The Influence of Starting Plant Material on Ni@C-Type Composites’ Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials’ Preparation
2.2. Characterization Methods
3. Results
3.1. BET Analysis
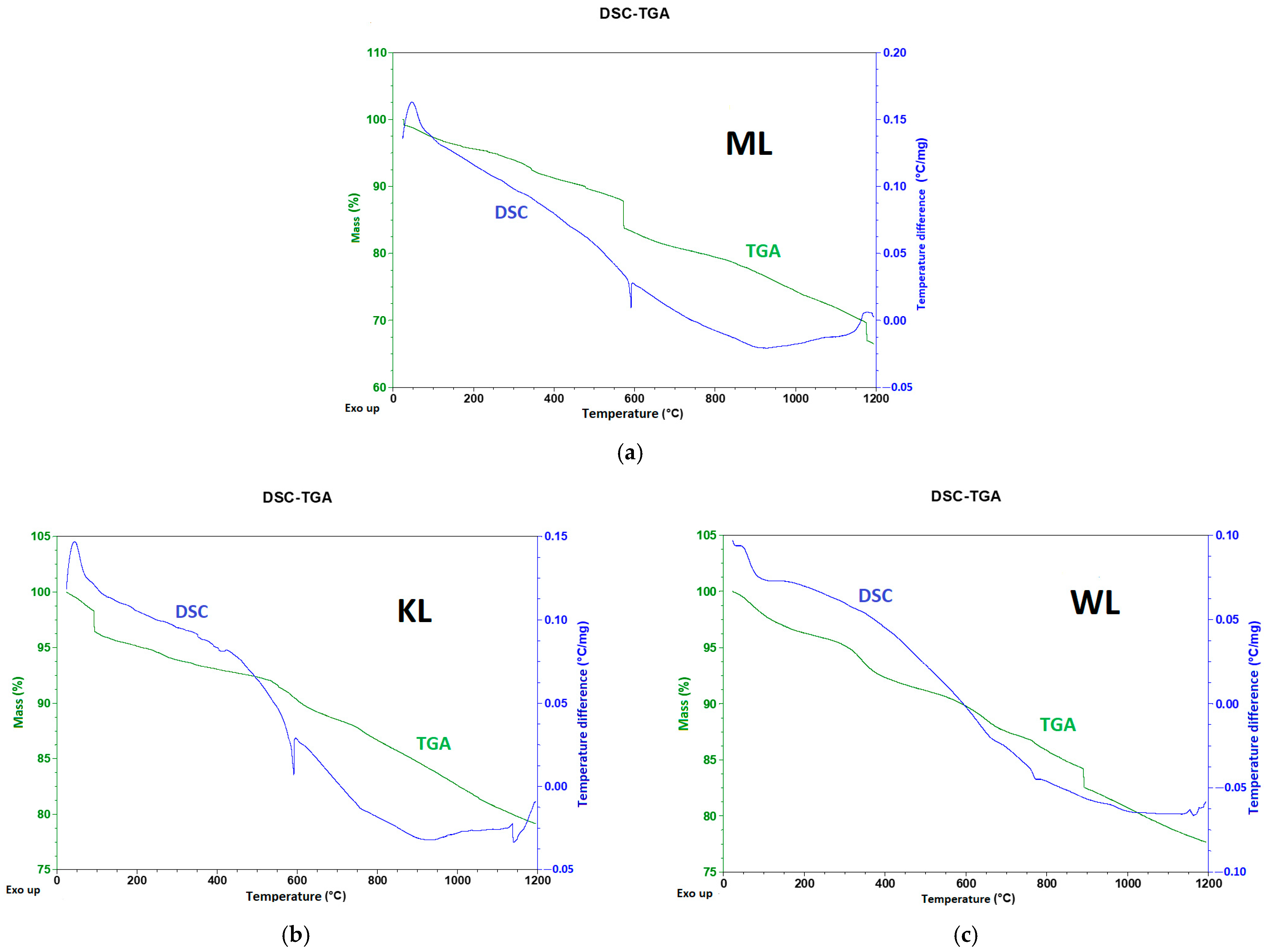
3.2. DSC-TGA Analysis
3.3. MP-AES Analysis
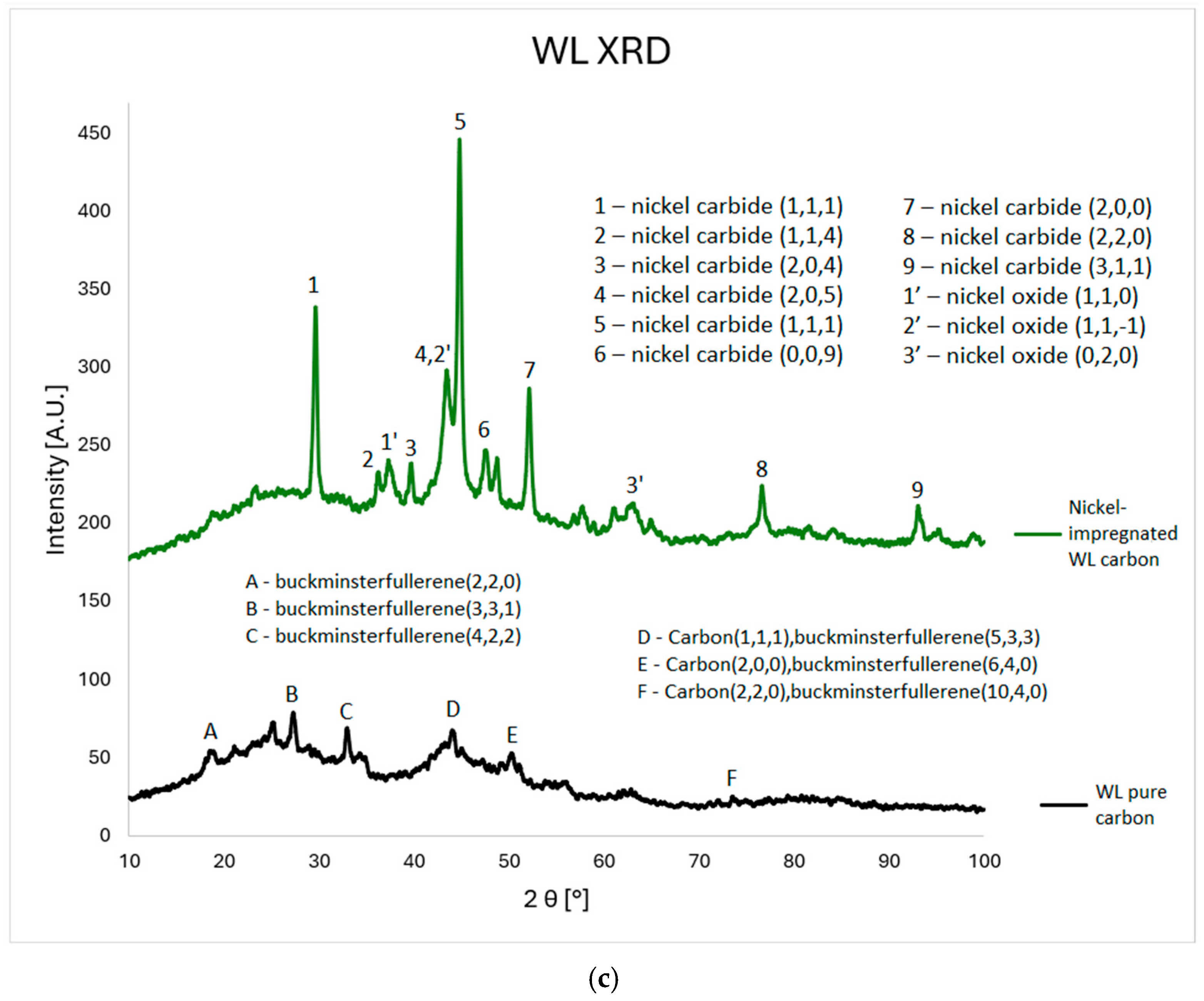
3.4. XRD Analysis
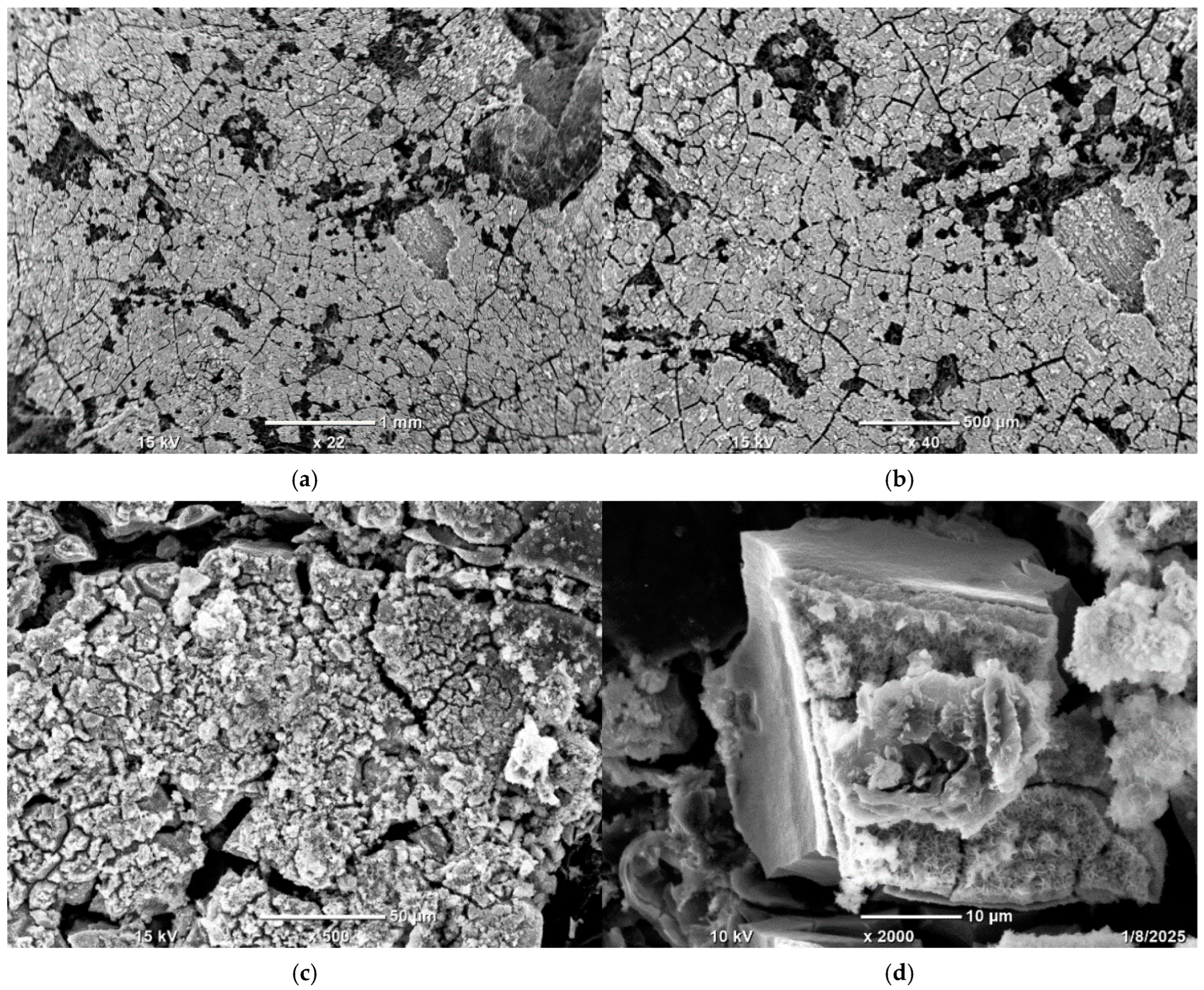
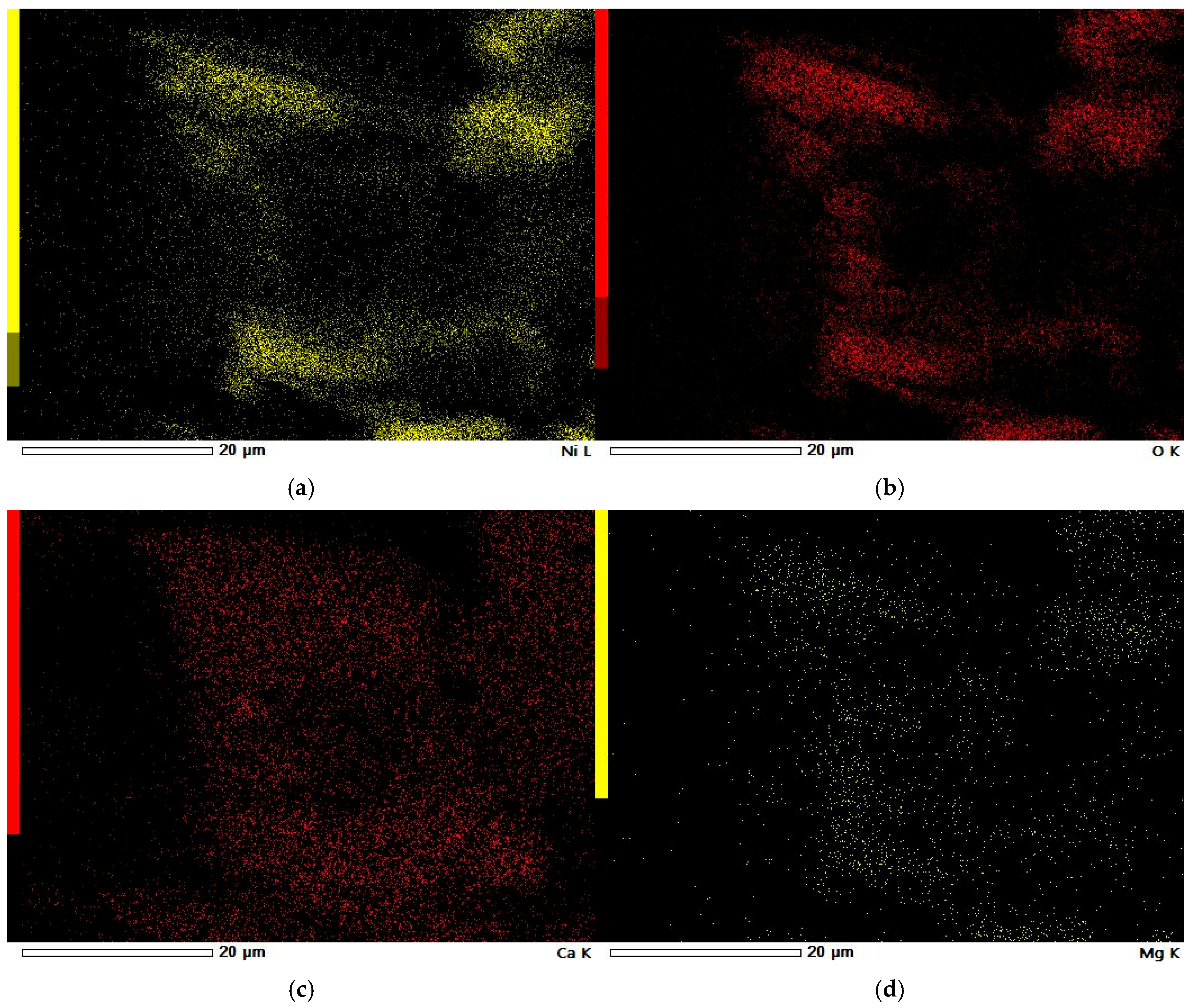
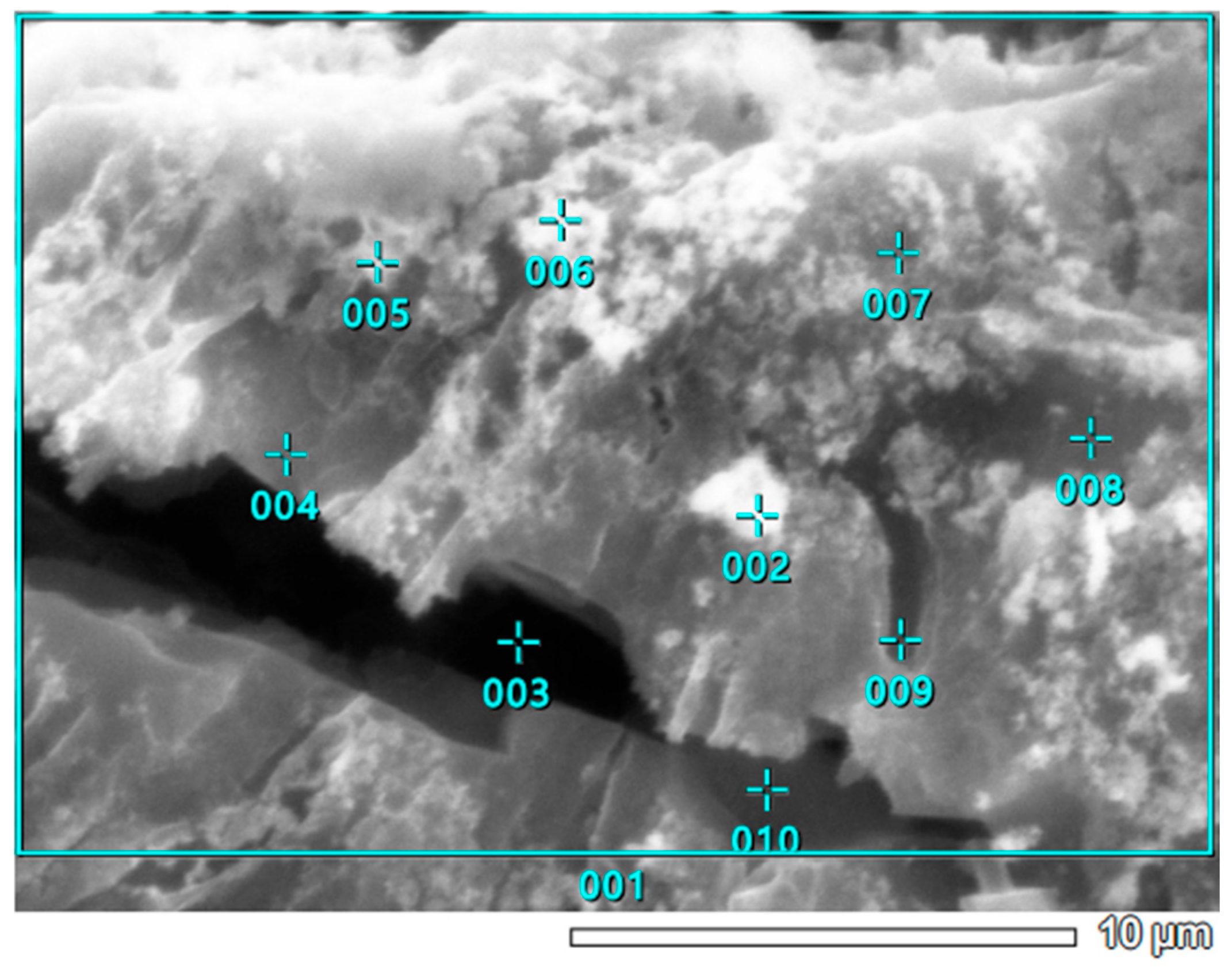
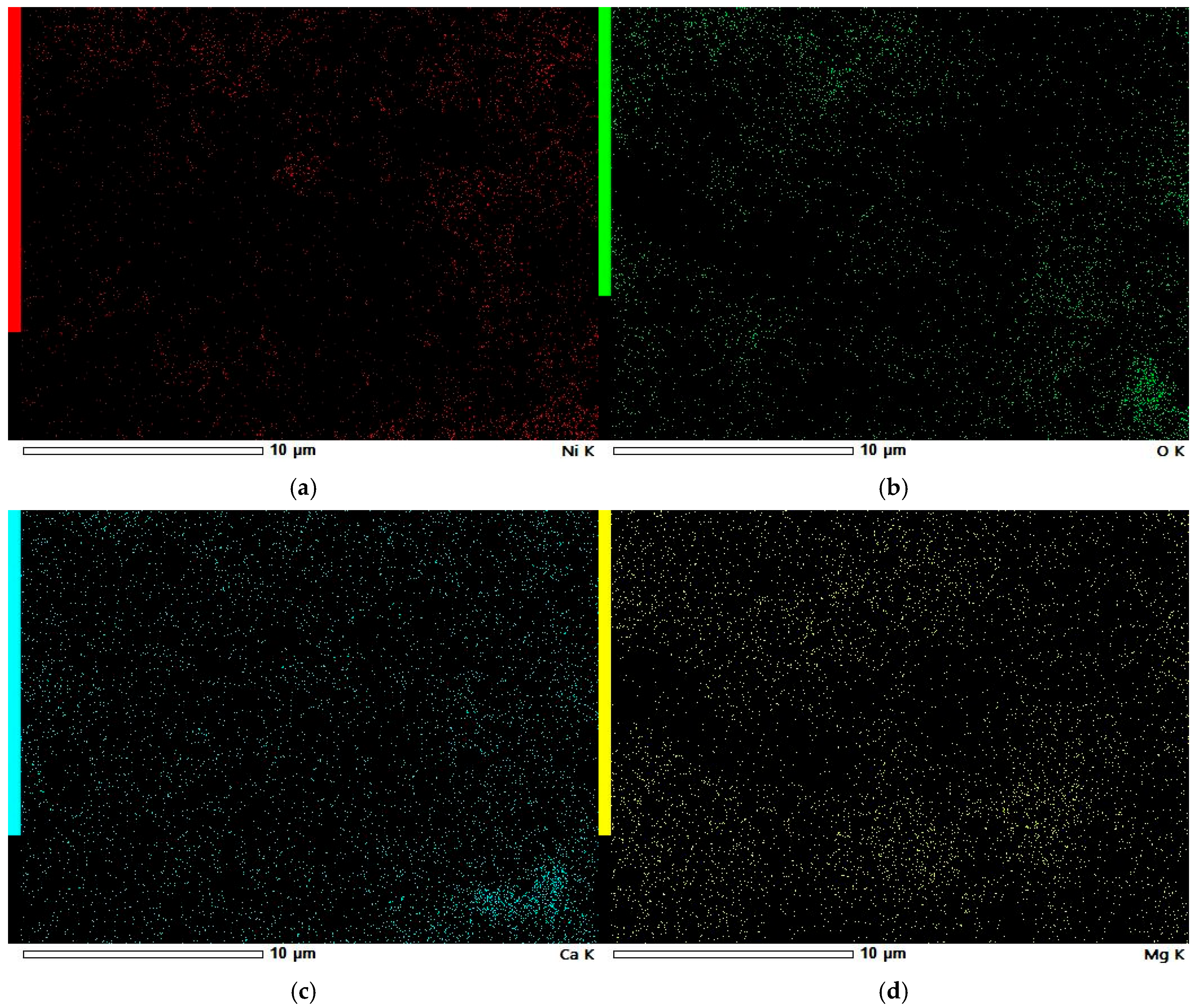
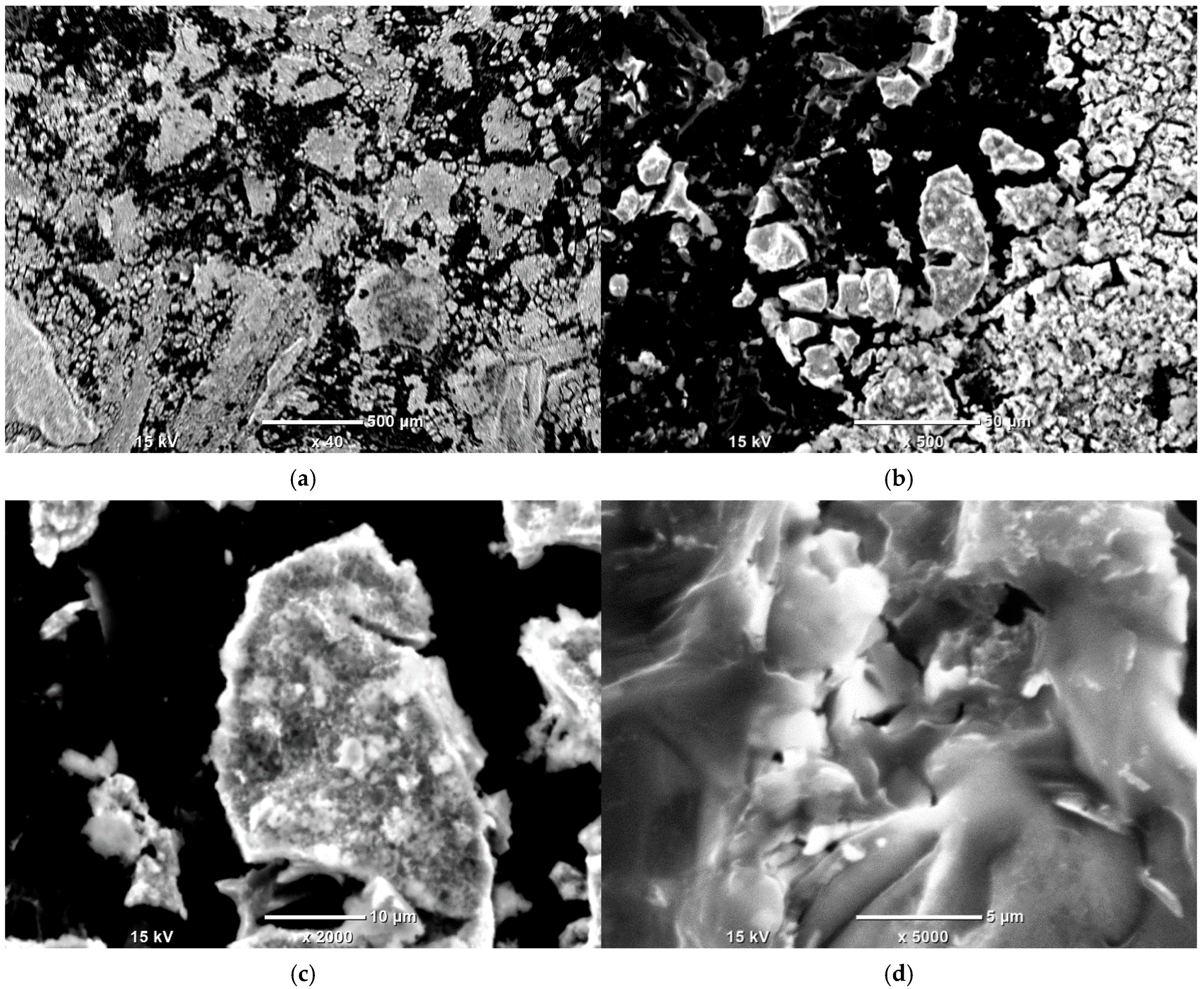
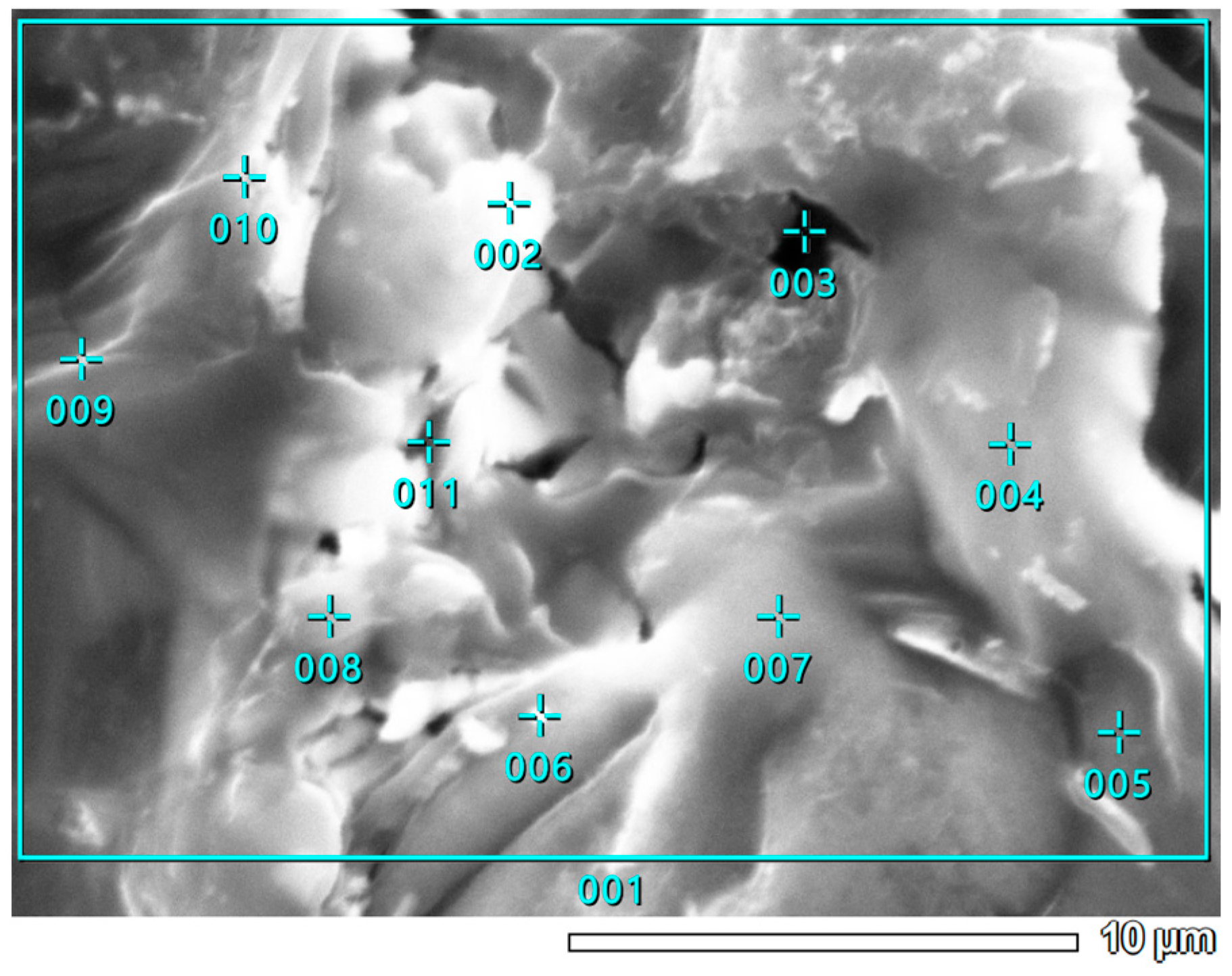
3.5. SEM-EDS Study
3.6. Ash Content
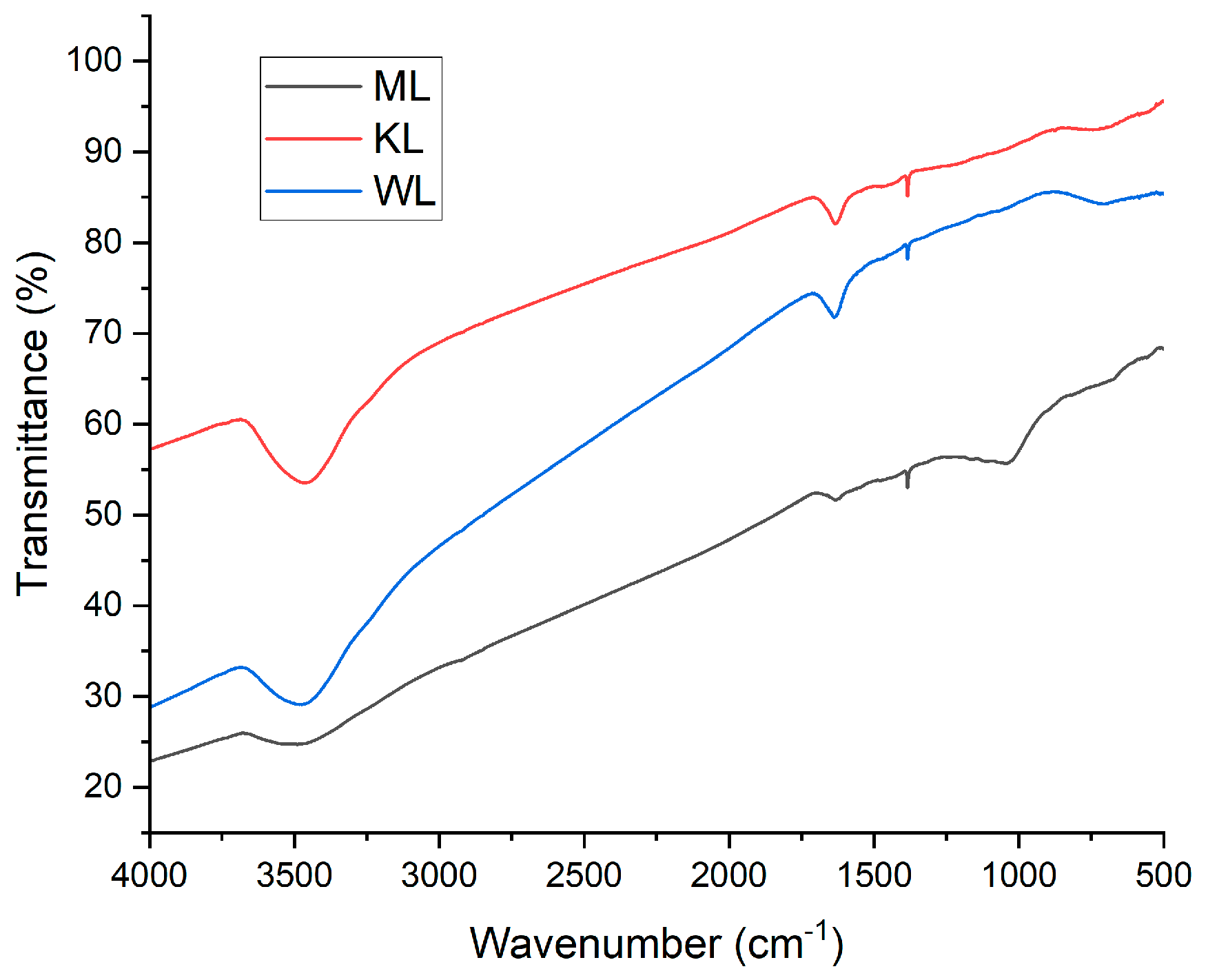
3.7. FT-IR Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Ayinla, R.T.; Dennis, J.; Zaid, H.; Sanusi, Y.; Usman, F.; Adebayo, L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Kleszyk, P.; Ratajczak, P.; Skowron, P.; Jagiello, J.; Abbas, Q.; Frąckowiak, E.; Béguin, F. Carbons with narrow pore size distribution prepared by simultaneous carbonization and self-activation of tobacco stems and their application to supercapacitors. Carbon 2015, 81, 148–157. [Google Scholar] [CrossRef]
- Balahmar, N.; Al-Jumialy, A.S.; Mokaya, R. Biomass to porous carbon in one step: Directly activated biomass for high performance CO2 storage. J. Mater. Chem. A Mater. 2017, 5, 12330–12339. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Yin, H.Y.; Lu, B.H.; Xu, Y.; Tang, D.Y.; Mao, X.H.; Xiao, W.; Wang, D.H.; Alshawabkeh, A.N. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Sci. Technol. 2014, 48, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Preparation and surface characterization of activated carbons from Euphorbia rigida by chemical activation with ZnCl2, K2CO3, NaOH and H3PO4. Appl. Surf. Sci. 2012, 261, 247–254. [Google Scholar] [CrossRef]
- Skodras, G.; Orfanoudaki, T.; Kakaras, E.; Sakellaropoulos, G.P. Production of Special Activated Carbon from Lignite for Environmental Purposes 8 2 0 (0 2) 0 0 0 6 2-0. 2002. Available online: www.elsevier.com/locate/fuproc (accessed on 3 June 2025).
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Rintala, V. Agro-Based Activated Carbon for Wastewater Treatment of Micropollutants Agricultural Feedstocks in Activated Carbon Production. Master’s Thesis, Aalto University, Aalto, Finland, 2025. [Google Scholar]
- Horikawa, T.; Kitakaze, Y.; Sekida, T.; Hayashi, J.; Katoh, M. Characteristics and humidity control capacity of activated carbon from bamboo. Bioresour. Technol. 2010, 101, 3964–3969. [Google Scholar] [CrossRef]
- Hafizi-Atabak, H.R.; Tuedeshki, H.G.; Shafaroudi, A.; Akbari, M.; Safaei-Ghomi, J.; Shariaty-Niassar, M. Production of Activated Carbon from Cellulose Wastes. J. Chem. Pet. Eng. 2013, 47, 13–25. [Google Scholar]
- Tan, I.; Ahmad, A.; Hameed, B. Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chem. Eng. J. 2008, 137, 462–470. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, M.H. Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities. Sustainability 2023, 15, 11574. [Google Scholar] [CrossRef]
- Saning, A.; Dubadi, R.; Chuenchom, L.; Dechtrirat, D.; Jaroniec, M. Microporous Carbons Obtained via Solvent-Free Mechanochemical Processing, Carbonization and Activation with Potassium Citrate and Zinc Chloride for CO2 Adsorption. Separations 2023, 10, 304. [Google Scholar] [CrossRef]
- Bao, H.; Islam, A.; Saha, B.B. Adsorption of HFO-1234ze(E) onto Steam-Activated Carbon Derived from Sawmill Waste Wood. Technologies 2024, 12, 104. [Google Scholar] [CrossRef]
- Elewa, A.M.; Amer, A.A.; Attallah, M.F.; Gad, H.A.; Al-Ahmed, Z.A.M.; Ahmed, I.A. Chemically Activated Carbon Based on Biomass for Adsorption of Fe(III) and Mn(II) Ions from Aqueous Solution. Materials 2023, 16, 1251. [Google Scholar] [CrossRef] [PubMed]
- Alhawtali, S.; El-Harbawi, M.; El Blidi, L.; Alrashed, M.M.; Alzobidi, A.; Yin, C.-Y. Date Palm Leaflet-Derived Carbon Microspheres Activated Using Phosphoric Acid for Efficient Lead (II) Adsorption. C-J. Carbon Res. 2024, 10, 26. [Google Scholar] [CrossRef]
- Alhawtali, S.; El-Harbawi, M.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Yin, C.-Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings 2023, 13, 1287. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, K.; Xue, M.; Zhang, Y.; Wang, J.; Liu, L. Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar. Bioengineering 2023, 10, 836. [Google Scholar] [CrossRef]
- Baikousi, M.; Gantzoudi, A.; Gioti, C.; Moschovas, D.; Giannakas, A.E.; Avgeropoulos, A.; Salmas, C.E.; Karakassides, M.A. Hydrogen Sulfide Removal via Sorption Process on Activated Carbon–Metal Oxide Composites Derived from Different Biomass Sources. Molecules 2023, 28, 7418. [Google Scholar] [CrossRef]
- Wang, M.; Cai, J.; Jiao, L.; Bu, Q. Biomass-Derived Co/MPC Nanocomposites for Effective Sensing of Hydrogen Peroxide via Electrocatalysis Reduction. Catalysts 2024, 14, 624. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, K.; Chen, Z.; Wang, Y.; Tan, Y.; Wang, J.; Yao, L.; Wang, L.; Sun, C.; Yang, J. Tuning the Electronic Structures of Mo-Based Sulfides/Selenides with Biomass-Derived Carbon for Hydrogen Evolution Reaction and Sodium-Ion Batteries. Catalysts 2024, 14, 627. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Karthik, N.; Sangaraju, S.; Choi, S.T.; Lee, Y.R. Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction. Catalysts 2023, 13, 542. [Google Scholar] [CrossRef]
- Yu, F.; Liu, S.; Liu, B. Exploring Deactivation Reasons of Biomass-Based Phosphorus-Doped Carbon as a Metal-Free Catalyst in the Catalytic Dehydroaromatization of n-Heptane. Molecules 2024, 29, 1288. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Q.; Li, F.; Sang, Y.; Hao, M.; Tang, X.; Qiu, F.; Zhang, H. Mitigating Co Metal Particle Agglomeration and Enhancing ORR Catalytic Activity through Nitrogen-Enriched Porous Carbon Derived from Biomass. Catalysts 2023, 13, 1118. [Google Scholar] [CrossRef]
- Dobele, G.; Volperts, A.; Plavniece, A.; Zhurinsh, A.; Upskuviene, D.; Balciunaite, A.; Niaura, G.; Colmenares-Rausseo, L.C.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction. Molecules 2024, 29, 2238. [Google Scholar] [CrossRef] [PubMed]
- Schmies, H.; Bengen, N.; Müller-Hülstede, J.; Ibitowa, O.A.; Wagner, P.; Wark, M. How Effective Is Graphitization of Biomasses for the Carbon Stability of Pt/C ORR Catalysts? Catalysts 2023, 13, 343. [Google Scholar] [CrossRef]
- Plavniece, A.; Kaare, K.; Simkunaitė, D.; Balciunaite, A.; Jasulaitiene, V.; Niaura, G.; Volperts, A.; Dobele, G.; Colmenares-Rausseo, L.C.; Kruusenberg, I.; et al. Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction. Catalysts 2024, 14, 92. [Google Scholar] [CrossRef]
- Murillo, H.A.; Juiña, E.; Vizuete, K.; Debut, A.; Echeverría, D.; Taco-Vasquez, S.; Ponce, S. Rapid Waste Motor Oil Conversion into Diesel-Range Hydrocarbons Using Hydrochar as Catalyst: Kinetic Study and Product Characterization. Recycling 2024, 9, 39. [Google Scholar] [CrossRef]
- Ibrahim, A.; Elsayed, I.; Hassan, E.B. Upgrading of Rice Straw Bio-Oil Using 1-Butanol over ZrO2-Fe3O4 Bimetallic Nanocatalyst Supported on Activated Rice Straw Biochar to Butyl Esters. Catalysts 2024, 14, 666. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef] [PubMed]
- Fałtynowicz, H.; Kaczmarczyk, J.; Kułażyński, M. Preparation and characterization of activated carbons from biomass material—Giant knotweed (Reynoutria sachalinensis). Open Chem. 2015, 13, 1150–1156. [Google Scholar] [CrossRef]
- Dudek, K.; Wojtaszek, K.; Żabiński, P. Ni-Doped Activated Carbon from Invasive Plants as a Potential Catalyst. Metals 2024, 14, 790. [Google Scholar] [CrossRef]
- Michałek, T.; Wojtaszek, K.; Youssif, M.M.; Żabiński, P.; Kołczyk-Siedlecka, K.; Kowalik, R.; Socha, R.P.; Hessel, V.; Wojnicki, M. Adsorption of Au(III), Pt(IV), Pd(II), and Rh(III) ions on activated carbon in a batch reactor supported by microwave radiation. Sci. Rep. 2025, 15, 5852. [Google Scholar] [CrossRef]
- de Oliveira, G.F.; de Andrade, R.C.; Trindade, M.A.G.; Andrade, H.M.C.; de Carvalho, C.T. Thermogravimetric and spectroscopic study (TG–DTA/FT–IR) of activated carbon from the renewable biomass source Babassu. Quim. Nova 2017, 40, 284–292. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Pastor-Villegas, J.; Perez-Florindo, A.; Duran-Valle, C.; Valenzuela-Calahorro, C. FT-IR study of rockrose and of char and activated carbon. J. Anal. Appl. Pyrolysis 1996, 36, 71–80. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Xu, X.; Li, Q.; Wang, Y. Adsorption and cosorption of ciprofloxacin and Ni(II) on activated carbon-mechanism study. J. Taiwan Inst. Chem. Eng. 2014, 45, 681–688. [Google Scholar] [CrossRef]
- Neyts, E.; Shibuta, Y.; Bogaerts, A. Bond switching regimes in nickel and nickel–carbon nanoclusters. Chem. Phys. Lett. 2010, 488, 202–205. [Google Scholar] [CrossRef]
- El-Sadaawy, M.; Abdelwahab, O. Adsorptive removal of nickel from aqueous solutions by activated carbons from doum seed (Hyphaenethebaica) coat. Alex. Eng. J. 2014, 53, 399–408. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dinh, K.N.; Gomes, V.G. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: A sustainable bifunctional electrocatalyst for efficient water splitting. Carbon 2020, 157, 515–524. [Google Scholar] [CrossRef]
- Sha, L.; Gao, P.; Wu, T.; Chen, Y. Chemical Ni–C Bonding in Ni–Carbon Nanotube Composite by a Microwave Welding Method and Its Induced High-Frequency Radar Frequency Electromagnetic Wave Absorption. ACS Appl. Mater. Interfaces 2017, 9, 40412–40419. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Chen, D.; Luo, J.; Huang, Z.; Hong, L.; Hu, Y. Direct synthesis of methane-rich gas from reed biomass pyrolysis volatiles over its biochar-supported Ni catalysts. Biomass Bioenergy 2021, 154, 106250. [Google Scholar] [CrossRef]
- Fatimah, I.; Wijayanti, H.K.; Ramanda, G.D.; Tamyiz, M.; Doong, R.-A.; Sagadevan, S. Nanocomposite of Nickel Nanoparticles-Impregnated Biochar from Palm Leaves as Highly Active and Magnetic Photocatalyst for Methyl Violet Photocatalytic Oxidation. Molecules 2022, 27, 6871. [Google Scholar] [CrossRef]
- Chai, D.-F.; Han, Y.; Zhang, W.; Dong, G.; Zhang, Z.; Bai, L.; Guo, D. Ni nanoparticles assembled on the surface of biomass-derived porous carbon as competitive candidates for the hydrogen evolution reaction. CrystEngComm 2023, 25, 2298–2306. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, S.; Xie, C.; Yu, H.; Liu, Y.; Yu, S.; Huang, L. Ni-doped mesoporous carbon obtained from hydrothermal carbonization of cellulose and their catalytic hydrogenation activity study. J. Mater. Sci. 2018, 53, 7900–7910. [Google Scholar] [CrossRef]
- Azuara, M.; Latorre, N.; Villacampa, J.I.; Sebastian, V.; Cazaña, F.; Romeo, E.; Monzón, A. Use of Ni Catalysts Supported on Biomorphic Carbon Derived From Lignocellulosic Biomass Residues in the Decomposition of Methane. Front. Energy Res. 2019, 7, 34. [Google Scholar] [CrossRef]
- Lv, P.; Liu, D.; Tian, B.; Ma, X.; Fan, M.; Xu, L. Preparation of biomass-derived porous carbon supported Ni nanoparticles for CO2 reforming of CH4. New J. Chem. 2020, 44, 12503–12513. [Google Scholar] [CrossRef]
- Tarifa, P.; Megías-Sayago, C.; Cazaña, F.; González-Martín, M.; Latorre, N.; Romeo, E.; Delgado, J.J.; Monzón, A. Highly active Ce- and Mg-promoted Ni catalysts supported on cellulose-derived carbon for low-temperature CO2 methanation. Energy Fuels 2021, 35, 17212–17224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |





| Material/Parameter | ML | KL | WL |
|---|---|---|---|
| Carbon yield [%] | 31 | 31 | 30 |
| Mass before pyrolysis [g] | 14.9825 | 13.7561 | 16.0704 |
| Mass after pyrolysis [g] | 4.6474 | 4.2604 | 4.8317 |
| Sample Name | ML | KL | WL |
|---|---|---|---|
| Specific surface area [m2/g] | 266 | 7 | 40 |
| dV/dlog (w) Pore Volume (cm3/g) | Average Width (nm) | Incremental Pore Volume (cm3/g) | Cumulative Pore Volume (cm3/g) | Incremental Pore Area (m2/g) | Cumulative Pore Area (m2/g) |
|---|---|---|---|---|---|
| 2.2796 × 10−2 | 181.2 | 0.0075 | 0.0075 | 0.2 | 0.2 |
| 4.0255 × 10−2 | 88.8 | 0.0124 | 0.0199 | 0.6 | 0.7 |
| 1.2942 × 10−1 | 43.7 | 0.0397 | 0.0595 | 3.6 | 4.4 |
| 9.3578 × 10−2 | 29.5 | 0.0139 | 0.0734 | 1.9 | 6.2 |
| 5.3035 × 10−2 | 21.7 | 0.0068 | 0.0802 | 1.3 | 7.5 |
| 2.9315 × 10−2 | 12.4 | 0.0077 | 0.0879 | 2.5 | 10.0 |
| 1.3922 × 10−2 | 8.1 | 0.0024 | 0.0903 | 1.2 | 11.1 |
| 1.0244 × 10−2 | 6.5 | 0.0008 | 0.0911 | 0.5 | 11.6 |
| 9.4051 × 10−3 | 5.6 | 0.0006 | 0.0916 | 0.4 | 12.0 |
| 9.7029 × 10−3 | 4.9 | 0.0006 | 0.0922 | 0.5 | 12.5 |
| 8.8048 × 10−3 | 4.3 | 0.0005 | 0.0926 | 0.4 | 12.9 |
| 9.3416 × 10−3 | 3.8 | 0.0005 | 0.0931 | 0.5 | 13.4 |
| 1.3367 × 10−2 | 3.4 | 0.0007 | 0.0938 | 0.8 | 14.2 |
| 1.6423 × 10−2 | 3.0 | 0.0008 | 0.0946 | 1.0 | 15.2 |
| 1.6695 × 10−2 | 2.7 | 0.0008 | 0.0953 | 1.1 | 16.3 |
| 2.2995 × 10−2 | 2.5 | 0.0011 | 0.0965 | 1.8 | 18.2 |
| 2.8047 × 10−2 | 2.2 | 0.0013 | 0.0978 | 2.4 | 20.6 |
| dV/dlog (w) Pore Volume (cm3/g) | Average Width (nm) | Incremental Pore Volume (cm3/g) | Cumulative Pore Volume (cm3/g) | Incremental Pore Area (m2/g) | Cumulative Pore Area (m2/g) |
|---|---|---|---|---|---|
| 9.8839 × 10−3 | 109.2 | 0.0046 | 0.0046 | 0.2 | 0.2 |
| 2.8576 × 10−2 | 67.8 | 0.0056 | 0.0102 | 0.3 | 0.5 |
| 2.2894 × 10−2 | 45.0 | 0.0040 | 0.0141 | 0.4 | 0.9 |
| 1.5178 × 10−2 | 31.3 | 0.0023 | 0.0165 | 0.3 | 1.1 |
| 1.8405 × 10−2 | 21.8 | 0.0029 | 0.0193 | 0.5 | 1.7 |
| 2.3890 × 10−2 | 12.2 | 0.0064 | 0.0257 | 2.1 | 3.8 |
| 8.1924 × 10−3 | 8.0 | 0.0014 | 0.0272 | 0.7 | 4.5 |
| 2.4600 × 10−3 | 6.4 | 0.0002 | 0.0273 | 0.1 | 4.6 |
| 1.8533 × 10−3 | 5.5 | 0.0001 | 0.0274 | 0.1 | 4.7 |
| 1.3253 × 10−4 | 4.8 | 0.0000 | 0.0275 | 0.0 | 4.7 |
| 4.0215 × 10−4 | 2.2 | 0.0001 | 0.0276 | 0.2 | 4.9 |
| Filtrate Name/Parameter | ML | KL | WL | S |
|---|---|---|---|---|
| Amount of Ni per 1 g of carbon [mg/g] | 165 | 15 | 117 | - |
| Amount of Ni per 1 m2 of carbon’s specific surface [mg/m2] | 0.6 | 15.9 | 0.9 | - |
| Ni concentration in the filtrate after impregnation [mmol/L] | 21 | 37 | 33 | 63 |
| Point Number | Chemical Element Concentration [Atomic %] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Si | S | K | Ca | Ni | Te | Total | |
| 001 | 64.42 | 1.74 | 3.15 | 30.69 | 100.00 | ||||
| 002 | 10.04 | 58.26 | 0.84 | 0.96 | 0.52 | 2.90 | 26.47 | 100.00 | |
| 003 | 4.98 | 35.67 | 1.11 | 0.66 | 3.44 | 54.14 | 100.00 | ||
| 004 | 8.58 | 61.47 | 0.91 | 0.98 | 0.41 | 2.84 | 24.81 | 100.00 | |
| 005 | 59.93 | 0.93 | 1.21 | 0.48 | 3.70 | 33.23 | 0.52 | 100.00 | |
| 006 | 42.57 | 0.69 | 1.26 | 0.53 | 3.85 | 51.09 | 100.00 | ||
| 007 | 43.80 | 0.80 | 1.01 | 0.84 | 3.84 | 49.70 | 100.00 | ||
| 008 | 9.59 | 57.77 | 0.68 | 0.82 | 0.60 | 3.14 | 27.39 | 100.00 | |
| 009 | 10.69 | 63.20 | 0.83 | 0.90 | 0.37 | 2.90 | 21.11 | 100.00 | |
| 010 | 0.00 | ||||||||
| Point Number | Chemical Element Concentration [Atomic %] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | S | Cl | K | Ca | Ni | Rb | Total | |
| 001 | 70.25 | 19.66 | 2.19 | 0.16 | 0.31 | 1.18 | 1.61 | 4.63 | 100.00 | |||
| 002 | 57.67 | 16.55 | 1.58 | 0.31 | 1.07 | 0.57 | 0.25 | 0.84 | 3.29 | 17.87 | 100.00 | |
| 003 | 80.55 | 1.73 | 1.00 | 5.68 | 7.32 | 3.72 | 100.00 | |||||
| 004 | 73.29 | 18.86 | 2.61 | 0.26 | 1.77 | 1.52 | 1.62 | 0.06 | 100.00 | |||
| 005 | 59.20 | 26.30 | 1.64 | 0.15 | 0.29 | 0.30 | 0.17 | 0.75 | 1.24 | 9.96 | 100.00 | |
| 006 | 57.48 | 29.14 | 1.25 | 0.31 | 0.49 | 0.27 | 0.09 | 0.66 | 1.63 | 8.69 | 100.00 | |
| 007 | 74.72 | 19.29 | 2.36 | 0.27 | 1.08 | 1.07 | 1.23 | 100.00 | ||||
| 008 | 63.84 | 21.12 | 4.77 | 3.45 | 3.51 | 3.31 | 100.00 | |||||
| 009 | 62.34 | 21.30 | 4.47 | 3.43 | 4.45 | 4.01 | 100.00 | |||||
| 010 | 74.13 | 17.35 | 2.15 | 0.25 | 0.16 | 1.12 | 1.97 | 2.38 | 100.00 | |||
| Point Number | Chemical Element Concentration [Atomic %] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Si | P | S | Cl | K | Ca | Ni | Zn | Mo | Total | |
| 001 | 59.84 | 22.88 | 0.85 | 3.79 | 0.28 | 0.87 | 1.24 | 3.13 | 7.14 | 100.00 | |||
| 002 | 41.27 | 39.49 | 1.72 | 2.31 | 0.63 | 2.99 | 11.59 | 100.00 | |||||
| 003 | 21.95 | 4.28 | 3.11 | 3.24 | 11.72 | 55.71 | 100.00 | ||||||
| 004 | 63.38 | 20.69 | 3.06 | 0.91 | 1.06 | 1.74 | 9.16 | 100.00 | |||||
| 005 | 51.24 | 24.20 | 17.29 | 0.22 | 3.03 | 1.25 | 0.51 | 2.27 | 100.00 | ||||
| 006 | 47.16 | 15.89 | 13.41 | 0.42 | 1.02 | 3.14 | 2.88 | 13.07 | 2.55 | 0.46 | 100.00 | ||
| 007 | 39.95 | 43.49 | 11.23 | 0.16 | 0.12 | 2.11 | 0.98 | 0.43 | 1.53 | 100.00 | |||
| 008 | 63.03 | 11.94 | 1.21 | 1.35 | 1.89 | 0.96 | 3.88 | 15.73 | 100.00 | ||||
| 009 | 70.98 | 19.65 | 2.27 | 0.40 | 0.47 | 1.37 | 4.87 | 100.00 | |||||
| 010 | 63.42 | 24.69 | 1.66 | 0.16 | 0.16 | 0.18 | 0.55 | 2.91 | 5.63 | 100.00 | |||
| Sample Name/Parameter | ML | KL | WL |
|---|---|---|---|
| Carbon mass [g] | 0.656 | 0.626 | 0.599 |
| Ash mass [g] | 0.186 | 0.118 | 0.120 |
| Ash content [%] | 28.4 | 18.9 | 20.0 |
| Carbon Name/ Parameter | ML (Maple) | KL (Knotweed) | WL (Willow) | 2p (Goldenrod) | FWB700 (Reed) |
|---|---|---|---|---|---|
| Specific surface area [m2/g] | 266 | 7 | 40 | 3 | 355 |
| Ni mass per 1 g of carbon [mg] | 165 | 105 | 117 | 76 | 155 |
| Ni mass per surface unit [mg/m2] | 0.6 | 15.9 | 0.9 | 22.4 | 0.4 |
| Fixed carbon [%] | 29.8 | 30.1 | 29.3 | 19.6 | 14.4 |
| Ash [%] | 28.4 | 18.9 | 20.0 | 29.7 | 6.2 |
| Volatile compounds [%] | 69 | 69 | 69 | 72 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, K.; Małecki, S.; Kornaus, K.; Żabiński, P. The Influence of Starting Plant Material on Ni@C-Type Composites’ Characteristics. Materials 2025, 18, 3784. https://doi.org/10.3390/ma18163784
Dudek K, Małecki S, Kornaus K, Żabiński P. The Influence of Starting Plant Material on Ni@C-Type Composites’ Characteristics. Materials. 2025; 18(16):3784. https://doi.org/10.3390/ma18163784
Chicago/Turabian StyleDudek, Kamil, Stanisław Małecki, Kamil Kornaus, and Piotr Żabiński. 2025. "The Influence of Starting Plant Material on Ni@C-Type Composites’ Characteristics" Materials 18, no. 16: 3784. https://doi.org/10.3390/ma18163784
APA StyleDudek, K., Małecki, S., Kornaus, K., & Żabiński, P. (2025). The Influence of Starting Plant Material on Ni@C-Type Composites’ Characteristics. Materials, 18(16), 3784. https://doi.org/10.3390/ma18163784







