Both Benzannulation and Heteroatom-Controlled Photophysical Properties in Donor–π–Acceptor Ionic Dyes: A Combined Experimental and Theoretical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Computational Details
3. Results and Discussion
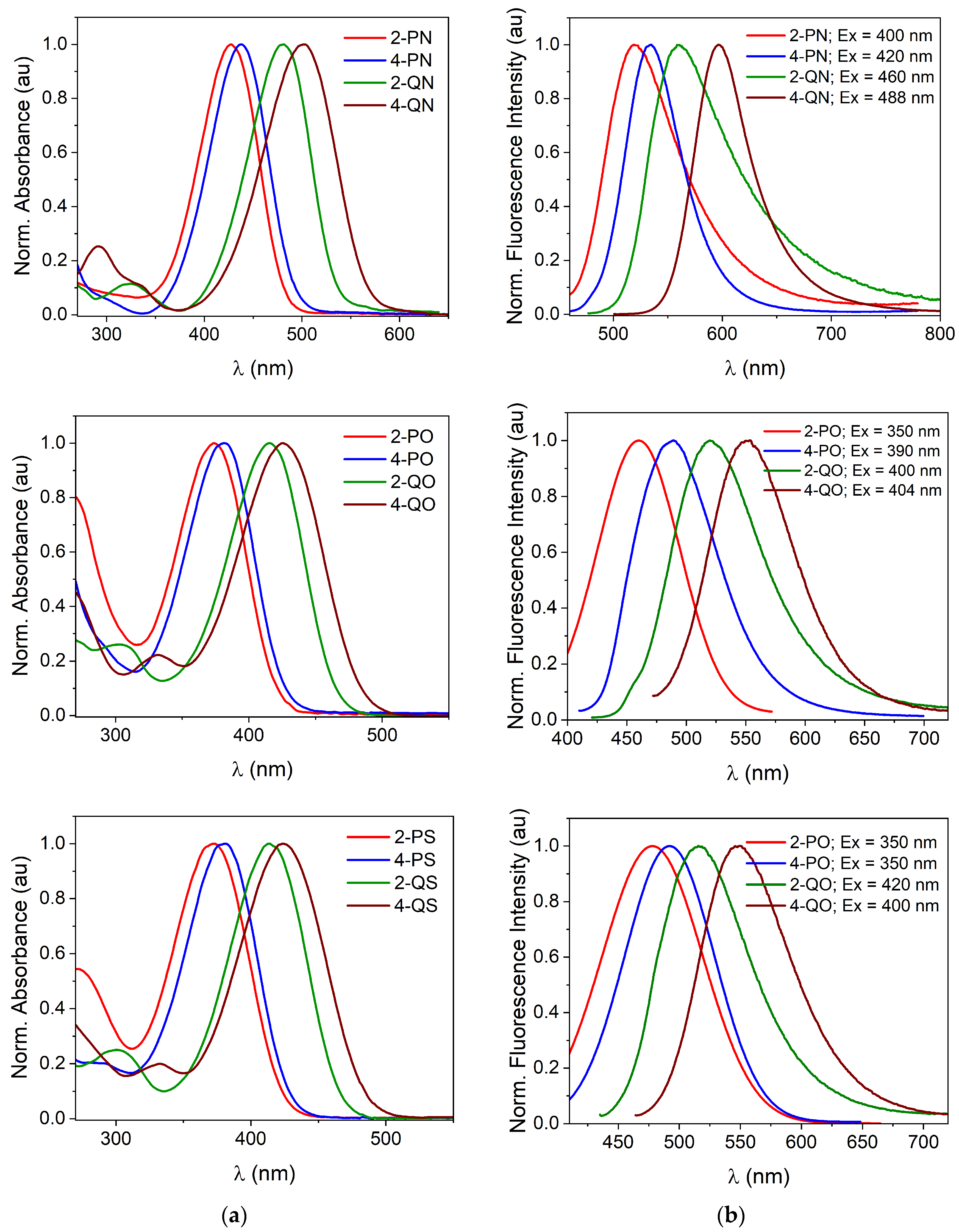
3.1. Spectral Properties
Spectroscopic Analysis
3.2. Theoretical Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akula, S.B.; Tingare, Y.S.; Su, C.; Chen, H.-S.; Li, W.-Q.; Lekphet, W.; Li, W.-R. Bridgehead nitrogen tripodal organic dyes having multiple donor-π-acceptor branches for solar cell applications. Dyes Pigm. 2021, 186, 108985. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.Y.; Jang, W.-D.; Jaung, J.Y. Effect of donor-π-acceptor structure on photochromism of dithienylethene-based dyes. Dyes Pigm. 2020, 177, 108315. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, T.; Islam, A.; Kwon, E.; Akhtaruzzaman, M.; Asao, N.; Han, L.; Alamry, K.A.; Kosa, S.A.; Asiri, A.M.; et al. Thieno[2,3-a]carbazole-based donor–π–acceptor organic dyes for efficient dye-sensitized solar cells. Tetrahedron 2014, 70, 6211–6216. [Google Scholar] [CrossRef]
- Chemate, S.; Sekar, N. Indole-Based NLOphoric Donor-π-Acceptor Styryl Dyes: Synthesis, Spectral Properties and Computational Studies. J. Fluoresc. 2016, 26, 2063–2077. [Google Scholar] [CrossRef]
- Mishra, A.; Bäuerle, P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem. Int. Ed. 2012, 51, 2020–2067. [Google Scholar] [CrossRef]
- Zayed, M.E.M.; El-Shishtawy, R.M.; Elroby, S.A.; Al-Footy, K.O.; Al-amshany, Z.M. Experimental and theoretical study of donor-π-acceptor compounds based on malononitrile. Chem. Cent. J. 2018, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Kothavale, S.; Katariya, S.; Sekar, N. NLOphoric rigid pyrazino-phenanthroline donor-π-acceptor compounds: Investigation of structural and solvent effects on non-linear optical properties using computational methods. Opt. Mater. 2018, 75, 379–389. [Google Scholar] [CrossRef]
- Irie, M. Diarylethenes for Memories and Switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef]
- Grabarz, A.M.; Jędrzejewska, B.; Zakrzewska, A.; Zaleśny, R.; Laurent, A.D.; Jacquemin, D.; Ośmiałowski, B. Photophysical Properties of Phenacylphenantridine Difluoroboranyls: Effect of Substituent and Double Benzannulation. J. Org. Chem. 2017, 82, 1529–1537. [Google Scholar] [CrossRef]
- Hanson, K.; Roskop, L.; Djurovich, P.I.; Zahariev, F.; Gordon, M.S.; Thompson, M.E. A Paradigm for Blue- or Red-Shifted Absorption of Small Molecules Depending on the Site of π-Extension. J. Am. Chem. Soc. 2010, 132, 16247–16255. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: New York, NY, USA, 1990. [Google Scholar]
- Naraso; Nishida, J.-I.; Ando, S.; Yamaguchi, J.; Itaka, K.; Koinuma, H.; Tada, H.; Tokito, S.; Yamashita, Y. High-Performance Organic Field-Effect Transistors Based on π-Extended Tetrathiafulvalene Derivatives. J. Am. Chem. Soc. 2005, 127, 10142–10143. [Google Scholar] [CrossRef]
- Adachi, M.; Nagao, Y. Design of Near-Infrared Dyes Based on π-Conjugation System Extension 2. Theoretical Elucidation of Framework Extended Derivatives of Perylene Chromophore. Chem. Mater. 2001, 13, 662–669. [Google Scholar] [CrossRef]
- Pastore, M.; Mosconi, E.; De Angelis, F.; Grätzel, M. A Computational Investigation of Organic Dyes for Dye-Sensitized Solar Cells: Benchmark, Strategies, and Open Issues. J. Phys. Chem. C 2010, 114, 7205–7212. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Zakrzewska, A.; Mlostoń, G.; Budzák, Š.; Mroczyńska, K.; Grabarz, A.M.; Kaczorowska, M.A.; Jacquemin, D.; Ośmiałowski, B. Synthesis and Photophysical Properties of Novel Donor–Acceptor N-(Pyridin-2-yl)-Substituted Benzo(thio)amides and Their Difluoroboranyl Derivatives. J. Phys. Chem. A 2016, 120, 4116–4123. [Google Scholar] [CrossRef]
- Deogratias, G.; Al-Qurashi, O.S.; Wazzan, N.; Pogrebnaya, T.; Pogrebnoi, A. Effects of heteroatoms in π-conjugated linkers on the optical and electronic properties of modified triphenylamine based dyes: Towards DSSCs’ applications. J. Mol. Model. 2020, 26, 288. [Google Scholar] [CrossRef]
- Kirenga, P.; Mkoma, S.L.; Mlowe, S.; Msambwa, Y.; Kiruri, L.W.; Jacob, F.R.; Mgaya, J.E.; Kinunda, G.A.; Deogratias, G. Influence of heteroatoms on the optoelectronic properties of triphenylamine-based dyes for DSSCs application: A computational approach. Comput. Theor. Chem. 2022, 1210, 113644. [Google Scholar] [CrossRef]
- Grabarz, A.M.; Jędrzejewska, B.; Skotnicka, A.; Murugan, N.A.; Patalas, F.; Bartkowiak, W.; Jacquemin, D.; Ośmiałowski, B. The impact of the heteroatom in a five-membered ring on the photophysical properties of difluoroborates. Dyes Pigm. 2019, 170, 107481. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, Q.; Fan, X.; Zhu, Q.; Jin, M. Heteroatom/Heterocycle-Substituted Ketone Dyes as Efficient Photoinitiators in Visible Light-Emitting Diode/Near Infrared Light Photopolymerization with Enhanced Two-Photon Lithography Capability. ACS Appl. Polym. Mater. 2024, 6, 5566–5575. [Google Scholar] [CrossRef]
- Geng, J.; Xu, D.; Chang, F.-F.; Tao, T.; Huang, W. From heterocyclic hydrazone to hydrazone-azomethine dyes: Solvent and pH induced hydrazone and azo-keto transformation for a family of pyrazolone-based heterocyclic dyes. Dyes Pigm. 2017, 137, 101–110. [Google Scholar] [CrossRef]
- Deogratias, G.; Al-Qurashi, O.S.; Wazzan, N.; Pogrebnaya, T.; Pogrebnoi, A. Effect of substituent in the acceptor on optical and electronic properties of triphenylamine based dyes: A density functional theory/time-dependent density functional theory investigation. Mater. Sci. Semicond. Process. 2022, 150, 106935. [Google Scholar] [CrossRef]
- Mustroph, H. Polymethine dyes. Phys. Sci. Rev. 2020, 5, 20190084. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Kabatc, J.; Pączkowski, J. 1,3-Bis[4-(p-aminostyryl)-pyridinyl]-propane dibromide derivatives: Synthesis and spectroscopic investigation. Dyes Pigm. 2007, 73, 361–367. [Google Scholar] [CrossRef]
- Phillips, A.P. Condensation of aromatic aldehydes with γ-picoline methiodide. J. Org. Chem. 1949, 14, 302–305. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Yun, H.S.; Kaplan, T.; Smith, J.C.; Foldes, F.F. Choline acetyltransferase inhibitors. Dimensional and substituent effects among styrylpyridine analogs. J. Med. Chem. 1970, 13, 221–224. [Google Scholar] [CrossRef]
- Fortuna, C.G.; Mazzucato, U.; Musumarra, G.; Pannacci, D.; Spalletti, A. Photochemistry and DNA-affinity of some stilbene and distyrylbenzene analogues containing pyridinium and imidazolium iodides. J. Photochem. Photobiol. A Chem. 2010, 216, 66–72. [Google Scholar] [CrossRef]
- Marri, E.; Mazzucato, U.; Fortuna, C.G.; Musumarra, G.; Spalletti, A. Photobehaviour of some 1-heteroaryl-2-(1-methylpyridinium-2-yl)ethene iodides (free and complexed with DNA). J. Photochem. Photobiol. A Chem. 2006, 179, 314–319. [Google Scholar] [CrossRef]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Scuseria, G.E.; Barone, V. Accurate excitation energies from time-dependent density functional theory: Assessing the PBE0 model. J. Chem. Phys. 1999, 111, 2889–2899. [Google Scholar] [CrossRef]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Muniappan, P.; Meenakshi, R.; Rajavel, G. PCM/TD-DFT analysis of 1-bromo-2,3-dichlorobenzene-A combined study of experimental (FT-IR and FT-Raman) and theoretical calculations. Spectrochim. Acta Part A 2013, 105, 497–508. [Google Scholar] [CrossRef]
- Liu, R.C.; Liu, Z.P. Polythiophene: Synthesis in aqueous medium and controllable morphology. Chin. Sci. Bull. 2009, 54, 2028–2032. [Google Scholar] [CrossRef]
- Bruce, G.T.; Cooksey, A.R.; Morgan, K.J. Aryl and heteroaryl substituent effects in reductions and solvolysis reactions. J. Chem. Soc. Perkin Trans. 1975, 6, 551–553. [Google Scholar] [CrossRef]
- Noyce, D.S.; Fike, S.A. Reactivity of thiazole in electrophilic reactions as determined from solvolysis rates. J. Org. Chem. 1973, 38, 3316–3318. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Barresi, V.; Consiglio, G.; Fortuna, C.G.; Longo, M.L.; Musumarra, G. Synthesis, spectroscopic characterization and in vitro antitumor activity of new trans 1-heteroaryl-2-(1-methylpyridinium-2-yl) ethylenes. Arkivoc 2003, 2003, 105–117. [Google Scholar] [CrossRef]
- Strehmel, B.; Seifert, H.; Rettig, W. Photophysical Properties of Fluorescence Probes. 2. A Model of Multiple Fluorescence for Stilbazolium Dyes Studied by Global Analysis and Quantum Chemical Calculations. J. Phys. Chem. B 1997, 101, 2232–2243. [Google Scholar] [CrossRef]
- Fromherz, P.; Heilemann, A. Twisted internal charge transfer in (aminophenyl)pyridinium. J. Phys. Chem. 1992, 96, 6864–6866. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Pietrzak, M.; Pączkowski, J. Solvent Effects on the Spectroscopic Properties of Styrylquinolinium Dyes Series. J. Fluoresc. 2010, 20, 73–86. [Google Scholar] [CrossRef]
- Pyszka, I.; Krawczyk, P.; Jędrzejewska, B. The Influence of the Alkylamino Group on the Solvatochromic Behavior of 5-(4-substituted-arylidene)-1,3-dimethylpyrimidine-2,4,6-triones: Synthesis, Spectroscopic and Computational Studies. Materials 2024, 17, 2447. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Krawczyk, P.; Pietrzak, M.; Gordel, M.; Matczyszyn, K.; Samoć, M.; Cysewski, P. Styryl dye possessing donor-π-acceptor structure—Synthesis, spectroscopic and computational studies. Dyes Pigm. 2013, 99, 673–685. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Kaczorowska, M.A.; Józefowicz, M.; Samoć, M. One- and two-photon-induced isomerization of styryl compounds possessing A-π-A′ structure. Dyes Pigm. 2016, 132, 237–247. [Google Scholar] [CrossRef]
- Zhan, C.-L.; Wang, D.-Y. Nonlinear dependence of solvent polarity effects on twisted intramolecular charge-transfer states and linear relation for electronic spectra in a stilbazolium-like dye. J. Photochem. Photobiol. A Chem. 2002, 147, 93–101. [Google Scholar] [CrossRef]
- Bradamante, S.; Facchetti, A.; Pagani, G.A. Heterocycles as donor and acceptor units in push–pull conjugated molecules. Part 1. J. Phys. Org. Chem. 1997, 10, 514–524. [Google Scholar] [CrossRef]
- Jelínková, V.; Dellai, A.; Verwaerde, L.; Rodriguez, V.; Fecková, M.; Vachtlová, M.; Podlesný, J.; Klikar, M.; Sempé, B.; Hugget, M.; et al. Property Tuning in N-Methylpyrrole Azo-Photoswitches via Modification of the Peripheral Substituents. Chem. Eur. J. 2025, 31, e202404221. [Google Scholar] [CrossRef]
- Vautrin, L.; Lambert, A.; Mahdhaoui, F.; El Abed, R.; Boubaker, T.; Ingrosso, F. Structural and Electronic Properties of Novel Azothiophene Dyes: A Multilevel Study Incorporating Explicit Solvation Effects. Molecules 2024, 29, 4053. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, G.B.; Krishna, M.M.G.; Periasamy, N. Time-resolved fluorescence studies of aminostyryl pyridinium dyes in organic solvents and surfactant solutions. J. Lumin. 2001, 92, 175–188. [Google Scholar] [CrossRef]
- Hammond, G.S.; Saltiel, J.; Lamola, A.A.; Turro, N.J.; Bradshaw, J.S.; Cowan, D.O.; Counsell, R.C.; Vogt, V.; Dalton, C. Mechanisms of Photochemical Reactions in Solution. XXII.1 Photochemical cis-trans Isomerization. J. Am. Chem. Soc. 1964, 86, 3197–3217. [Google Scholar] [CrossRef]
- Ikeda, N.; Mataga, N.; Steiner, U.; Abdel-Kader, M.H. Picosecond laser photolysis studies upon photochemical isomerization and protolytic reaction of a stilbazolium betaine. Chem. Phys. Lett. 1983, 95, 66–71. [Google Scholar] [CrossRef]
- Catalán, J. Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C. A Generalized Solvent Acidity Scale: The Solvatochromism of o-tert-Butylstilbazolium Betaine Dye and Its Homomorph o,o′-Di-tert-butylstilbazolium Betaine Dye. Liebigs Ann. 1997, 1997, 1941–1949. [Google Scholar] [CrossRef]
- Catalán, J.; López, V.; Pérez, P.; Martin-Villamil, R.; Rodríguez, J.-G. Progress towards a generalized solvent polarity scale: The solvatochromism of 2-(dimethylamino)-7-nitrofluorene and its homomorph 2-fluoro-7-nitrofluorene. Liebigs Ann. 1995, 1995, 241–252. [Google Scholar] [CrossRef]
- Catalán, J.; Reichardt, C. Analysis of the Solvatochromism of 9,9′-Biaryl Compounds Using a Pure Solvent Dipolarity Scale. J. Phys. Chem. A 2012, 116, 4726–4734. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Koelsch, C.F. The Condensation of α-Picoline Methiodide with Aromatic Aldehydes. J. Am. Chem. Soc. 1944, 66, 2126. [Google Scholar] [CrossRef]
- Acheson, R.M.; Harrison, D.R. The synthesis, spectra, and reactions of some S-alkylthiophenium salts. J. Chem. Soc. C Org. 1970, 1764–1784. [Google Scholar] [CrossRef]
- Xie, X.; Zuffo, M.; Teulade-Fichou, M.-P.; Granzhan, A. Identification of optimal fluorescent probes for G-quadruplex nucleic acids through systematic exploration of mono- and distyryl dye libraries. Beilstein J. Org. Chem. 2019, 15, 1872–1889. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.F. The methiodides of the condensation products of some cyclic aldehydes with quinaldine and alpha picoline, and their possible value as indicators in acidimetry. J. Am. Chem. Soc. 1920, 42, 2309–2314. [Google Scholar] [CrossRef]
- Abbotto, A.; Beverina, L.; Bozio, R.; Bradamante, S.; Ferrante, C.; Pagani, G.A.; Signorini, R. Push–Pull Organic Chromophores for Frequency-Upconverted Lasing. Adv. Mater. 2000, 12, 1963–1967. [Google Scholar] [CrossRef]


| No | Structural Formula | Solvent | (nm) | ε (M−1 cm−1) | (nm) | ΦFL (%) | ΔνSS (cm−1) |
|---|---|---|---|---|---|---|---|
| 2-PN | 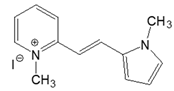 | DMSO | 427 | 31,000 | 519 | 0.19 | 4151 |
| DMF | 427 | 34,300 | 518 | 0.16 | 4114 | ||
| MeAc | 427 | 35,400 | 516 | 0.19 | 4039 | ||
| EtOA | 422 | – | 506 | 0.12 | 3934 | ||
| THF | 428 | – | 517 | 0.41 | 4022 | ||
| 1,4-Dx | 419 | – | 498 | 0.97 | 3786 | ||
| 4-PN | 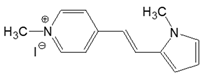 | DMSO | 438 | 37,700 | 534 | 1.22 | 4104 |
| DMF | 437 | 36,300 | 531 | 0.87 | 4051 | ||
| MeAc | 437 | 42,500 | 529 | 0.74 | 3980 | ||
| EtOAc | 435 | – | 521 | 0.24 | 3795 | ||
| THF | 439 | – | 527 | 0.41 | 3804 | ||
| 1,4-Dx | 435 | – | 518 | 0.57 | 3683 | ||
| 2-QN | 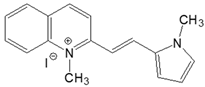 | DMSO | 482 | 50,800 | 560 | 0.40 | 2890 |
| DMF | 480 | 49,800 | 556 | 0.25 | 2848 | ||
| MeAc | 478 | 46,400 | 552 | 0.44 | 2805 | ||
| EtOAc | 479 | – | 547 | 0.23 | 2595 | ||
| THF | 483 | – | 549 | 1.60 | 2489 | ||
| 1,4-Dx | 484 | – | 548 | 1.87 | 2413 | ||
| 4-QN | 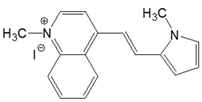 | DMSO | 502 | 37,600 | 597 | 1.51 | 3170 |
| DMF | 500 | 32,000 | 594 | 1.02 | 3165 | ||
| MeAc | 498 | 37,700 | 588 | 0.46 | 3074 | ||
| EtOAc | 496 | – | 582 | 0.22 | 2979 | ||
| THF | 499 | – | 586 | 0.71 | 2975 | ||
| 1,4-Dx | 497 | – | 582 | 1.51 | 2939 | ||
| 2-PO | 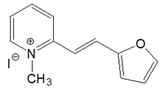 | DMSO | 374 | 24,600 | 466 | 0.03 | 5279 |
| DMF | 372 | 26,100 | 459 | 0.02 | 5095 | ||
| MeAc | 371 | 22,400 | 456 | 0.03 | 5024 | ||
| EtOAc | 371 | – | 448 | 0.01 | 4633 | ||
| THF | 372 | – | 448 | 0.11 | 4560 | ||
| 1,4-Dx | 371 | – | 446 | 0.12 | 4533 | ||
| 4-PO | 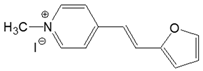 | DMSO | 381 | 31,200 | 488 | 0.23 | 5755 |
| DMF | 380 | 29,200 | 481 | 0.17 | 5526 | ||
| MeAc | 380 | 33,600 | 477 | 0.17 | 5351 | ||
| EtOAc | 376 | – | 460 | 0.04 | 4857 | ||
| THF | 379 | – | 466 | 0.08 | 4926 | ||
| 1,4-Dx | 375 | – | 455 | 0.41 | 4689 | ||
| 2-QO | 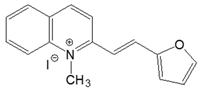 | DMSO | 414 | 28,900 | 522 | 0.32 | 4998 |
| DMF | 413 | 33,300 | 518 | 0.22 | 4908 | ||
| MeAc | 412 | 29,300 | 509 | 0.16 | 4625 | ||
| EtOAc | 412 | – | 499 | 0.05 | 4237 | ||
| THF | 411 | – | 499 | 0.22 | 4291 | ||
| 1,4-Dx | 413 | – | 497 | 0.44 | 4092 | ||
| 4-QO | 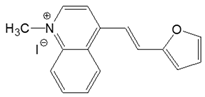 | DMSO | 425 | 26,100 | 551 | 0.40 | 5381 |
| DMF | 422 | 38,600 | 544 | 0.37 | 5314 | ||
| MeAc | 420 | 27,700 | 538 | 0.29 | 5222 | ||
| EtOAc | 417 | – | 530 | 0.06 | 5113 | ||
| THF | 420 | – | 533 | 0.27 | 5048 | ||
| 1,4-Dx | 418 | – | 529 | 0.70 | 5020 | ||
| 2-PS |  | DMSO | 372 | 20,700 | 478 | 0.02 | 5981 |
| DMF | 372 | 20,500 | 477 | 0.01 | 5917 | ||
| MeAc | 370 | 23,800 | 472 | 0.01 | 5841 | ||
| EtOAc | 365 | – | 454 | 0.01 | 5371 | ||
| THF | 370 | – | 459 | 0.03 | 5241 | ||
| 1,4-Dx | 366 | – | 458 | 0.13 | 5488 | ||
| 4-PS | 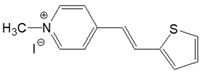 | DMSO | 381 | 21,700 | 492 | 0.08 | 5922 |
| DMF | 379 | 25,800 | 489 | 0.05 | 5935 | ||
| MeAc | 378 | 21,600 | 480 | 0.04 | 5622 | ||
| EtOAc | 373 | – | 457 | 0.02 | 4928 | ||
| THF | 379 | – | 474 | 0.05 | 5288 | ||
| 1,4-Dx | 373 | – | 458 | 0.32 | 4976 | ||
| 2-QS | 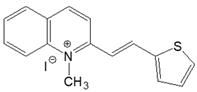 | DMSO | 414 | 38,100 | 515 | 0.10 | 4737 |
| DMF | 413 | 40,500 | 512 | 0.08 | 4682 | ||
| MeAc | 410 | 40,000 | 508 | 0.07 | 4705 | ||
| EtOAc | 410 | – | 498 | 0.03 | 4310 | ||
| THF | 410 | – | 506 | 0.05 | 4627 | ||
| 1,4-Dx | 412 | – | 480 | 0.04 | 3439 | ||
| 4-QS | 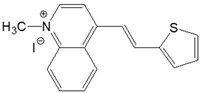 | DMSO | 424 | 29,100 | 546 | 0.32 | 5270 |
| DMF | 424 | 30,400 | 543 | 0.20 | 5169 | ||
| MeAc | 420 | 28,100 | 535 | 0.13 | 5118 | ||
| EtOAc | 414 | – | 528 | 0.05 | 4927 | ||
| THF | 422 | – | 530 | 0.12 | 4829 | ||
| 1,4-Dx | 413 | – | 510 | 0.10 | 4605 |
| Dye | EHOMO | ELUMO | ΔEGAP | Dye | EHOMO | ELUMO | ΔEGAP |
|---|---|---|---|---|---|---|---|
| 2-PN | −4.5314 | −2.0946 | 2.4367 | 4-PN | −4.5700 | −2.2035 | 2.3665 |
| 2-QN | −4.5414 | −2.2324 | 2.3091 | 4-QN | −4.5442 | −2.3232 | 2.2209 |
| 2-PO | −4.5586 | −2.7129 | 1.8456 | 4-PO | −4.4522 | −2.7233 | 1.7289 |
| 2-QO | −4.4220 | −2.7810 | 1.6410 | 4-QO | −4.6979 | −2.4707 | 2.2272 |
| 2-PS | −4.5746 | −2.7304 | 1.8443 | 4-PS | −4.4707 | −2.7361 | 1.7346 |
| 2-QS | −4.4421 | −2.8008 | 1.6413 | 4-QS | −4.7055 | −2.5099 | 2.1956 |
| No | τ1 | α1 | τ2 | α2 | τ3 | α3 | τav | χ2 | avkr | avknr | knr/kr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-PN | 0.07 | 89.0 | 1.47 | 11.0 | 0.224 | 1.43 | 8.48 | 4.46 | 525.3 | ||
| 4-PN | 0.08 | 97.5 | 1.69 | 2.5 | 0.120 | 1.47 | 101.0 | 8.21 | 81.0 | ||
| 2-QN | 0.03 | 92.0 | 6.6 | 8.0 | 0.556 | 1.52 | 7.20 | 1.79 | 249.0 | ||
| 4-QN | 0.08 | 99.5 | 3.32 | 0.5 | 0.096 | 1.57 | 157.0 | 10.2 | 65.2 | ||
| 2-PO | 0.04 | 62.2 | 0.98 | 21.2 | 5.33 | 16.6 | 1.117 | 1.11 | 0.27 | 0.90 | 3332.3 |
| 4-PO | 0.06 | 97.2 | 1.77 | 2.8 | 0.108 | 1.55 | 21.3 | 9.25 | 433.8 | ||
| 2-QO | 0.06 | 91.5 | 1.9 | 8.5 | 0.216 | 1.22 | 14.8 | 4.61 | 311.5 | ||
| 4-QO | 0.12 | 62.2 | 2.02 | 37.8 | 0.838 | 1.30 | 4.77 | 1.19 | 249.0 | ||
| 2-PS | 0.05 | 66.2 | 0.83 | 17.1 | 4.23 | 16.7 | 0.881 | 1.02 | 0.23 | 1.13 | 4999.0 |
| 4-PS | 0.04 | 87.0 | 0.89 | 6.3 | 4.34 | 6.7 | 0.382 | 1.02 | 2.10 | 2.62 | 1249.0 |
| 2-QS | 0.07 | 77.9 | 1.09 | 11.0 | 4.04 | 11.1 | 0.623 | 1.08 | 1.61 | 1.60 | 999.0 |
| 4-QS | 0.06 | 85.0 | 0.84 | 7.7 | 4.41 | 7.3 | 0.438 | 1.15 | 7.31 | 2.28 | 311.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, P.; Jędrzejewska, B. Both Benzannulation and Heteroatom-Controlled Photophysical Properties in Donor–π–Acceptor Ionic Dyes: A Combined Experimental and Theoretical Study. Materials 2025, 18, 4676. https://doi.org/10.3390/ma18204676
Krawczyk P, Jędrzejewska B. Both Benzannulation and Heteroatom-Controlled Photophysical Properties in Donor–π–Acceptor Ionic Dyes: A Combined Experimental and Theoretical Study. Materials. 2025; 18(20):4676. https://doi.org/10.3390/ma18204676
Chicago/Turabian StyleKrawczyk, Przemysław, and Beata Jędrzejewska. 2025. "Both Benzannulation and Heteroatom-Controlled Photophysical Properties in Donor–π–Acceptor Ionic Dyes: A Combined Experimental and Theoretical Study" Materials 18, no. 20: 4676. https://doi.org/10.3390/ma18204676
APA StyleKrawczyk, P., & Jędrzejewska, B. (2025). Both Benzannulation and Heteroatom-Controlled Photophysical Properties in Donor–π–Acceptor Ionic Dyes: A Combined Experimental and Theoretical Study. Materials, 18(20), 4676. https://doi.org/10.3390/ma18204676






