickel content on the structure parameters and magnetic properties of austenitic ductile iron castings. Arch. Civ. Mech. Eng. 2025, 25, 117. [Google Scholar] [CrossRef]
- Yaakob, K.I.; Maarof, M.R.; Rosnizan, M.A.; Zalani, M.R.; Ahmad, A.H. Evaluation of dendritic structure of modified ductile Ni-resist. J. Adv. Res. Appl. Mech. 2023, 130, 159–167. [Google Scholar] [CrossRef]
- Yaakob, K.I.; Maarof, M.R.; Rosnizan, M.A.; Zalani, M.R.; Ahmad, A.H. Evaluation of graphite size of modified ductile Ni-resist with higher manganese. J. Adv. Res. Appl. Mech. 2023, 130, 130–139. [Google Scholar] [CrossRef]
- Rosnizan, M.A.; Zalani, M.R.; Yaakob, K.I.; Syairah, F.; Maarof, M.R. Evaluation of modified ductile Ni-resist alloy solidification with higher chromium and manganese. J. Adv. Res. Appl. Mech. 2023, 130, 27–37. [Google Scholar] [CrossRef]
- Fatahalla, N.; AbuElEzz, A.; Semeida, M. C, Si and Ni as alloying elements to vary carbon equivalent of austenitic ductile cast iron: Microstructure and mechanical properties. Mater. Sci. Eng. A 2009, 504, 81–89. [Google Scholar] [CrossRef]
- EN 13835:2002; Founding—Austenitic Cast Irons. European Committee for Standardization: Brussels, Belgium, 2002.
- ISO 945-1:2019; Microstructure of Cast Irons—Part 1: Graphite Classification by Visual Analysis. International Organization for Standardization: Geneva, Switzerland, 2019.
- Bork, M.; Górny, M.; Palumbo, G.; Angella, G.; Marosz, J. Microstructure, mechanical parameters, and corrosion resistance of austenitic ductile iron castings. Arch. Civ. Mech. Eng. 2025, 25, 268. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; He, Y.; Li, Y.; Huang, Z.; Liu, G.; Geng, Q. Microstructure and crystallography of M7C3 carbide in chromium cast iron. Mater. Chem. Phys. 2015, 161, 65–73. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Chairuangsri, T.; Brown, A.; Brydson, R.; Edmonds, D.V.; Pearce, J.T.H. Microstructural and crystallographical study of carbides in 30 wt% Cr cast irons. Acta Mater. 2005, 53, 4143–4154. [Google Scholar] [CrossRef]
- Eshed, E.; Choudhuri, D.; Osovski, S. M7C3: The story of a misunderstood carbide. Acta Mater. 2022, 235, 117985. [Google Scholar] [CrossRef]
- Gorunov, A.I. Investigation of M7C3, M23C6 and M3C carbides synthesized on austenitic stainless steel and carbon fibers using laser metal deposition. Surf. Coat. Technol. 2020, 401, 126294. [Google Scholar] [CrossRef]
- He, L. Hexagonal close-packed nickel or Ni3C? J. Magn. Magn. Mater. 2010, 322, 1991–1993. [Google Scholar] [CrossRef]
- Ryś, M. Modeling of Damage Evolution and Martensitic Transformation in Austenitic Steel at Cryogenic Temperature. Arch. Mech. Eng. 2015, 62, 523–537. [Google Scholar] [CrossRef]
- Warlimont, H.; Martienssen, W. (Eds.) Springer Handbook of Materials Data, 2nd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Granta Selector 2023 R1Version, 23.1.1. Database; Ansys: Canonsburg, PA, USA, 2023.





| Cast Iron | Composition [%] | CE% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Ni | Si | Mn | Mg | S | Fe | ||
| 1 | 2.74 | 0.00 | 21.14 | 2.27 | 1.99 | 0.05 | 0.02 | Balance | 4.22 |
| 2 | 2.82 | 0.00 | 25.03 | 2.00 | 2.05 | 0.05 | 0.02 | Balance | 4.38 |
| 3 | 2.50 | 0.00 | 28.04 | 2.11 | 1.90 | 0.05 | 0.02 | Balance | 4.19 |
| 4 | 2.47 | 0.00 | 34.32 | 2.00 | 2.02 | 0.05 | 0.01 | Balance | 4.37 |
| 5 | 2.71 | 2.70 | 21.25 | 2.00 | 1.01 | 0.05 | 0.01 | Balance | 4.14 |
| 6 | 2.64 | 2.53 | 24.67 | 1.94 | 0.95 | 0.05 | 0.02 | Balance | 4.18 |
| 7 | 2.82 | 2.67 | 28.67 | 2.03 | 1.02 | 0.05 | 0.01 | Balance | 4.52 |
| 8 | 2.30 | 2.46 | 35.80 | 1.87 | 1.01 | 0.05 | 0.02 | Balance | 4.23 |
| Electrical Conductivity, MS/m | |||||||
|---|---|---|---|---|---|---|---|
| 21% Ni | 25% Ni | 28% Ni | 35% Ni | ||||
| 0% Cr | 2.5% Cr | 0% Cr | 2.5% Cr | 0% Cr | 2.5% Cr | 0% Cr | 2.5% Cr |
| 1.69 | 1.63 | 1.41 | 1.33 | <0.5 | <0.5 | <0.5 | <0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bork, M.; Górny, M.; Gondek, Ł.; Morgiel, J.; Morgiel, K. The Evaluation of Thermal Stability, Electric Conductivity and Carbide Morphology of Austenitic Ductile Iron Castings. Materials 2025, 18, 4734. https://doi.org/10.3390/ma18204734
Bork M, Górny M, Gondek Ł, Morgiel J, Morgiel K. The Evaluation of Thermal Stability, Electric Conductivity and Carbide Morphology of Austenitic Ductile Iron Castings. Materials. 2025; 18(20):4734. https://doi.org/10.3390/ma18204734
Chicago/Turabian StyleBork, Magdalena, Marcin Górny, Łukasz Gondek, Jerzy Morgiel, and Krzysztof Morgiel. 2025. "The Evaluation of Thermal Stability, Electric Conductivity and Carbide Morphology of Austenitic Ductile Iron Castings" Materials 18, no. 20: 4734. https://doi.org/10.3390/ma18204734
APA StyleBork, M., Górny, M., Gondek, Ł., Morgiel, J., & Morgiel, K. (2025). The Evaluation of Thermal Stability, Electric Conductivity and Carbide Morphology of Austenitic Ductile Iron Castings. Materials, 18(20), 4734. https://doi.org/10.3390/ma18204734







