Physicochemical Properties of Hematite Nanoparticles Obtained via Thermogravimetric Conversion of Biosynthesized Nanomaghemite
Abstract
1. Introduction
2. Materials and Methods
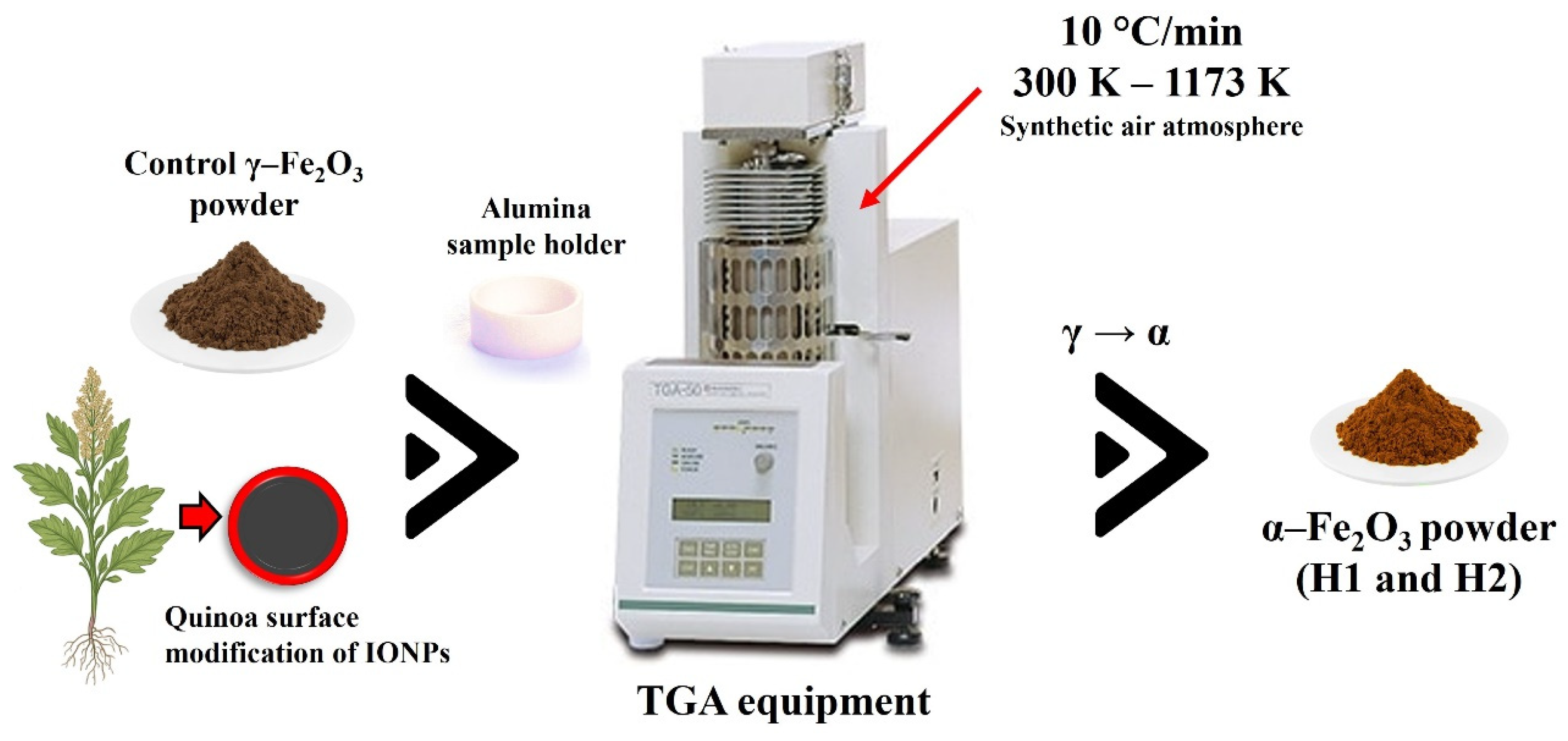
2.1. α-Fe2O3 Preparation Using the Shimadzu Thermogravimetry Equipment
2.2. Characterization
3. Results and Discussions
3.1. TGA and α-Fe2O3 Crystallization
3.2. X-Ray Diffraction and Rietveld Analysis
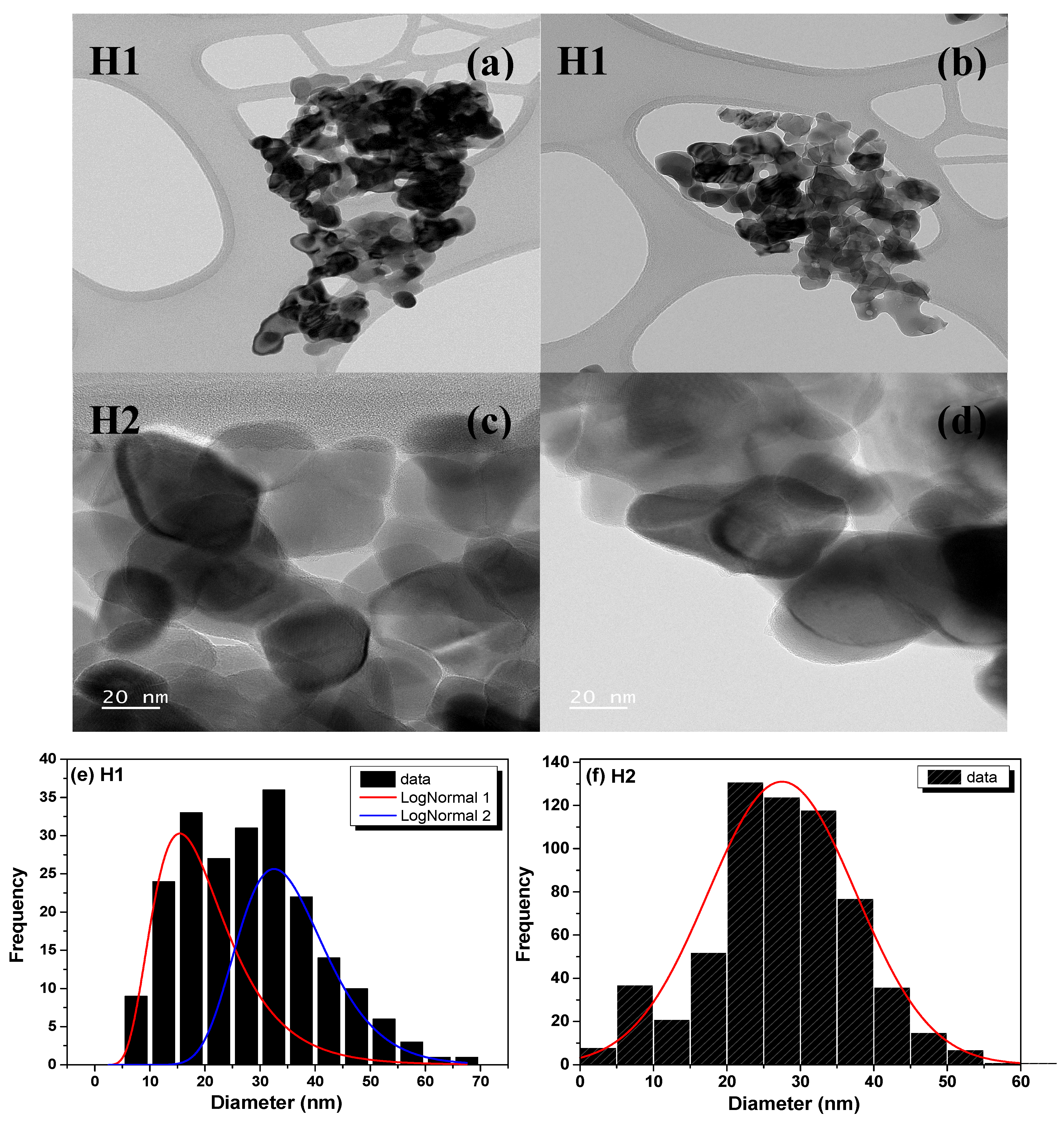
3.3. TEM Analysis
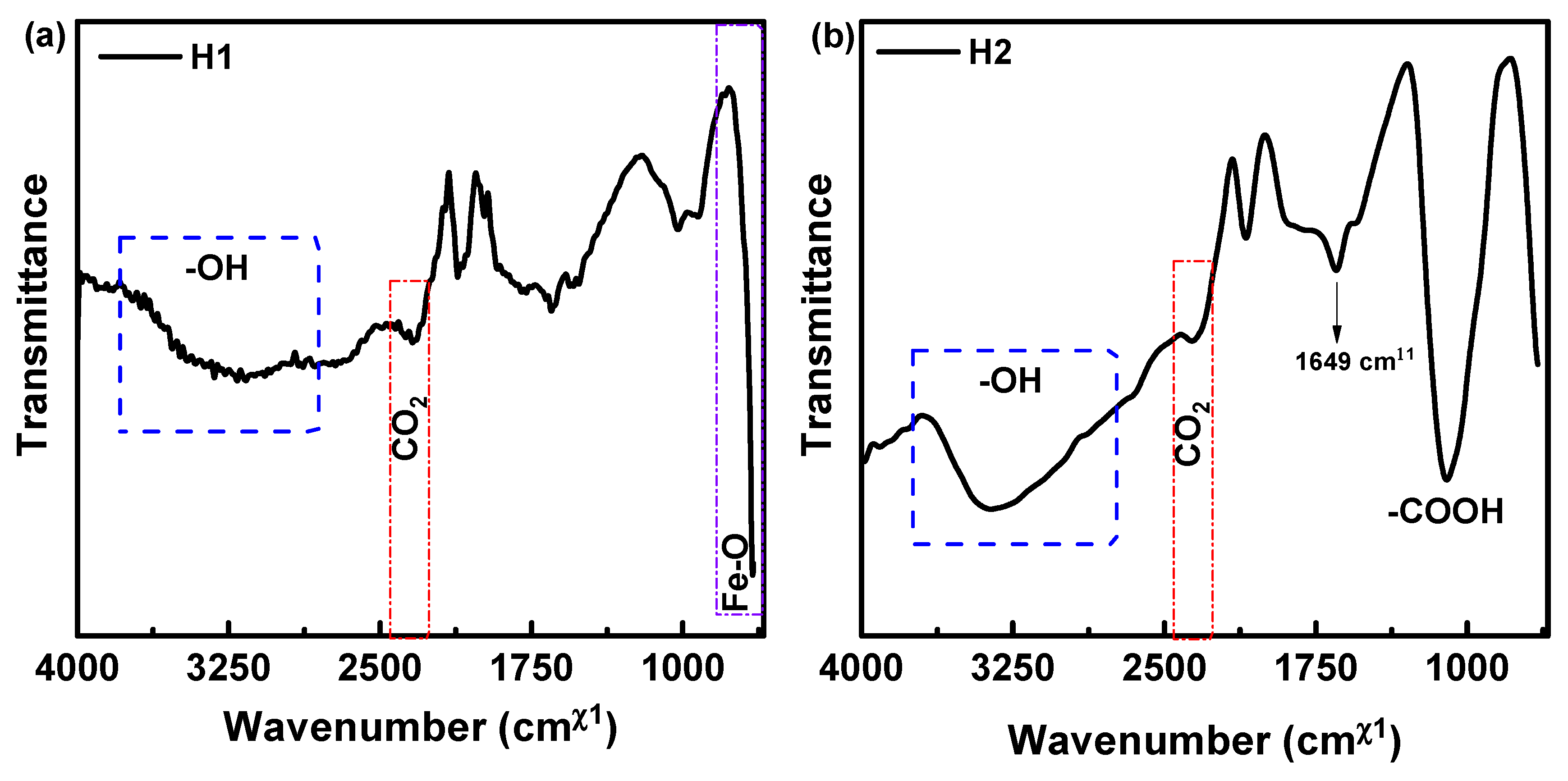
3.4. ATR-FTIR Analysis
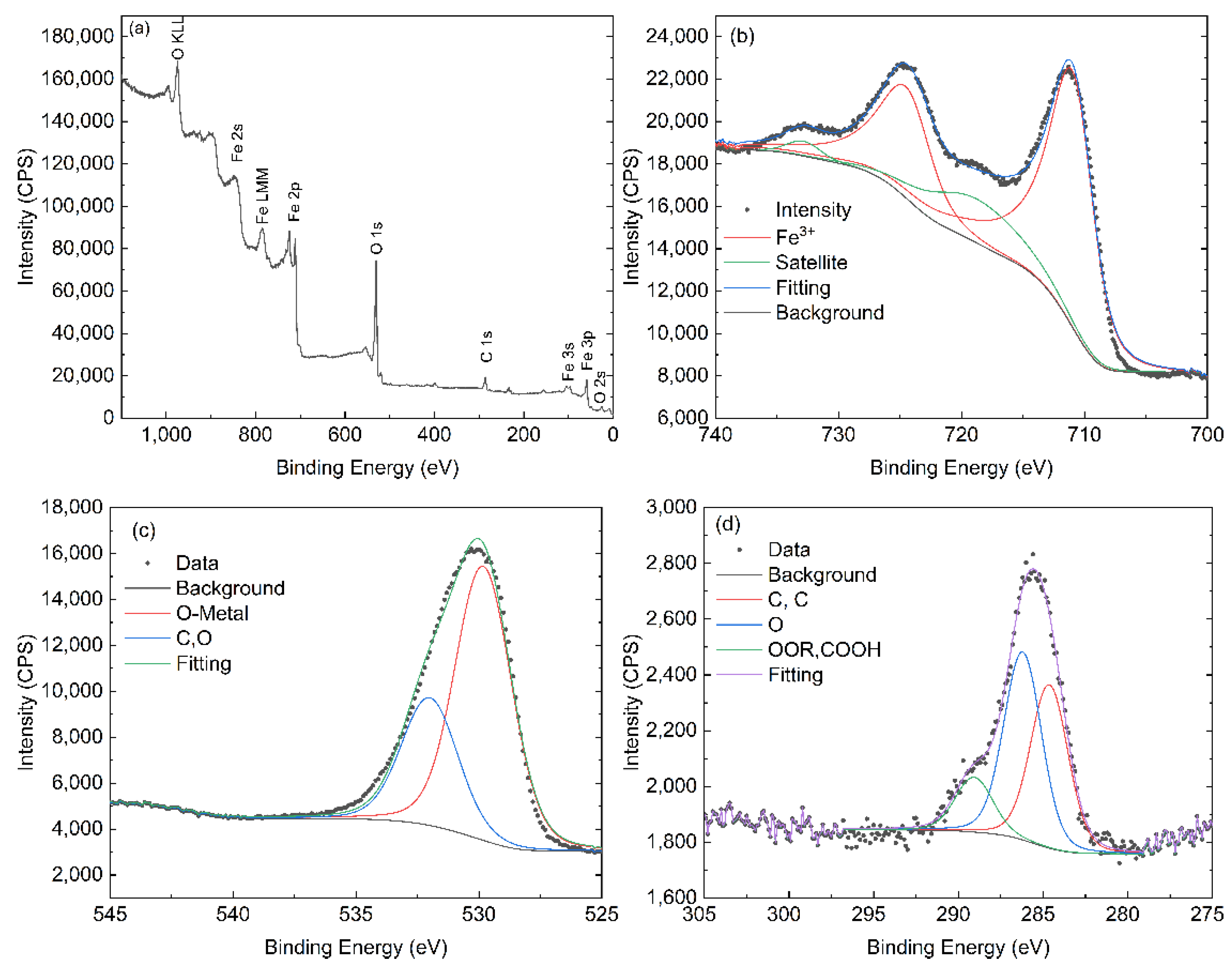
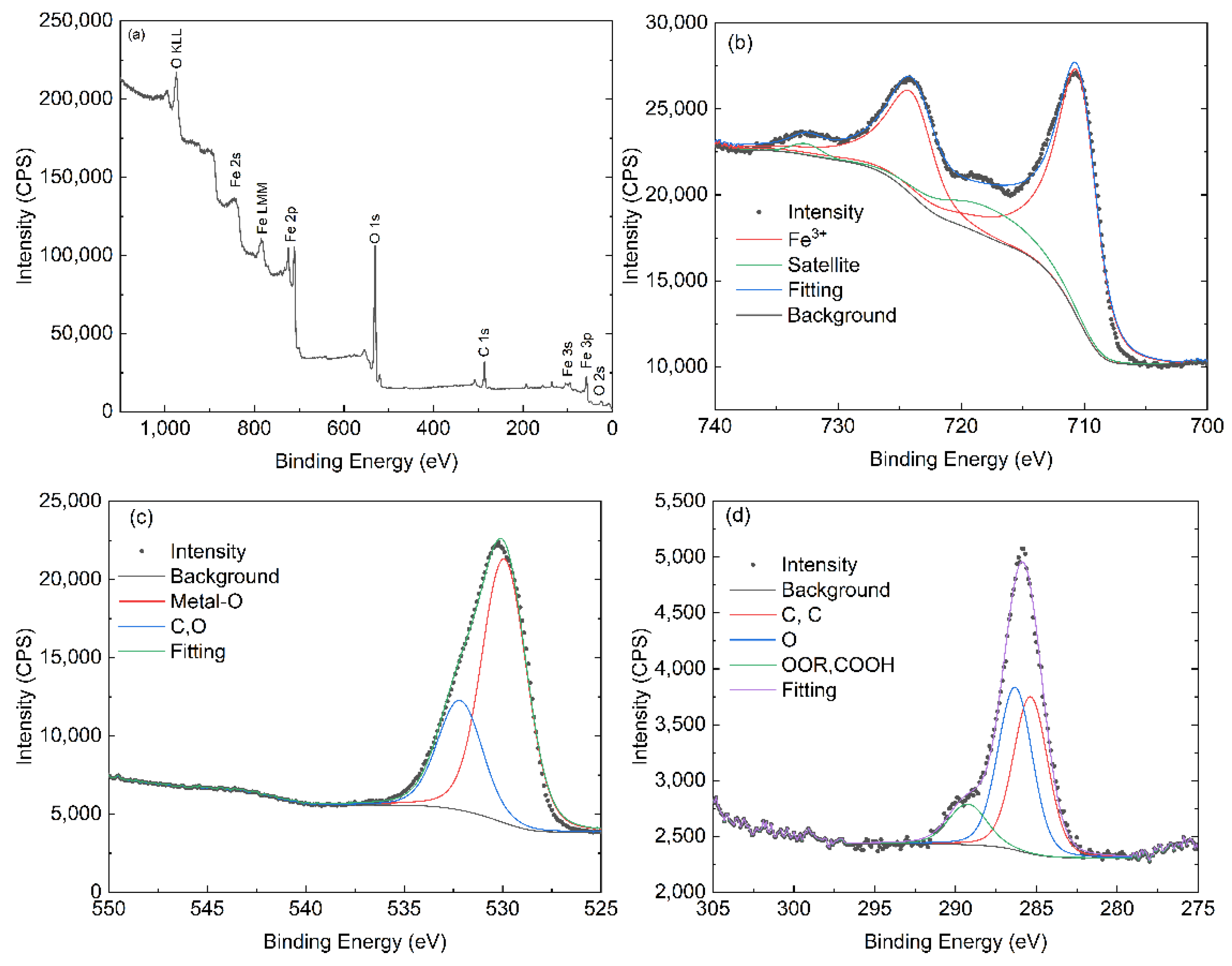
3.5. XPS Analysis
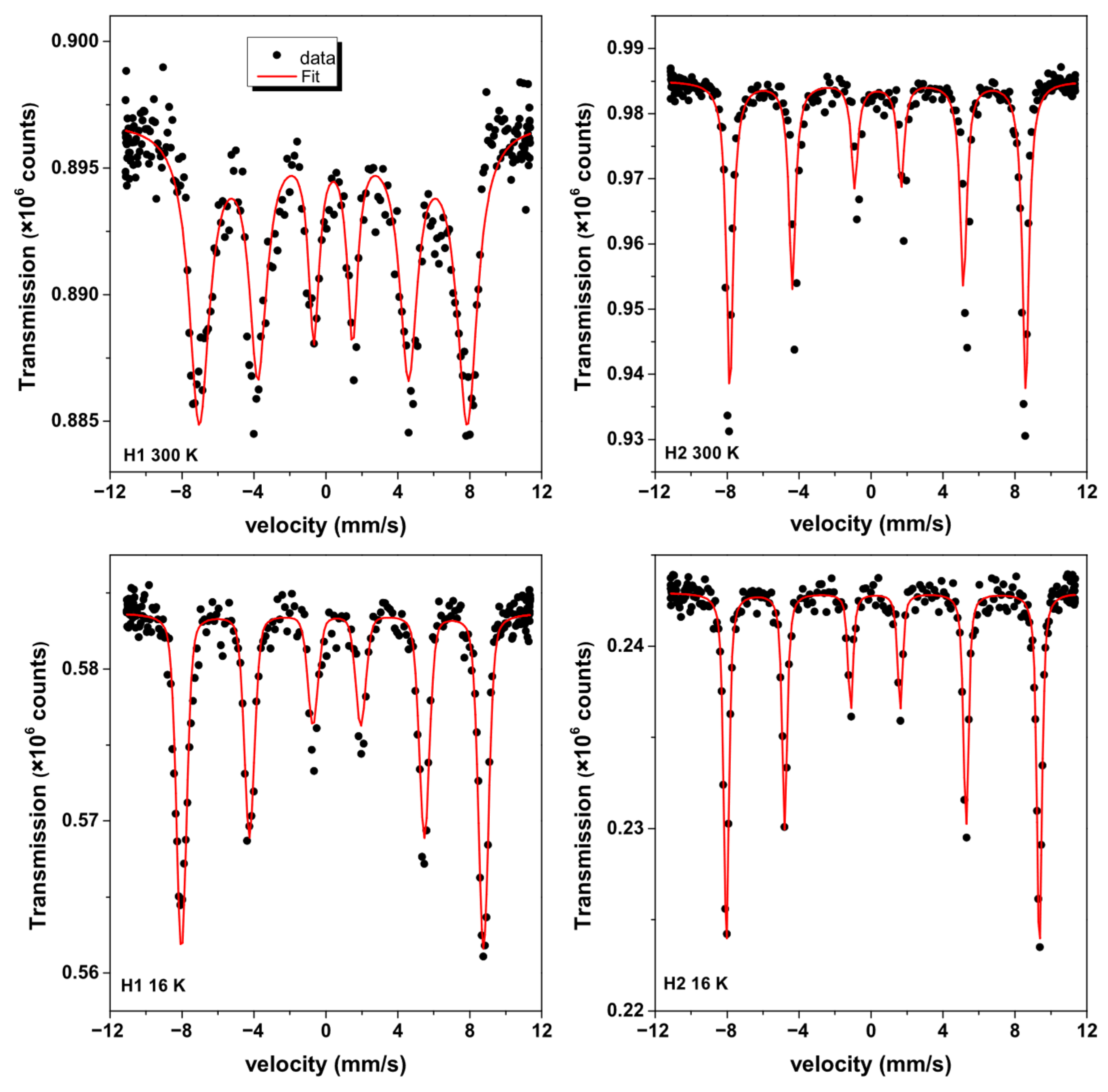
3.6. 57Fe Mössbauer Analysis of Nanohematites
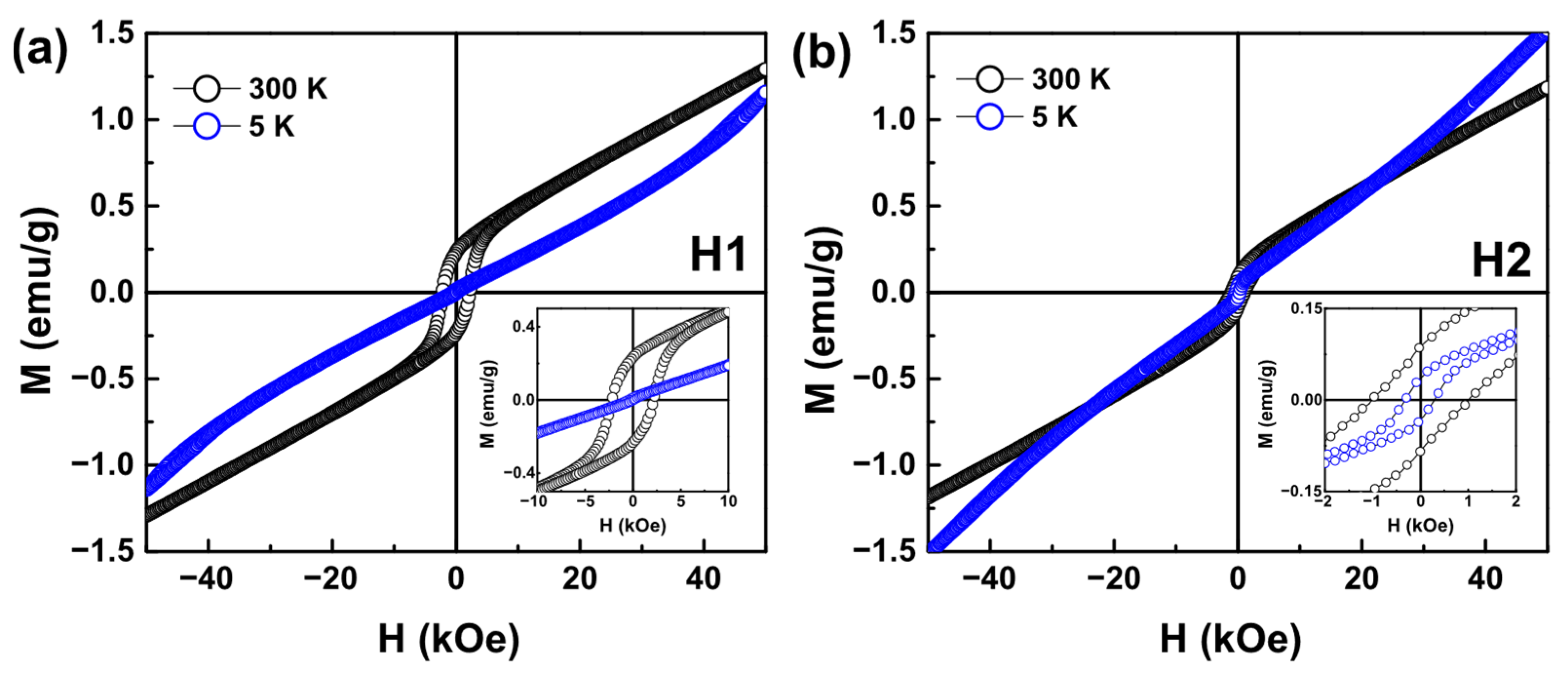
3.7. VSM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhateria, R.; Singh, R. A Review on Nanotechnological Application of Magnetic Iron Oxides for Heavy Metal Removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent Trends in Preparation and Biomedical Applications of Iron Oxide Nanoparticles. J. Nanobiotech. 2024, 22, 24. [Google Scholar] [CrossRef]
- Meijer, J.M.; Rossi, L. Preparation, Properties, and Applications of Magnetic Hematite Microparticles. Soft Matter 2021, 17, 2354–2368. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structures, Properties, Reactions, Occurences and Uses, 2nd ed.; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Chen, W.; She, A.-S.; Ji, M.-H.; Shi, H.-Y.; Yang, Y.; Pu, Y.-H.; Chen, R.; Yang, W.-H.; Chen, Y.-X.; Lu, C.-Z. Optimizing Charge Separation and Transport: Enhanced Photoelectrochemical Water Splitting in α-Fe2O3/CZTS Nanorod Arrays. Catalysts 2024, 14, 812. [Google Scholar] [CrossRef]
- Mandal, B.K.; Chauhan, P.S.; Das, R. Photocatalytic Activity of Green Synthesized Canted Ferromagnetic Hematite Iron Oxide Nanoparticles for Methyl Orange Removal in Wastewater Remediation. J. Magn. Magn. Mater. 2025, 628, 173182. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef]
- Bødker, F.; Hansen, M.F.; Koch, C.B.; Lefmann, K.; Mørup, S. Magnetic Properties of Hematite Nanoparticles. Phys. Rev. B 2000, 61, 6826–6838. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J.; Berquó, T.S. Morin Transition in Hematite: Size Dependence and Thermal Hysteresis. Geochem. Geophys. Geosyst. 2008, 9, 10. [Google Scholar] [CrossRef]
- Vinayagam, R.; Patnaik, Y.; Brijesh, P.; Prabhu, D.; Quadras, M.; Pai, S.; Narasimhan, M.K.; Kaviyarasu, K.; Varadavenkatesan, T.; Selvaraj, R. Superparamagnetic Hematite Spheroids Synthesis, Characterization, and Catalytic Activity. Chemosphere 2022, 294, 133730. [Google Scholar] [CrossRef] [PubMed]
- Lemine, O.M.; Madkhali, N.; Hjiri, M.; All, N.A.; Aida, M.S. Comparative Heating Efficiency of Hematite (α-Fe2O3) and Nickel Ferrite Nanoparticles for Magnetic Hyperthermia Application. Ceram. Int. 2020, 46, 28821–28827. [Google Scholar] [CrossRef]
- Paulson, E.; Jothibas, M. Significance of Thermal Interfacing in Hematite (α-Fe2O3) Nanoparticles Synthesized by Sol-Gel Method and Its Characteristics Properties. Surf. Interfaces 2021, 26, 101432. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Di Iorio, E.; Song, X.; Jiang, Z.; Liu, Q.; Angelico, R. Influence of Hydrothermal Synthesis Conditions on Size, Morphology and Colloidal Properties of Hematite Nanoparticles. Nano-Struct. Nano-Objects 2015, 2, 19–27. [Google Scholar] [CrossRef]
- Tahir, M.; Fakhar-e-Alam, M.; Atif, M.; Mustafa, G.; Ali, Z. Investigation of Optical, Electrical and Magnetic Properties of Hematite α-Fe2O3 Nanoparticles via Sol-Gel and Co-Precipitation Method. J. King Saud. Univ. Sci. 2023, 35, 102695. [Google Scholar] [CrossRef]
- Darezereshki, E.; Bakhtiari, F.; Alizadeh, M.; Vakylabad, A.B.; Ranjbar, M. Direct Thermal Decomposition Synthesis and Characterization of Hematite (α-Fe2O3) Nanoparticles. Mater. Sci. Semicond. Process. 2012, 15, 91–97. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Sarani, M. Biosynthesis, Magnetic and Cytotoxic Studies of Hematite Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 767–774. [Google Scholar] [CrossRef]
- Chen, Y.H. Thermal Properties of Nanocrystalline Goethite, Magnetite, and Maghemite. J. Alloys Compd. 2013, 553, 194–198. [Google Scholar] [CrossRef]
- Marcos-Carrillo, M.d.P.; Checca-Huaman, N.-R.; Passamani, E.C.; Ramos-Guivar, J.A. Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Chenopodium quinoa Extract. Nanomaterials 2024, 14, 1607. [Google Scholar] [CrossRef]
- Chakraborty, A.R.; Zohora Toma, F.T.; Alam, K.; Yousuf, S.B.; Hossain, K.S. Influence of Annealing Temperature on Fe2O3 Nanoparticles: Synthesis Optimization and Structural, Optical, Morphological, and Magnetic Properties Characterization for Advanced Technological Applications. Heliyon 2024, 10, e40000. [Google Scholar] [CrossRef]
- Rueda-Vellasmin, R.; Checca-Huaman, N.-R.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Mössbauer Studies of Core-Single-Shell and Core-Double-Shell Polymer Functionalized Magnetic Nanoparticles. Hyperfine Interact. 2022, 243, 27. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Pielichowska, K.; Nowicka, K. Analysis of Nanomaterials and Nanocomposites by Thermoanalytical Methods. Thermochim. Acta 2019, 675, 140–163. [Google Scholar] [CrossRef]
- N, S.; Mahalakshmi, S. A Study on the Oxidative Property Using Thermogravimetric Analysis of NiO Nanoparticles Prepared by a Simple Wet Chemical Method. Chem. Phys. Impact 2024, 8, 100613. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of Nanocrystal Sizes: A Comparison of TEM, SAXS, and XRD Studies of Highly Monodisperse CoPt3 Particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef]
- Weidenthaler, C. Pitfalls in the Characterization of Nanoporous and Nanosized Materials. Nanoscale 2011, 3, 792–810. [Google Scholar] [CrossRef]
- Nasiri, S.; Rabiei, M.; Palevicius, A.; Janusas, G.; Vilkauskas, A.; Nutalapati, V.; Monshi, A. Modified Scherrer Equation to Calculate Crystal Size by XRD with High Accuracy, Examples Fe2O3, TiO2 and V2O5. Nano Trends 2023, 3, 100015. [Google Scholar] [CrossRef]
- Khanam, J.; Hasan, M.R.; Biswas, B.; Ahmed, M.F.; Mostofa, S.; Akhtar, U.S.; Hossain, M.K.; Quddus, M.S.; Ahmed, S.; Sharmin, N.; et al. Effect of low temperature calcination on micro structure of hematite nanoparticles synthesized from waste iron source. Heliyon 2024, 10, e41030. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic Properties of Hematite (α-Fe2O3) Nanoparticles Synthesized by Sol-Gel Synthesis Method: The Influence of Particle Size and Particle Size Distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F., Jr.; Madden, A.S. Size-Dependent Structural Transformations of Hematite Nanoparticles. 1. Phase Transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Segura, R.; Vásquez, G.; Colson, E.; Gerbaux, P.; Frischmon, C.; Nesic, A.; García, D.E.; Cabrera-Barjas, G. Phytostimulant Properties of Highly Stable Silver Nanoparticles Obtained with Saponin Extract from Chenopodium Quinoa. J. Sci. Food Agric. 2020, 100, 4987–4994. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghan, R.; Hatefi, P.; Chelgani, S.C. A Comparative Study between the Adsorption Mechanisms of Sodium Co-Silicate and Conventional Depressants for the Reverse Anionic Hematite Flotation. Sep. Sci. Technol. 2022, 57, 141–158. [Google Scholar] [CrossRef]
- Ramos-Pacheco, B.S.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Palomino-Rincón, H.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Moscoso-Moscoso, E.; Aiquipa-Pillaca, Á.S. Effect of Germination on the Physicochemical Properties, Functional Groups, Content of Bioactive Compounds, and Antioxidant Capacity of Different Varieties of Quinoa (Chenopodium quinoa Willd.) Grown in the High Andean Zone of Peru. Foods 2024, 13, 417. [Google Scholar] [CrossRef]
- Torres Vargas, O.L.; García Salcedo, A.J.; Ariza Calderón, H. Physical-Chemical Characterization of Quinoa (Chenopodium quinoa Willd.), Amaranth (Amaranthus caudatus L.), and Chia (Salvia hispanica L.) Flours and Seeds. Acta Agron. 2018, 67, 215–222. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.R.; Crist, B.V.; Lahiri, N.; Rosso, K.M. Origin of the Complex Main and Satellite Features in Fe 2p XPS of Fe2O3. Phys. Chem. Chem. Phys. 2022, 24, 4562–4575. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Flak, D.; Chen, Q.; Mun, B.S.; Liu, Z.; Rękas, M.; Braun, A. In Situ Ambient Pressure XPS Observation of Surface Chemistry and Electronic Structure of α-Fe2O3 and γ-Fe2O3 Nanoparticles. Appl. Surf. Sci. 2018, 455, 1019–1028. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Ren, G.; Deng, Y.; Yang, X. A Novel Fabrication of Hematite Nanoparticles via Recycling of Titanium Slag by Pyrite Reduction Technology. Nanomaterials 2024, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Kirik, N.; Krylov, A.; Boronin, A.; Koshcheev, S.; Solovyov, L.; Rabchevskii, E.; Shishkina, N.; Anshits, A. The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen. Materials 2023, 16, 4466. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2022, 16, 59. [Google Scholar] [CrossRef]
- Ahmad, S.; Ni, H.; Al-Mubaddel, F.S.; Rizk, M.A.; Ben Ammar, M.; Khan, A.U.; Almarhoon, Z.M.; Alanazi, A.A.; Zaki, M.E.A. Magnetic Properties of Different Phases Iron Oxide Nanoparticles Prepared by Micro Emulsion-Hydrothermal Method. Sci. Rep. 2025, 15, 878. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.R.; Lahiri, N.; Ilton, E.S.; Rosso, K.M. Analysis of the Fe 2p XPS for Hematite α Fe2O3: Consequences of Covalent Bonding and Orbital Splittings on Multiplet Splittings. J. Chem. Phys. 2020, 152, 014704. [Google Scholar] [CrossRef] [PubMed]
- Blume, M.; Tjon, J.A. Mössbauer Spectra in a Fluctuating Environment. Phys. Rev. 1968, 165, 446–456. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; López, E.O.; Greneche, J.-M.; Jochen Litterst, F.; Passamani, E.C. Effect of EDTA Organic Coating on the Spin Canting Behavior of Maghemite Nanoparticles for Lead (II) Adsorption. Appl. Surf. Sci. 2021, 538, 148021. [Google Scholar] [CrossRef]
- Morrish, A.H. Canted Antiferromagnetism: Hematite; World Scientific: Singapore, 1995; ISBN 978-981-02-2007-5. [Google Scholar]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and Dynamic Magnetic Properties of Spherical Magnetite Nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]


| Sample | Phase | Caglioti Parameters | Lattice Parameters (Å) | DRietveld (nm) | Weight Fraction (%) | Statistical Parameters | ||
|---|---|---|---|---|---|---|---|---|
| U | V | W | ||||||
| H1 | α-Fe2O3 | 0.011 | −0.055 | 0.058 | a = 5.034(1) b = 5.034(1) c = 13.745(1) α = β = 90° γ = 120° | 54(1) | 100 | Rp = 74.2% Rwp = 34.1% Rexp = 19.9% χ2 = 2.94 |
| H2 | α-Fe2O3 | 0.021 | −0.062 | 0.057 | a = 5.031(1) b = 5.031(1) c = 13.737(1) α = β = 90° γ = 120° | 56(1) | 100 | Rp = 73.8% Rwp = 34.0% Rexp = 19.9% χ2 = 2.91 |
| H1 | |||
| Miller Plane | 2θ (°) | d(nm) | dref. (nm) |
| (104) | 33.17 | 0.2700 | 0.2703 |
| (110) | 35.68 | 0.2517 | 0.2519 |
| H2 | |||
| Miller Plane | 2θ (°) | d(nm) | dref. (nm) |
| (104) | 33.19 | 0.2702 | 0.2703 |
| (110) | 35.67 | 0.2516 | 0.2519 |
| Sample/Reference | Fe 2p3/2 (eV) | Satellite (eV) | O 1s (eV) | Notes |
|---|---|---|---|---|
| Literature (α-Fe2O3, Fe3+) [34,35,36] | 709.9–711.0 | ~718.9 | 529.2–530.0 (lattice O); 530.9–531.4 (–OH) | Classical α-Fe2O3 XPS signature |
| H1 (this work) | 710.4 | 718.7 | 529.8 | Very close to classical α-Fe2O3; Fe3+ well defined; lattice oxygen dominant |
| H2 (this work) | 710.0 | 717.7 | 529.9 | Fe3+ state preserved, but satellite shifted ~1 eV lower; suggests subtle changes in surface/electronic environment |
| Sample | IS (mm/s) ±0.01 | Bhf (T) ±0.5 | ε (mm/s) ±0.01 | LW (mm/s) ±0.01 | R.A.A. (%) ±1 |
|---|---|---|---|---|---|
| H1-300 K | 0.41 | 47.5 | 0.00 | 0.63 | 100 |
| H1-16 K | 0.49 | 52.2 | −0.24 | 0.54 | 100 |
| H2-300 K | 0.38 | 51.1 | −0.19 | 0.35 | 100 |
| H2-16 K | 0.46 | 54.1 | 0.42 | 0.18 | 100 |
| Sample | T | Hc | Ms | Mr |
|---|---|---|---|---|
| (K) | ±0.1 (kOe) | ±0.01 (emu/g) | ±0.01 (emu/g) | |
| H1 | 300 | 2.2 | 0.32 | 0.23 |
| 5 | 0.3 | - | - | |
| H2 | 300 | 1.0 | 0.23 | 0.08 |
| 5 | 0.3 | - | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Guivar, J.A.; Marcos-Carrillo, M.d.P.; Rueda-Vellasmin, R.; Manrique-Castillo, E.V.; Checca-Huaman, N.-R.; Santos, B.L.D.; Macedo, W.A.A.; Passamani, E.C. Physicochemical Properties of Hematite Nanoparticles Obtained via Thermogravimetric Conversion of Biosynthesized Nanomaghemite. Materials 2025, 18, 4677. https://doi.org/10.3390/ma18204677
Ramos-Guivar JA, Marcos-Carrillo MdP, Rueda-Vellasmin R, Manrique-Castillo EV, Checca-Huaman N-R, Santos BLD, Macedo WAA, Passamani EC. Physicochemical Properties of Hematite Nanoparticles Obtained via Thermogravimetric Conversion of Biosynthesized Nanomaghemite. Materials. 2025; 18(20):4677. https://doi.org/10.3390/ma18204677
Chicago/Turabian StyleRamos-Guivar, Juan A., Mercedes del Pilar Marcos-Carrillo, Renzo Rueda-Vellasmin, Erich V. Manrique-Castillo, Noemi-Raquel Checca-Huaman, Bruno L. D. Santos, Waldemar A. A. Macedo, and Edson C. Passamani. 2025. "Physicochemical Properties of Hematite Nanoparticles Obtained via Thermogravimetric Conversion of Biosynthesized Nanomaghemite" Materials 18, no. 20: 4677. https://doi.org/10.3390/ma18204677
APA StyleRamos-Guivar, J. A., Marcos-Carrillo, M. d. P., Rueda-Vellasmin, R., Manrique-Castillo, E. V., Checca-Huaman, N.-R., Santos, B. L. D., Macedo, W. A. A., & Passamani, E. C. (2025). Physicochemical Properties of Hematite Nanoparticles Obtained via Thermogravimetric Conversion of Biosynthesized Nanomaghemite. Materials, 18(20), 4677. https://doi.org/10.3390/ma18204677






