Tunable Fly Ash-Based Geopolymer Fibers for Multivariate Heavy-Metal Adsorption: Optimization and Mechanistic Insights
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Geopolymer Preparation
2.2. Fabrication of the Geopolymer–PES Composite Fibers
2.3. Characterization of Composite Fibers
2.4. Adsorption Experiments
3. Results and Discussion
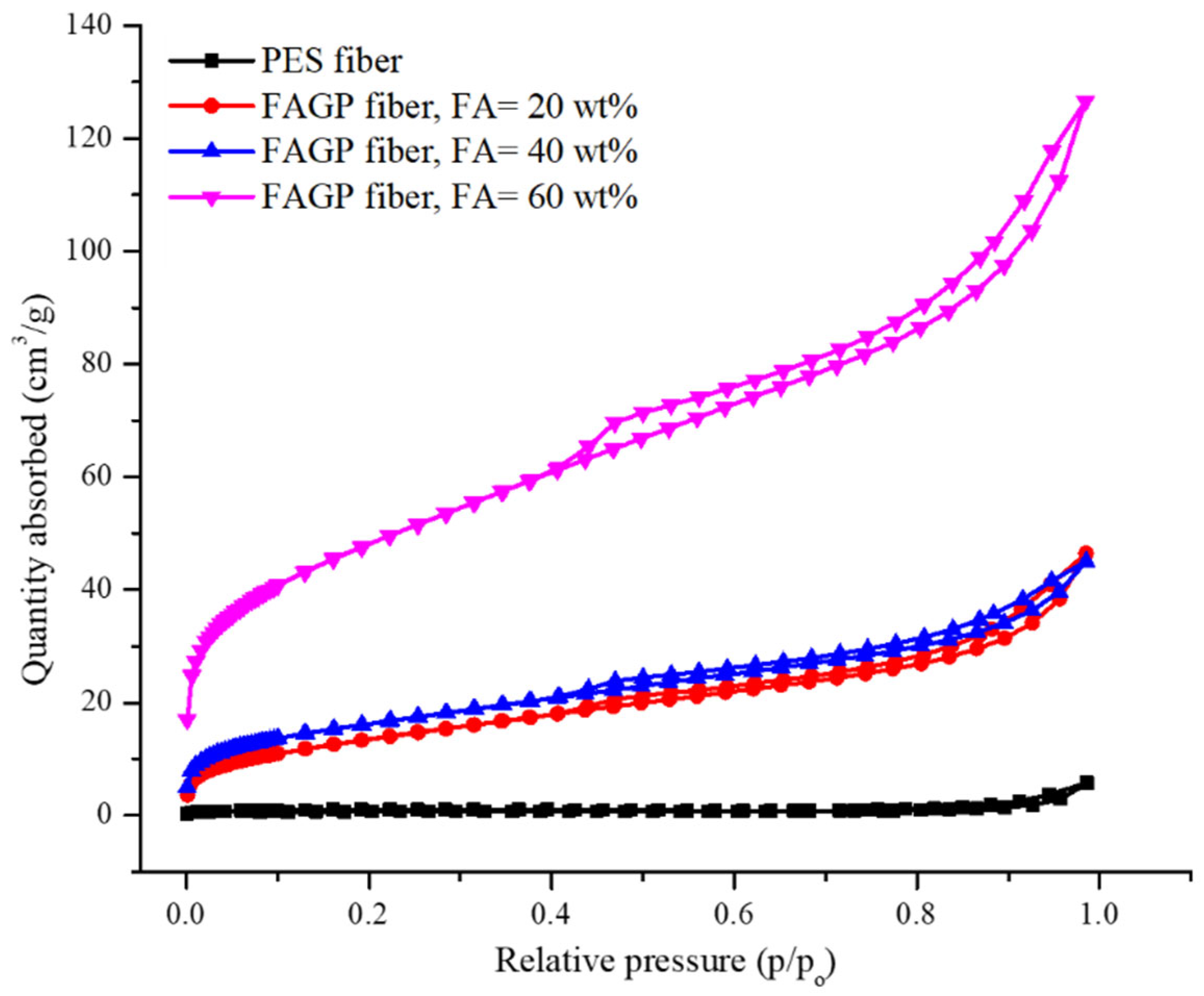
3.1. Fiber Properties and Morphology
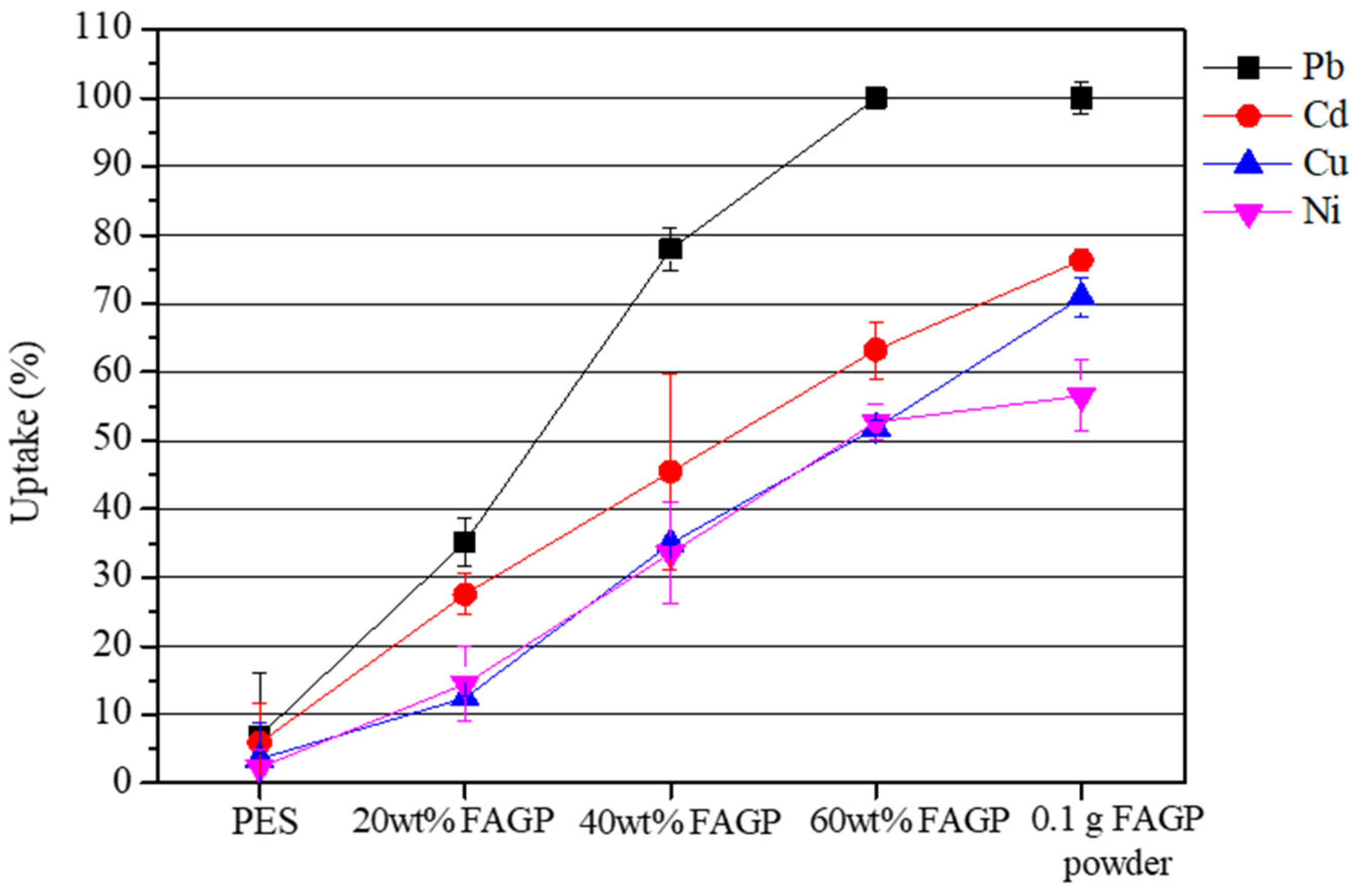
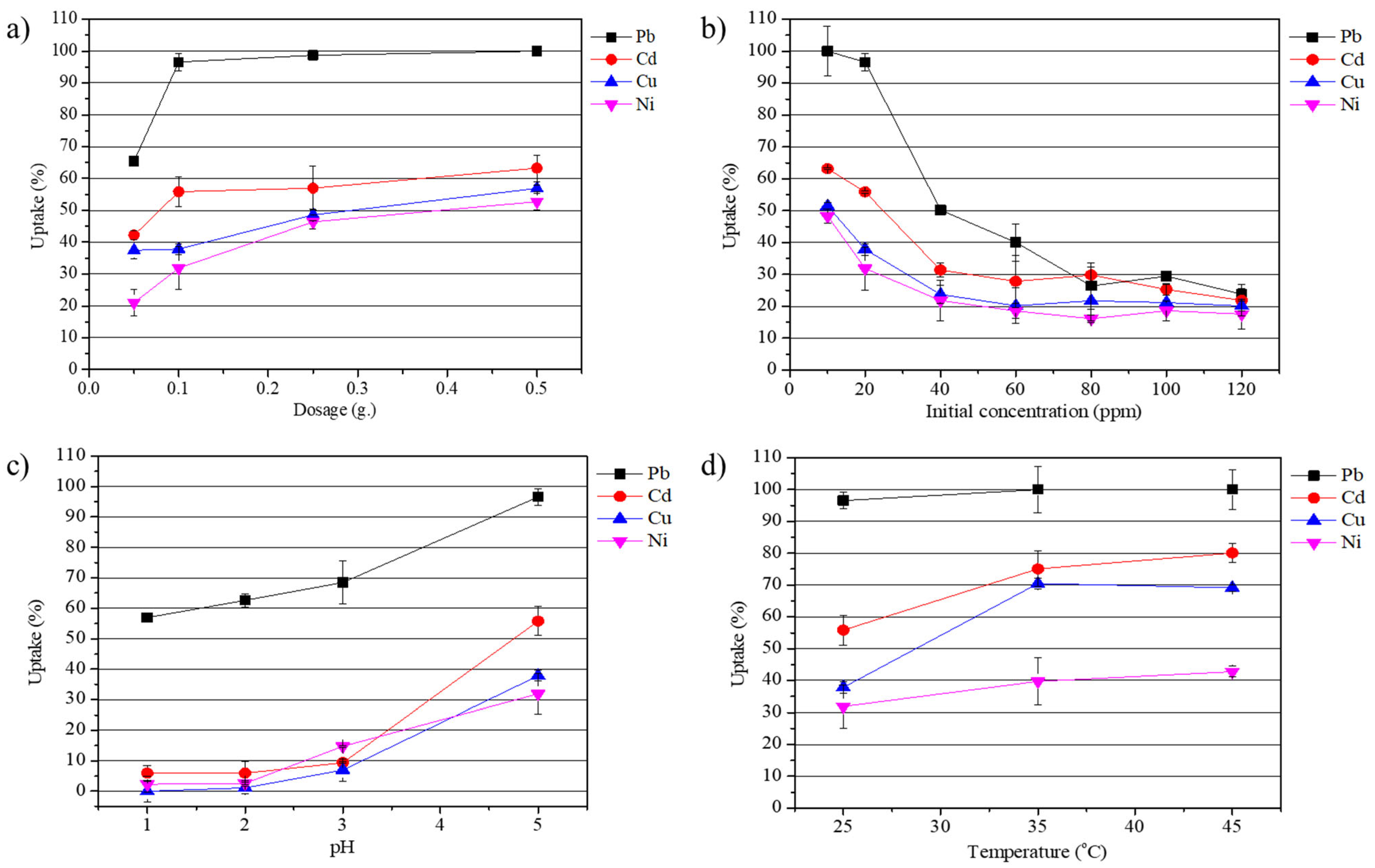
3.2. Adsorption Performance Under Different Conditions
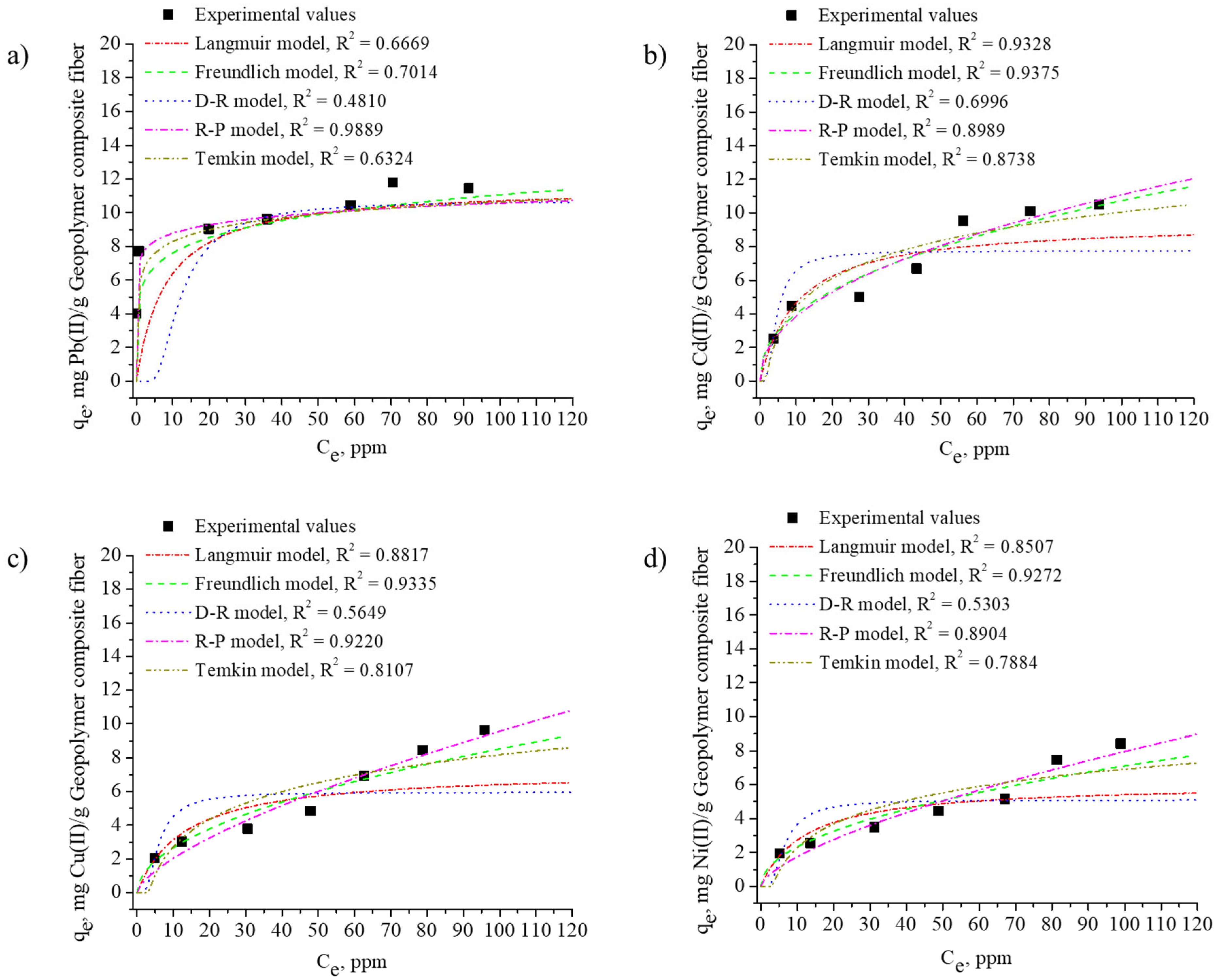
3.3. Isotherm Model Analysis
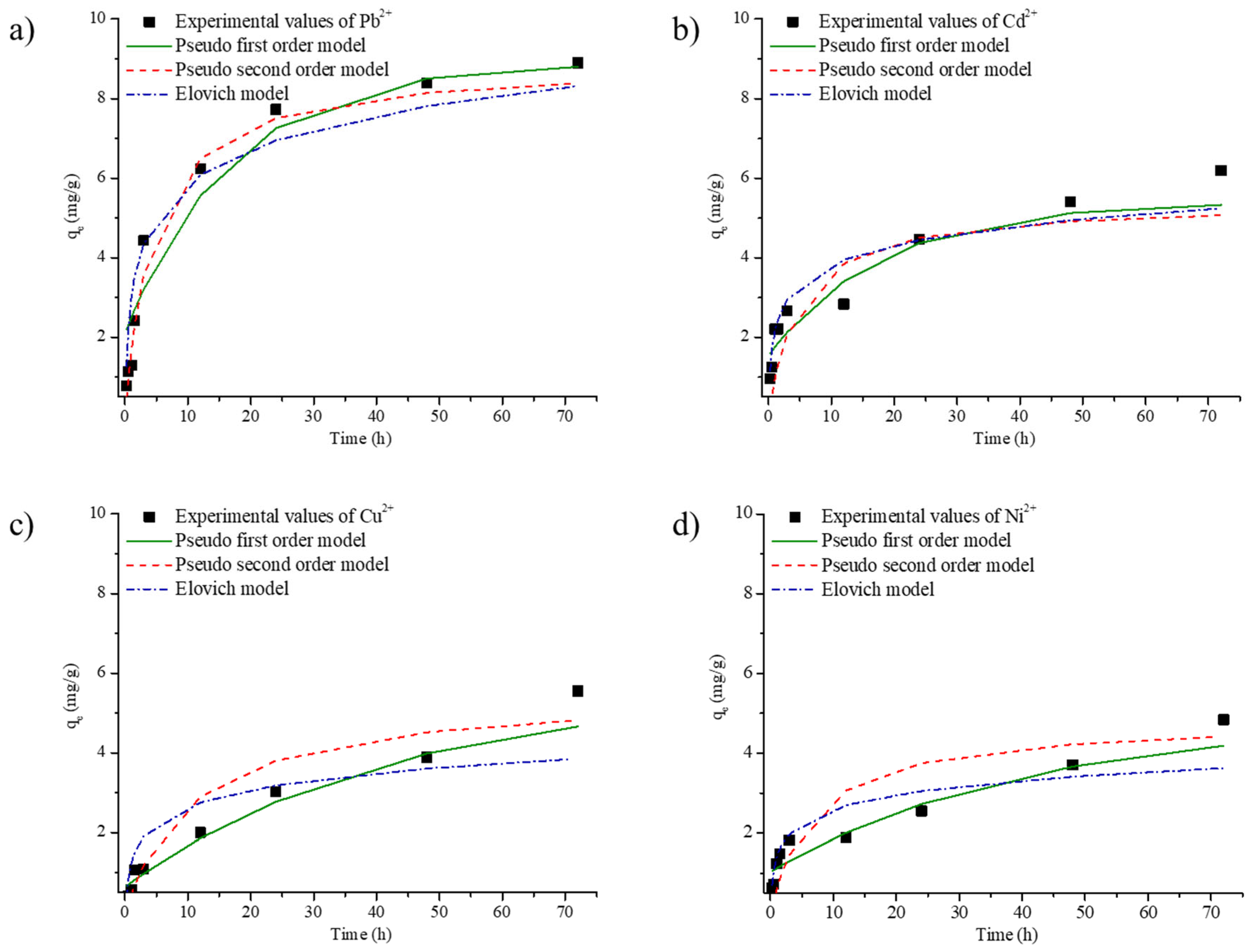
3.4. Kinetics and Intraparticle Diffusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siyal, A.A.; Shamsuddin, M.R.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Low, A. Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. J. Clean. Prod. 2019, 229, 232–243. [Google Scholar] [CrossRef]
- Al-Shahrani, S.S. Treatment of wastewater contaminated with cobalt using Saudi activated bentonite. Alex. Eng. J. 2014, 53, 205–211. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Fugetsu, B.; Tanaka, S. Selective elimination of lead(II) ions by alginate/polyurethane composite foams. J. Hazard. Mater. 2009, 162, 423–429. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J.; Rao, L.A.K. Removal and recovery of heavy metals from electroplating wastewater by using Kyanite as an adsorbent. J. Hazard. Mater. 2001, 87, 127–137. [Google Scholar] [CrossRef]
- Jalayeri, H.; Salarirad, M.M.; Ziaii, M. Behavior and mechanism of various components of soil in Cu (II) adsorption from aqueous solution. Desalin. Water Treat. 2016, 57, 8494–8503. [Google Scholar] [CrossRef]
- Nogawa, K.; Kobayashi, E.; Okubo, Y.; Suwazono, Y. Environmental cadmium exposure, adverse effects and preventive measures in Japan. Biometals 2004, 17, 581–587. [Google Scholar] [CrossRef]
- Kara, İ.; Yilmazer, D.; Akar, S.T. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- López, F.J.; Satoshi, S.; Tagaya, M.; Kobayashi, T. Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
- Chiarle, S.; Ratto, M.; Rovatti, M. Mercury removal from water by ion exchange resins adsorption. Water Res. 2000, 34, 2971–2978. [Google Scholar] [CrossRef]
- Bapat, S.A.; Jaspal, D.K. Parthenium hysterophorus: Novel adsorbent for the removal of heavy metals and dyes. Glob. J. Environ. Sci. 2016, 2, 135–144. [Google Scholar]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vazquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sindhunata; Van Deventer, J.; Lukey, G.; Xu, H. Effect of curing temperature and silicate concentration on fly-ash-based geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, X.; Zhang, W.; Tong, Z. Preparation of geopolymer precursors by sol–gel method and their characterization. J. Mater. Sci. 2009, 44, 3991–3996. [Google Scholar] [CrossRef]
- Tavor, D.; Wolfson, A.; Shamaev, A.; Shvarzman, A. Recycling of industrial wastewater by its immobilization in geopolymer cement. Ind. Eng. Chem. Res. 2007, 46, 6801–6805. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lukey, G. A comparative study of kaolinite versus metakaolinite in fly ash based geopolymers containing immobilized metals. Chem. Eng. Commun. 2004, 191, 531–549. [Google Scholar] [CrossRef]
- Al-Zboon, K.; Al-Harahsheh, M.S.; Hani, F.B. Fly ash-based geopolymer for Pb removal from aqueous solution. J. Hazard. Mater. 2011, 188, 414–421. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Mohan, D.; Pittman, C.U., Jr. Arsenate adsorption on three types of granular schwertmannite. Water Res. 2013, 47, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, Z.; Feng, C.; Zhu, D.; Yang, Y.; Sugiura, N. Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. J. Hazard. Mater. 2011, 186, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2020, 244, 118844. [Google Scholar] [CrossRef]
- Papa, E.; Landi, E.; Miccio, F.; Medri, V. K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials 2022, 15, 1008. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of highly efficient porous inorganic polymer microspheres for the adsorptive removal of Pb2+ from wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. Porous fly ash-based geopolymer composite fiber as an adsorbent for removal of heavy metal ions from wastewater. Mater. Lett. 2019, 236, 30–33. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Capito, J.A.; Vilando, A.C.; Lu, M.-C. Effect of EDTA and CH2O on copper recovery from simulated electroless copper plating spent rinse water by unseeded fluidized-bed granulation process. Sep. Purif. Technol. 2020, 253, 117460. [Google Scholar] [CrossRef]
- Piedra, E.; Álvarez, J.R.; Luque, S. Hexavalent chromium removal from chromium plating rinsing water with membrane technology. Desalin. Water Treat. 2015, 53, 1431–1439. [Google Scholar] [CrossRef]
- Snukiškis, J.; Kaušpėdienė, D. Integrated removal of nonionic surfactant and cobalt(II) from plating rinse water. Colloids Surf. A 2005, 253, 27–32. [Google Scholar] [CrossRef]
- Revathi, M.; Saravanan, M.; Chiya, A.B.; Velan, M. Removal of Copper, Nickel, and Zinc Ions from Electroplating Rinse Water. CLEAN—Soil Air Water 2012, 40, 66–79. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Aghababai Beni, A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring Techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.-y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef]
- Nakamoto, K.; Ohshiro, M.; Kobayashi, T. Mordenite zeolite—Polyethersulfone composite fibers developed for decontamination of heavy metal ions. J. Environ. Chem. Eng. 2017, 5, 513–525. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef]
- Djukić, A.; Jovanović, U.; Tuvić, T.; Andrić, V.; Grbović Novaković, J.; Ivanović, N.; Matović, L. The potential of ball-milled Serbian natural clay for removal of heavy metal contaminants from wastewaters: Simultaneous sorption of Ni, Cr, Cd and Pb ions. Ceram. Int. 2013, 39, 7173–7178. [Google Scholar] [CrossRef]
- Ding, P.; Song, W.; Wang, C.; Yu, Z. Adsorption of Cu(II) and Pb(II) from aqueous solutions onto a metakaolin-based geopolymer. Desalin. Water Treat. 2019, 158, 164–173. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Qiu, X.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. Novel porous phosphoric acid-based geopolymer foams for adsorption of Pb(II), Cd(II) and Ni(II) mixtures: Behavior and mechanism. Ceram. Int. 2023, 49, 7030–7039. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Lai, S.H.; Ling, T.-C. Synthesis of porous geopolymer sphere for Ni(II) removal. Ceram. Int. 2021, 47, 29055–29063. [Google Scholar] [CrossRef]



| Chemical Compound (%) | PES | Washed FAGP Powder | 20 wt% FAGP Fiber | 40 wt% FAGP Fiber | 60 wt% FAGP Fiber |
|---|---|---|---|---|---|
| SiO2 | - | 44.80 | 5.44 | 25.30 | 42.40 |
| Al2O3 | - | 16.30 | 2.13 | 6.36 | 9.89 |
| Fe2O3 | - | 12.40 | 1.15 | 3.89 | 6.39 |
| CaO | - | 7.64 | 0.44 | 1.65 | 2.65 |
| MgO | - | 1.49 | 0.16 | 0.57 | 0.77 |
| SO3 | 20.70 | 0.10 | 20.30 | 18.10 | 13.20 |
| Na2O | - | 0.80 | 0.12 | 0.23 | 0.42 |
| CO2 | 79.10 | 13.80 | 69.90 | 52.40 | 40.00 |
| Samples | Obvious Specific Volume (cm3/g) | Tensile Strength (MPa) | Surface Area (m2/g) |
|---|---|---|---|
| PES | 0.70 | 5.83 | 27.39 |
| 20 wt% FAGP | 0.62 | 4.17 | 50.05 |
| 40 wt% FAGP | 0.60 | 2.35 | 57.50 |
| 60 wt% FAGP | 0.53 | 1.40 | 71.67 |
| FAGP powder | - | - | 85.01 |
| Isotherm Model | Parameter | Metal | |||
|---|---|---|---|---|---|
| Pb2+ | Cd2+ | Cu2+ | Ni2+ | ||
| Langmuir | qm | 11.574 | 9.343 | 7.246 | 6.068 |
| KL | 0.123 | 0.098 | 0.076 | 0.082 | |
| R2 | 0.6669 | 0.9328 | 0.8817 | 0.8507 | |
| RL | 0.082 | 0.098 | 0.120 | 0.110 | |
| Freundlich | KF | 5.249 | 1.497 | 0.835 | 0.762 |
| 1/n | 0.162 | 0.428 | 0.505 | 0.485 | |
| R2 | 0.7014 | 0.6996 | 0.9335 | 0.9272 | |
| Redlich–Peterson (R–P) | KRP | 8.40 × 106 | 27,493.8 | 11,319.9 | 5640.06 |
| a | 1.15 × 106 | 20,586.1 | 26,018 | 14,686.6 | |
| g | 0.920 | 0.540 | 0.329 | 0.342 | |
| R2 | 0.8289 | 0.8989 | 0.9220 | 0.8904 | |
| Dubinin–Radushkevich (D–R) | qm | 10.710 | 7.752 | 5.964 | 5.105 |
| β | 2 × 10−5 | 3 × 10−6 | 5 × 10−6 | 6 × 10−6 | |
| R2 | 0.4810 | 0.6996 | 0.5649 | 0.5303 | |
| E | 158.114 | 408.248 | 316.228 | 288.675 | |
| Temkin | AT | 1.002 | 0.998 | 0.997 | 0.998 |
| b | 2433.316 | 1010.196 | 1042.002 | 1230.977 | |
| B | 1.018 | 2.453 | 2.378 | 2.013 | |
| R2 | 0.6324 | 0.8738 | 0.8107 | 0.7884 | |
| qe of Experimentally value (mg/g) | qe | 11.78 | 10.79 | 9.64 | 8.42 |
| Metal | Intraparticle Diffusion | ||
|---|---|---|---|
| kp (mg/g h0.5) | C (mg/g) | R2 | |
| Pb2+ | 1.684 | 0.1204 | 0.9907 |
| Cd2+ | 0.681 | 0.932 | 0.9527 |
| Cu2+ | 0.596 | 0.034 | 0.9869 |
| Ni2+ | 0.404 | 0.063 | 0.9618 |
| Kinetic Model | Parameter | Metal | |||
|---|---|---|---|---|---|
| Pb2+ | Cd2+ | Cu2+ | Ni2+ | ||
| Pseudo-first order | k1 (min−1) | 0.0001 | 0.0001 | 0.0003 | 0.0004 |
| qm (mg/g) | 6.794 | 3.856 | 4.908 | 3.806 | |
| R2 | 0.9501 | 0.8960 | 0.9775 | 0.9506 | |
| Pseudo-second order | k2 (g/mg min) | 0.0004 | 0.0005 | 0.0002 | 0.0005 |
| qm (mg/g) | 9.327 | 6.208 | 5.550 | 4.670 | |
| R2 | 0.9977 | 0.6878 | 0.9125 | 0.9157 | |
| Elovich | β | 0.8030 | 1.3869 | 1.6339 | 1.9409 |
| α | 0.2295 | 0.2395 | 0.0772 | 0.1347 | |
| R2 | 0.8966 | 0.8759 | 0.7779 | 0.8380 | |
| qe of Experimentally value (mg/g) | qe | 8.899 | 6.192 | 5.551 | 4.840 |
| Adsorbent Materials | Metal Ion | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| FAGP Composite Fibers | Pb2+ | 11.78 | This study |
| (60 wt%) | Cd2+ | 10.79 | |
| Cu2+ | 9.64 | ||
| Ni2+ | 8.42 | ||
| Magnetic activated carbon | Pb2+ | 253.20 | [42] |
| powder | Cd2+ | 73.30 | |
| Natural clay powder | Pb2+ | 9.91 | [43] |
| Cd2+ | 9.45 | ||
| Ni2+ | 10.20 | ||
| Metakaolin-based | Pb2+ | 312.50 | [44] |
| Geopolymers powder | Cu2+ | 178.60 | |
| Geopolymer foams | Pb2+ | 11.99 | [45] |
| Cd2+ | 2.81 | ||
| Ni2+ | 6.16 | ||
| Geopolymer spheres | Ni2+ | 19.94 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Zhou, Y.; Liao, S.; Onutai, S. Tunable Fly Ash-Based Geopolymer Fibers for Multivariate Heavy-Metal Adsorption: Optimization and Mechanistic Insights. Materials 2025, 18, 4698. https://doi.org/10.3390/ma18204698
Luo G, Zhou Y, Liao S, Onutai S. Tunable Fly Ash-Based Geopolymer Fibers for Multivariate Heavy-Metal Adsorption: Optimization and Mechanistic Insights. Materials. 2025; 18(20):4698. https://doi.org/10.3390/ma18204698
Chicago/Turabian StyleLuo, Gongming, Yuanbing Zhou, Shuangquan Liao, and Sujitra Onutai. 2025. "Tunable Fly Ash-Based Geopolymer Fibers for Multivariate Heavy-Metal Adsorption: Optimization and Mechanistic Insights" Materials 18, no. 20: 4698. https://doi.org/10.3390/ma18204698
APA StyleLuo, G., Zhou, Y., Liao, S., & Onutai, S. (2025). Tunable Fly Ash-Based Geopolymer Fibers for Multivariate Heavy-Metal Adsorption: Optimization and Mechanistic Insights. Materials, 18(20), 4698. https://doi.org/10.3390/ma18204698







