Leakage-Proof and High-Conductivity Composite Phase Change Material Using Low-Melting-Point-Alloy-Encapsulated Copper Foam/Paraffin for Superior Thermal Homogeneity in Lithium-Ion Battery Modules
Highlights
- A novel LMA-encapsulated CPCM was developed using a PA/CF matrix, solving leakage issues effectively.
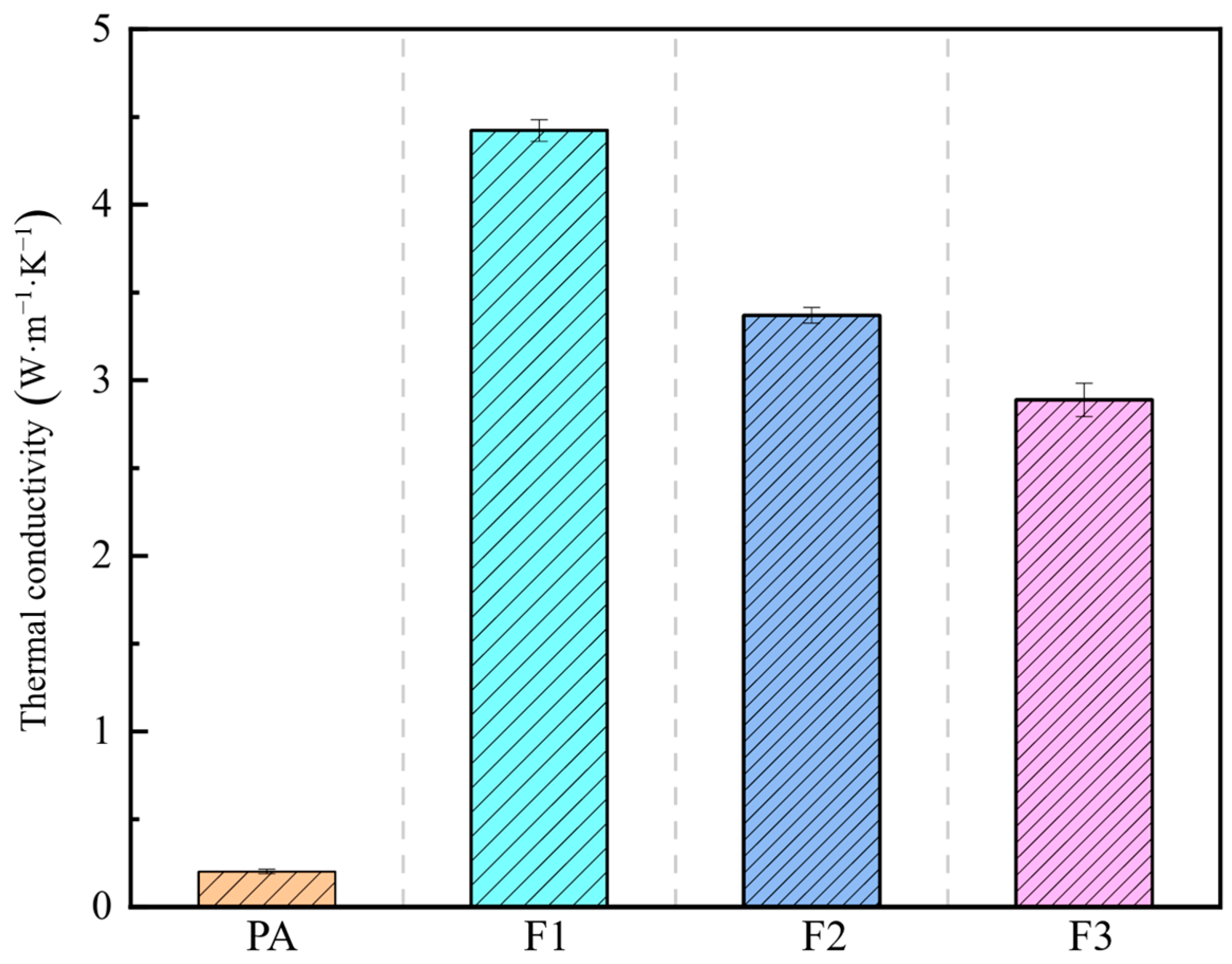
- Thermal conductivity reached 4.42 W·m−1·K−1 with 10 PPI copper foam, a significant enhancement.
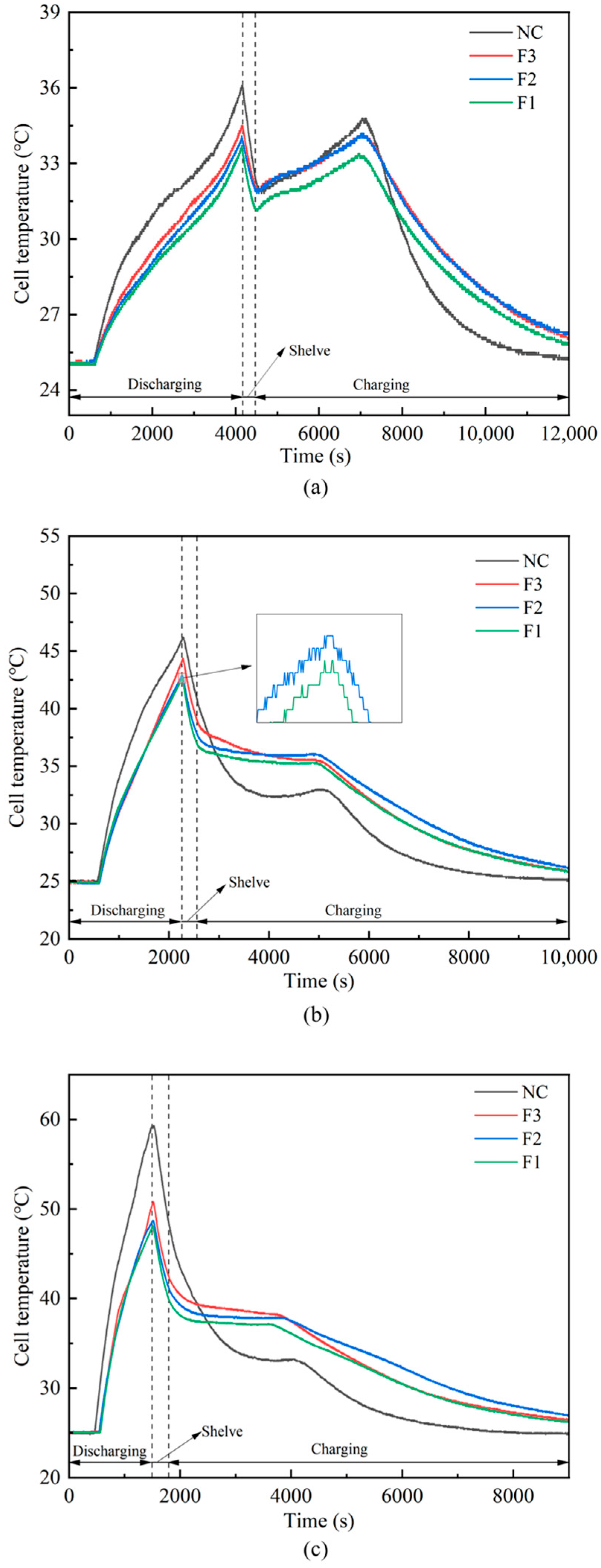
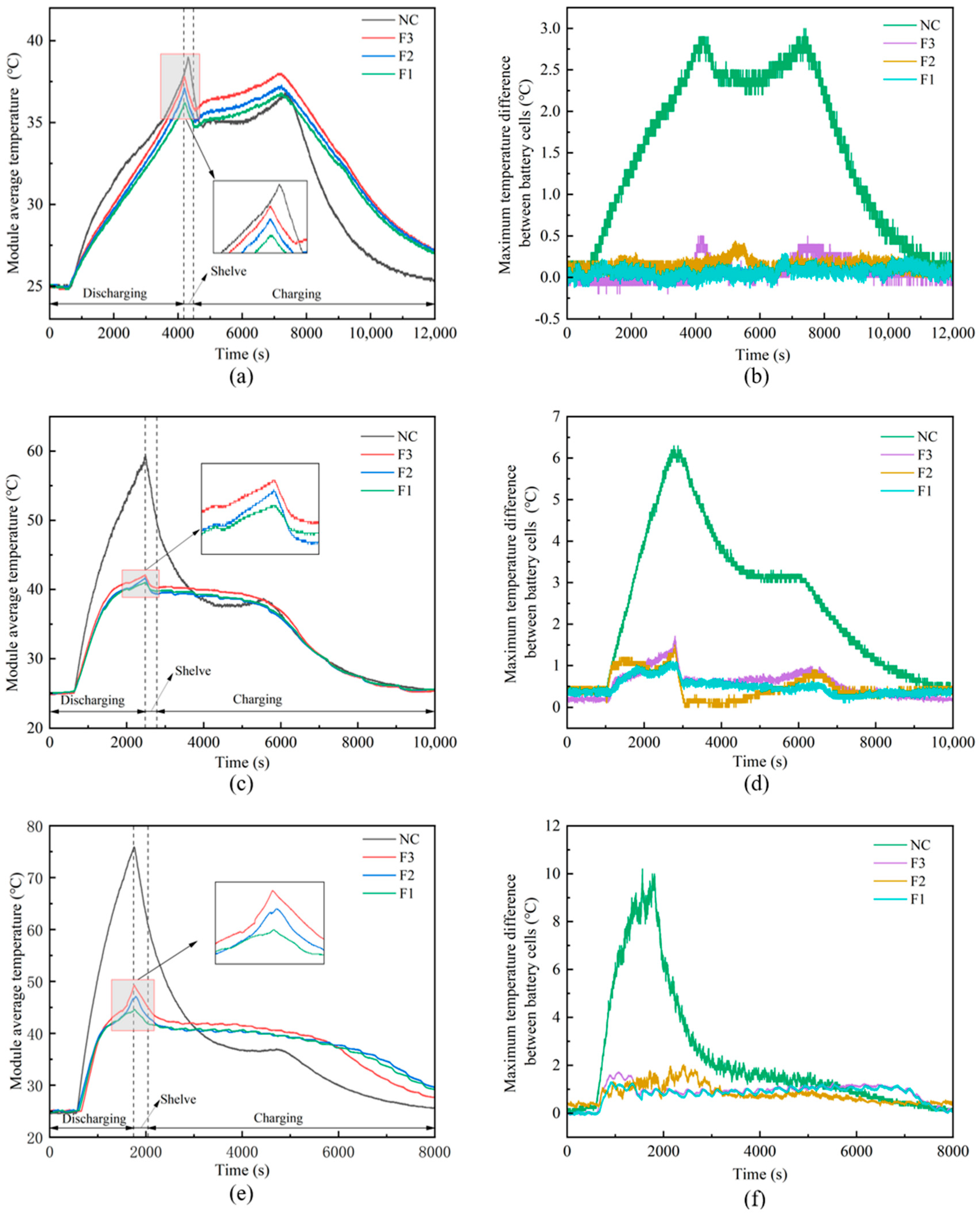
- Maximum battery temperature was reduced by 31.3 °C (from 75.9 °C to 44.6 °C) at a 3C discharge rate.
- The maximum temperature difference between cells was drastically reduced to a safe 1.2 °C, ensuring uniformity.
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation Method
2.3. Material Characterization
2.3.1. Structure and Impregnation Effect
2.3.2. Thermophysical Properties
2.4. Experimental Setup
3. Results and Discussion
3.1. Performance Characterization of CPCM
3.1.1. Structural Analysis of Copper Foam and Paraffin/Copper Foam Composite Materials
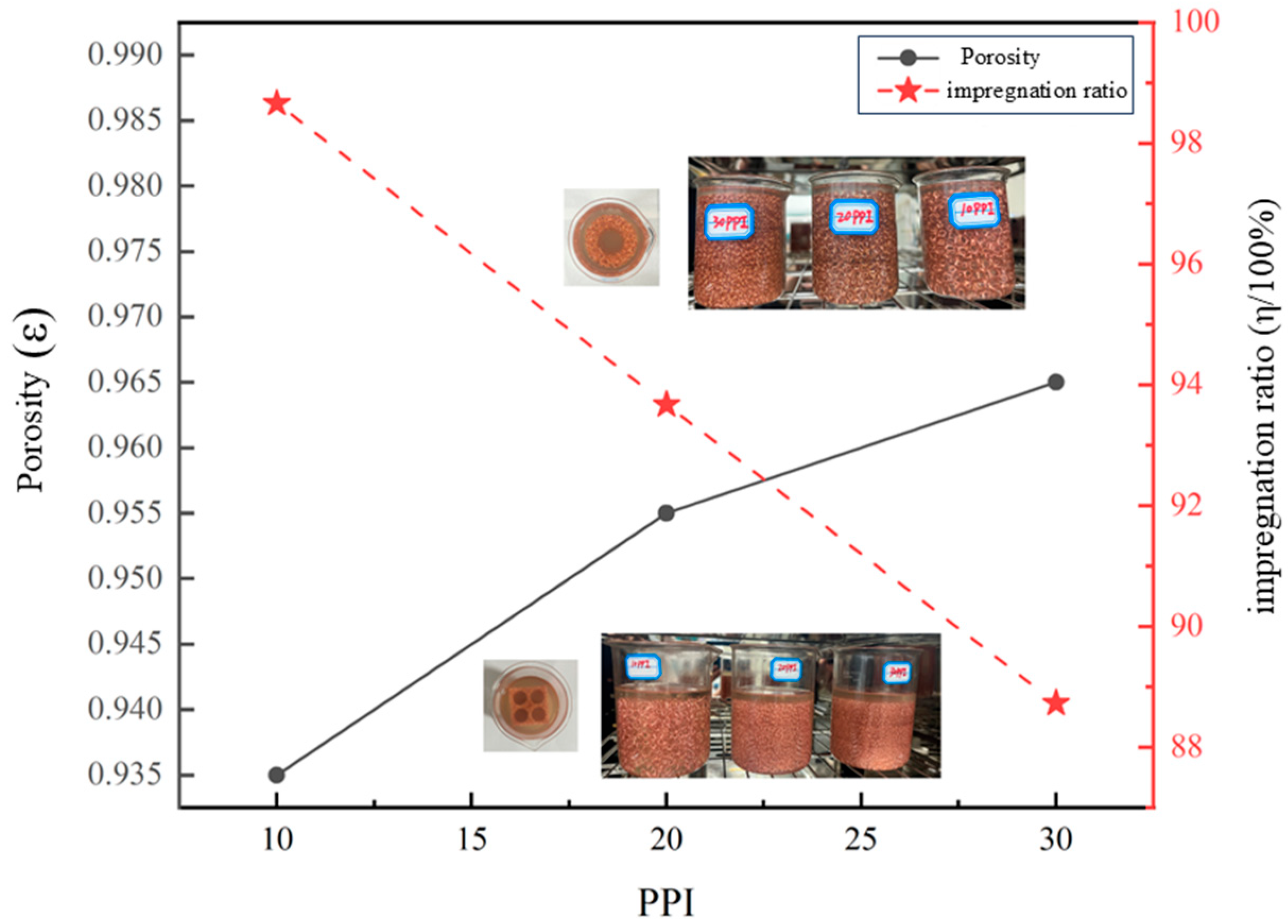
3.1.2. Analysis of Impregnation Effect
3.1.3. Thermal Conductivity
3.1.4. Infrared Thermographic Analysis
3.1.5. Anti-Leakage Performance
3.2. Thermal Management Performance and Analysis of CPCM
3.2.1. Single Cell
3.2.2. Battery Module
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Weng, J.; Jossen, A.; Stefanopoulou, A.; Li, J.; Feng, X.; Offer, G. Fast-charging lithium-ion batteries require a systems engineering approach. Nat. Energy 2025. [Google Scholar] [CrossRef]
- Jiangyi, H.; Fan, W. Design and testing of a small orchard tractor driven by a power battery. Eng. Agric. 2023, 43, e20220195. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, L.; Chen, L.; Zou, R.; Cai, Y. Fuzzy adaptive energy management strategy for a hybrid agricultural tractor equipped with HMCVT. Agriculture 2022, 12, 1986. [Google Scholar] [CrossRef]
- Mao, N.; Gadkari, S.; Wang, Z.; Zhang, T.; He, T.; Cai, Q. A systematic investigation of thermal runaway characteristics, inflame evolution and post-mortem analysis caused by overcharging for lithium-ion batteries with different cathode materials. J. Energy Storage 2023, 74, 109421. [Google Scholar] [CrossRef]
- Thakur, A.K.; Sathyamurthy, R.; Velraj, R.; Saidur, R.; Pandey, A.K.; Ma, Z.; Singh, P.; Hazra, S.K.; Sharshir, S.W.; Prabakaran, R.; et al. A state-of-the art review on advancing battery thermal management systems for fast-charging. Appl. Therm. Eng. 2023, 226, 120303. [Google Scholar] [CrossRef]
- Sun, T.; Yan, Y.; Wang, X.; Rasool, G.; Zhang, K.; Li, T. A comprehensive study on heat transfer mechanism and thermal runaway suppression of the lithium-ion battery. Int. J. Heat Mass Transf. 2025, 245, 127027. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.; Wu, B.; Song, M.; Wu, X. An air-cooled system with a control strategy for efficient battery thermal management. Appl. Therm. Eng. 2024, 236, 121578. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Li, M.; Ren, F.; Feng, Y. A manifold channel liquid cooling system with low-cost and high temperature uniformity for lithium-ion battery pack thermal management. Therm. Sci. Eng. Prog. 2023, 41, 101857. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, R.; Wang, S.; Huang, D. Heat transfer characteristics and influencing factors of immersion coupled direct cooling for battery thermal management. J. Energy Storage 2023, 62, 106821. [Google Scholar] [CrossRef]
- Sinha, R.K.; Kumar, A.; Maurya, A.; Kumar, A. Thermal management of counter terminal lithium-ion battery using Bio-based PCM. Therm. Sci. Eng. Prog. 2024, 54, 102854. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, H.; Kou, Z.; Sun, Y.; Zhao, Y.; Zhao, L.; Chen, M. Aloe vera-inspired boron nitride/expanded graphite/melamine phosphate polymer composites for battery temperature management. Energy 2025, 336, 138447. [Google Scholar] [CrossRef]
- Vikram, S.; Vashisht, S.; Rakshit, D.; Wan, M.P. Recent advancements and performance implications of hybrid battery thermal management systems for Electric Vehicles. J. Energy Storage 2024, 90, 111814. [Google Scholar] [CrossRef]
- Guan, J.; Chen, M. An overview of phase change materials on battery application: Modification methods and thermal management systems. J. Energy Storage 2024, 103, 114268. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; He, T.; Mao, N. A numerical study on a hybrid battery thermal management system based on PCM and wavy microchannel liquid cooling. Renew. Energy 2024, 235, 121273. [Google Scholar] [CrossRef]
- Jang, D.S.; Yun, S.; Hong, S.H.; Cho, W.; Kim, Y. Performance characteristics of a novel heat pipe-assisted liquid cooling system for the thermal management of lithium-ion batteries. Energy Convers. Manag. 2022, 251, 115001. [Google Scholar] [CrossRef]
- Nasiri, M.; Hadim, H. Thermal management of Li-ion batteries using phase change materials: Recent advances and future challenges. J. Energy Storage 2025, 111, 115440. [Google Scholar] [CrossRef]
- Ni, R.; Zhang, D.; Wang, R.; Xie, Z.; Wang, Y. Prevention and suppression effects of phase change material on thermal runaway in batteries. Case Stud. Therm. Eng. 2023, 48, 103160. [Google Scholar] [CrossRef]
- Lamrani, B.; Lebrouhi, B.E.; Khattari, Y.; Kousksou, T. A simplified thermal model for a lithium-ion battery pack with phase change material thermal management system. J. Energy Storage 2021, 44, 103377. [Google Scholar] [CrossRef]
- Yan, J.; Li, K.; Chen, H.; Wang, Q.; Sun, J. Experimental study on the application of phase change material in the dynamic cycling of battery pack system. Energy Convers. Manag. 2016, 128, 12–19. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Fang, X.; Zhang, Z.; Xu, T.; Gao, X.; Wang, S. Experimental and numerical investigation of the application of phase change materials in a simulative power batteries thermal management system. Appl. Energy 2014, 121, 104–113. [Google Scholar] [CrossRef]
- Patel, J.R.; Rathod, M.K. Phase change material selection using simulation-oriented optimization to improve the thermal performance of lithium-ion battery. J. Energy Storage 2022, 49, 103974. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Ruprecht, J.; Liew, W.Y.; Jiang, Z.-T. A Binary Salt Mixture LiCl–LiOH for Thermal Energy Storage. Materials 2023, 16, 1434. [Google Scholar] [CrossRef]
- Pilali, E.; Soltani, M.; Hatefi, M.; Shafiei, S.; Salimi, M.; Amidpour, M. Passive thermal management systems with phase change material-based methods for lithium-ion batteries: A state-of-the-art review. J. Power Sources 2025, 632, 236345. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Wang, Z.; Cheng, X. A review of power battery cooling technologies. Renew. Sustain. Energy Rev. 2025, 213, 115494. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.C.; Du, X.; Zhang, Z.Y.; Wang, H. NH4Al(SO4)2.12H2O-Na2SO4 eutectic-based phase change materials with excellent light absorbance, enhanced thermal conductivity, and high latent heat performance. J. Energy Storage 2024, 99, 113349. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Y.; Ouyang, D.; Weng, J.; Zhao, L.; Wang, J.; Chen, Y. Research progress of enhancing battery safety with phase change materials. Renew. Sustain. Energy Rev. 2024, 189, 113921. [Google Scholar] [CrossRef]
- Iasiello, M.; Mameli, M.; Filippeschi, S.; Bianco, N. Metal foam/PCM melting evolution analysis: Orientation and morphology effects. Appl. Therm. Eng. 2021, 187, 116572. [Google Scholar] [CrossRef]
- Singh, D.; Kim, T.; Zhao, W.; Yu, W.; France, D.M. Development of graphite foam infiltrated with MgCl2 for a latent heat based thermal energy storage (LHTES) system. Renew. Energy 2016, 94, 660–667. [Google Scholar] [CrossRef]
- Chen, M.; Gong, Y.; Zhao, L.; Chen, Y. Phase change material with outstanding thermal stability and mechanical strength for battery thermal management. J. Energy Storage 2024, 104, 114565. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Zhou, D.; Tian, Y. Experimental study on thermal conductivity of paraffin-based shape-stabilized phase change material with hybrid carbon nano-additives. Renew. Energy 2020, 146, 2637–2645. [Google Scholar] [CrossRef]
- Zhu, Q.; Ong, P.J.; Goh, S.H.A.; Yeo, R.J.; Wang, S.; Liu, Z.; Loh, X.J. Recent advances in graphene-based phase change composites for thermal energy storage and management. Nano Mater. Sci. 2024, 6, 115–138. [Google Scholar] [CrossRef]
- Lv, Y.; Situ, W.; Yang, X.; Zhang, G.; Wang, Z. A novel nanosilica-enhanced phase change material with anti-leakage and anti-volume-changes properties for battery thermal management. Energy Convers. Manag. 2018, 163, 250–259. [Google Scholar] [CrossRef]
- Ge, H.; Li, H.; Mei, S.; Liu, J. Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area. Renew. Sustain. Energy Rev. 2013, 21, 331–346. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M.; Radhika, N. Thermal characteristics enhancement of Paraffin Wax Phase Change Material (PCM) for thermal storage applications. Renew. Energy 2024, 222, 119986. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Effective thermal conductivity of open-cell metal foams impregnated with pure paraffin for latent heat storage. Int. J. Therm. Sci. 2014, 81, 94–105. [Google Scholar] [CrossRef]
- Huang, X.; Lin, Y.; Alva, G.; Fang, G. Thermal properties and thermal conductivity enhancement of composite phase change materials using myristyl alcohol/metal foam for solar thermal storage. Sol. Energy Mater. Sol. Cells 2017, 170, 68–76. [Google Scholar] [CrossRef]
- Jin, H.-Q.; Fan, L.-W.; Liu, M.-J.; Zhu, Z.-Q.; Yu, Z.-T. A pore-scale visualized study of melting heat transfer of a paraffin wax saturated in a copper foam: Effects of the pore size. Int. J. Heat Mass Transf. 2017, 112, 39–44. [Google Scholar] [CrossRef]
- Liu, H.; Ahmad, S.; Shi, Y.; Zhao, J. A parametric study of a hybrid battery thermal management system that couples PCM/copper foam composite with helical liquid channel cooling. Energy 2021, 231, 120869. [Google Scholar] [CrossRef]
- Hussain, A.; Shahid, H.; Ali, I.; Ali, H.M. Passive thermal management system of lithium-ion batteries employing metal foam/pcm composite for the development of electric vehicles. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 505–522. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Xu, C.; Wu, W.; Zhang, W.; Hou, J.; Rui, X.; Chen, Y.; Fan, L.; Feng, X.; et al. Multi-objective optimization of side plates in a large format battery module to mitigate thermal runaway propagation. Int. J. Heat Mass Transf. 2022, 186, 122395. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Wang, Z.; Zhang, Y.; Wang, H.; Zou, D. Micro-encapsulation of a low-melting-point alloy phase change material and its application in electronic thermal management. J. Clean. Prod. 2023, 417, 138058. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Liu, X. Experimental investigation on the thermal management performance of heat sink using low melting point alloy as phase change material. Renew. Energy 2020, 146, 1578–1587. [Google Scholar] [CrossRef]
- Tianrui, H.; Yuming, X.; Wenyuan, Z.; Zhaolong, H. Analysis of copper foam/low melting point alloy composite phase change material. Appl. Therm. Eng. 2022, 204, 117934. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhan, W.; Chen, Y.; Chen, M. Flame retardant composite phase change materials with MXene for lithium-ion battery thermal management systems. J. Energy Storage 2024, 86, 111293. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, D.; Yang, W.; Li, C.; Liu, H.; Qiang, W.; Yang, X.; Zhao, G.; Bi, C.; Wang, T.; et al. Phosphorus-nitrogen based flame retardant polyurethane composite phase change materials for battery thermal safety system. Appl. Therm. Eng. 2025, 258, 124763. [Google Scholar] [CrossRef]
- Zhi, M.; Fan, R.; Zheng, L.; Yue, S.; Pan, Z.; Sun, Q.; Liu, Q. Experimental investigation on hydrated salt phase change material for lithium-ion battery thermal management and thermal runaway mitigation. Energy 2024, 307, 132685. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Gong, Y.; Chen, M. A Novel Paraffin Wax/Expanded Graphite/Bacterial Cellulose Powder Phase Change Materials for the Dependable Battery Safety Management. Batteries 2024, 10, 363. [Google Scholar] [CrossRef]
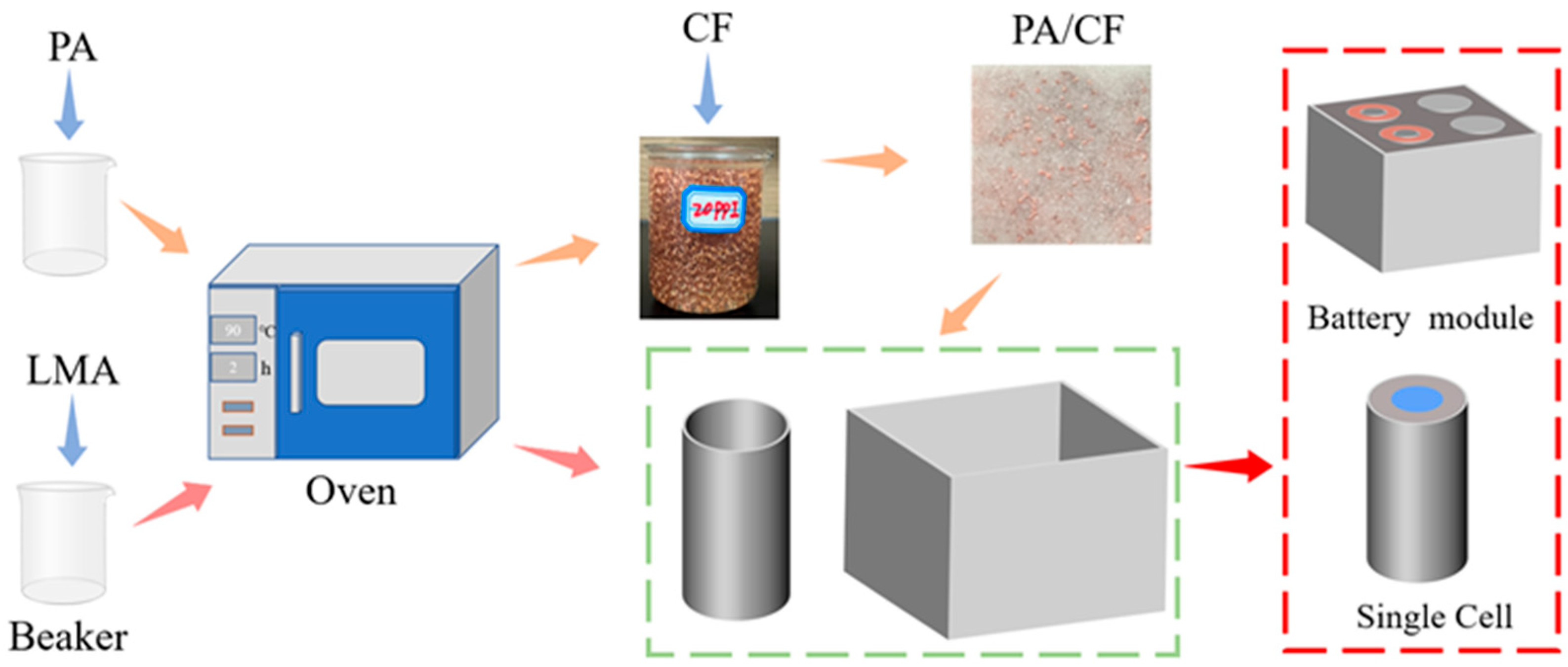
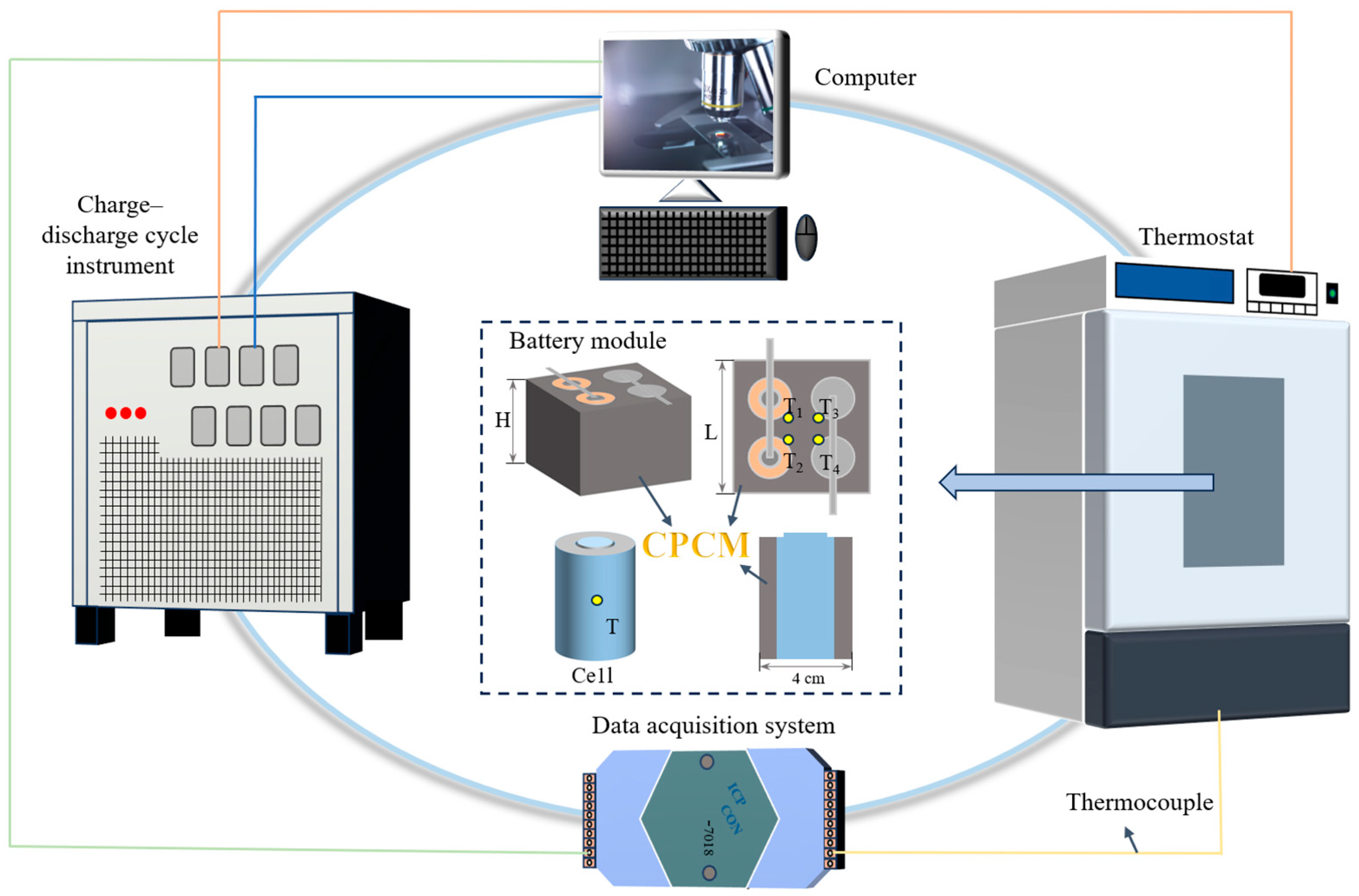
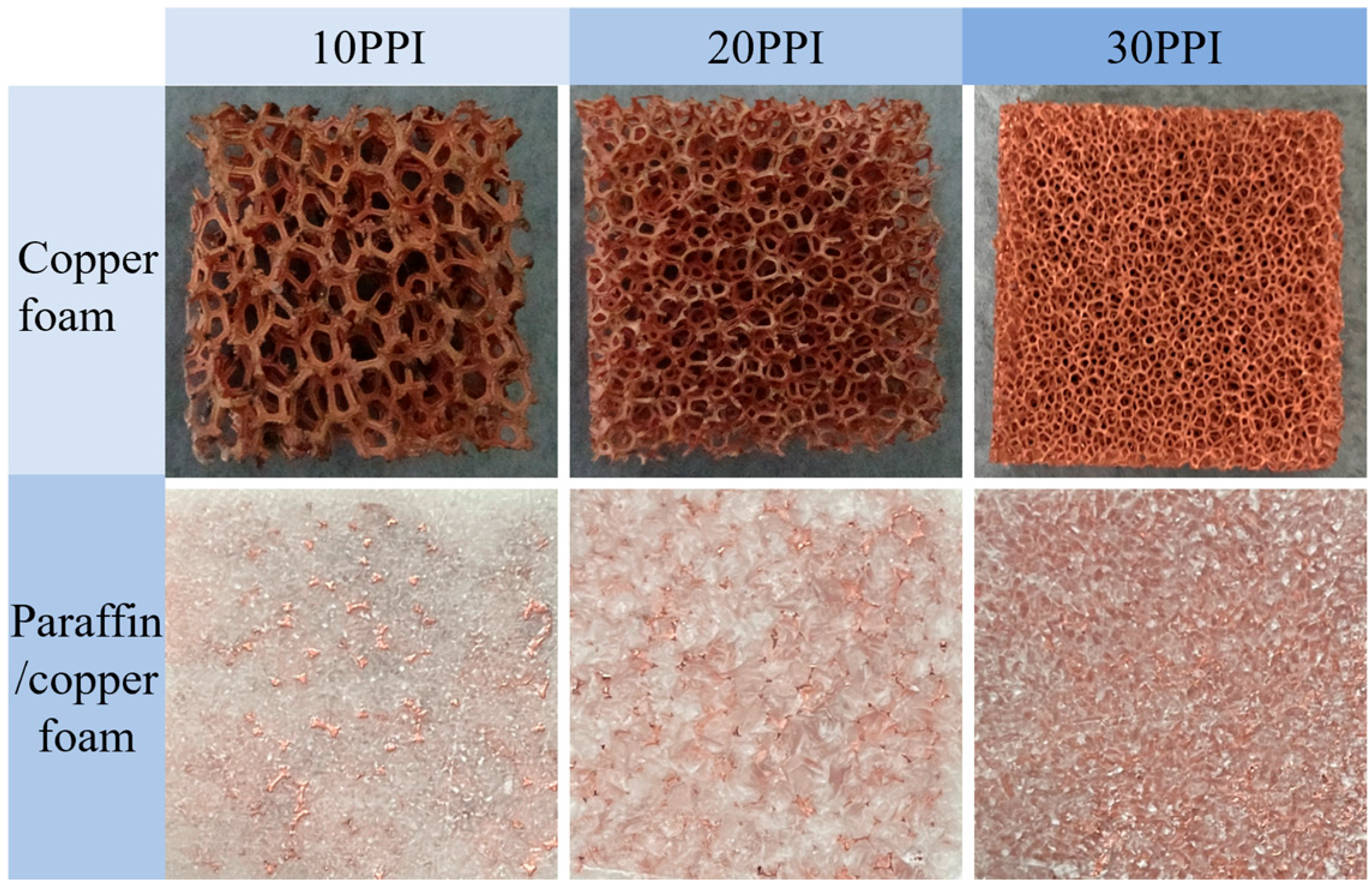


| Step | Condition (Cell) | Condition (Battery Module) |
|---|---|---|
| Rest | 10 min | 10 min |
| Constant Current (CC) Discharge | Cut-off: 2.75 V Current: 3.2 A (1C), 6.4 A (2C), 9.6 A (3C) | Cut-off: 5.0 V Current: 6.4 A (1C), 12.8 A (2C), 19.2 A (3C) |
| Rest | 5 min | 5 min |
| Charge (CC-CV) | 4.2 V, 3.2 A | 8.4 V, 6.4 A |
| Total Cycles | 2 | 2 |
| Copper Foam Pore Size | mbefore (g) | mafter (g) | (g/cm3) | ε | mtheoretical (g) | mactual (g) | η (%) |
|---|---|---|---|---|---|---|---|
| 10 PPI | 5.873 | 24.322 | 0.367 | 0.935 | 18.7 | 18.449 | 98.658 |
| 20 PPI | 4.674 | 21.493 | 0.229 | 0.955 | 19.1 | 17.891 | 93.670 |
| 30 PPI | 3.446 | 20.571 | 0.215 | 0.965 | 19.3 | 17.125 | 88.731 |
| CPCM System | Tmax (°C) | ΔTmax (°C) | Discharge Rate | Reference |
|---|---|---|---|---|
| polyethylene glycol, expanded graphite/ammonium polyphosphate (APP)/MXene/Zinc hydroxy stannate (ZHS) | 57.03 | 5 | 3C | [46] |
| Polyethylene glycol (PEG)/EG/Diphenylmethane diisocyanate (MDI)/Melamine (MA)/9,10-dihydro-9-oxa-10-phospha-phenanthrene-10-oxide (DOPO) (PMDM) | 55 | 5.5 | 2C | [47] |
| polyethylene glycol (PEG)/hexamethylene diisocyanate (HDI)/EG/hexagonal boron nitride (H-BN)/carbon nanotubes (CNTs)/silicon carbide (SiC) | 50.2 | 3 | 3C | [30] |
| KAl(SO4)2·12H2O hydrated salts/Na2SO4·10H2O/EG/open-cell polyurethane foam | 52.47 | 2.2 | 1.5C | [48] |
| PA/EG/bacterial cellulose (BC) | 47 | 4 | 3C | [49] |
| PA/CF/LMA (This work) | 44.6 | 1.2 | 3C | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhao, J.; Ouyang, D.; Chen, M. Leakage-Proof and High-Conductivity Composite Phase Change Material Using Low-Melting-Point-Alloy-Encapsulated Copper Foam/Paraffin for Superior Thermal Homogeneity in Lithium-Ion Battery Modules. Materials 2025, 18, 4604. https://doi.org/10.3390/ma18194604
He S, Zhao J, Ouyang D, Chen M. Leakage-Proof and High-Conductivity Composite Phase Change Material Using Low-Melting-Point-Alloy-Encapsulated Copper Foam/Paraffin for Superior Thermal Homogeneity in Lithium-Ion Battery Modules. Materials. 2025; 18(19):4604. https://doi.org/10.3390/ma18194604
Chicago/Turabian StyleHe, Shengzhi, Jiajun Zhao, Dongxu Ouyang, and Mingyi Chen. 2025. "Leakage-Proof and High-Conductivity Composite Phase Change Material Using Low-Melting-Point-Alloy-Encapsulated Copper Foam/Paraffin for Superior Thermal Homogeneity in Lithium-Ion Battery Modules" Materials 18, no. 19: 4604. https://doi.org/10.3390/ma18194604
APA StyleHe, S., Zhao, J., Ouyang, D., & Chen, M. (2025). Leakage-Proof and High-Conductivity Composite Phase Change Material Using Low-Melting-Point-Alloy-Encapsulated Copper Foam/Paraffin for Superior Thermal Homogeneity in Lithium-Ion Battery Modules. Materials, 18(19), 4604. https://doi.org/10.3390/ma18194604







