Application of Rapeseed Oil Cake from Biodiesel Production in Methane Co-Digestion with Microalgal Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
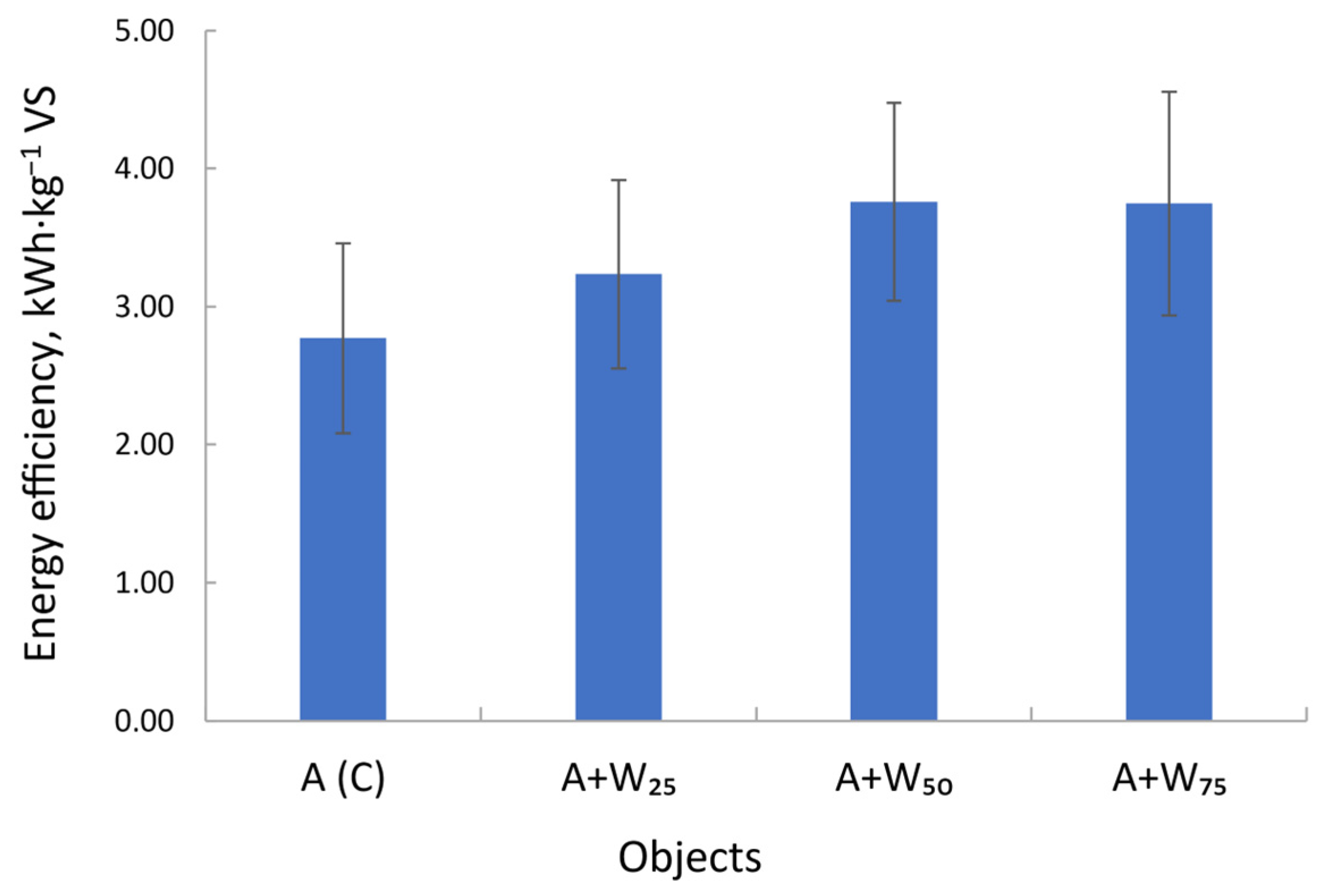
- 1 g of VS·L−1 microalgal biomass—control sample A (C);
- 1 g of VS·L−1 microalgal biomass and 0.25 g of VS·L−1 rapeseed oil cake (A + W25);
- 1 g of VS·L−1 microalgal biomass and 0.50 g of VS·L−1 rapeseed oil cake (A + W50);
- 1 g of VS·L−1 microalgal biomass and 0.75 g of VS·L−1 rapeseed oil cake (A + W75).
3. Discussion of Results
3.1. Biogas Production
3.2. The Content of Methane and Other Gases
3.3. Hydrogen Sulfide Content
3.4. Methane Fermentation Process Conditions
3.5. Energy Potential of the Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass energy and biofuels: Perspective, potentials, and challenges in the energy transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Shahzad, H.M.A.; Asim, Z.; Khan, S.J.; Almomani, F.; Mahmoud, K.A.; Mustafa, M.R.U.; Rasool, K. Thermochemical and biochemical conversion of agricultural waste for bioenergy production: An updated review. Discov. Environ. 2024, 2, 134. [Google Scholar] [CrossRef]
- Wu, R. Biomass Conversion Technologies: Transforming Organic Matter into Energy and Materials. In Biomass Based Products; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Trif-Tordai, G.; Ionel, I. Waste Biomass as Alternative Bio-Fuel—Co-Firing versus Direct Combustion. In Alternative Fuel; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical Conversion of Biomass for Syngas Production: Current Status and Future Trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability 2023, 15, 11238. [Google Scholar] [CrossRef]
- Abrar, I.; Ashfaq, A.; Rumaisa, T.; Ammara, W.; Farrukh, J.; Forruque, A.S.; Chaouki, G.; Young-Kwon, P. Techno-Economical Evaluation of Bio-Oil Production via Biomass Fast Pyrolysis Process: A Review. Front. Energy Res. 2021, 9, 770355. [Google Scholar] [CrossRef]
- Shyam, S.; Ahmed, S.; Joshi, S.J.; Sarma, H. Biochar as a Soil amendment: Implications for soil health, carbon sequestration, and climate resilience. Discov. Soil 2025, 2, 18. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Liang, M.; Guo, Z.; Cai, Y.; Zhu, C.; Wang, Z.; Wang, S.; Xu, J.; Ying, H. Physical-Chemical-Biological Pretreatment for Biomass Degradation and Industrial Applications: A Review. Waste 2024, 2, 451–473. [Google Scholar] [CrossRef]
- Sambasivam, K.M.; Kuppan, P.; Laila, L.S.; Shashirekha, V.; Tamilarasan, K.; Abinandan, S. Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications. Energies 2023, 16, 7589. [Google Scholar] [CrossRef]
- Farouk, S.M.; Tayeb, A.M.; Abdel-Hamid, S.M.S.; Osman, R.M. Recent advances in transesterification for sustainable biodiesel production, challenges, and prospects: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2024, 31, 12722–12747. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. [Google Scholar] [CrossRef]
- Suchocki, T. Energy Utilization of Rapeseed Biomass in Europe: A Review of Current and Innovative Applications. Energies 2024, 17, 6177. [Google Scholar] [CrossRef]
- Brown, J.; Lindstrom, J.K.; Ghosh, A.; Rollag, S.A.; Brown, R.C. Production of sugars from lignocellulosic biomass via biochemical and thermochemical routes. Front. Energy Res. 2024, 12, 1347373. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Biogas production from different lignocellulosic biomass sources: Advances and perspectives. 3 Biotech 2018, 8, 233. [Google Scholar] [CrossRef]
- Begum, Y.A.; Ahmed, M.M.; Begum, S.; Alharthi, N.; Sabagh, A.E.; Islam, M.R. A Review on Waste Biomass-to-Energy: Integrated Thermochemical and Biochemical Conversion for Resource Recovery. Environ. Sci. Adv. 2024, 3, 1197–1216. [Google Scholar] [CrossRef]
- Neri, A.; Bernardi, B.; Zimbalatti, G.; Benalia, S. An Overview of Anaerobic Digestion of Agricultural By-Products and Food Waste for Biomethane Production. Energies 2023, 16, 6851. [Google Scholar] [CrossRef]
- Pavičić, J.; Novak Mavar, K.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
- Ngo, T.; Ball, A.S.; Shahsavari, E. The Current Status, Potential Benefits and Future Prospects of the Australian Biogas Sector. J. Sustain. Bioenergy Syst. 2021, 11, 14–32. [Google Scholar] [CrossRef]
- He, Y.; Li, M.; Perumal, V.; Feng, X.; Fang, J.; Xie, J.; Sievert, S.M.; Wang, F. Genomic and enzymatic evidence for acetogenesis among multiple lineages of the archaeal phylum Bathyarchaeota widespread in marine sediments. Nat. Microbiol. 2016, 1, 16035. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.L.U.; Iqbal, A.; Chang, C.-C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef]
- Kumar, D.J.P.; Mishra, R.K.; Chinnam, S.; Binnal, P.; Dwivedi, N. A comprehensive study on anaerobic digestion of organic solid waste: A review on configurations, operating parameters, techno-economic analysis and current trends. Biotechnol. Notes 2024, 5, 33–49. [Google Scholar] [CrossRef]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B. Impacts of Anaerobic Co-Digestion on Different Influencing Parameters: A Critical Review. Sustainability 2022, 14, 9387. [Google Scholar] [CrossRef]
- Gebreegziabher, B.W.; Dubale, A.A.; Adaramola, M.S.; Morken, J. Advancing Anaerobic Digestion of Biodiesel Byproducts: A Comprehensive Review. Bioenergy Res. 2025, 18, 15. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic digestion of microalgal biomass for bioenergy production, removal of nutrients and microcystin: Current status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.F.; Meers, E.; Mangini, S. The potential of microalgae for carbon capture and sequestration. EFB Bioecon. J. 2025, 4, 100067. [Google Scholar] [CrossRef]
- Hasan, M.M.; Mofijur, M.; Uddin, M.N.; Kabir, Z.; Badruddin, I.A.; Khan, T.M.Y. Insights into anaerobic digestion of microalgal biomass for enhanced energy recovery. Front. Energy Res. 2024, 12, 1355686. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Krička, T.; Matin, A.; Voća, N.; Jurišić, V.; Bilandžija, N. Reuse of rapeseed by-products from biodiesel production. Span. J. Agric. Res. 2015, 13, e0207. [Google Scholar] [CrossRef][Green Version]
- PN-EN 12879:2004; Characterization of Sludges—Loss on Ignition. Polish Committee for Standardization: Warsaw, Poland, 2004.[Green Version]
- Hawrot-Paw, M.; Koniuszy, A.; Ratomski, P.; Sąsiadek, M.; Gawlik, A. Biogas Production from Arthrospira platensis Biomass. Energies 2023, 16, 3971. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Comparison of biogas production from anaerobic digestion of microalgae species belonged to various taxonomic groups. Arch. Environ. Prot. 2020, 46, 33–40. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Bartkowska, I. Co-Fermentation of Microalgae Biomass and Miscanthus × giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Appl. Sci. 2022, 12, 7291. [Google Scholar] [CrossRef]
- Alharbi, R.M. Anaerobic co-digestion of cow manure and microalgae to increase biogas production: A sustainable bioenergy source. J. King Saud Univ. Sci. 2024, 36, 103380. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Huang, Y.; Liang, T.; Lu, J.; Zhang, L.; Qi, C. Anaerobic Co-Digestion of Food Waste and Microalgae at Variable Mixing Ratios: Enhanced Performance, Kinetic Analysis, and Microbial Community Dynamics Investigation. Appl. Sci. 2024, 14, 4387. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Jabłoński, S.J.; Biernacki, P.; Steinigeweg, S.; Łukaszewicz, M. Continuous mesophilic anaerobic digestion of manure and rape oilcake—Experimental and modelling study. Waste Manag. 2015, 35, 105–110. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.C.; Giglio-Jacamino, A.; Ferrer, I. Co-Digestion Strategies to Enhance Microalgae Anaerobic Digestion: A Review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic Co-Digestion: Current Status and Perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Östbring, K.; Tullberg, C.; Burri, S.; Malmqvist, E.; Rayner, M. Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract. Foods 2019, 8, 627. [Google Scholar] [CrossRef]
- Östbring, K.; Malmqvist, E.; Nilsson, K.; Rosenlind, I.; Rayner, M. The Effects of Oil Extraction Methods on Recovery Yield and Emulsifying Properties of Proteins from Rapeseed Meal and Press Cake. Foods 2020, 9, 19. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Elsamadony, M.; Mostafa, A.; Fujii, M.; Tawfik, A.; Pant, D. Advances towards Understanding Long-Chain Fatty Acids-Induced Inhibition and Overcoming Strategies for Efficient Anaerobic Digestion. Water Res. 2021, 190, 116732. [Google Scholar] [CrossRef]
- Dagnew, M.; Parker, W. Impact of AnMBR Operating Conditions on Anaerobic Digestion of Waste Activated Sludge. Water Environ. Res. 2021, 93, 703–713. [Google Scholar] [CrossRef]
- Olsson, J.; Forkman, T.; Gentili, F.G.; Zambrano, J.; Schwede, S.; Thorin, E.; Nehrenheim, E. Anaerobic Co-Digestion of Sludge and Microalgae Grown in Municipal Wastewater—A Feasibility Study. Water Sci. Technol. 2018, 77, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.M.; Greses, S.; Hagen, L.H.; Schiml, V.C.; Pope, P.B.; González-Fernández, C.; Arntzen, M.Ø. Anaerobic digestion of microalgae: Microbial response and recovery after organic loading disturbances. mSystems 2025, 10, e01674-24. [Google Scholar] [CrossRef] [PubMed]
- de Castro, I.M.P.; de Alencar Neves, T.; Rosa, A.P.; da Cunha, F.F.; Passos, F. Long-term assessment of anaerobic co-digestion of food waste and microalgae: Process stabilization, methane yield and agronomic properties of digestate. Algal Res. 2025, 86, 103947. [Google Scholar] [CrossRef]
- Ferreira, L.O.; Astals, S.; Passos, F. Anaerobic co-digestion of food waste and microalgae in an integrated treatment plant. J. Chem. Technol. Biotechnol. 2022, 97, 1545–1554. [Google Scholar] [CrossRef]
- Ehouman, A.D.; Kouakou, A.R.; Coubaly, M.; Konan, G.R.; Bamba, A.; Niamien, P.M.; Yao, B. Reduction of the corrosive character of a biogas: Elimination of hydrogen sulfide by filtration on activated carbon based on palm kernel shell. J. Mater. Environ. Sci. 2023, 14, 1078–1095. [Google Scholar]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Kijewski, Ł.; Groth, D.; Skwierawska, M.; Budzyński, W.S. The effect of sulfur fertilization on macronutrient concentrations in the post-harvest biomass of rapeseed (Brassica napus L. ssp. oleifera Metzg). J. Elem. 2015, 20, 585–597. [Google Scholar] [CrossRef]
- Mutegoa, E.; Sahini, M.G. Approaches to mitigation of hydrogen sulfide during anaerobic digestion process—A review. Heliyon 2023, 9, e19768. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič Višnjevec, A.; Tamayo Tenorio, A.; Steenkjær Hastrup, A.C.; Hansen, N.M.L.; Peeters, K.; Schwarzkopf, M. Glucosinolates and Isothiocyantes in Processed Rapeseed Determined by HPLC-DAD-qTOF. Plants 2021, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Okoro, O.V.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. ChemEngineering 2019, 3, 76. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Q.; Li, Y. A Review on Sulfur Transformation during Anaerobic Digestion of Organic Solid Waste: Mechanisms, Influencing Factors and Resource Recovery. Sci. Total Environ. 2023, 865, 161193. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef]
- Vongvichiankul, C.; Deebao, J.; Khongnakorn, W. Relationship between pH, Oxidation Reduction Potential (ORP) and Biogas Production in Mesophilic Screw Anaerobic Digester. Energy Procedia 2017, 138, 877–882. [Google Scholar] [CrossRef]
- Cheenakula, D.; Hoffstadt, K.; Krafft, S.; Reinecke, D.; Klose, H.; Kuperjans, I.; Grömping, M. Anaerobic digestion of algal–bacterial biomass of an Algal Turf Scrubber system. Biomass Convers. Biorefin. 2022, 13, 13605–13619. [Google Scholar] [CrossRef]

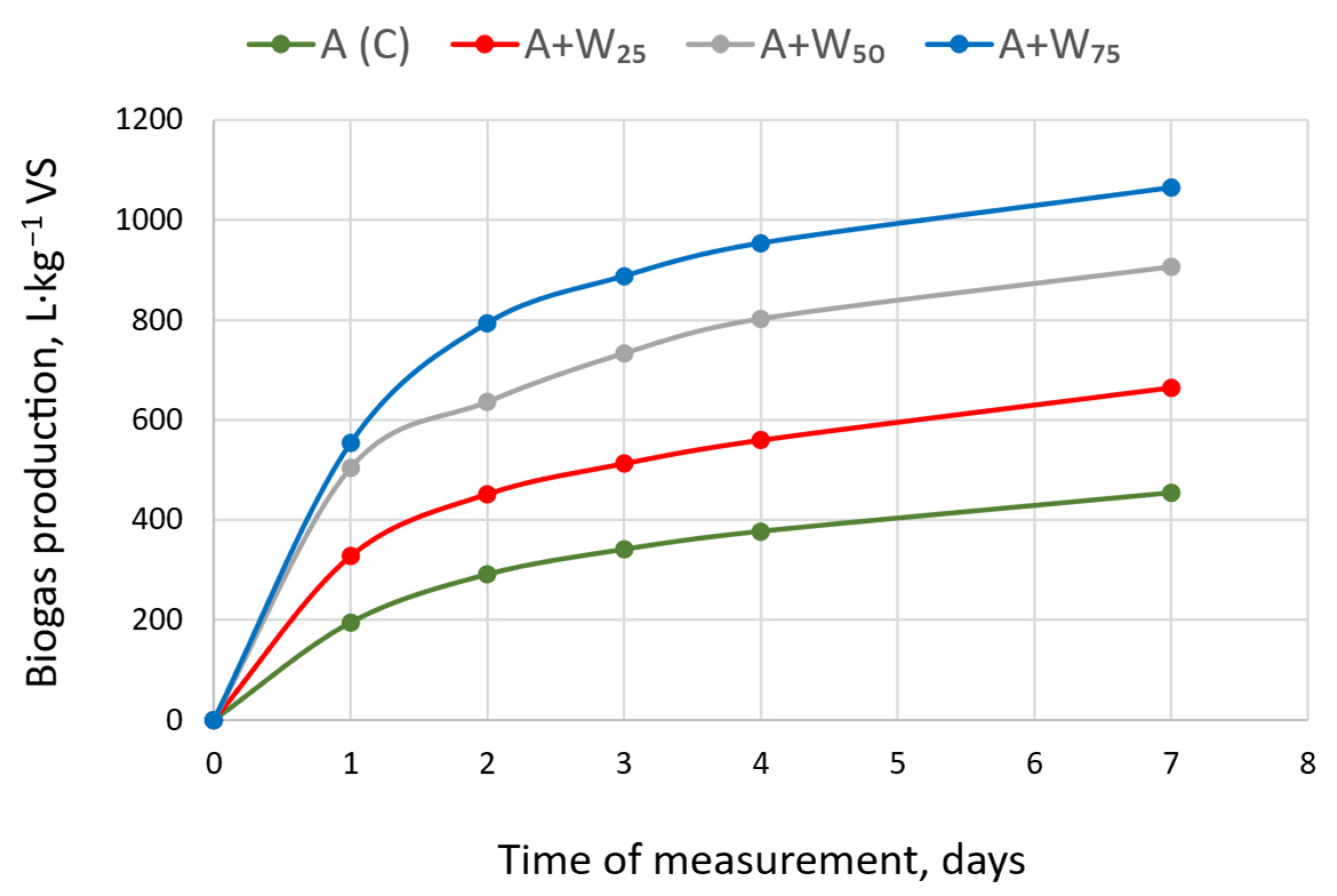
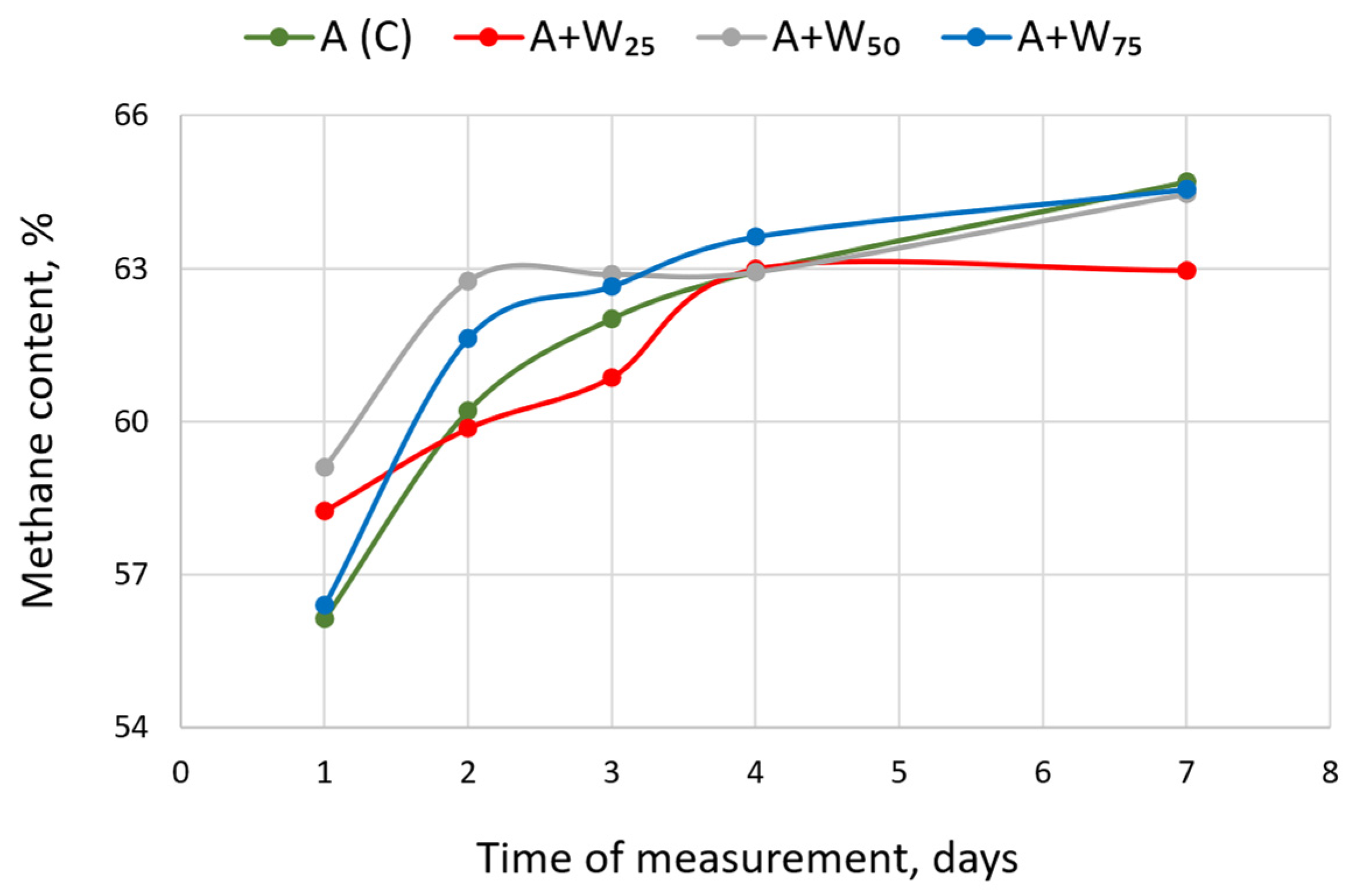

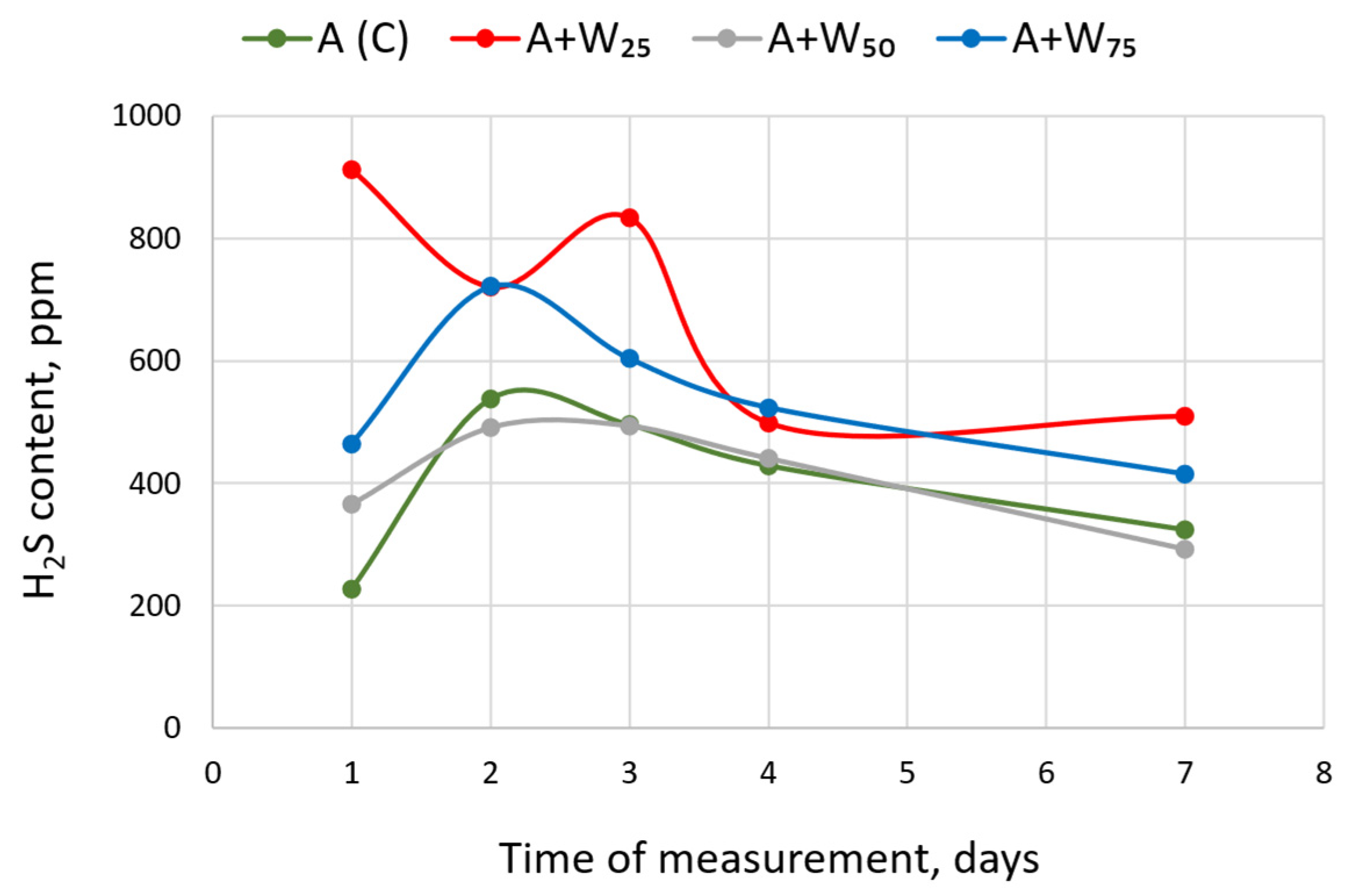
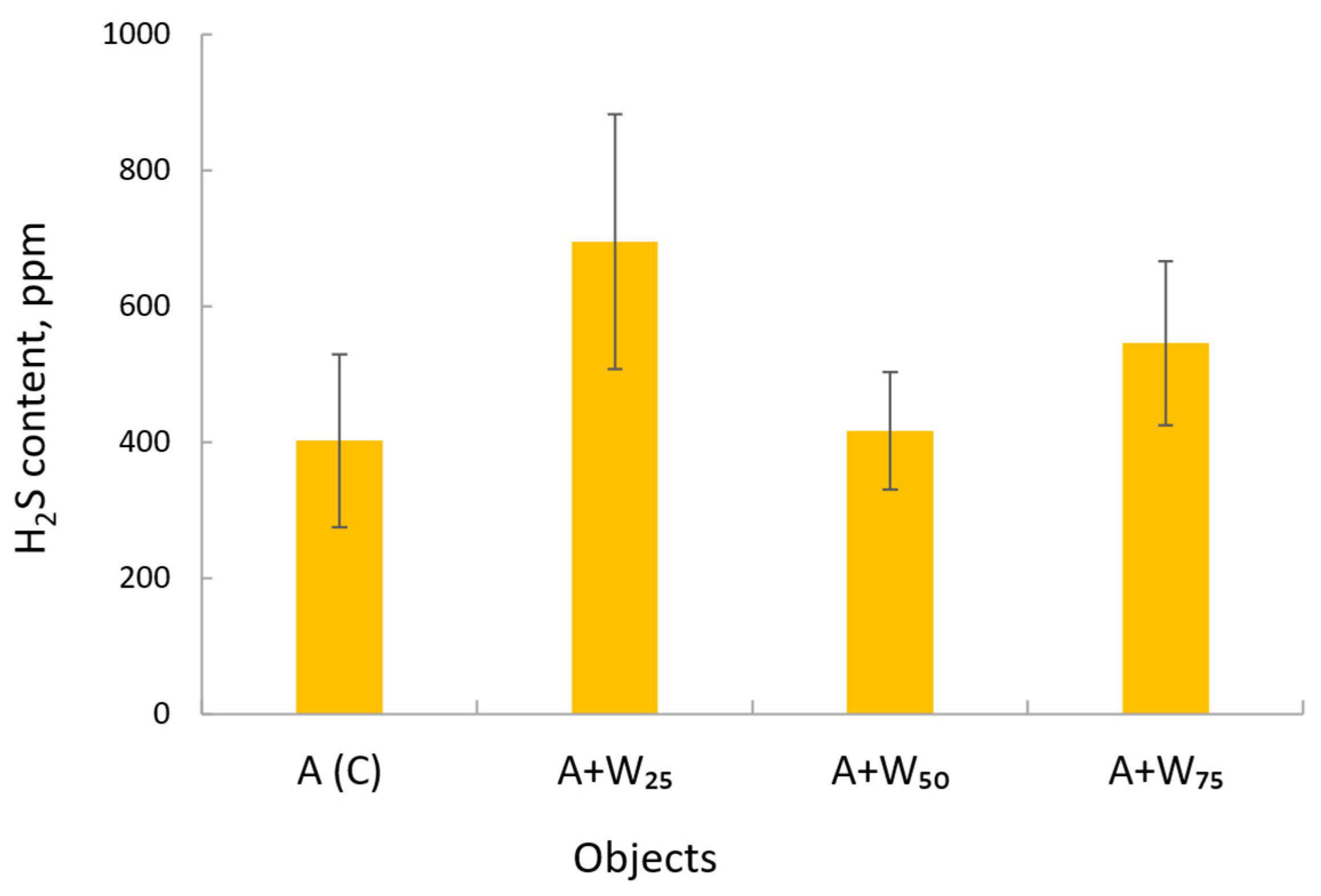
| Variant | Parameter | Day of Measurement | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | ||
| A + C | pH | 6.96 | 6.96 | 6.96 | 6.97 | 6.97 |
| REDOX [mV] | −307.1 | −303.4 | −334.4 | −334.9 | −333.7 | |
| Temperature [°C] | 37.18 | 37.18 | 37.18 | 37.18 | 37.78 | |
| A + W25 | pH | 6.95 | 6.96 | 6.96 | 6.96 | 6.96 |
| REDOX [mV] | −321.7 | −323.3 | −331.3 | −336.0 | −318.8 | |
| Temperature [°C] | 37.78 | 37.26 | 37.18 | 37.18 | 37.18 | |
| A + W50 | pH | 7.04 | 7.04 | 7.04 | 7.05 | 7.06 |
| REDOX [mV] | −316.0 | −307.0 | −319.0 | −314.0 | −323.0 | |
| Temperature [°C] | 37.00 | 37.00 | 37.00 | 37.00 | 37.00 | |
| A + W75 | pH | 7.01 | 7.03 | 7.04 | 7.04 | 7.05 |
| REDOX [mV] | −305.0 | −304.0 | −304.0 | −308.0 | −327.0 | |
| Temperature [°C] | 37.00 | 37.00 | 37.00 | 37.00 | 37.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Drzewicka, W. Application of Rapeseed Oil Cake from Biodiesel Production in Methane Co-Digestion with Microalgal Biomass. Materials 2025, 18, 4542. https://doi.org/10.3390/ma18194542
Hawrot-Paw M, Drzewicka W. Application of Rapeseed Oil Cake from Biodiesel Production in Methane Co-Digestion with Microalgal Biomass. Materials. 2025; 18(19):4542. https://doi.org/10.3390/ma18194542
Chicago/Turabian StyleHawrot-Paw, Małgorzata, and Wiktoria Drzewicka. 2025. "Application of Rapeseed Oil Cake from Biodiesel Production in Methane Co-Digestion with Microalgal Biomass" Materials 18, no. 19: 4542. https://doi.org/10.3390/ma18194542
APA StyleHawrot-Paw, M., & Drzewicka, W. (2025). Application of Rapeseed Oil Cake from Biodiesel Production in Methane Co-Digestion with Microalgal Biomass. Materials, 18(19), 4542. https://doi.org/10.3390/ma18194542







