Pea Pod Valorization: A Green Processing Route to Obtain Cellulosic Reinforcements for Compression Molded Polylactic Acid Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Extraction
2.3. Preparation of PLA Biocomposites
2.4. Characterization of Cellulosic Reinforcements and Manufactured Biocomposites
2.4.1. Cellulosic Reinforcements
2.4.2. Characterization of PLA Biocomposites
3. Results and Discussion
3.1. Characterization of the Cellulosic Reinforcements
3.1.1. Composition and Yield
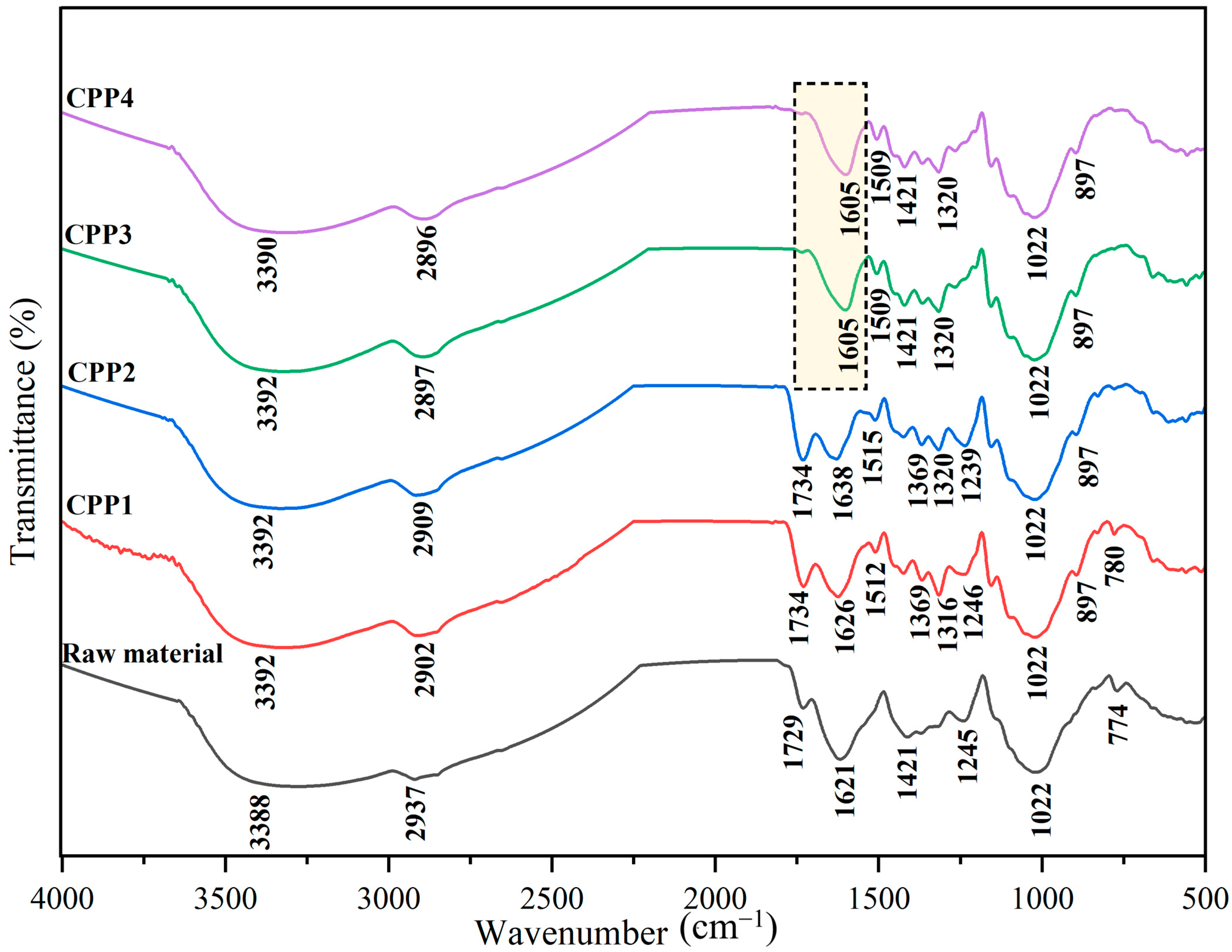
3.1.2. FTIR Analyses
3.1.3. Crystallinity
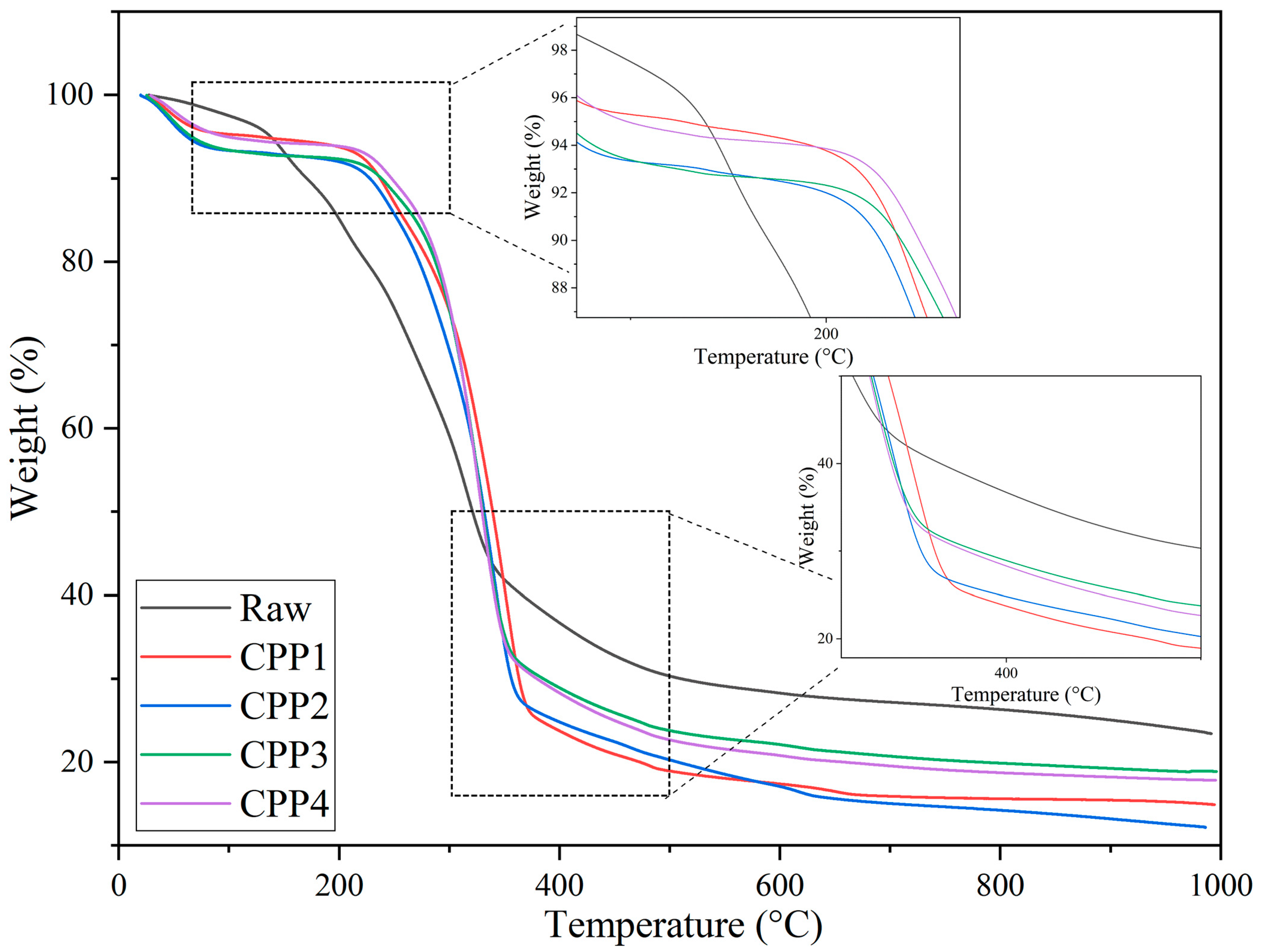
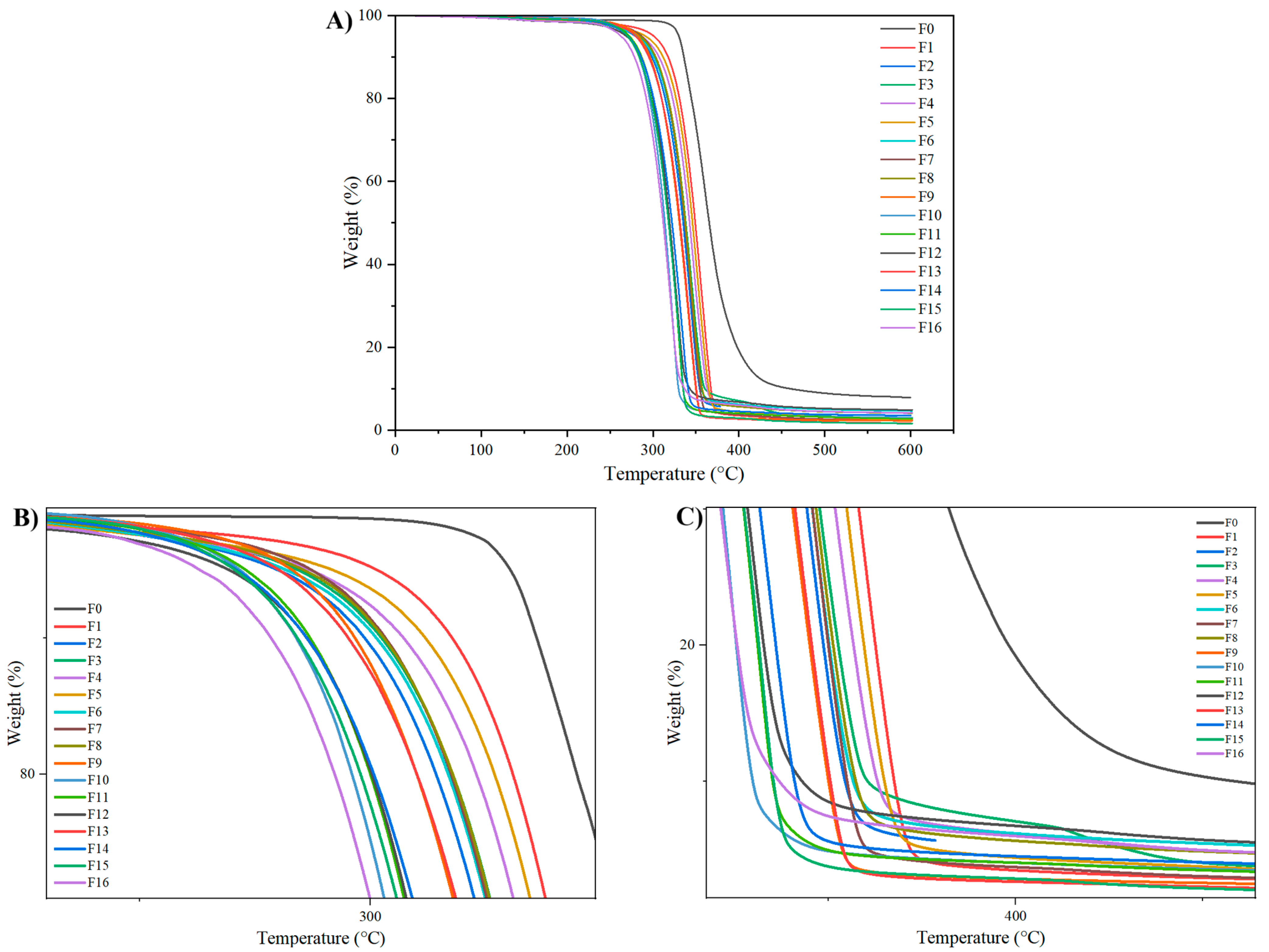
3.1.4. Thermogravimetric Analysis
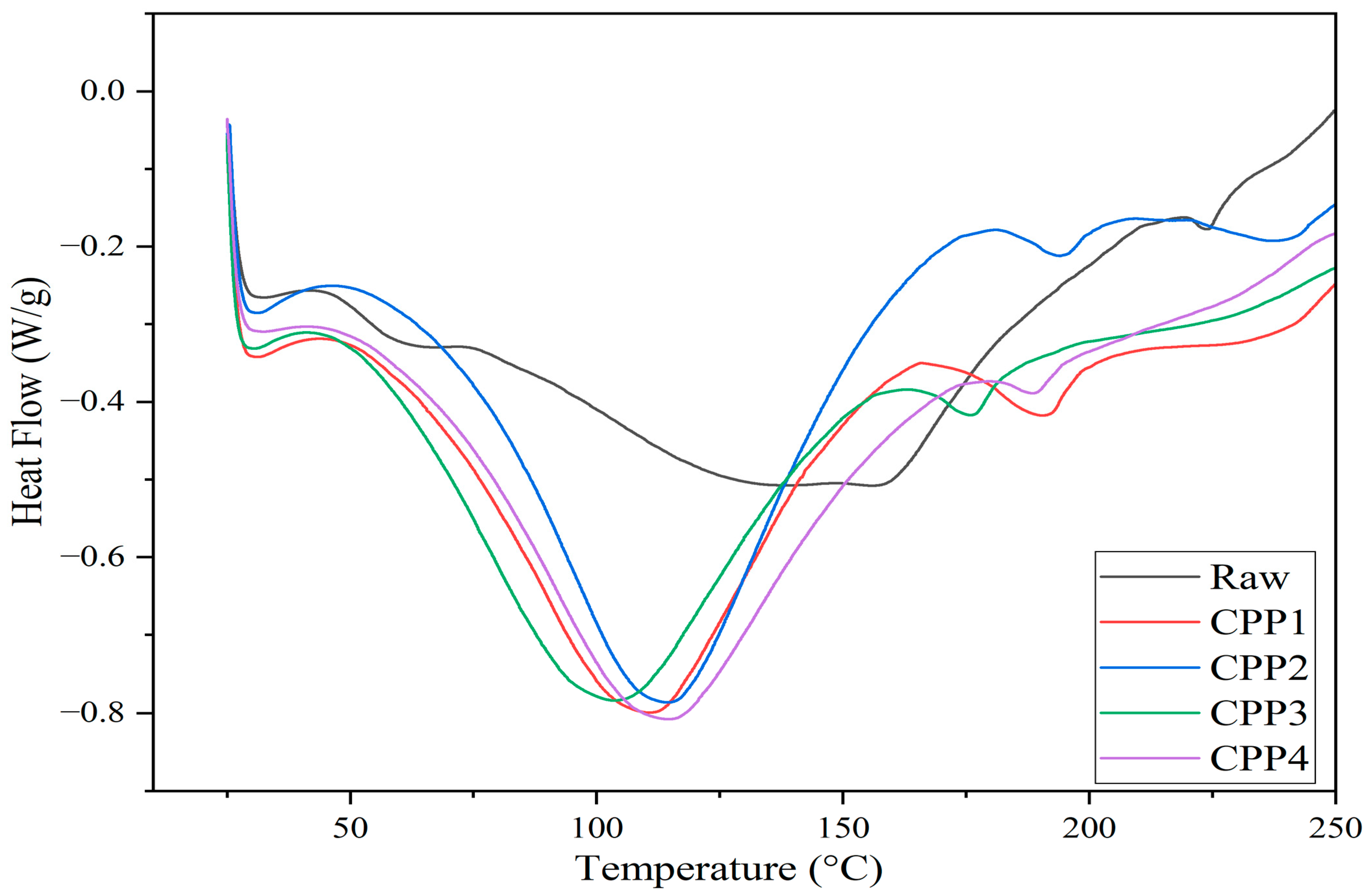
3.1.5. DSC Analysis
3.2. Biocomposites Characterization
3.2.1. FTIR Analysis
3.2.2. Thermal Analysis
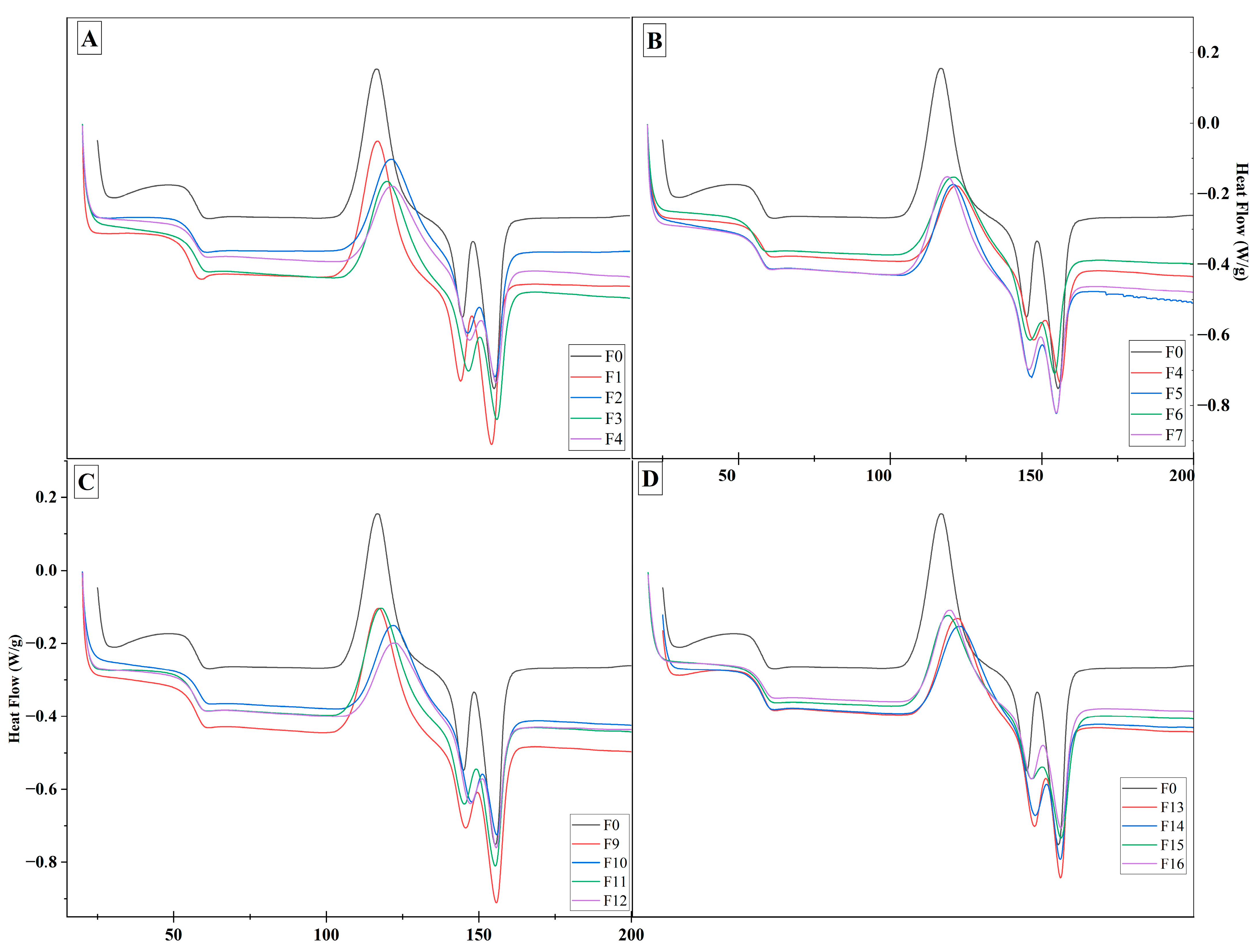
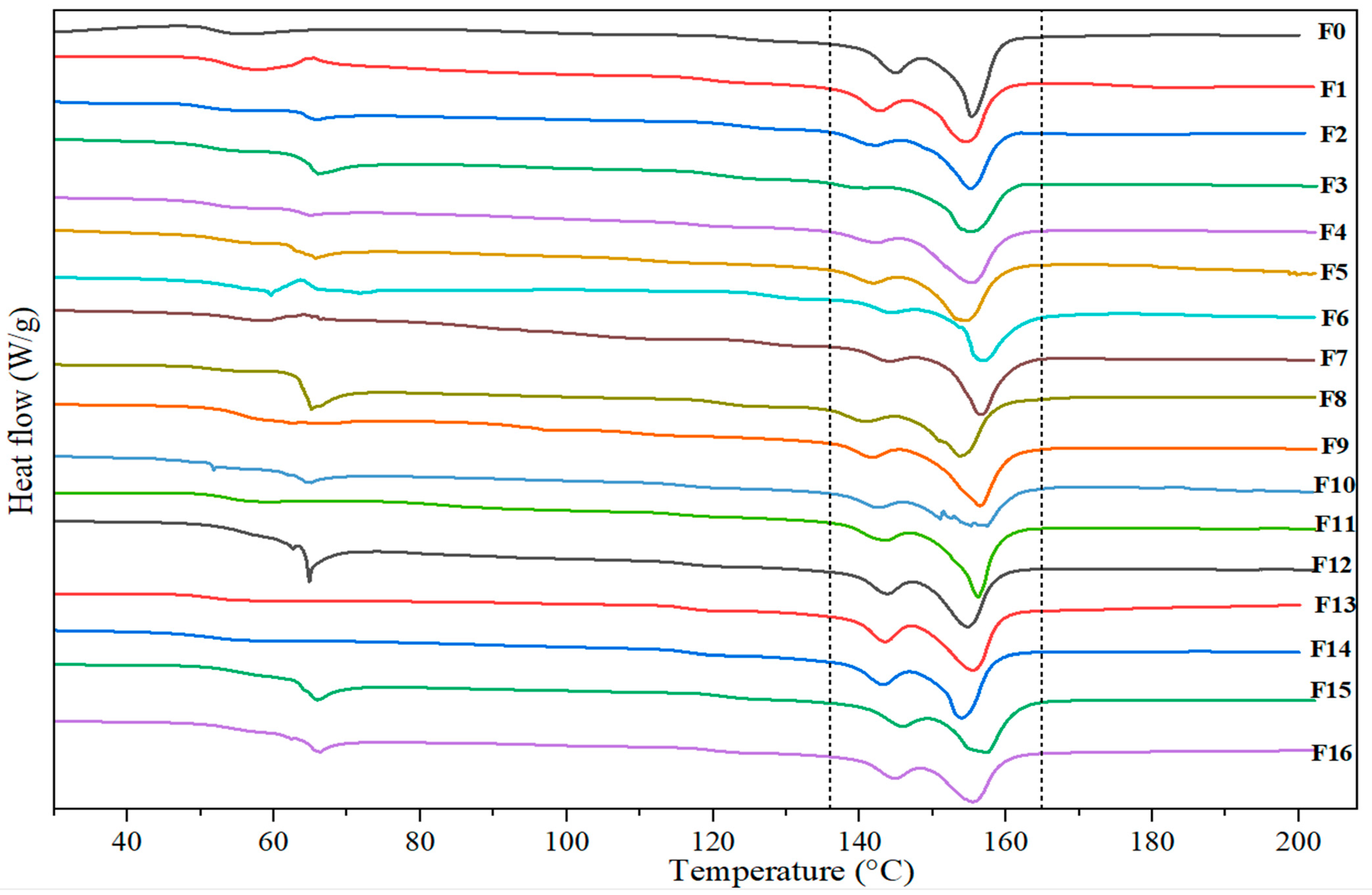
3.2.3. DSC Analysis
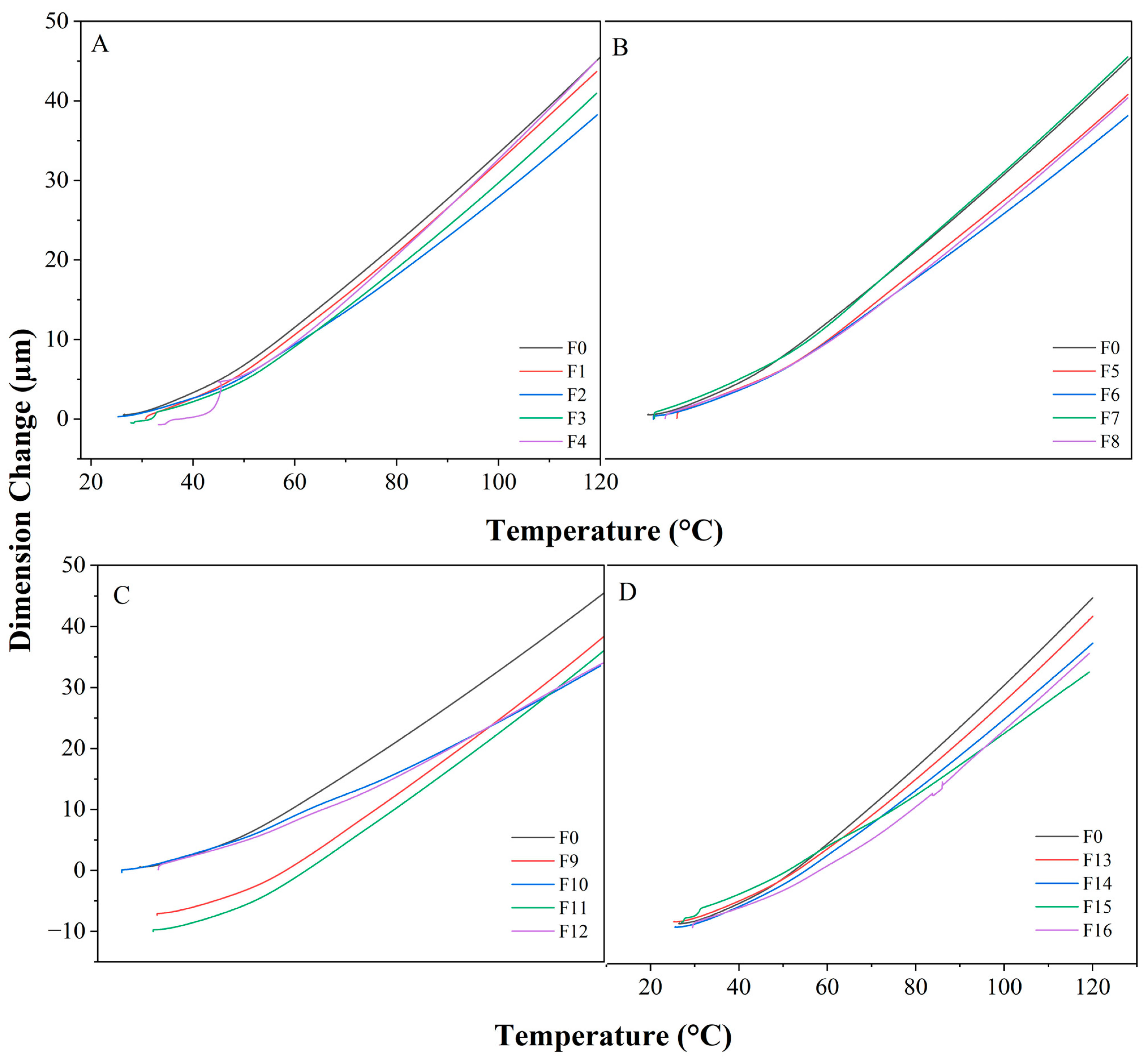
3.2.4. TMA Analysis
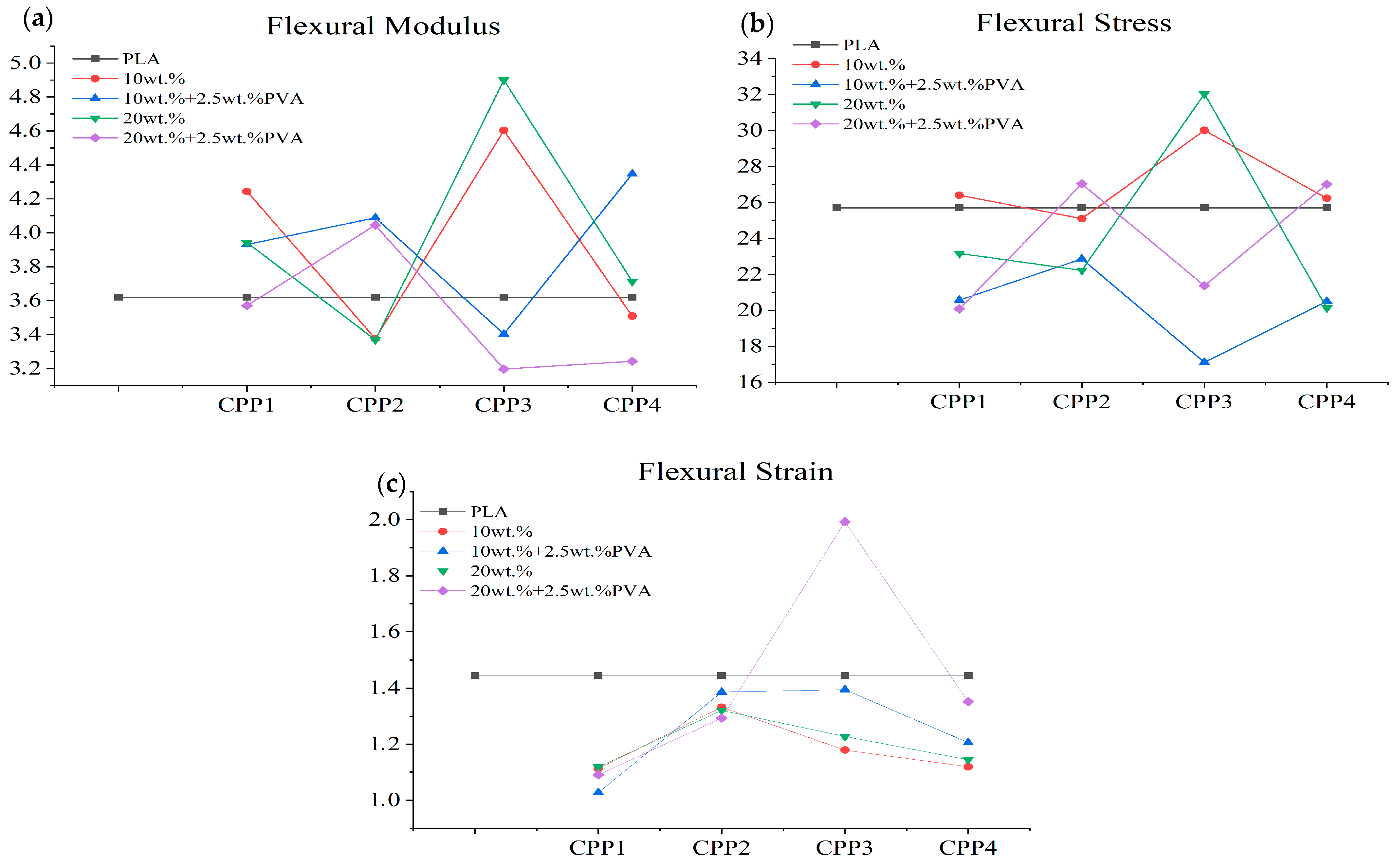
3.2.5. Mechanical Behavior
3.2.6. Water Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Samples | Flexural Modulus (GPa) | Flexural Strength (MPa) | Flexural Strain (%) |
|---|---|---|---|
| F0 | 3.620 ± 0.102 | 25.710 ± 3.973 | 1.445 ± 0.093 |
| F1 | 4.244 ± 0.249 | 26.407 ± 3.179 | 1.112 ± 0.057 |
| F2 | 3.942 ± 0.074 | 23.168 ± 2.536 | 1.119 ± 0.046 |
| F3 | 3.931 ± 0.404 | 20.576 ± 2.297 | 1.027 ± 0.117 |
| F4 | 3.571 ± 0.176 | 20.086 ± 1.832 | 1.091 ± 0.119 |
| F5 | 3.375 ± 0.417 | 25.104 ± 3.583 | 1.332 ± 0.131 |
| F6 | 3.369 ± 0.189 | 22.225 ± 1.609 | 1.320 ± 0.059 |
| F7 | 4.088 ± 0.201 | 22.863 ± 2.820 | 1.386 ± 0.224 |
| F8 | 4.045 ± 0.322 | 27.036 ± 0.867 | 1.293 ± 0.096 |
| F9 | 4.603 ± 0.750 | 30.018 ± 1.085 | 1.179 ± 0.150 |
| F10 | 4.900 ± 0.215 | 32.051 ± 2.997 | 1.228 ± 0.085 |
| F11 | 3.403 ± 0.154 | 17.108 ± 1.574 | 1.395 ± 0.316 |
| F12 | 3.197 ± 0.568 | 21.371 ± 0.387 | 1.993 ± 0.499 |
| F13 | 3.509 ± 0.143 | 26.235 ± 0.733 | 1.119 ± 0.040 |
| F14 | 3.713 ± 0.216 | 20.137 ± 1.086 | 1.144 ± 0.253 |
| F15 | 3.619 ± 0.127 | 19.890 ± 2.474 | 1.131 ± 0.145 |
| F16 | 3.700 ± 0.234 | 18.969 ± 2.084 | 1.117 ± 0.060 |
| Water Absorption (%) | |||||
|---|---|---|---|---|---|
| Time (Days) | 1 | 2 | 7 | 14 | 21 |
| F0 | 0.708 | 0.934 | 1.522 | 2.631 | 3.319 |
| F1 | 1.062 | 1.411 | 3.875 | 6.291 | 6.637 |
| F2 | 2.271 | 3.166 | 9.040 | 11.944 | 9.975 |
| F3 | 1.908 | 3.197 | 7.665 | 9.457 | 10.050 |
| F4 | 2.472 | 4.395 | 10.235 | 14.016 | 14.494 |
| F5 | 4.453 | 7.806 | 18.951 | 21.669 | 22.222 |
| F6 | 3.505 | 4.091 | 11.132 | 16.568 | 20.570 |
| F7 | 3.486 | 6.203 | 12.306 | 15.526 | 19.326 |
| F8 | 2.320 | 4.061 | 11.114 | 14.417 | 16.368 |
| F9 | 0.442 | 0.724 | 3.340 | 6.207 | 7.953 |
| F10 | 2.237 | 2.907 | 6.483 | 11.178 | 12.331 |
| F11 | 2.211 | 3.225 | 6.515 | 9.196 | 9.196 |
| F12 | 5.795 | 11.534 | 21.149 | 23.833 | 28.992 |
| F13 | 1.955 | 2.273 | 3.388 | 5.822 | 5.844 |
| F14 | 2.717 | 3.103 | 5.509 | 7.560 | 8.359 |
| F15 | 0.285 | 1.318 | 6.185 | 7.130 | 4.048 |
| F16 | 0.459 | 1.973 | 3.128 | 6.656 | 6.698 |
| Sample | Relation | Flexural Modulus (GPa) | Flexural Strength (MPa) | Flexural Strain (%) | Results | References |
|---|---|---|---|---|---|---|
| PLA | 100 | 3 | 8.4 | -- | The flexural modulus and strength increase by 25% and 80%, respectively, with 20% of MFC. Flexural strength increases by 130% with 1% CNC. At 5% CNC, the flexural modulus increases to 175% | [10] |
| PLA-cellulose microfibers (MFC) | 80:20 | 3.8 | 16.5 | -- | ||
| PLA-MFC-cellulose nanocrystals (CNC) | 80:19:1 80:15:5 | 5.3 5.7 | 19.2 26.5 | -- | ||
| PLA | 100 | 1.17 | 15.24 | 40.30 | At 1% CNF-Ac into PLA films, the strain increased by more than 60 percent. CNF-Ac to 3%, the tensile strength and tensile modulus increase. | [99] |
| PLA/acetylated cellulose nanofiber (CNF-Ac)-1 | 1% CNF-Ac | 1.13 | 16.70 | 64.77 | ||
| PLA/CNF-Ac-3 | 3% CNF-Ac | 1.20 | 33.06 | 188.86 | ||
| PLA/microcrystalline cellulose (MCC)-3 | 3% MCC | 1.19 | 15.88 | 38.47 | ||
| PLA | 100 | 14.72 | 10.32 | At 1 wt% NFC, the tensile strain increases to 87.9% compared with PLA. When NFC is at 5 wt%, tensile strength reaches a 98.8% boost. | [90] | |
| PLA/nanofibrillated cellulose (NFC)-3wt% | 3 wt% NFC | 27.08 | 17.95 | |||
| PLA/NFC-5wt% | 5 wt% NFC | 29.26 | 18.67 | |||
| PLA/NFC-10wt% | 10 wt% NFC | 26.30 | 15.71 | |||
| PLA | 100 | 3.3 | 57.7 | 6.8 | 20 wt% of MFC improved the modulus of amorphous PLA from 3.3 GPa to 5.2 GPa and the tensile strength. | [100] |
| PLA/microfibrillated cellulose (MFC) 3wt% | 3 wt% MFC | 3.8 | 61.4 | 2.7 | ||
| PLA/MFC 5wt% | 5 wt% MFC | 3.9 | 63.4 | 2.5 | ||
| PLA/MFC 10wt% | 10 wt% MFC | 4.5 | 65.4 | 2.2 | ||
| PLA/MFC 20wt% | 20 wt% MFC | 5.2 | 70.2 | 1.9 |
References
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Guerrero, V.H. Opportunities and Challenges in the Application of Bioplastics: Perspectives from Formulation, Processing, and Performance. Polymers 2024, 16, 2561. [Google Scholar] [CrossRef]
- Galera Manzano, L.M.; Ruz Cruz, M.Á.; Moo Tun, N.M.; Valadez González, A.; Mina Hernandez, J.H. Effect of Cellulose and Cellulose Nanocrystal Contents on the Biodegradation, under Composting Conditions, of Hierarchical PLA Biocomposites. Polymers 2021, 13, 1855. [Google Scholar] [CrossRef]
- Chavhan, G.R.; Wankhade, L.N. Improvement of the Mechanical Properties of Hybrid Composites Prepared by Fibers, Fiber-Metals, and Nano-Filler Particles—A Review. Mater. Today Proc. 2020, 27, 72–82. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sundarakannan, R.; John, K.M.; Joel Johnson, R.D.; Prasath, K.A.; Ajith, S.; Arumugaprabu, V.; Uthayakumar, M. Recent Advancement in the Natural Fiber Polymer Composites: A Comprehensive Review. J. Clean. Prod. 2020, 277, 124109. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B.; Zuriyati, A.; Khan, A. Morphological, Chemical and Thermal Analysis of Cellulose Nanocrystals Extracted from Bamboo Fibre; Elsevier: Amsterdam, The Netherlands, 2020; Volume 160. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and Characterization of Natural Cellulose Fibers/Thermoset Polymer Composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Ruz-Cruz, M.A.A.; Herrera-Franco, P.J.J.; Flores-Johnson, E.A.A.; Moreno-Chulim, M.V.V.; Galera-Manzano, L.M.M.; Valadez-González, A. Thermal and Mechanical Properties of PLA-Based Multiscale Cellulosic Biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Sabaruddin, F.A.; Atikah, M.S.N.; Ibrahim, R.; Asyraf, M.R.M.; Huzaifah, M.R.M.; Saifulazry, S.O.A.; Ainun, Z.M.A. Reuse and Recycle of Biobased Packaging Products. In Bio-Based Packaging: Material, Environmental and Economic Aspects; Wiley: Hoboken, NJ, USA, 2021; pp. 413–426. [Google Scholar] [CrossRef]
- Campaña, O.; Guerrero; Hugo, V. Caracterización Mecánica y Térmica de Ácido Poliláctico (PLA) Reforzado Con Polvo de Bambú (PB). Rev. Politec. 2018, 42, 8. [Google Scholar]
- Safdari, F.; Bagheriasl, D.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Rheological, Mechanical, and Thermal Properties of Polylactide/Cellulose Nanofiber Biocomposites. Polym. Compos. 2018, 39, 1752–1762. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Yang, S.; Song, X.; Wang, S. Combining Organosolv Pretreatment with Mechanical Grinding of Sugarcane Bagasse for the Preparation of Nanofibrillated Cellulose in a Novel Green Approach. Bioresources 2018, 14, 313–335. [Google Scholar] [CrossRef]
- Wijekoon, N.K.; Appuhamillage, G.A.; Dassanayake, R.S.; Liyanage, R.N.; Mapage, D.; Wijenayake, A.; Lokuge, E.L.; Rajapaksha, S.M.; Abeygunawardane, G.A.; Senarath, N.D.D.D. Facile Fabrication of 3D-Printed Cellulosic Fiber/Polylactic Acid Composites as Low-Cost and Sustainable Acoustic Panels. Sustain. Chem. Environ. 2024, 8, 100–168. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The Suitability of Banana Leaf Residue as Raw Material for the Production of High Lignin Content Micro/Nano Fibers: From Residue to Value-Added Products. Ind. Crops Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A Comparative Study of the Suitability of Different Cereal Straws for Lignocellulose Nanofibers Isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Lecoublet, M.; Ragoubi, M.; Leblanc, N.; Koubaa, A. Sustainable 3D-Printed Cellulose-Based Biocomposites and Bio-Nano-Composites: Analysis of Dielectric Performances. Ind. Crops Prod. 2024, 221, 119–332. [Google Scholar] [CrossRef]
- Bahrami, P.; Morsali, P.; Abbasi, D.; Hemmati, F.; Mohammadi-Roshandeh, J. Environmentally-Friendly Solvent Pulping and Grafting Strategies for Better Foamability of Poly(Lactic Acid)/Agricultural Waste Biocomposites. J. Reinf. Plast. Compos. 2024, 75, 1. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Yang, Q.; Song, X.; Qin, C.; Wang, S.; Li, K. Effects of Residual Lignin on Mechanical Defibrillation Process of Cellulosic Fiber for Producing Lignocellulose Nanofibrils. Cellulose 2018, 25, 6479–6494. [Google Scholar] [CrossRef]
- Chihaoui, B.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Lignin-Containing Cellulose Fibrils as Reinforcement of Plasticized PLA Biocomposites Produced by Melt Processing Using PEG as a Carrier. Ind. Crops Prod. 2022, 175, 114–287. [Google Scholar] [CrossRef]
- Linares, M.G.; Delgado Aguilar, M.; Tarrés, J.; Aguado, R.; Pereira, M.; Valerio, O. Impact of Lignocellulosic Nanofiber Source on the Performance of Polylactic Acid. J. Appl. Polym. Sci. 2024, 141, e56088. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, J.S. Effects of Surface Treatment of Ramie Fibers in a Ramie/Poly(Lactic Acid) Composite. Fibers Polym. 2012, 13, 217–223. [Google Scholar] [CrossRef]
- Verma, N.; Mukesh, C.B.; Vivek, K. Pea Peel Waste: A Lignocellulosic Waste and Its Utility in Cellulase Production. Bioresources 2011, 6, 1505–1519. [Google Scholar] [CrossRef]
- Nimbalkar, P.R.; Khedkar, M.A.; Chavan, P.V.; Bankar, S.B. Biobutanol Production Using Pea Pod Waste as Substrate: Impact of Drying on Saccharification and Fermentation. Renew. Energy 2018, 117, 520–529. [Google Scholar] [CrossRef]
- Fatima, R.; Fatima, F.; Altemimi, A.B.; Bashir, N.; Sipra, H.M.; Hassan, S.A.; Mujahid, W.; Shehzad, A.; Abdi, G.; Aadil, R.M. Bridging Sustainability and Industry through Resourceful Utilization of Pea Pods- A Focus on Diverse Industrial Applications. Food Chem. 2024, 23, 101–518. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Herrera-Barrios, A.; Puello-Mendez, J.; Pasqualino, J.C.; Lambis-Miranda, H.A. Agro-Industrial Waste from Cocoa Pod Husk (Theobroma cacao L.), as a Potential Raw Material for Preparation of Cellulose Nanocrystals. Chem. Eng. Trans. 2022, 92, 205–210. [Google Scholar] [CrossRef]
- ASTM E872-82(2019); Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM D1106-21; Test Method for Acid-Insoluble Lignin in Wood. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM D1107-21; Test Method for Ethanol-Toluene Solubility of Wood. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM D1109-21; Test Method for 1 % Sodium Hydroxide Solubility of Wood. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- MDI Materials Data. JADE Pro; MDI Materials Data: Livermore, CA, USA, 2025. [Google Scholar]
- Tejedor, J.; Cevallos, P.D.; Coro, E.S.; Pontón, P.I.; Guamán, M.; Guerrero, V.H. Effects of Annealing on the Mechanical, Thermal, and Physical Properties of 3D-Printed PLA Aged in Salt Water. Mech. Adv. Mater. Struct. 2024, 32, 2307–2321. [Google Scholar] [CrossRef]
- Pontón, P.I.; Prisco, L.P.; Marinkovic, B.A. Effects of Low Contents of A2M3O12 Submicronic Thermomiotic-like Fillers on Thermal Expansion and Mechanical Properties of HDPE-Based Composites. Polym. Compos. 2018, 39, 1821–1833. [Google Scholar] [CrossRef]
- ASTM D790-17; Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM D792-20; Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Penjumras, P.; Rahman, R.A.; Talib, R.A.; Abdan, K. Mechanical Properties and Water Absorption Behaviour of Durian Rind Cellulose Reinforced Poly(Lactic Acid) Biocomposites. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 343–349. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.; Ek, M. Esterification and Hydrolysis of Cellulose Using Oxalic Acid Dihydrate in a Solvent-Free Reaction Suitable for Preparation of Surface-Functionalised Cellulose Nanocrystals with High Yield. Green. Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef]
- Zambrano-Mite, L.F.; Villasana, Y.; Bejarano, M.L.; Luciani, C.; Niebieskikwiat, D.; Álvarez, W.; Cueva, D.F.; Aguilera-Pesantes, D.; Orejuela-Escobar, L.M. Optimization of Microfibrillated Cellulose Isolation from Cocoa Pod Husk via Mild Oxalic Acid Hydrolysis: A Response Surface Methodology Approach. Heliyon 2023, 9, e17258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhang, X.; Lin, Q.; Xin, F.; Sun, R.; Wang, X.; Ren, J. A New Approach to Recycle Oxalic Acid during Lignocellulose Pretreatment for Xylose Production. Biotechnol. Biofuels 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Ren, J.; Wang, W.; Li, H.; Lin, Q.; Yan, Y.; Sun, R.; Liu, G. Production of Xylo-Sugars from Corncob by Oxalic Acid-Assisted Ball Milling and Microwave-Induced Hydrothermal Treatments. Ind. Crops Prod. 2016, 79, 137–145. [Google Scholar] [CrossRef]
- Xu, W.; Grénman, H.; Liu, J.; Kronlund, D.; Li, B.; Backman, P.; Peltonen, J.; Willför, S.; Sundberg, A.; Xu, C. Mild Oxalic-Acid-Catalyzed Hydrolysis as a Novel Approach to Prepare Cellulose Nanocrystals. ChemNanoMat 2017, 3, 109–119. [Google Scholar] [CrossRef]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, C.D.M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Luo, J.; Huang, K.; Xu, Y.; Fan, Y. A Comparative Study of Lignocellulosic Nanofibrils Isolated from Celery Using Oxalic Acid Hydrolysis Followed by Sonication and Mechanical Fibrillation. Cellulose 2019, 26, 5237–5246. [Google Scholar] [CrossRef]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef]
- Bastida, G.A.; Schnell, C.N.; Mocchiutti, P.; Solier, Y.N.; Inalbon, M.C.; Zanuttini, M.Á.; Galván, M.V. Effect of Oxalic Acid Concentration and Different Mechanical Pre-Treatments on the Production of Cellulose Micro/Nanofibers. Nanomaterials 2022, 12, 2908. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Qian, X.; Zuo, L.; Yue, X. Effects of Supplementary Alkali after Alkaline Peroxide on the Properties of Bleached Kraft Pine Fluff. Bioresources 2016, 11, 336–353. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Jumaidin, R.; Ilyas, R.A.; Selamat, M.Z.; Alamjuri, R.H.; Md Yusof, F.A. Influence of Alkali Treatment on the Mechanical, Thermal, Water Absorption, and Biodegradation Properties of Cymbopogan citratus Fiber-Reinforced, Thermoplastic Cassava Starch–Palm Wax Composites. Polymers 2022, 14, 2769. [Google Scholar] [CrossRef]
- Gudayu, A.D.; Steuernagel, L.; Meiners, D.; Gideon, R. Effect of Surface Treatment on Moisture Absorption, Thermal, and Mechanical Properties of Sisal Fiber. J. Ind. Text. 2022, 51, 2853–2873. [Google Scholar] [CrossRef]
- Delgado-Orti, C.; Navas-Martos, F.J.; Rodríguez-Liébana, J.A.; La Rubia, M.D.; Jurado-Contreras, S. Development of PLA–Waste Paper Biocomposites with High Cellulose Content. Polymers 2024, 16, 2000. [Google Scholar] [CrossRef]
- Bhiri, F.; Abidi, S.; Bouallegue, A.; Bensidhom, G.; Kallel, F.; Ellouz Chaabouni, S.; Trabelsi, A.B.H. Valorization of Pea By-Products for the Isolation of Cellulosic Micro Bers: Extraction and Physico- Chemical Characterization. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Alshahrani, H.; Arun Prakash, V.R. Characterisation of Microcrystalline Cellulose from Waste Green Pea Pod Sheath and Its Sunn Hemp Fibre-Polyester Composite: A Step towards Greener Manufacturing. Physiol. Plant 2024, 176, e14166. [Google Scholar] [CrossRef]
- Salim, M.H.; Abdellaoui, Y.; Ait Benhamou, A.; Ablouh, E.H.; El Achaby, M.; Kassab, Z. Influence of Cellulose Nanocrystals from Pea Pod Waste on Mechanical, Thermal, Biodegradability, and Barrier Properties of Chitosan-Based Films. Cellulose 2022, 29, 5117–5135. [Google Scholar] [CrossRef]
- Mamudu, U.; Kabyshev, A.; Bekmyrza, K.; Kuterbekov, K.A.; Baratova, A.; Omeiza, L.A.; Lim, R.C. Extraction, Preparation and Characterization of Nanocrystalline Cellulose from Lignocellulosic Simpor Leaf Residue. Molecules 2025, 30, 1622. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Pistor, V.J.A. Structural Characteristics and Thermal Properties of Native Cellulose. In Cellulose—Fundamental Aspects; InTech: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and Characterization of Cellulose Nanocrystals from Jackfruit Peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Kulandaivel, N.; Muralikannan, R.; KalyanaSundaram, S. Extraction and Characterization of Novel Natural Cellulosic Fibers from Pigeon Pea Plant. J. Nat. Fibers 2020, 17, 769–779. [Google Scholar] [CrossRef]
- Bhiri, F.; Abidi, S.; Bouallegue, A.; Bensidhom, G.; Kallel, F. A Renewable Cellulose Source: Isolation and Characterization of Cellulose Microfibers from Pea Pod Waste. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions; Springer: Cham, Switzerland, 2024; pp. 41–44. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical Properties of Biodegradable Composites from Poly Lactic Acid (PLA) and Microcrystalline Cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.M.; Tayeb, A.M. Production of Biodegradable Plastic from Agricultural Wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Dosen, A.; Blanton, T.N. PDF-5+: A Comprehensive Powder Diffraction FileTM for Materials Characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- Mostafa, S.; Bockisch, F.J. Mechanical Properties of Some Bioplastics Under Different Soil Types for Use as a Biodegradable Drip Tubes. Agric. Eng. Int. CIGR J. 2010, 12, 1–16. [Google Scholar]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; El Achaby, M. Cellulosic Materials from Pea (Pisum sativum) and Broad Beans (Vicia faba) Pods Agro-Industrial Residues. Mater. Lett. 2020, 280, 128539. [Google Scholar] [CrossRef]
- Singh, S.; Bhardwaj, S.; Meda, R.S.; Verma, C.; Chhajed, M.; Ghosh, K.; Maji, P.K. Insights into Thermal Degradation Kinetics and Liquid Crystalline Behavior of Cellulose Nanocrystals from the Waste of Cajanus cajan (Pigeon Pea). Int. J. Biol. Macromol. 2023, 242, 124507. [Google Scholar] [CrossRef]
- Lapuz, A.R.; Tsuchikawa, S.; Inagaki, T.; Ma, T.; Migo, V. Production of Nanocellulose Film from Abaca Fibers. Crystals 2022, 12, 601. [Google Scholar] [CrossRef]
- Al-Awa, Z.F.A.; Sangor, F.I.M.S.; Babili, S.B.; Saud, A.; Saleem, H.; Zaidi, S.J. Effect of Leaf Powdering Technique on the Characteristics of Date Palm-Derived Cellulose. ACS Omega 2023, 8, 18930–18939. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Keer, L.M. Influence of Surface Modification on the Microstructure and Thermo-Mechanical Properties of Bamboo Fibers. Materials 2015, 8, 6597–6608. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Wolski, K.; Zaborski, M. Universal Approach of Cellulose Fibres Chemical Modification Result Analysis via Commonly Used Techniques. Polym. Bull. 2019, 76, 2147–2162. [Google Scholar] [CrossRef]
- Gorgun, E.; Ali, A.; Islam, M.S. Biocomposites of Poly(Lactic Acid) and Microcrystalline Cellulose: Influence of the Coupling Agent on Thermomechanical and Absorption Characteristics. ACS Omega 2024, 9, 11523–11533. [Google Scholar] [CrossRef]
- Raisipour-Shirazi, A.; Ahmadi, Z.; Garmabi, H. Polylactic Acid Nanocomposites Toughened with Nanofibrillated Cellulose: Microstructure, Thermal, and Mechanical Properties. Iran. Polym. J. 2018, 27, 785–794. [Google Scholar] [CrossRef]
- Espinach, F.X.; Boufi, S.; Delgado-Aguilar, M.; Julián, F.; Mutjé, P.; Méndez, J.A. Composites from Poly(Lactic Acid) and Bleached Chemical Fibres: Thermal Properties. Compos. B Eng. 2018, 134, 169–176. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Sugarcane Bagasse for Sustainable Development of Thermoset Biocomposites. J. Polym. Res. 2024, 31, 317–320. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M. Green Esterification: A New Approach to Improve Thermal and Mechanical Properties of Poly(Lactic Acid) Composites Reinforced by Cellulose Nanocrystals. J. Appl. Polym. Sci. 2018, 135, 46468. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Hua, X.; Zhang, J. Thermal-Oxidation Degradation of Polylactic Acid/Cellulose Nanocrystal Composites: Effects of Surface Chemistry. Ind Crops Prod 2023, 202. [Google Scholar] [CrossRef]
- Tan, L.; Liu, L.; Liu, C.; Wang, W. Improvement in the Performance of the Polylactic Acid Composites by Using Deep Eutectic Solvent Treated Pulp Fiber. J. Renew. Mater. 2021, 9, 1897–1911. [Google Scholar] [CrossRef]
- Bhiogade, A.; Kannan, M. Studies on Thermal and Degradation Kinetics of Cellulose Micro/Nanoparticle Filled Polylactic Acid (PLA) Based Nanocomposites. Polym. Polym. Compos. 2021, 29 (Suppl. 9), S85–S98. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Vuluga, Z.; Vitelaru, C.; Damian, C.M. The Effect of Cellulose Nanofibers on the Crystallinity and Nanostructure of Poly(Lactic Acid) Composites. J. Mater. Sci. 2016, 51, 9771–9791. [Google Scholar] [CrossRef]
- Fiore, V.; Botta, L.; Scaffaro, R.; Valenza, A.; Pirrotta, A. PLA Based Biocomposites Reinforced with Arundo donax Fillers. Compos. Sci. Technol. 2014, 105, 110–117. [Google Scholar] [CrossRef]
- Wach, R.A.; Wolszczak, P.; Adamus-Wlodarczyk, A. Enhancement of Mechanical Properties of FDM-PLA Parts via Thermal Annealing. Macromol. Mater. Eng. 2018, 303, 1800169. [Google Scholar] [CrossRef]
- Rahman, M.M.; Afrin, S.; Haque, P.; Islam, M.M.; Islam, M.S.; Gafur, M.A. Preparation and Characterization of Jute Cellulose Crystals-Reinforced Poly(l-Lactic Acid) Biocomposite for Biomedical Applications. Int. J. Chem. Eng. 2014, 2014, 842147. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the Crystallinity and Mechanical Properties of PLA by Nucleation and Annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Ungtrakul, T.; Chandrasekar, M.; Senthil Muthu Kumar, T.; Parameswaranpillai, J.; Mohit, H.; Aravind, D.; Rajini, N.; Siengchin, S.; Natarajan, V. Analysis of the Thermal Properties in Short Sansevieria Cylindrica Fibre/PLA Composites Processed by Twin Screw Extruder Followed by Hot Press Molding Technique. Mater. Res. Express 2024, 11, 035506. [Google Scholar] [CrossRef]
- Hong, J.; Kim, D.S. Preparation and Physical Properties of Polylactide/Cellulose Nanowhisker/Nanoclay Composites. Polym. Compos. 2013, 34, 293–298. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Deng, Q.; Lin, D.; Wang, Y. Fabrication and Properties of Green Cellulose Nanofibers/Polylactic Acid Composites. Appl. Mech. Mater. 2012, 174–177, 885–892. [Google Scholar] [CrossRef]
- Kuciel, S.; Mazur, K.; Hebda, M. The Influence of Wood and Basalt Fibres on Mechanical, Thermal and Hydrothermal Properties of PLA Composites. J. Polym. Environ. 2020, 28, 1204–1215. [Google Scholar] [CrossRef]
- Bondeson, D.; Oksman, K. Polylactic Acid/Cellulose Whisker Nanocomposites Modified by Polyvinyl Alcohol. Compos. Part A Appl. Sci. Manuf. 2007, 38, 2486–2492. [Google Scholar] [CrossRef]
- Chu, H.; Chen, Z.; Chen, Y.; Wei, D.; Liu, Y.; Zhao, H. Mechanical Properties and Crystallinity of Specific PLA/Cellulose Composites by Surface Modification of Nanofibrillated Cellulose. Polymers 2024, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. Thermo-Mechanical Properties of Microfibrillated Cellulose-Reinforced Partially Crystallized PLA Composites. Cellulose 2010, 17, 771–778. [Google Scholar] [CrossRef]
- Mahmoud, Y.; Belhanche-Bensemra, N.; Safidine, Z. Impact of Microcrystalline Cellulose Extracted from Walnut and Apricots Shells on the Biodegradability of Poly (Lactic Acid). Front. Mater. 2022, 9, 2296–8016. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; He, X.; Zhao, J.; Zhang, W.; Lu, C. Effective Dispersion and Crosslinking in PVA/Cellulose Fiber Biocomposites via Solid-State Mechanochemistry. Int. J. Biol. Macromol. 2015, 72, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Gupta, M.K. Water Absorption Behavior of Cellulosic Fibres Polymer Composites: A Review on Its Effects and Remedies. J. Ind. Text. 2020, 51, 7480S–7512S. [Google Scholar] [CrossRef]
- El Assimi, T.; Blažic, R.; Vidović, E.; Raihane, M.; El Meziane, A.; Baouab, M.H.V.; Khouloud, M.; Beniazza, R.; Kricheldorf, H.; Lahcini, M. Polylactide/Cellulose Acetate Biocomposites as Potential Coating Membranes for Controlled and Slow Nutrients Release from Water-Soluble Fertilizers. Prog. Org. Coat. 2021, 156, 106–255. [Google Scholar] [CrossRef]
- Wan Ishak, W.H.; Rosli, N.A.; Ahmad, I. Influence of Amorphous Cellulose on Mechanical, Thermal, and Hydrolytic Degradation of Poly(Lactic Acid) Biocomposites. Sci. Rep. 2020, 10, 11342. [Google Scholar] [CrossRef]
- Xie, L.; Huang, Z.; Meng, H.; Shi, X.; Xie, J. Immunomodulation Effect of Polysaccharides from Liquid Fermentation of Monascus purpureus 40269 via Membrane TLR-4 to Activate the MAPK and NF-ΚB Signaling Pathways. Int. J. Biol. Macromol. 2022, 201, 480–491. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhani, A.; Hosseinzadeh, J.; Dadashi, S.; Mousavi, M. A Study of Morphological, Thermal, Mechanical and Barrier Properties of PLA Based Biocomposites Prepared with Micro and Nano Sized Cellulosic Fibers. Cellul. Chem. Technol. 2015, 49, 597–605. [Google Scholar]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The Effect of Crystallization of PLA on the Thermal and Mechanical Properties of Microfibrillated Cellulose-Reinforced PLA Composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]




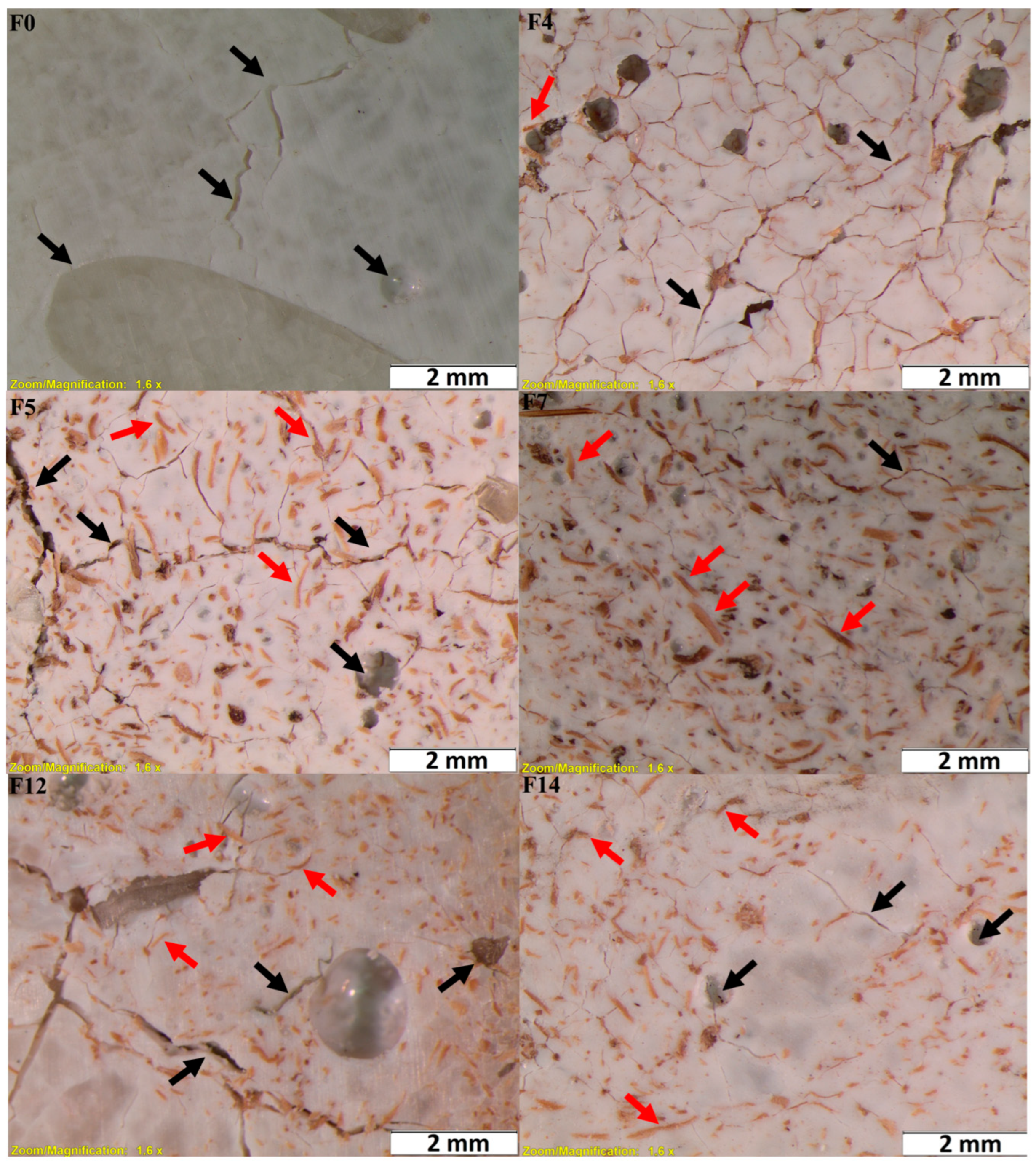
| Formulation | Composition (wt.%) | |||
|---|---|---|---|---|
| PLA | Cellulose Reinforcement | Treatment | PVA Compatibilizer | |
| F0 | 100 | 0 | - | 0 |
| F1 | 90 | 10 | CPP1 0.5 M of OA | 0 |
| F2 | 80 | 20 | 0 | |
| F3 | 87.5 | 10 | 2.5 | |
| F4 | 77.5 | 20 | 2.5 | |
| F5 | 90 | 10 | CPP2 0.75 M of OA | 0 |
| F6 | 80 | 20 | 0 | |
| F7 | 87.5 | 10 | 2.5 | |
| F8 | 77.5 | 20 | 2.5 | |
| F9 | 90 | 10 | CPP3 0.5 M of OA + 5% of KOH | 0 |
| F10 | 80 | 20 | 0 | |
| F11 | 87.5 | 10 | 2.5 | |
| F12 | 77.5 | 20 | 2.5 | |
| F13 | 90 | 10 | CPP4 0.75 M of OA + 5% of KOH | 0 |
| F14 | 80 | 20 | 0 | |
| F15 | 87.5 | 10 | 2.5 | |
| F16 | 77.5 | 20 | 2.5 | |
| Samples | Crystallinity Index (%) | °C | °C | Char Residue at 800 °C (%) |
|---|---|---|---|---|
| Raw material | - | 227.01 | 315.77 | 26.29 |
| CPP1 | 65.59 | 283.91 | 351.60 | 15.60 |
| CPP2 | 64.41 | 283.11 | 341.30 | 14.21 |
| CPP3 | 91.14 | 285.63 | 328.37 | 19.87 |
| CPP4 | 92.62 | 284.32 | 327.93 | 18.73 |
| Formulation | A1448/A1743 | A867/A1743 |
|---|---|---|
| F0 | 0.250 | 0.0810 |
| F1 | 0.266 | 0.0857 |
| F2 | 0.217 | 0.0684 |
| F3 | 0.257 | 0.0846 |
| F4 | 0.220 | 0.0654 |
| F5 | 0.223 | 0.0670 |
| F6 | 0.245 | 0.0786 |
| F7 | 0.222 | 0.0677 |
| F8 | 0.238 | 0.0667 |
| F9 | 0.252 | 0.0820 |
| F10 | 0.219 | 0.0662 |
| F11 | 0.231 | 0.0682 |
| F12 | 0.248 | 0.0790 |
| F13 | 0.255 | 0.0831 |
| F14 | 0.225 | 0.0703 |
| F15 | 0.214 | 0.0606 |
| F16 | 0.243 | 0.0731 |
| Sample | Td10 (°C) | T d50 (°C) | T d90 (°C) | Char Residue at 600 °C (%) |
|---|---|---|---|---|
| F0 | 331.09 | 365.5 | 459.6 | 1.416 |
| F1 | 317.7 | 349.17 | 367.46 | 0.702 |
| F2 | 298.77 | 335.36 | 354.39 | 0.654 |
| F3 | 302.43 | 338.25 | 360.42 | 0.5629 |
| F4 | 307.2 | 342.73 | 363.02 | 1.152 |
| F5 | 311.93 | 346.06 | 364.89 | 0.416 |
| F6 | 301.25 | 336.89 | 356.51 | 1.310 |
| F7 | 303.2 | 337.02 | 354.9 | 0.402 |
| F8 | 302.79 | 337.85 | 357.26 | 0.806 |
| F9 | 296.64 | 330.7 | 349.92 | 0.593 |
| F10 | 283.96 | 313.85 | 329.73 | 0.543 |
| F11 | 286.89 | 318.79 | 335 | 0.801 |
| F12 | 286.24 | 319.54 | 342.95 | 1.478 |
| F13 | 290.11 | 328.12 | 346.7 | 1.83 |
| F14 | 288.10 | 325.49 | 344.48 | 2.11 |
| F15 | 284.22 | 318.22 | 335.23 | 0.438 |
| F16 | 278.48 | 312.03 | 336.83 | 1.156 |
| Samples | 1st Heating | ||||
|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) | ||
| Tm1 | Tm2 | ||||
| F0 | 51.87 | 140.63 | 152.96 | 31.20 | 33.55 |
| F1 | 53.46 | 138.04 | 149.68 | 29.28 | 34.98 |
| F2 | 50.26 | 136.46 | 149.99 | 22.35 | 30.04 |
| F3 | 51.74 | 134.57 | 150.52 | 21.52 | 27.47 |
| F4 | 51.94 | 140.95 | 148.21 | 23.97 | 29.86 |
| F5 | 53.22 | 137.08 | 149.04 | 25.29 | 28.64 |
| F6 | 55.14 | 139.98 | 154.02 | 20.61 | 33.99 |
| F7 | 53.99 | 139.60 | 152.81 | 21.93 | 25.33 |
| F8 | 51.19 | 135.83 | 149.11 | 25.51 | 30.43 |
| F9 | 55.41 | 137.19 | 149.66 | 26.35 | 30.48 |
| F10 | 51.68 | 137.59 | 150.41 | 23.99 | 35.42 |
| F11 | 53.15 | 137.89 | 152.45 | 25.97 | 29.48 |
| F12 | 56.57 | 139.43 | 149.60 | 24.59 | 36.03 |
| F13 | 51.75 | 139.29 | 149.13 | 28.51 | 29.38 |
| F14 | 51.88 | 138.57 | 151.14 | 25.10 | 38.32 |
| F15 | 55.05 | 141.16 | 152.01 | 26.42 | 32.09 |
| F16 | 53.87 | 140.33 | 150.11 | 23.89 | 36.66 |
| Sample | T1 (°C) | α1 [μm (m·°C)−1] 30–50 °C |
|---|---|---|
| F0 | 49.96 | 95.38 |
| F1 | 49.22 | 90.38 |
| F2 | 49.33 | 75.53 |
| F3 | 46.02 | 85.83 |
| F4 | 47.94 | 78.75 |
| F5 | 44.53 | 77.71 |
| F6 | 50 | 76.01 |
| F7 | 49.65 | 83.35 |
| F8 | 46.55 | 65.04 |
| F9 | 49.82 | 76.71 |
| F10 | 50 | 81.54 |
| F11 | 49.31 | 90.97 |
| F12 | 48.92 | 57.72 |
| F13 | 50 | 92.04 |
| F14 | 50 | 95.33 |
| F15 | 48.37 | 81.75 |
| F16 | 48.15 | 50.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrete-Bolagay, D.; Guerrero, V.H.; Galeas, S.; Tejedor, J.; Pontón, P.I.; Dosen, A. Pea Pod Valorization: A Green Processing Route to Obtain Cellulosic Reinforcements for Compression Molded Polylactic Acid Biocomposites. Materials 2025, 18, 4608. https://doi.org/10.3390/ma18194608
Negrete-Bolagay D, Guerrero VH, Galeas S, Tejedor J, Pontón PI, Dosen A. Pea Pod Valorization: A Green Processing Route to Obtain Cellulosic Reinforcements for Compression Molded Polylactic Acid Biocomposites. Materials. 2025; 18(19):4608. https://doi.org/10.3390/ma18194608
Chicago/Turabian StyleNegrete-Bolagay, Daniela, Victor H. Guerrero, Salomé Galeas, Jennifer Tejedor, Patricia I. Pontón, and Anja Dosen. 2025. "Pea Pod Valorization: A Green Processing Route to Obtain Cellulosic Reinforcements for Compression Molded Polylactic Acid Biocomposites" Materials 18, no. 19: 4608. https://doi.org/10.3390/ma18194608
APA StyleNegrete-Bolagay, D., Guerrero, V. H., Galeas, S., Tejedor, J., Pontón, P. I., & Dosen, A. (2025). Pea Pod Valorization: A Green Processing Route to Obtain Cellulosic Reinforcements for Compression Molded Polylactic Acid Biocomposites. Materials, 18(19), 4608. https://doi.org/10.3390/ma18194608








