Fabrication of Novel n-n Heterojunction Bi2O2CO3/AgVO3 Photocatalytic Materials with Visible-Light-Driven Photocatalytic Activity Enhancement
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Bi2O2CO3/AgVO3
2.3. Material Characterizations
2.4. Photocatalytic Performance Evaluation
3. Results and Discussion
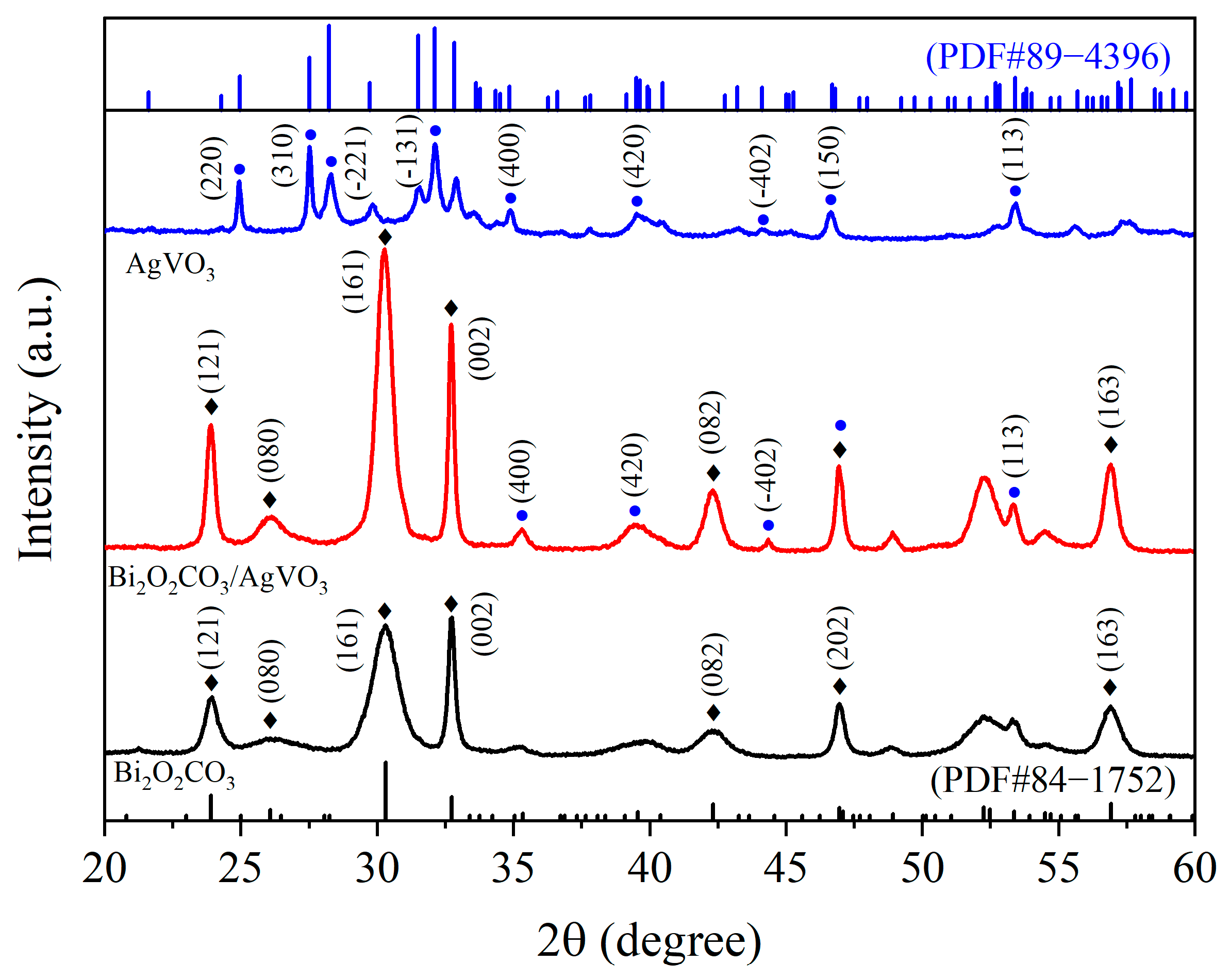
3.1. XRD Analysis
3.2. SEM, EDS, and TEM Analyses
3.3. FT-IR Characterization
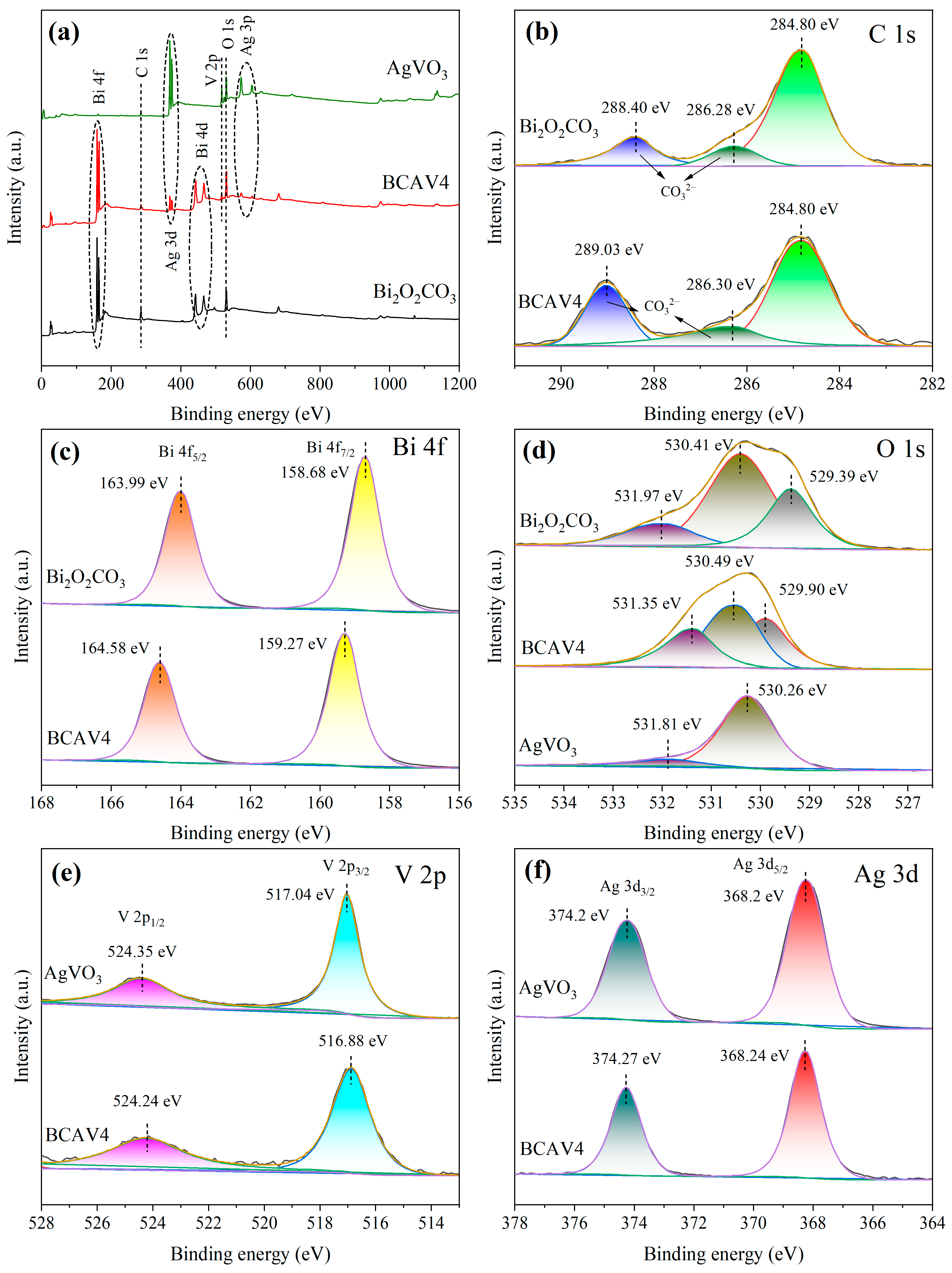
3.4. XPS Analysis
3.5. Optical Properties
3.6. Photoluminescence Analysis
3.7. Photocatalytic Degradation Performance
3.8. Effect of Reaction Parameters on the Photocatalytic Activity of Bi2O2CO3/AgVO3
3.8.1. Photocatalyst Dosage
3.8.2. Initial Concentration of Contaminants
3.9. Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, S.H.; Zhu, L.L.; Li, Y.; Jia, C.M.; Sun, S.Y. The Adsorption Capacity of GONs/CMC/Fe3O4 Magnetic Composite Microspheres and Applications for Purifying Dye Wastewater. Materials 2017, 10, 58. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Ravi, G.; Al-Sehemi, A.G.; Velauthapillai, D. Investigation of optimum Mn dopant level on TiO2 for dye degradation. Chemosphere 2022, 306, 135574. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Wu, J.B.; Chen, X.P.; Li, A.J.; Xing, T.L.; Chen, G.Q. Preparation of CS-LS/AgNPs Composites and Photocatalytic Degradation of Dyes. Materials 2024, 17, 1214. [Google Scholar] [CrossRef]
- Perovic, K.; dela Rosa, F.M.; Kovacic, M.; Kusic, H.; Stangar, U.L.; Fresno, F.; Dionysiou, D.D.; Bozic, A.L. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef]
- Borges, M.E.; Navarro, S.; Carmona, H.D.; Esparza, P. Natural Volcanic Material as a Sustainable Photocatalytic Material for Pollutant Degradation under Solar Irradiation. Materials 2022, 15, 3996. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.M.; Han, H.T.; Wang, Y.X.; Liu, S.T.; Zhao, J.Y.; Meng, X.C.; Li, Z.Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Xue, L.; Du, Y.; Sun, X.Y.; Jiang, N.; Qu, J.N.; Zhao, J.H. Preparation of a Bi4O5I2/Bi2O2CO3 p-n heterojunction with enhanced photocatalytic degradation performance by a one-pot solvothermal method. Mater. Sci. Semicond. Process. 2022, 141, 106447. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.; Zhang, J.; Yu, J.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Luo, Y.; Xu, Y.; Liu, J.; Lin, Y. (002) Oriented Bi2O2CO3 Nanosheets with Enhanced Photocatalytic Performance for Toluene Removal in Air. Catalysts 2020, 10, 389. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, D.; Zhang, Q.; Zhang, Y.; Cao, J.-j.; Shen, Z.; Ho, W.; Lee, S.C. Synthesis of a Bi2O2CO3/ZnFe2O4 heterojunction with enhanced photocatalytic activity for visible light irradiation-induced NO removal. Appl. Catal. B 2018, 234, 70–78. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, M.Q.; Zhao, N.N.; Kan, P.F. Controlled synthesis of Bi2O2CO3 nanorods with enhanced photocatalytic performance. CrystEngComm 2021, 23, 3671–3680. [Google Scholar] [CrossRef]
- Madhusudan, P.; Yu, J.; Wang, W.; Cheng, B.; Liu, G. Facile synthesis of novel hierarchical graphene–Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans. 2012, 41, 14345–14353. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.K.; Liu, T.; Peng, Y.; Xu, B.G. Br-Doped Bi2O2CO3 exposed (001) crystal facets with enhanced photocatalytic activity. CrystEngComm 2017, 19, 5001–5007. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, H.; Shen, M.; Yao, Y.; Gao, Q. Rationally designed a g-C3N4/BiOI/Bi2O2CO3 composite with promoted photocatalytic activity. J. Alloys Compd. 2021, 853, 157307. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, D.; Liu, X.; Yu, C.; Yang, K.; Liu, M. Hierarchical δ-Bi2O3/Bi2O2CO3 composite microspheres: Phase transformation fabrication, characterization and high photocatalytic performance. Res. Chem. Intermed. 2018, 44, 5995–6010. [Google Scholar] [CrossRef]
- Qiu, F.; Li, W.; Wang, F.; Li, H.; Liu, X.; Ren, C. Preparation of novel p-n heterojunction Bi2O2CO3/BiOBr photocatalysts with enhanced visible light photocatalytic activity. Colloids Surf. A 2017, 517, 25–32. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, Q.; Huang, J.; Song, D.; Chen, L.; Chen, Q.; Zhai, C.; Wang, B.; Klemeš, J.J.; Tao, H. Visible-light driven p–n heterojunction formed between α-Bi2O3 and Bi2O2CO3 for efficient photocatalytic degradation of tetracycline. RSC Adv. 2023, 13, 1594–1605. [Google Scholar] [CrossRef]
- Wang, Y.; Lang, X.; Liu, X.; Kang, Y. Construction of visible-light driven AgVO3/BiOBr p-n heterojunction for improving visible-light photocatalytic performance. Mater. Lett. 2022, 325, 132849. [Google Scholar] [CrossRef]
- Ren, C.; Li, X.Y.; Luo, Q.W.; Liu, R.P.; Yang, Z.; Xu, L.C. Electronic structure and optical absorption properties of β-AgVO3 with vacancy defects. Acta Phys. Sin. 2017, 66, 157101. [Google Scholar]
- Sasikumar, K.; Ju, H. Recent Advances in Vanadate-Based Materials for Photocatalytic Hydrogen Production. Molecules 2025, 30, 789. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Lin, M.; Huang, C.; Lei, Z.; Cao, H.; Qi, F.; Ouyang, X.; Yun, Z. Efficient visible-light-driven S-scheme AgVO3/Ag2S heterojunction photocatalyst for boosting degradation of organic pollutants. Environ. Pollut. 2023, 325, 121436. [Google Scholar] [CrossRef]
- Alzahrani, K.A.; Ismail, A.A. Highly efficient AgVO3/WO3 photocatalyst n-n heterojunction toward visible-light induced degradation antibiotic. J. Ind. Eng. Chem. 2023, 124, 270–278. [Google Scholar] [CrossRef]
- Bavani, T.; Madhavan, J.; Prasad, S.; AlSalhi, M.S.; Aljaffreh, M.; Vijayanand, S. Fabrication of novel AgVO3/BiOI nanocomposite photocatalyst with photoelectrochemical activity towards the degradation of Rhodamine B under visible light irradiation. Environ. Res. 2021, 200, 111365. [Google Scholar] [CrossRef]
- Bavani, T.; Madhavan, J.; Prasad, S.; AlSalhi, M.S.; AlJaafreh, M.J. A straightforward synthesis of visible light driven BiFeO3/AgVO3 nanocomposites with improved photocatalytic activity. Environ. Pollut. 2021, 269, 116067. [Google Scholar] [CrossRef]
- Senthil, R.A.; Sun, M.; Pan, J.; Osman, S.; Khan, A.; Sun, Y. Facile fabrication of a new BiFeWO6/α-AgVO3 composite with efficient visible-light photocatalytic activity for dye-degradation. Opt. Mater. 2019, 92, 284–293. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Mousavi, M.; Ghasemi, J.B.; Van Le, Q.; Delbari, S.A.; Namini, A.S.; Asl, M.S.; Shokouhimehr, M.; Mohammadi, M. Novel p-n Heterojunction Nanocomposite: TiO2 QDs/ZnBi2O4 Photocatalyst with Considerably Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2020, 124, 27519–27528. [Google Scholar] [CrossRef]
- Sun, M.; Senthil, R.A.; Pan, J.; Osman, S.; Khan, A. A Facile Synthesis of Visible-Light Driven Rod-on-Rod like α-FeOOH/α-AgVO3 Nanocomposite as Greatly Enhanced Photocatalyst for Degradation of Rhodamine B. Catalysts 2018, 8, 392. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Liu, G.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Synthesis of carnation flower-like Bi2O2CO3 photocatalyst and its promising application for photoreduction of Cr(VI). Adv. Powder Technol. 2022, 33, 103481. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Yi, Z.; Wang, S.; Wu, X.; Li, R.; Yang, H. Comparative investigation on synthesis, morphological tailoring and photocatalytic activities of Bi2O2CO3 nanostructures. Colloids Surf. A 2022, 644, 128758. [Google Scholar] [CrossRef]
- Liang, N.; Wang, M.; Jin, L.; Huang, S.; Chen, W.; Xu, M.; He, Q.; Zai, J.; Fang, N.; Qian, X. Highly Efficient Ag2O/Bi2O2CO3 p-n Heterojunction Photocatalysts with Improved Visible-Light Responsive Activity. ACS Appl. Mater. Interfaces 2014, 6, 11698–11705. [Google Scholar] [CrossRef]
- Liu, X.; Cai, L. Novel indirect Z-scheme photocatalyst of Ag nanoparticles and polymer polypyrrole co-modified BiOBr for photocatalytic decomposition of organic pollutants. Appl. Surf. Sci. 2018, 445, 242–254. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, H.; Li, H.; Liu, X.; Ren, C.; Wang, F.; Li, W. Novel Ag2CrO4/Bi2O2CO3 heterojunction: Simple preparation, wide visible-light absorption band and excellent photocatalytic activity. Chem. Phys. 2019, 517, 60–66. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, L.; Liang, J.; Pan, Y.; Zhang, J.; Wang, H.; Leng, L.; Wu, Z.; Guan, R.; Zeng, G. In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation. Chem. Eng. J. 2019, 356, 371–381. [Google Scholar] [CrossRef]
- Li, J.; Han, M.; Guo, Y.; Wang, F.; Meng, L.; Mao, D.; Ding, S.; Sun, C. Hydrothermal synthesis of novel flower-like BiVO4/Bi2Ti2O7 with superior photocatalytic activity toward tetracycline removal. Appl. Catal. A 2016, 524, 105–114. [Google Scholar]
- Kostjukova, L.O.; Leontieva, S.V.; Kostjukov, V.V. Vibronic absorption spectrum and electronic properties of methylene blue in aqueous solution: TD-DFT study. J. Mol. Liq. 2021, 336, 116369. [Google Scholar] [CrossRef]
- Tchaikovskaya, O.; Chaidonova, V.; Bocharnikova, E.; Telminov, E.; Dmitrieva, N.; Ezhov, D. Solvent effect on the Spectra of Methylene Green and Methylene Blue. J. Fluoresc. 2023, 33, 685–695. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Chen, S.; Xie, Y.; Ma, Y.; Luo, Y.; Ling, Y.; Ye, J. CdS/ZnIn2S4 type II heterojunctions improve photocatalytic hydrogen production: Faster electron–hole separation and wider visible light utilization. Sustain. Energy Fuels 2022, 6, 4893–4902. [Google Scholar] [CrossRef]










| Photocatalyst | Pseudo-First-Order Kinetic Model and Parameters | ||
|---|---|---|---|
| Kinetic Equations | Kinetic Constants (min−1) | R2 | |
| Bi2O2CO3 | y = 0.7081 + 0.0090x | 0.0090 | 0.9853 |
| AgVO3 | y = 0.1190 + 0.0081x | 0.0081 | 0.9998 |
| BCAV2 | y = 0.8406 + 0.0136x | 0.0136 | 0.9998 |
| BCAV3 | y = 0.6782 + 0.0169x | 0.0169 | 0.9999 |
| BCAV4 | y = 0.7664 + 0.0226x | 0.0226 | 0.9997 |
| BCAV5 | y = 0.9484 + 0.0206x | 0.0206 | 0.9039 |
| BCAV6 | y = 0.6676 + 0.0191x | 0.0191 | 0.9861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, W.; Yuan, H.; Huang, S. Fabrication of Novel n-n Heterojunction Bi2O2CO3/AgVO3 Photocatalytic Materials with Visible-Light-Driven Photocatalytic Activity Enhancement. Materials 2025, 18, 4705. https://doi.org/10.3390/ma18204705
Hua W, Yuan H, Huang S. Fabrication of Novel n-n Heterojunction Bi2O2CO3/AgVO3 Photocatalytic Materials with Visible-Light-Driven Photocatalytic Activity Enhancement. Materials. 2025; 18(20):4705. https://doi.org/10.3390/ma18204705
Chicago/Turabian StyleHua, Weijie, Huixin Yuan, and Songhua Huang. 2025. "Fabrication of Novel n-n Heterojunction Bi2O2CO3/AgVO3 Photocatalytic Materials with Visible-Light-Driven Photocatalytic Activity Enhancement" Materials 18, no. 20: 4705. https://doi.org/10.3390/ma18204705
APA StyleHua, W., Yuan, H., & Huang, S. (2025). Fabrication of Novel n-n Heterojunction Bi2O2CO3/AgVO3 Photocatalytic Materials with Visible-Light-Driven Photocatalytic Activity Enhancement. Materials, 18(20), 4705. https://doi.org/10.3390/ma18204705






