Evaluation of Factors Affecting Fluoride Release from Fluoride Varnishes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Information Sources, Search Strategy and Study Design
- PubMed: (“fluoride release”[Title/Abstract]) AND (“varnish”[Title/Abstract]);
- Scopus: TITLE-ABS-KEY (“fluoride release” AND varnish);
- Web of Science (WoS): TS = (“fluoride release” AND varnish);
- Embase: (‘fluoride release’:ab,ti) AND (varnish:ab,ti);
- Cochrane Library: (“fluoride release” in Title, Abstract, Keywords) AND varnish.
2.3. Eligibility Criteria
- Investigation of evaluation the fluoride release from dental varnishes;
- Only research articles;
- In vitro studies;
- Studies conducted only on human teeth;
- Studies in English;
- Full-text articles.
- Evaluation of properties other than fluoride release;
- In vivo studies;
- Studies conducted on animal teeth or synthetic samples;
- Clinical reports;
- Review articles;
- Editorial papers;
- Full text not accessible;
- Duplicated publications.
2.4. Data Collection Process and Data Items
2.5. Protocol
2.6. Risk of Bias and Quality Assessment
3. Results
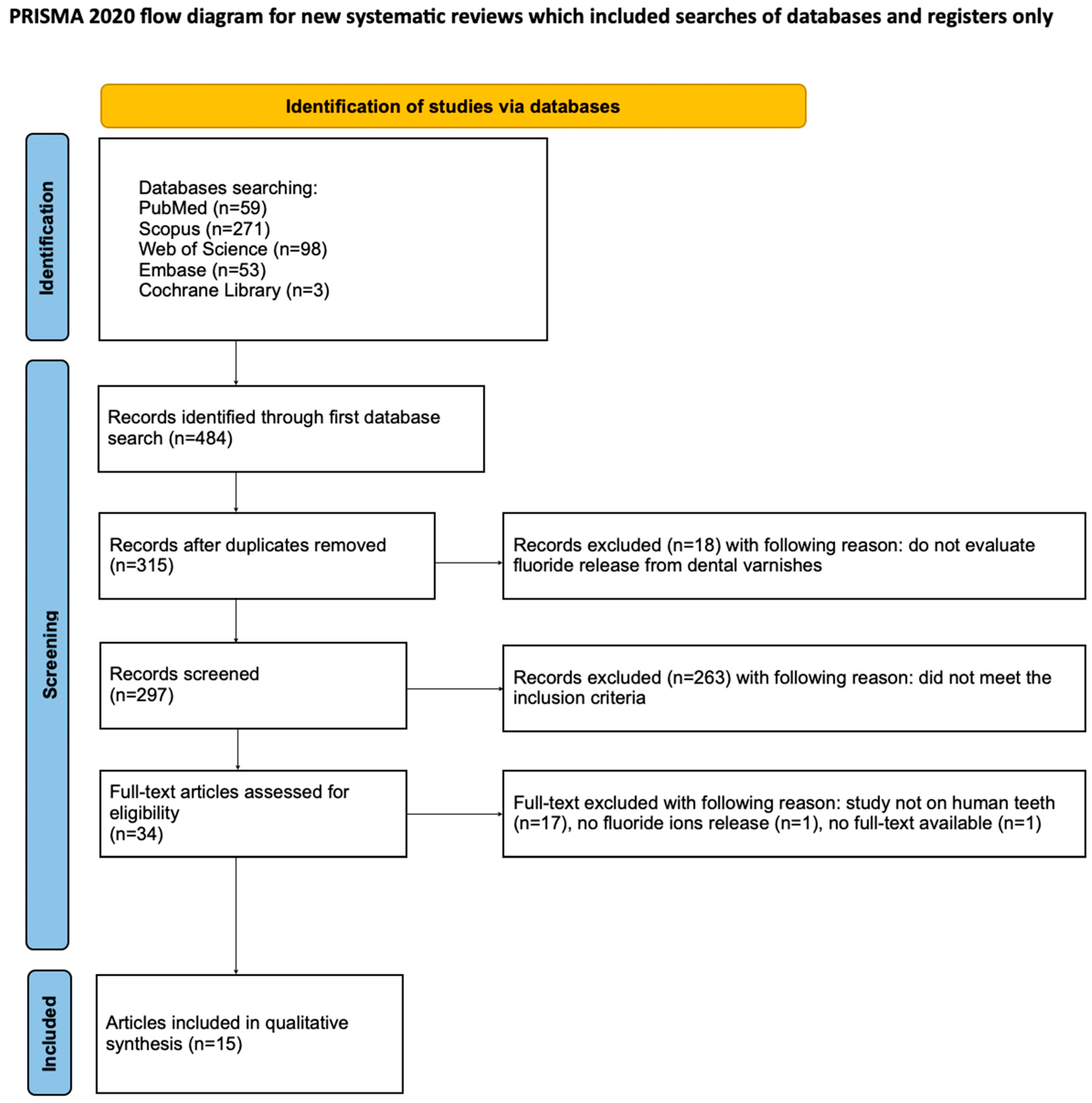
3.1. Study Selection
3.2. General Characteristics of the Included Studies
3.3. Main Study Outcomes
3.3.1. Sample Size/Volume
3.3.2. Storage Conditions
3.3.3. Measurement Methods and Time
3.3.4. Fluoride Release Results
3.3.5. Summary of Commercial Fluoride Varnishes
3.3.6. Additional Findings
3.4. Quality Assessment of Individual Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thakur, T.; Lahiri, P.K.; Karmakar, M.; Sarvaiya, B.; Datta, P.; Saha, R. Comparison of the Fluoride Release of Silver Diamine Fluoride, Fluoride Varnish, Acidulated Phosphate Fluoride Gel on Extracted Teeth over Various Time Intervals in Artificial Saliva. J. Pharm. Res. Int. 2021, 33, 55–63. [Google Scholar] [CrossRef]
- Attiguppe, P.; Malik, N.; Ballal, S.; Naik, S.V. CPP-ACP and Fluoride: A Synergism to Combat Caries. Int. J. Clin. Pediatr. Dent. 2019, 12, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Ortega, C.C.; Rodil, S.E.; Silva-Bermudez, P.; Delgado-Cardona, A.; Almaguer-Flores, A.; Prado-Prone, G. Fluoride Casein Phosphopeptide and Tri-Calcium Phosphate Treatments for Enamel Remineralization: Effects on Surface Properties and Biofilm Resistance. Dent. J. 2025, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Şen Yavuz, B.; Yilmaz, M.A.; Oktay, N.Ş.; Kargül, B. The Effect of a Single Application of Different Fluoride Varnishes on Enamel Subsurface Lesions In Vitro. 2022. Available online: https://www.researchgate.net/publication/357270330_THE_EFFECT_OF_SINGLE_APPLICATION_OF_DIFFERENT_FLUORIDE_VARNISHES_ON_ENAMEL_SUBSURFACE_LESIONS_IN_VITRO (accessed on 19 August 2025).
- Nassar, Y.; Brizuela, M. The Role of Fluoride on Caries Prevention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Piszko, P.J.; Kulus, M.; Piszko, A.; Kiryk, J.; Kiryk, S.; Kensy, J.; Małyszek, A.; Michalak, M.; Dobrzyński, W.; Matys, J.; et al. The Influence of Calcium Ions and pH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study. Gels 2025, 11, 486. [Google Scholar] [CrossRef]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef]
- Kritikou, K.; Vinereanu, A.; Munteanu, A. Fluoride in Dentistry: Brief Updated Guidelines for Caries Prevention. Rom. J. Dent. Med. 2022, XXV, 97–109. [Google Scholar]
- Olczak-Kowalczyk, D.; Mielczarek, A.; Jackowska, T.; Mielnik-Błaszczak, M.; Turska-Szybka, A.; Opydo-Szymaczek, J.; Jurczak, A.; Kaczmarek, U. Fluoride agents in the prevention and treatment of dental caries and erosion in children, adolescents and adults—Recommendations of Polish Experts. Update of recommendations: Individual fluoride prevention in children and adolescents—Recommendations of Polish Experts. Nowa Stomatol. 2022, 27, 35–59. [Google Scholar]
- Manjunathappa, T.H.; Devegowda, D.; Mysore, N.K.; Vishwanath, P.; Narayana, P.S. Association between Drinking Water Fluoride and the Serum Alkaline Phosphatase and Phosphate Levels in Pregnant Women and Newborn Infants. Dent. Med. Probl. 2023, 60, 569–575. [Google Scholar] [CrossRef]
- Bronckers, A.L.J.J.; Lyaruu, D.M.; DenBesten, P.K. The Impact of Fluoride on Ameloblasts and the Mechanisms of Enamel Fluorosis. J. Dent. Res. 2009, 88, 877–893. [Google Scholar] [CrossRef]
- Peyron, M.; Matsson, L.; Birkhed, D. Progression of Approximal Caries in Primary Molars and the Effect of Duraphat Treatment. Scand. J. Dent. Res. 1992, 100, 314–318. [Google Scholar] [CrossRef]
- Sirivichayakul, P.; Jirarattanasopha, V.; Phonghanyudh, A.; Tunlayadechanont, P.; Khumsub, P.; Duangthip, D. The Effectiveness of Topical Fluoride Agents on Preventing Development of Approximal Caries in Primary Teeth: A Randomized Clinical Trial. BMC Oral Health 2023, 23, 349. [Google Scholar] [CrossRef]
- Szczepańska, J.; Olczak-Kowalczyk, D.; Kaczmarek, U.; Wydanie, I. (Eds.) Współczesna Stomatologia Wieku Rozwojowego; Med Tour Press International: Otwock, Poland, 2017; ISBN 978-83-87717-26-1. [Google Scholar]
- Lee, Y. Diagnosis and Prevention Strategies for Dental Caries. J. Lifestyle Med. 2013, 3, 107–109. [Google Scholar]
- Hong, Y.C.; Chow, L.C.; Brown, W.E. Enhanced Fluoride Uptake from Mouthrinses. J. Dent. Res. 1985, 64, 82–84. [Google Scholar] [CrossRef]
- Dobrzyński, W.; Nikodem, A.; Diakowska, D.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M.; Mikulewicz, M. Comparison of the Fluoride Ion Release from Nanofluoroapatite-Modified Orthodontic Cement under Different pH Conditions—An in Vitro Study. Acta Bioeng. Biomech. 2023, 25, 159–176. [Google Scholar] [CrossRef]
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Diakowska, D.; Nienartowicz, J.; Blicharski, T.; Nagel, S.; Sikora, M.; Wiglusz, K.; Watras, A.; et al. Comparison of the Fluoride Ion Release from Composite and Compomer Materials under Varying pH Conditions—Preliminary In Vitro Study. Appl. Sci. 2022, 12, 12540. [Google Scholar] [CrossRef]
- Kosior, P.; Klimas, S.; Nikodem, A.; Wolicka, J.; Diakowska, D.; Watras, A.; Wiglusz, R.J.; Dobrzyński, M. An in Vitro Examination of Fluoride Ions Release from Selected Materials—Resin-Modified Glass-Ionomer Cement (Vitremer) and Nanohybrid Composite Material (Tetric EvoCeram). Acta Bioeng. Biomech. 2023, 25, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pal, M.; Rawat, S.; Grewal, M.S.; Garg, H.; Chauhan, D.; Ahlawat, P.; Tandon, S.; Khurana, R.; Pahuja, A.K.; et al. Radiation-Induced Dental Caries, Prevention and Treatment—A Systematic Review. Natl. J. Maxillofac. Surg. 2015, 6, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Lubojanski, A.; Piesiak-Panczyszyn, D.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Mielan, B.; Wiglusz, R.J.; Watras, A.; Dobrzynski, M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials 2023, 16, 1242. [Google Scholar] [CrossRef]
- Sidhu, S.; Thomas, A.M.; Kundra, R.; Cherian, J.M. Assessment of Fluoride Release Pattern from Different Fluoride Varnishes—An in Vitro Study. J. Pharm. Res. Int. 2020, 32, 104–115. [Google Scholar] [CrossRef]
- Diniz, M.; Campos, P.; Sanabe, M.; Duarte, D.; Santos, M.; Guaré, R.; Duque, C.; Lussi, A.; Rodrigues, J. Effectiveness of Fluorescence-Based Methods in Monitoring Progression of Noncavitated Caries-like Lesions on Smooth Surfaces. Oper. Dent. 2015, 40, E230–E241. [Google Scholar] [CrossRef]
- Weintraub, J.A.; Ramos-Gomez, F.; Jue, B.; Shain, S.; Hoover, C.I.; Featherstone, J.D.B.; Gansky, S.A. Fluoride Varnish Efficacy in Preventing Early Childhood Caries. J. Dent. Res. 2006, 85, 172–176. [Google Scholar] [CrossRef]
- Jullien, S. Prophylaxis of Caries with Fluoride for Children under Five Years. BMC Pediatr. 2021, 21, 351. [Google Scholar] [CrossRef]
- Munteanu, A.; Holban, A.-M.; Păuna, M.-R.; Imre, M.; Farcașiu, A.-T.; Farcașiu, C. Review of Professionally Applied Fluorides for Preventing Dental Caries in Children and Adolescents. Appl. Sci. 2022, 12, 1054. [Google Scholar] [CrossRef]
- Chow, L.C.; Guo, M.K.; Hsieh, C.C.; Hong, Y.C. Apatitic Fluoride Increase in Enamel from a Topical Treatment Involving Intermediate CaHPO4·2H2O Formation, an in Vivo Study. Caries Res. 1981, 15, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, A.; Cakirer, B. A Comparative Evaluation of Casein Phosphopeptide-Amorphous Calcium Phosphate and Fluoride on the Shear Bond Strength of Orthodontic Brackets. Eur. J. Orthod. 2011, 33, 282–287. [Google Scholar] [CrossRef]
- Virupaxi, S.G.; Roshan, N.M.; Poornima, P.; Nagaveni, N.B.; Neena, I.E.; Bharath, K.P. Comparative Evaluation of Longevity of Fluoride Release From Three Different Fluoride Varnishes—An Invitro Study. J. Clin. Diagn. Res. JCDR 2016, 10, ZC33–ZC36. [Google Scholar] [CrossRef]
- Castillo, J.L.; Milgrom, P. Fluoride Release from Varnishes in Two in Vitro Protocols. J. Am. Dent. Assoc. 1939 2004, 135, 1696–1699. [Google Scholar] [CrossRef]
- Baik, A.; Alamoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride Varnishes for Preventing Occlusal Dental Caries: A Review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef]
- Saveanu, C.I.; Dragos, O.; Anistoroaei, D.; Bobu, L.I.; Saveanu, A.E.; Armencia, A.; Solomon, S.M.; Tanculescu, O. Xylitol Fluoride Varnish: In Vitro Effect Analysis on Enamel by Atomic Force Microscopy. Biomedicines 2022, 10, 1900. [Google Scholar] [CrossRef]
- Jabir, E.; McGrade, C.; Quinn, G.; McGarry, J.; Nic Iomhair, A.; Kelly, N.; Srinivasan, M.; Watson, S.; McKenna, G.J. Evaluating the Effectiveness of Fluoride Varnish in Preventing Caries amongst Long-Term Care Facility Residents. Gerodontology 2022, 39, 250–256. [Google Scholar] [CrossRef]
- Sharma, P.R. Allergic Contact Stomatitis from Colophony. Dent. Update 2006, 33, 440–442. [Google Scholar] [CrossRef]
- Isaksson, M.; Bruze, M.; Björkner, B.; Niklasson, B. Contact Allergy to Duraphat. Scand. J. Dent. Res. 1993, 101, 49–51. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-Preventive Effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF Varnishes on Sound Dentin and Artificial Dentin Caries in Vitro. Caries Res. 2018, 52, 199–211. [Google Scholar] [CrossRef]
- Xu, J.; Shi, H.; Luo, J.; Yao, H.; Wang, P.; Li, Z.; Wei, J. Advanced Materials for Enamel Remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. [Google Scholar] [CrossRef]
- Bhat, D.V.; Awchat, K.L.; Singh, P.; Jha, M.; Arora, K.; Mitra, M. Evaluation of Remineralizing Potential of CPP-ACP, CPP-ACP + F and β TCP + F and Their Effect on Microhardness of Enamel Using Vickers Microhardness Test: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2022, 15, S221–S225. [Google Scholar] [CrossRef]
- Monjarás-Ávila, A.J.; Hardan, L.; Cuevas-Suárez, C.E.; Alonso, N.V.Z.; Fernández-Barrera, M.Á.; Moussa, C.; Jabr, J.; Bourgi, R.; Haikel, Y. Systematic Review and Meta-Analysis of Remineralizing Agents: Outcomes on White Spot Lesions. Bioengineering 2025, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Paula, V.; Modesto, A.; Santos, K.; Gleiser, R. Antimicrobial Effects of the Combination of Chlorhexidine and Xylitol. Br. Dent. J. 2010, 209, E19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nascimento, M.M.; Alvarez, A.J.; Huang, X.; Hanway, S.; Perry, S.; Luce, A.; Richards, V.P.; Burne, R.A. Arginine Metabolism in Supragingival Oral Biofilms as a Potential Predictor of Caries Risk. JDR Clin. Transl. Res. 2019, 4, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lu, Q.; Tian, Y.; Zhou, X.; Cheng, L.; Ren, B. Effect of Toothpaste Containing Arginine on Dental Plaque-A Randomized Controlled in Situ Study. J. Dent. 2017, 67, 88–93. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Kensy, J.; Michalak, M.; Matys, J.; Dobrzyński, M. Fluoride Release from Two Commercially Available Dental Fluoride Gels—In Vitro Study. Gels 2025, 11, 135. [Google Scholar] [CrossRef]
- Marko, T.; Ivana, Š.; Kristina, P. Comparison of Fluoride Ion Release from Fluoride Gel in Various Solvents. Acta Stomatol. Croat. 2020, 54, 147–154. [Google Scholar] [CrossRef]
- Piesiak-Pańczyszyn, D.; Zakrzewski, W.; Piszko, A.; Piszko, P.J.; Dobrzyński, M. Review on Fluoride Varnishes Currently Recommended in Dental Prophylaxis. Polym. Med. 2023, 53, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Poza-Pascual, A.; Serna-Muñoz, C.; Pérez-Silva, A.; Martínez-Beneyto, Y.; Cabello, I.; Ortiz-Ruiz, A.J. Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 10049. [Google Scholar] [CrossRef] [PubMed]
- Oleniacz-Trawińska, M.; Kotela, A.; Kensy, J.; Kiryk, S.; Dobrzyński, W.; Kiryk, J.; Gerber, H.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Affecting Fluoride Release from Compomer Restorative Materials: A Systematic Review. Materials 2025, 18, 1627. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef]
- Rajewska, J.; Kowalski, J.; Matys, J.; Dobrzyński, M.; Wiglusz, R.J. The Use of Lactide Polymers in Bone Tissue Regeneration in Dentistry—A Systematic Review. J. Funct. Biomater. 2023, 14, 83. [Google Scholar] [CrossRef]
- Rygas, J.; Matys, J.; Wawrzyńska, M.; Szymonowicz, M.; Dobrzyński, M. The Use of Graphene Oxide in Orthodontics—A Systematic Review. J. Funct. Biomater. 2023, 14, 500. [Google Scholar] [CrossRef]
- Małyszek, A.; Zawiślak, I.; Kulus, M.; Watras, A.; Kensy, J.; Kotela, A.; Styczyńska, M.; Janeczek, M.; Matys, J.; Dobrzyński, M. Fluoride Content in Infusions of Selected Teas Available on the Polish Market—An In Vitro Study. Foods 2025, 14, 2452. [Google Scholar] [CrossRef]
- Klimas, S.; Kiryk, S.; Kiryk, J.; Kotela, A.; Kensy, J.; Michalak, M.; Rybak, Z.; Matys, J.; Dobrzyński, M. The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies. Materials 2025, 18, 3187. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Quasi-Experimental Studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef]
- Mainente, M.P.; Naves, P.A.; de Campos, P.H.; Rodrigues, M.C.; Diniz, M.B.; Zaroni, W.C.d.S.; Cardoso, C.d.A.B. Inhibition of Incipient Caries Lesion Progression by Different Fluoridated Varnishes. Braz. Dent. J. 2024, 35, e245616. [Google Scholar] [CrossRef]
- Paiva, M.F.; Delbem, A.C.B.; Veri, I.V.; Sampaio, C.; Wiegand, A.; Pessan, J.P. Fluoride Varnishes Supplemented with Nano-Sized Sodium Trimetaphosphate Reduce Enamel Erosive Wear in Vitro. J. Dent. 2023, 138, 104726. [Google Scholar] [CrossRef]
- Park, S.-A.; Son, J.; Kim, A.-J.; Oh, S.; Bae, J.-M. Effect of Adhesive Components in Experimental Fluoride Varnish on Fluoride Release within 30 Days in Vitro Study. Dent. Mater. J. 2024, 43, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.; Bijle, M.N.; Abdallah, N.M.A.; Yiu, C.K.Y. Enamel Remineralization Potential and Antimicrobial Effect of a Fluoride Varnish Containing Calcium Strontium Silicate. J. Dent. 2023, 138, 104731. [Google Scholar] [CrossRef] [PubMed]
- Fathollah, S.; Abbasi, H.; Akhoundi, S.; Naeimabadi, A.; Emamjome, S. Cold Plasma Enamel Surface Treatment to Increase Fluoride Varnish Uptake. Sci. Rep. 2022, 12, 4657. [Google Scholar] [CrossRef] [PubMed]
- Larpbunphol, N.; Chanamuangkon, T.; Thamrongananskul, N. The Fluoride Release, Abrasion Resistance, and Cytotoxicity to hGFs of a Novel Cyanoacrylate-Based Fluoride Varnish Compared with Conventional Fluoride Varnish. Dent. Mater. J. 2022, 41, 757–766. [Google Scholar] [CrossRef]
- Kusuma Eriwati, Y.; Putriani, D.; Geraldine, K.; Hermansyah, H. Fluoride and Calcium Release from Peppermint-Flavored Fluoride Varnish Containing Dicalcium-Phosphate-Dihydrate Coated with Xylitol. Saudi Dent. J. 2022, 34, 68–73. [Google Scholar] [CrossRef]
- Geraldine, K.; Putriani, D.; Hermansyah, H.; Eriwati, Y.K. Optimization of Fluoride Release by Addition of Flavor Oils in Fast Release Fluoride Varnish. AIP Conf. Proc. 2021, 2344, 020006. [Google Scholar] [CrossRef]
- Geraldine, K.; Putriani, D.; Eriwati, Y.K.; Hermansyah, H. Modification of Fast Release Fluoride Varnish by Addition of Plant-Based Extracts as Antibacterial Agents; American Institute of Physics Inc.: Depok, Indonesia, 2021; p. 020007. [Google Scholar]
- Soares-Yoshikawa, A.L.; Varanda, T.; Iwamoto, A.S.; Kantovitz, K.R.; Puppin-Rontani, R.M.; Pascon, F.M. Fluoride Release and Remineralizing Potential of Varnishes in Early Caries Lesions in Primary Teeth. Microsc. Res. Tech. 2021, 84, 1012–1021. [Google Scholar] [CrossRef]
- Varma, V.; Hegde, K.S.; Bhat, S.S.; Sargod, S.S.; Rao, H.A. Comparative Evaluation of Remineralization Potential of Two Varnishes Containing CPP-ACP and Tricalcium Phosphate: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2019, 12, 233–236. [Google Scholar] [CrossRef]
- Bezerra, N.V.F.; Martins, M.L.; Leite, K.L.D.F.; Medeiros, M.M.D.D.; Almeida, L.D.F.D.D.; Padilha, W.W.N.; Cavalcanti, Y.W. In Vitro Evaluation of Fluoride in Saliva After Topical Application of Professional Use Products. Pesqui. Bras. Em Odontopediatria Clínica Integr. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Tork-Zaban, R.; Malek-Afzali, B.; Zaban, M.T.; Rezvani, Y. Comparing Fluoride Release in Preventa and Enamel Pro in Laboratory Environment. Biosc. Biotech. Res. Comm. 2017, 10, 208–212. [Google Scholar] [CrossRef]
- Bolis, C.; Härtli, G.P.; Lendenmann, U. Fluoride Varnishes--Is There a Correlation Between Fluoride Release and Deposition on Enamel? Oral Health Prev. Dent. 2015, 13, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F. Fluoride Release from Fluoride Varnishes under Acidic Conditions. J. Clin. Pediatr. Dent. 2014, 39, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F.; Hara, A.T.; Martinez-Mier, E.A.; Zero, D.T. Laboratory Investigations into the Potential Anticaries Efficacy of Fluoride Varnishes. Pediatr. Dent. 2014, 36, 291–295. [Google Scholar]
- Attin, T.; Lennon, A.M.; Yakin, M.; Becker, K.; Buchalla, W.; Attin, R.; Wiegand, A. Deposition of Fluoride on Enamel Surfaces Released from Varnishes Is Limited to Vicinity of Fluoridation Site. Clin. Oral Investig. 2007, 11, 83–88. [Google Scholar] [CrossRef]
- Jablonowski, B.L.; Bartoloni, J.A.; Hensley, D.M.; Vandewalle, K.S. Fluoride Release from Newly Marketed Fluoride Varnishes. Quintessence Int. Berl. Ger. 1985 2012, 43, 221–228. [Google Scholar]
- Singh, V.; Naik, S.; Vashisth, P.; Sharma, S.; Chandak, A.; Murry, J.N. Comparative Evaluation of Longevity of Fluoride Release from Three Different Fluoride Varnishes: An Observational Study. Int. J. Clin. Pediatr. Dent. 2024, 17, 341–345. [Google Scholar] [CrossRef]
- Nahum, S.-V.E.; Rogelio José, S.-V.; Edith, L.-C.; Hugo, T.-R.V.; Ulises, V.-E.; Alejandra, M.-V.A. Fluoride Release and Recharge in Conventional Varnishes, Compared to a Giomer and a Resin Modified Glass Ionomer. J. Dent. Mater. Technol. 2021, 10, 62–70. [Google Scholar] [CrossRef]
- Asian, J.; Quenta, E.; Castillo, J.L. Do Viscosity and Wettability of Fluoride Varnishes Affect Their Fluoride Release? J. Clin. Exp. Dent. 2021, 13, e221–e226. [Google Scholar] [CrossRef]
- Okuyama, K.; Matsuda, Y.; Yamamoto, H.; Suzuki, K.; Shintani, K.; Saito, T.; Hayashi, M.; Tamaki, Y. Fluoride Retention in Root Dentin Following Surface Coating Material Application. J. Funct. Biomater. 2023, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Piesiak-Panczyszyn, D.; Watras, A.; Wiglusz, R.J.; Dobrzynski, M. In Vitro Comparison of the Fluoride Ion Release from the First- and Second-Generation Fluoride Varnishes. Appl. Sci. 2023, 13, 7327. [Google Scholar] [CrossRef]
- Rirattanapong, P.; Vongsavan, K.; Saengsirinavin, C.; Jantarakam, N. A 3-month study of fluoride release from different calcium phosphate fluoride varnishes on primary teeth. S. A. J. Trop. Med. Public Health 2016, 47, 1098–1104. [Google Scholar]
- Ritwik, P.; Aubel, J.D.; Xu, X.; Fan, Y.; Hagan, J. Evaluation of Short-Term Fluoride Release from Fluoride Varnishes. J. Clin. Pediatr. Dent. 2012, 36, 275–278. [Google Scholar] [CrossRef]
- Castillo, J.L.; Milgrom, P.; Kharasch, E.; Izutsu, K.; Fey, M. Evaluation of Fluoride Release from Commercially Available Fluoride Varnishes. J. Am. Dent. Assoc. 1939 2001, 132, 1389–1392; quiz 1459–1460. [Google Scholar] [CrossRef]
- Tuloglu, N.; Bayrak, S.; Tunc, E.S.; Ozer, F. Effect of Fluoride Varnish with Added Casein Phosphopeptide-Amorphous Calcium Phosphate on the Acid Resistance of the Primary Enamel. BMC Oral Health 2016, 16, 103. [Google Scholar] [CrossRef]
- Iranparvar, P.; Ghasemi, A.; Iranparvar, P. Adhesion of Glass Ionomer Cements to Primary Dentin Using a Universal Adhesive. Dent. Med. Probl. 2024, 61, 93–98. [Google Scholar] [CrossRef]
- Sleibi, A.; Tappuni, A.; Mills, D.; Davis, G.R.; Baysan, A. Comparison of the Efficacy of Different Fluoride Varnishes on Dentin Remineralization During a Critical pH Exposure Using Quantitative X-Ray Microtomography. Oper. Dent. 2018, 43, E308–E316. [Google Scholar] [CrossRef]
- Shen, P.; Bagheri, R.; Walker, G.D.; Yuan, Y.; Stanton, D.P.; Reynolds, C.; Reynolds, E.C. Effect of Calcium Phosphate Addition to Fluoride Containing Dental Varnishes on Enamel Demineralization. Aust. Dent. J. 2016, 61, 357–365. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Shen, P.; Yuan, Y.; Reynolds, E.C. Ion Release from Calcium and Fluoride Containing Dental Varnishes. Aust. Dent. J. 2014, 59, 100–105. [Google Scholar] [CrossRef]
- Yimcharoen, V.; Rirattanapong, P.; Kiatchallermwong, W. The Effect of Casein Phosphopeptide Toothpaste versus Fluoride Toothpaste on Remineralization of Primary Teeth Enamel. S. A. J. Trop. Med. Public Health 2011, 42, 1032–1040. [Google Scholar]
- Erkmen Almaz, M.; Ulusoy, N.B.; Akbay Oba, A.; Dokumacı, A. Remineralization Effect of NaF, NaF with TCP, NaF with CPP-ACP and NaF with CXP Varnishes on Newly Erupted First Permanent Molars: A Randomized Controlled Trial. Int. J. Dent. Hyg. 2024, 22, 703–710. [Google Scholar] [CrossRef]
- Zimmer, S.; Robke, F.J.; Roulet, J.F. Caries Prevention with Fluoride Varnish in a Socially Deprived Community. Community Dent. Oral Epidemiol. 1999, 27, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Yow, L.; Liaw, L.H.; Macleay, L.; Zavar, R.B.; Orenstein, A.; Wright, W.H.; Andrews, J.J.; Berns, M.W. Ablation of Bone and Methacrylate by a Prototype Mid-Infrared Erbium:YAG Laser. Lasers Surg. Med. 1988, 8, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Leyda, A.M.; Forner, L. CPP-ACP and CPP-ACFP versus Fluoride Varnish in Remineralisation of Early Caries Lesions. A Prospective Study. Eur. J. Paediatr. Dent. 2015, 16, 181–186. [Google Scholar]
- AlAmoudi, S.A.; Pani, S.C.; AlOmari, M. The Effect of the Addition of Tricalcium Phosphate to 5% Sodium Fluoride Varnishes on the Microhardness of Enamel of Primary Teeth. Int. J. Dent. 2013, 2013, 486358. [Google Scholar] [CrossRef]
- Anani, H.; Elaaser, D.; Niazy, M.; Jamil, W.; Elsharqawy, D. Evaluation of the Remineralization and Antibacterial Effect of Natural versus Synthetic Materials on Deep Carious Dentin: A Randomized Controlled Trial. Dent. Med. Probl. 2023, 60, 87–97. [Google Scholar] [CrossRef]
- Sköld, L.; Sundquist, B.; Eriksson, B.; Edeland, C. Four-Year Study of Caries Inhibition of Intensive Duraphat Application in 11-15-Year-Old Children. Community Dent. Oral Epidemiol. 1994, 22, 8–12. [Google Scholar] [CrossRef]
- Bergström, E.-K.; Birkhed, D.; Granlund, C.; Sköld, U.M. Approximal Caries Increment in Adolescents in a Low Caries Prevalence Area in Sweden after a 3.5-Year School-Based Fluoride Varnish Programme with Bifluorid 12 and Duraphat. Community Dent. Oral Epidemiol. 2014, 42, 404–411. [Google Scholar] [CrossRef]
- Bayrak, S.; Tuloglu, N.; Bicer, H.; Sen Tunc, E. Effect of Fluoride Varnish Containing CPP-ACP on Preventing Enamel Erosion. Scanning 2017, 2017, 1897825. [Google Scholar] [CrossRef]
- Kooshki, F.; Fatemi, S.; Darvishghaderi, S.; Vahedi, P. Comparison of the Effects of Fluoride Varnish Containing Silver Nanoparticles and Conventional Fluoride Varnish on the Surface Microhardness of Tooth Enamel. Dent. Med. Probl. 2024, 61, 241–247. [Google Scholar] [CrossRef]
- Kim, H.-N.; Kim, J.-B.; Jeong, S.-H. Remineralization Effects When Using Different Methods to Apply Fluoride Varnish in Vitro. J. Dent. Sci. 2018, 13, 360–366. [Google Scholar] [CrossRef]
- Stecksén-Blicks, C.; Renfors, G.; Oscarson, N.D.; Bergstrand, F.; Twetman, S. Caries-Preventive Effectiveness of a Fluoride Varnish: A Randomized Controlled Trial in Adolescents with Fixed Orthodontic Appliances. Caries Res. 2007, 41, 455–459. [Google Scholar] [CrossRef]
- Petersson, L.G.; Twetman, S.; Dahlgren, H.; Norlund, A.; Holm, A.-K.; Nordenram, G.; Lagerlöf, F.; Söder, B.; Källestål, C.; Mejàre, I.; et al. Professional Fluoride Varnish Treatment for Caries Control: A Systematic Review of Clinical Trials. Acta Odontol. Scand. 2004, 62, 170–176. [Google Scholar] [CrossRef]
- de Sousa, F.S.d.O.; Dos Santos, A.P.P.; Nadanovsky, P.; Hujoel, P.; Cunha-Cruz, J.; de Oliveira, B.H. Fluoride Varnish and Dental Caries in Preschoolers: A Systematic Review and Meta-Analysis. Caries Res. 2019, 53, 502–513. [Google Scholar] [CrossRef] [PubMed]
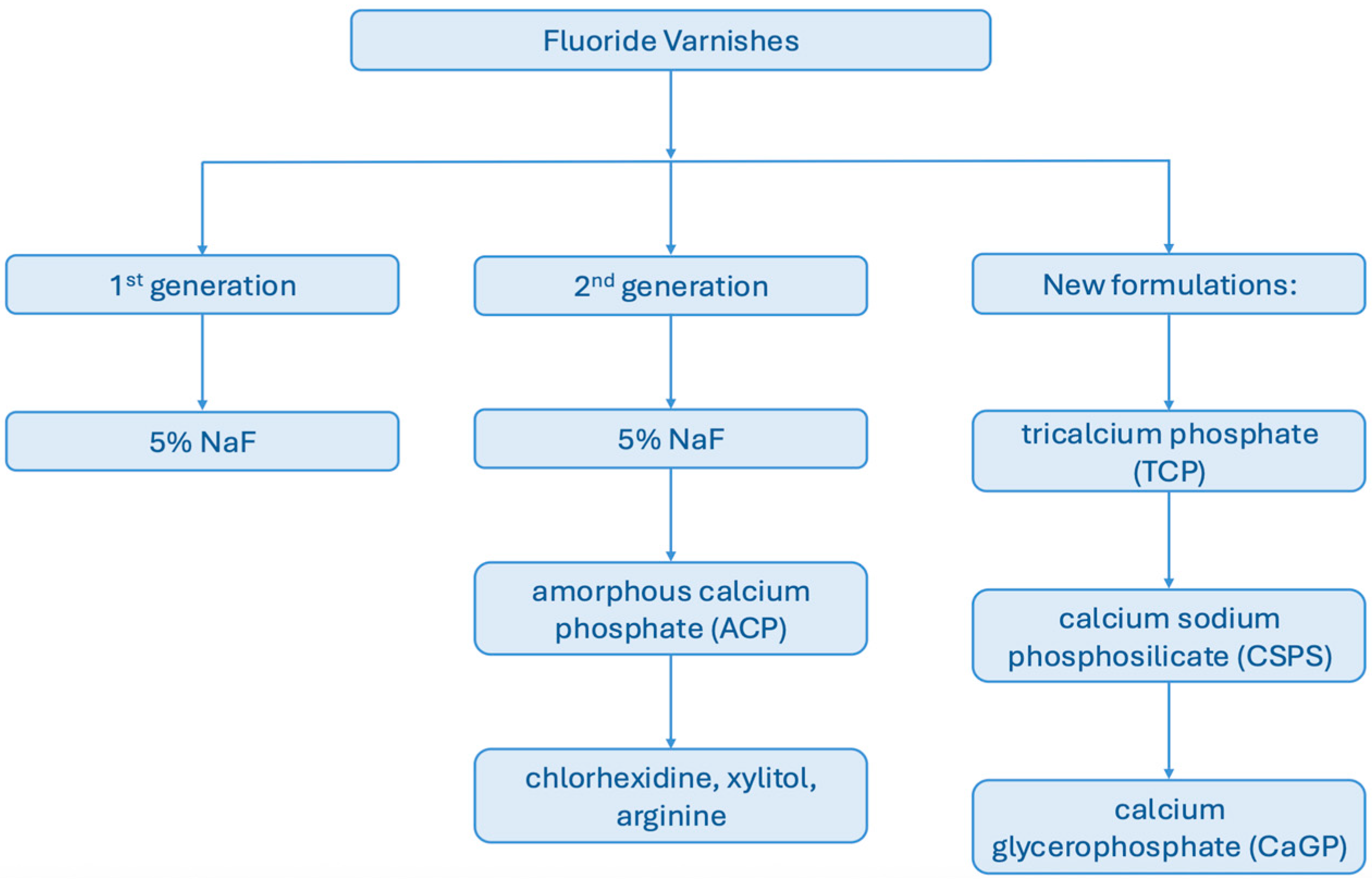

| Fluoride Varnish | Additive(s) | Main Positive Findings | Main Limitations/Negatives | References |
|---|---|---|---|---|
| Duraphat | NaF (5%), no additive | Sustained release (up to 28 weeks), widely studied, clinical benchmark | Rapid decline after initial release, less effect on remineralization vs. bioactive formulations | [30,75,76,78,79,81] |
| Fluor Protector | Amine fluoride | Stable compound, moderate release | Lowest cumulative release in several studies, limited remineralization | [1,2,4,29,75,76] |
| Clinpro White Varnish | fTCP | High cumulative release (20.26 mg/L), sustained over months | Initial burst followed by decline, variable performance | [22,75,76,78,79] |
| Clinpro XT | fTCP + resin-modified base | Most stable, long-term release, good substantivity | Requires light-curing, less studied clinically | [29,77] |
| MI Varnish | CPP–ACP | High cumulative release, superior microhardness recovery, antibacterial effect | Performance depends on medium pH, limited long-term data | [2,4,78] |
| Enamel Pro | ACP | High fluoride release, ability to reverse demineralization | Lower long-term release vs. TCP products | [4,22,74,79,80] |
| Embrace Varnish | Xylitol–calcium phosphate | Fluoride release demonstrated | Lowest cumulative release among tested products | [22,78] |
| Bifluorid 10 | NaF + CaF2 | Very high release in first 14 days | Short-term effect, limited long-term data | [1] |
| Omni Vanish XT | NaF (resin-based) | Sustained release beyond 48 h, extended profile | Limited independent studies | [80] |
| Duraflor | NaF | High initial release, widely used | Release stops earlier than Duraphat (~19 weeks) | [81] |
| Other formulations (Mahidol, Varnal, etc.) | TCP/experimental bases | Variable effects, some strong initial release | Limited replication, early-stage data | [76,79] |
| Authors | Study/Samples Design | Fluoride Varnish | Storage Conditions | Measurement Time and Method | Total Fluoride Released | Additional Findings |
|---|---|---|---|---|---|---|
| Barrera-Ortega [3] | In vitro, 120 human third molars | - β-TCP-F (ClinproTM White Varnish, St. Paul, MN, USA) | Demineralizing solution (2.2 mM CaCl2, 2.2 mM NaH2PO4, and 0.05 M CH3COOH) for 96 h at 37 °C, pH 4.4 | 5, 10, 15 days Method: 10 surfaces of each group were randomly chosen and immersed in deionized water at 37 °C Fluoride-Ion Selective Electrode (F-ISE) (Orion Star A-214) | After 15 days CPP-ACP-F showed higher fluoride concentrations (ppm) in remineralizing solutions than β-TCP-F. | β-TCP-F varnish and CPP-ACP-F paste treatments demonstrated the ability to counteract surface modifications on human enamel caused by in vitro demineralization. |
| Singh [74] | In vitro 72 healthy permanent maxillary anterior teeth. | - Fluor Protector (Ivoclar Vivadent, New York, USA) - Enamelast varnish (Ultradent Products, Cologne, Germany) - Enamel Pro varnish (Premier Dental, Pennsylvania, USA) - control group | In artificial saliva with pH 7.2 at room temperature. | 1, 3, and 6 months Metrohm 940 Professional IC Vario | Fluoride release (ppm) Day 1 Group I 0.56 ± 0.09 Group II 1.38 ± 0.21 Group III 3.47 ± 0.19 1 month Group I 0.36 ± 0.06 Group II 1.24 ± 0.13 Group III 1.57 ± 0.12 3 months Group I 0.11 ± 0.02 Group II 0.26 ± 0.06 Group III 0.32 ± 0.08 6 months Group I 0.03 ± 0.01 Group II 0.16 ± 0.06 Group III 0.09 ± 0.03 | Enamel Pro released the maximum amount of fluoride in artificial saliva for up to 3 months. |
| Okuyama [77] | In vitro, 6 disc- shaped specimens (10 × 1 mm) | - PRG Barrier Coat (Shofu, Kyoto, Japan), - Clinpro XT varnish (3M, Minnestota, USA), - Fuji IX EXTRA (GC, Japan), - Clearfil Mega Bond (Kuraray Noritake Dental, Osaka, Japan) | - 8 mL of deionized water - 37 °C - distilled water changed every day for 7 days and weekly up to 28 days | -TISAB III -Fluoride-selective electrode | After 7 days Clinpro XT varnish ~200 μg/cm2, followed by Fuji IX EXTRA, PRG Barrier Coat and Clearfil Mega Bond. Clinpro XT varnish: release increased over time reaching ~380 μg/cm2 | After 7 days no significant difference in fluoride release between the Fuji IX EXTRA and PRG Barrier Coat groups. Clinpro XT varnish released the most fluoride, whereas Clearfil Mega Bond released the least. |
| Pańczyszyn [78] | In vitro, 45 human molars, free from caries, demineralization, and enamel defects | - Duraphat (Colgate Oral Care, Sydney, NSW, Australia), - MI Varnish (GC, Tokyo, Japan), - Embrace Varnish (Pulpdent, Watertown, MA, USA) | - 5 mL of artificial saliva (NaCl, KCl, urea, Na2S·9H2O, NaH2PO4·2H2O, CaCl2·2H2O) - pH = 4; 5; 7 - 37 °C | -measurement after 1, 2, 24, 48 and 168 h -ORION 9609 Model ion selective electrode with the CPI-551 Elmetron microcomputer | (a) Duraphat pH = 4: 9.753 ppm pH = 5: 7.513 ppm pH = 7: 9.276 ppm (b) MI Varnish pH = 4: 11.52 ppm pH = 5: 9.297 ppm pH = 7: 6.470 ppm (c) Embrace Vanish: pH = 4: 6.826 ppm pH = 5: 5.724 ppm pH = 7: 4.821 ppm | MI Varnish demonstrated the highest cumulative fluoride release, independent of the environmental pH; Embrace Varnish exhibited the lowest fluoride release. |
| Yildiz [4] | In vitro, 48 caries- free human molars | -MI Varnish (GC, America, USA) -Clinpro White Varnish (3M ESPE, MN, USA) -Duraphat (Colgate-Palmolive, NSW, Australia) -Fluor Protector (Ivoclar Vivadent, NY, USA) -Enamel Pro (Premier Dental, PA, USA) | - 10 mL of artificial saliva - 37 °C - Sample incubated for 2, 24, 48 h, and 7 days, with daily saliva renewal. After 7 days, they were rinsed, the fluoride varnish was removed and incubated again in fresh saliva for 24 h. | -TISAB III buffer added to the solution -Ion-selective electrode | MI Varnish: -2 h: 6.72 (3.44) ppm -24 h: 22.66 (6.79) ppm -48 h: 0.76 (0.26) ppm -7 days: 0.084 (0.11) ppm Clinpro White Varnish: -2 h: 0.62 (0.27) ppm -24 h: 5.07 (3.87) ppm -48 h: 2.22 (0.85) ppm -7 days: 0.48 (0.16) ppm Duraphat: -2 h: 2.3 ± 0.54 ppm -24 h: 12.81 ± 4.85 ppm -48 h: 2.70 ± 0.90 ppm -7 days: 0.69 ± 0.31 ppm Fluor Protector: -2 h: 0.37 ± 0.096 ppm -24 h: 0.42 ± 0.15 ppm -48 h: 0.05 ± 0.03 ppm -7 days: 0.05 ± 0.03 ppm Enamel Pro Varnish: -2 h: 2.33 ± 0.94 -24 h: 12.30 ± 5.10 -48 h: 5.52 ± 2.64 -7 days: 0.62 ± 0.31 | MI Varnish showed the highest surface microhardness recovery. All varnishes significantly improved enamel microhardness compared to the control. No significant differences were found between varnishes, except MI Varnish performed better than Fluor Protector. |
| Nahum [75] | In vitro, 40 human premolars and molars; rectangular blocks | -Duraphat 2.26% (Colgate Palmolive, New York, USA) -Clinpro White Varnish 2.26% (3M ESPE, Minnesota, USA) -Fluor Protector 0.1% (Ivoclar-Vivadent, Schaan, Liechtenstein) Single application. | In plastic bottles, 5 ml deionized water, 37 °C; On each measurement day, the sample was rinsed with 1 mL of deionized water before being placed in a new container. | -ion selective electrode for sodium fluoride (model 1011, Hanna Instruments, USA) and potentiometer (model HI 3222, Hanna Instruments); measured in: 1, 2, 5, 15 and 30 days. After recharging measured in 24, 48, 72 h | -Duraphat—9.51 ppm -Clinpro White Varnish—10.16 ppm -Fluor Protector—5.01 ppm After recharging: -Duraphat—3.0 ppm -Clinpro White Varnish—3.02 ppm -Fluor Protector—3.0 ppm | DP and CWV released the highest amount of fluoride on day 1 and throughout the study. |
| Asian [76] | In vitro, 44 enamel blocks 5 × 5 mm from human premolars | -Duraphat (Colgate-Palmolive, New York, NY, USA) -ClinproTM White Varnish (3M ESPE, Minnesota, USA) -Fluor Protector (Ivoclar Vivadent, Amherst, New York, USA) -Varnal (Biodinámica, Paraná, Brasil)-control Single application: 30 mg of fluoride varnish; DP and Clinpro-37.5 μmol of fluoride; Fluor Protector 1.58 μmol of fluoride | 20 mL-buffer calcium phosphate solution; pH = 6.0; temperature 5 °C | -ion analyzer (Versa Star A329, Orion, Ther- mo Scientific) and a fluoride selective electrode (Plus Model 9606 VPN, Orion, Thermo Scientific) Measurements taken daily in the first week, once a week for the remaining six weeks. | For 6 weeks: -Duraphat—5.9266 ppm -ClinproTM White Varnish—2.2148 ppm -Fluor Protector—0.407 ppm -Varnal control—0.21196 ppm | Duraphat released the highest amount of fluoride over 6 weeks. The highest viscosity and the lowest wettability- Duraphat The higher the viscosity and lower the wettability, the better the varnish’s ability to release fluoride. |
| Thakur [1] | In vitro, 96 human premolars | -38% Silver Diamine Fluoride (e-SDF, India) -Bifluorid 10 (Voco, Germany) -1.23% Acidulated phosphate fluoride gel Fluocal Gel (Septodont, France) -control sample (no varnish) | In plastic containers, artificial saliva, 500 mL, pH = 7, room temperature, | Ion-sensitive electrode Measurement after: -24 h, -7 days -14 days | For 14 days: -38% Silver Diamine Fluoride: 9.06 ppm -Bifluorid 10: 19.5 ppm -1.23% Acidulated phosphate fluoride gel Fluocal Gel: 3.42 ppm -control sample (no varnish): 0.64 ppm | Varnish Bifluorid released highest amount of fluoride for 14 days. |
| Sidhu [22] | In vitro 75 extracted, caries-free premolar human teeth | Type: -MI Varnish (CPP-ACP) (GC, Tokyo, Japan) -Clinpro White Varnish (f-TCP) (3M ESPE, Minnesota, USA) -Embrace Varnish (Xylitol-coated calcium and phosphate) (Pulpdent, Watertown, MA, USA) -Enamel Pro Varnish (ACP) (Premier Dental, Pennsylvania, USA) Fluoride concentration: 5% NaF Application protocol: Applied varnish to a 3 × 3 mm window on the tooth surface; immersed in 50 mL artificial saliva. | - 50 mL of artificial saliva in plastic containers; replaced at specific intervals. | Time points: -1 day, -1 month, -3 months, -6 months. SPADNS method with a spectrophotometer (570 nm). | MI Varnish 1 Day: 1.52 mgF−/L 1 Month: 6.45 mgF−/L 3 Months: 6.95 mgF−/L 6 Months: 3.50 mgF−/L Total: 18.42 mgF−/L Clinpro White Varnish 1 Day: 1.76 mgF−/L 1 Month: 7.60 mgF−/L 3 Months: 8.05 mgF−/L 6 Months: 2.85 mgF−/L Total: 20.26 mgF−/L Embrace Varnish 1 Day: 1.52 mgF−/L 1 Month: 6.71 mgF−/L 3 Months: 6.55 mgF−/L 6 Months: 1.95 mgF−/L Total: 16.73 mgF−/L Enamel Pro Varnish 1 Day: 1.35 mgF−/L 1 Month: 7.20 mgF−/L 3 Months: 7.30 mgF−/L 6 Months: 2.95 mgF−/L Total: 18.50 mgF−/L | Clinpro White Varnish: highest cumulative fluoride release over 6 months (20.26 mgF−/L). MI Varnis: highest substantivity, released the most fluoride by 6 months (3.5 mgF−/L at 6-month mark). Embrace Varnish: lowest cumulative release and fastest depletion. |
| Attiguppe [2] | In vitro 24 extracted human premolar teeth | -MI Varnish: 5% NaF + CPP–ACP (GC, Tokyo, Japan) -Fluor Protector (Ivoclar Vivadent, Amherst, New York, USA) Application: varnish and fluor protector varnish was applied on 5 mm × 1 mm surface | - 30 mL of artificial saliva at 37 °C; - the saliva was replaced at each time point | Fluoride ion-selective electrode measurements: -after 30 min, -daily for the first 7 days -weekly up to 1 month | Cumulative fluoride release: -MI varnish: 4.19 ± 0.41 ppm -Fluor Protector: 3.2 ± 0.19 ppm | MI varnish released more fluoride than Fluor Protector. MI varnish resulted in lower lesion depth (79.78 μm vs. 119.2 μm), showing better demineralization resistance. MI varnish showed a larger inhibition zone against Streptococcus mutans (24.75 mm vs. 15.25 mm). |
| Virupaxi [29] | In vitro, 24 extracted human primary anterior teeth | - Clinpro XT Varnish (3M ESPE, Minnesota, USA); - Fluoritop SR (ICPA, Mumbai, India); - Fluor Protector (Ivoclar Vivadent, Amherst, New York, USA) Application protocol: Teeth coated with varnishes 3 × 3 mm window. Fluorprotector and Fluoritop SR applied using the supplied brush, while Clinpro XT was mixed as directed and light-cured for 20 s. | Artificial saliva (pH 7.2) at room temperature; medium renewed at: 1 day, 1 month, 3 months, and 6 months | Ion-selective electrode (ISE) with TISAB III buffer Fluoride concentration measured at 1 week, 1 month, 3 months, and 6 months | Clinpro XT Varnish 9.78 ± 4.11 ppm Fluoritop SR 0.61 ± 0.36 ppm Fluorprotector 0.17 ± 0.02 ppm | Clinpro XT demonstrated the most stable and sustained fluoride release compared to Fluoritop SR and Fluorprotector; favourable profile for long-term remineralization due to glass ionomer base. |
| Rirattanapong [79] | In vitro, 25 extracted sound human primary incisors | - Duraphat: (5%NaF) (Colgate Oral Pharmaceuticals New York, NY, USA) - Clinpro White: (5%NaF) + (TCP) (Premier Dental, Hannover, Germany) - Enamel Pro: (5%NaF) + (ACP) (3M ESPE, West Palm Beach, FL, USA) - Mahidol varnish: (5%NaF) + (TCP) (Mahidol University, Thailand) Application: Approximately 30 mg of the assigned fluoride varnish was applied to each prepared tooth (5 × 5 mm window). | - 60 mL of artificial saliva at room temperature; - maintained on a laboratory shaker to simulate oral conditions | Fluoride ion-selective electrode (Orion 96-09) with TISAB III Fluoride release assessed at 2, 4, 8, 12, 24, and 48 h, then weekly for 3 months | Duraphat: 11.42 ± 0.67 Clinpro White: 11.19 ± 0.38 Enamel Pro: 3.72 ± 0.27 Mahidol varnish: 8.36 ± 0.41 | All varnishes released significantly more fluoride than control. Mahidol varnish had the highest release in the first 24 h (0.87 ppm). After 3 months, fluoride release order: Duraphat = Clinpro White > Mahidol > Enamel Pro > Control. Duraphat showed lower initial release but more sustained levels over time. |
| Ritwik [80] | In vitro study, 50 extracted permanent human teeth divided into 5 groups (n = 10). Varnish application (5 × 5 mm window). | Single application, all containing 5% NaF (22,600 ppm): - Enamel Pro Varnish (EP), -Colgate PreviDent (CP), -Omni Vanish (OV), -Omni Vanish XT (OVXT). | Medium: 3 mL artificial saliva, pH 7.2, room temperature | Time points: 1, 2, 4, 8, 12, 24, and 48 h Measurements with ion selective electrode with TISAB III. Duration: 48 h | At 1 h: -Premier Enamel Pro: 1730.2 ppm (highest initial release) -Omni Vanish XT: 487.1 ppm -Colgate PreviDent: 163.5 ppm -Omni Vanish: 45.8 ppm Mean hourly release rate: -Premier Enamel Pro: 358.467 ppm/hour (±124.712) -Omni Vanish XT: 188.676 ppm/hour (±106.484) -Colgate PreviDent: 52.244 ppm/hour (±10.081) -Omni Vanish: 18.470 ppm/hour (±5.959) | EP had highest fluoride release in first 8 h. OVXT had highest sustained release after initial 4 h period. All varnishes showed significantly different fluoride release profiles (p < 0.0001). |
| Castillo [30] | In vitro study, 14 primary molar slabs (5 × 5 mm) from exfoliated teeth collected in nonfluorinated water communities (Lima, Peru), divided into three groups: 5 samples single application, 5 samples three applications, 4 unpainted controls. Slabs painted with 30 mg fluoride varnish. | Duraphat (Colgate-Palmolive, New York, USA)) 5% NaF (2.26% fluoride, equivalent to 35.7 micromoles fluoride per 30 mg application). 1: one application at baseline 3: at baseline, day 2, day 4 in one week | Medium: 20 mL buffered calcium phosphate solution (1.5 mM calcium nitrate, 1.0 mM sodium phosphate monobasic, 0.35 mM MES buffer), pH 6.0, room temperature | Duration: 6 months (21 weeks—stopped due to fungal growth). Weekly measurements with ion selective electrode with TISAB solution (low-level TISAB and TISAB III) | Total release (21 weeks): -single application: 23.7 ± 1.6 μmol (64.9% of applied fluoride released) -three applications: 34.9 ± 0.3 μmol (31.9% of applied fluoride released) | Three-application protocol showed 47% higher total fluoride release compared to single one. Three-application samples released more fluoride in weeks 8–21 and had slower release rate indicating longer availability of fluoride. |
| Castillo [81] | In vitro study, 23 primary molar slabs (5 × 5 mm) from exfoliated teeth collected in nonfluorinated water communities (Lima, Peru), divided into three groups: 9 samples Duraphat, 9 samples Duraflor, 5 untreated controls. Slabs painted with 30 mg fluoride varnish | -Duraphat 5% NaF (Colgate-Palmolive Co., New York, USA) -Duraflor 5% NaF (Pharmascience Inc., Montreal, Canada)) Protocol: single application with varnish from 9 different tubes of each product to assess inter-tube variability. | Medium: 20 mL buffered calcium phosphate solution, pH 6.0, room temperature. | Duration: 6 months (24 weeks). Weekly measurements with ion selective electrode with TISAB | Total Release over 24 weeks: -Duraphat: 25.1 ± 4.9 μmol (67% of applied fluoride released) -Duraflor: 20.2 ± 14.7 μmol (56% of applied fluoride released) | Initial 3 weeks: Duraflor had higher release rate but from week 4 onwards Duraphat had higher release rate. Weeks 4–24: no difference in release rates between products (p < 0.18) Physical prosperities: -Duraphat: more viscous, dries faster -Duraflor: less viscous Duraphat released fluoride until week 28 while Duraflor until week 19. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyński, M.; Kotela, A.; Klimas, S.; Majchrzak, Z.; Kensy, J.; Laszczyńska, M.; Michalak, M.; Rybak, Z.; Fast, M.; Matys, J. Evaluation of Factors Affecting Fluoride Release from Fluoride Varnishes: A Systematic Review. Materials 2025, 18, 4603. https://doi.org/10.3390/ma18194603
Dobrzyński M, Kotela A, Klimas S, Majchrzak Z, Kensy J, Laszczyńska M, Michalak M, Rybak Z, Fast M, Matys J. Evaluation of Factors Affecting Fluoride Release from Fluoride Varnishes: A Systematic Review. Materials. 2025; 18(19):4603. https://doi.org/10.3390/ma18194603
Chicago/Turabian StyleDobrzyński, Maciej, Agnieszka Kotela, Sylwia Klimas, Zuzanna Majchrzak, Julia Kensy, Marzena Laszczyńska, Mateusz Michalak, Zbigniew Rybak, Magdalena Fast, and Jacek Matys. 2025. "Evaluation of Factors Affecting Fluoride Release from Fluoride Varnishes: A Systematic Review" Materials 18, no. 19: 4603. https://doi.org/10.3390/ma18194603
APA StyleDobrzyński, M., Kotela, A., Klimas, S., Majchrzak, Z., Kensy, J., Laszczyńska, M., Michalak, M., Rybak, Z., Fast, M., & Matys, J. (2025). Evaluation of Factors Affecting Fluoride Release from Fluoride Varnishes: A Systematic Review. Materials, 18(19), 4603. https://doi.org/10.3390/ma18194603












