Tunable Bandgap in Cobalt-Doped FeS2 Thin Films for Enhanced Solar Cell Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Characterization
3. Results and Discussion
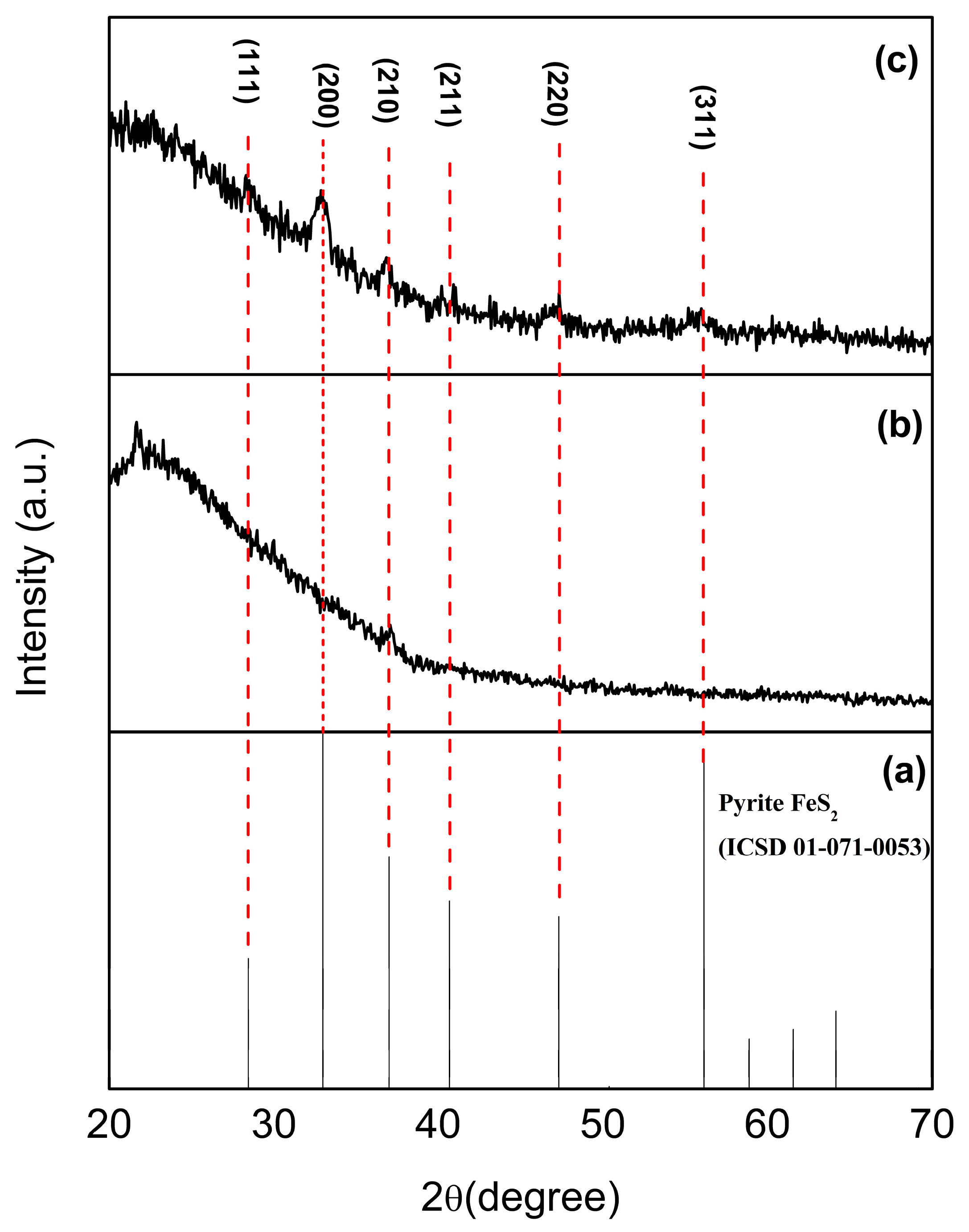
3.1. Structural Characterization by XRD Measurements
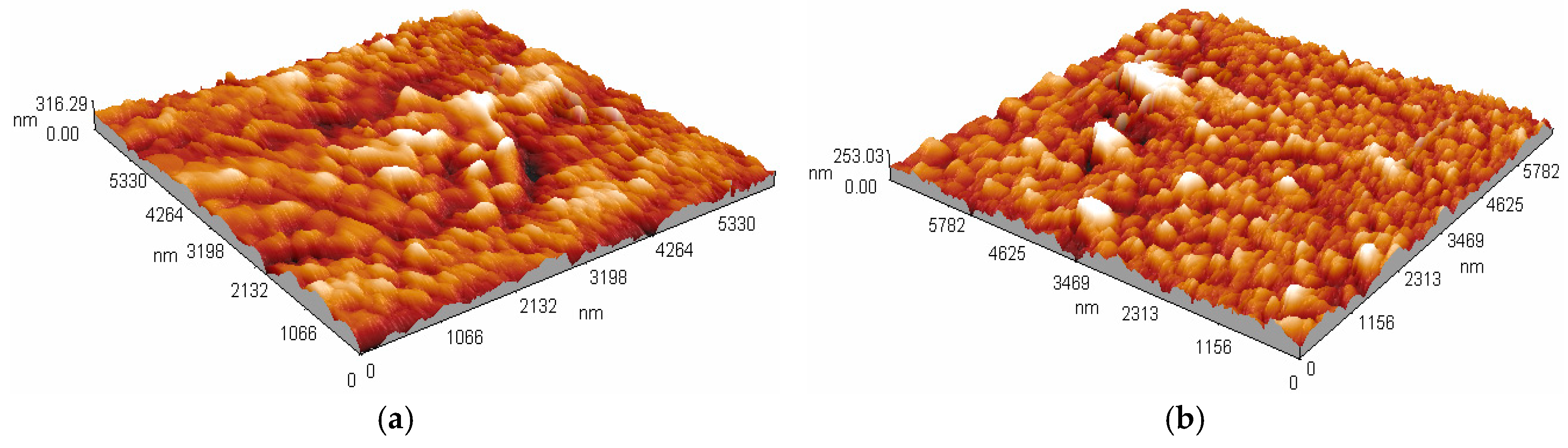
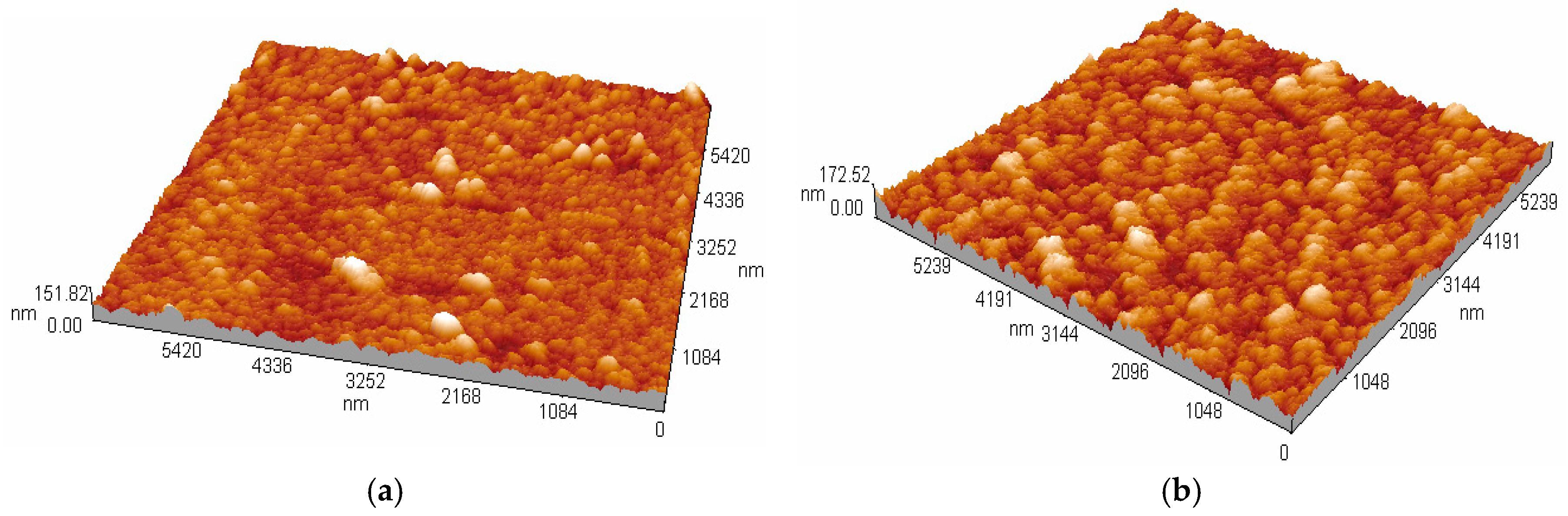
3.2. Thickness and Surface Analysis
3.3. Morphology and Surface Composition Analysis
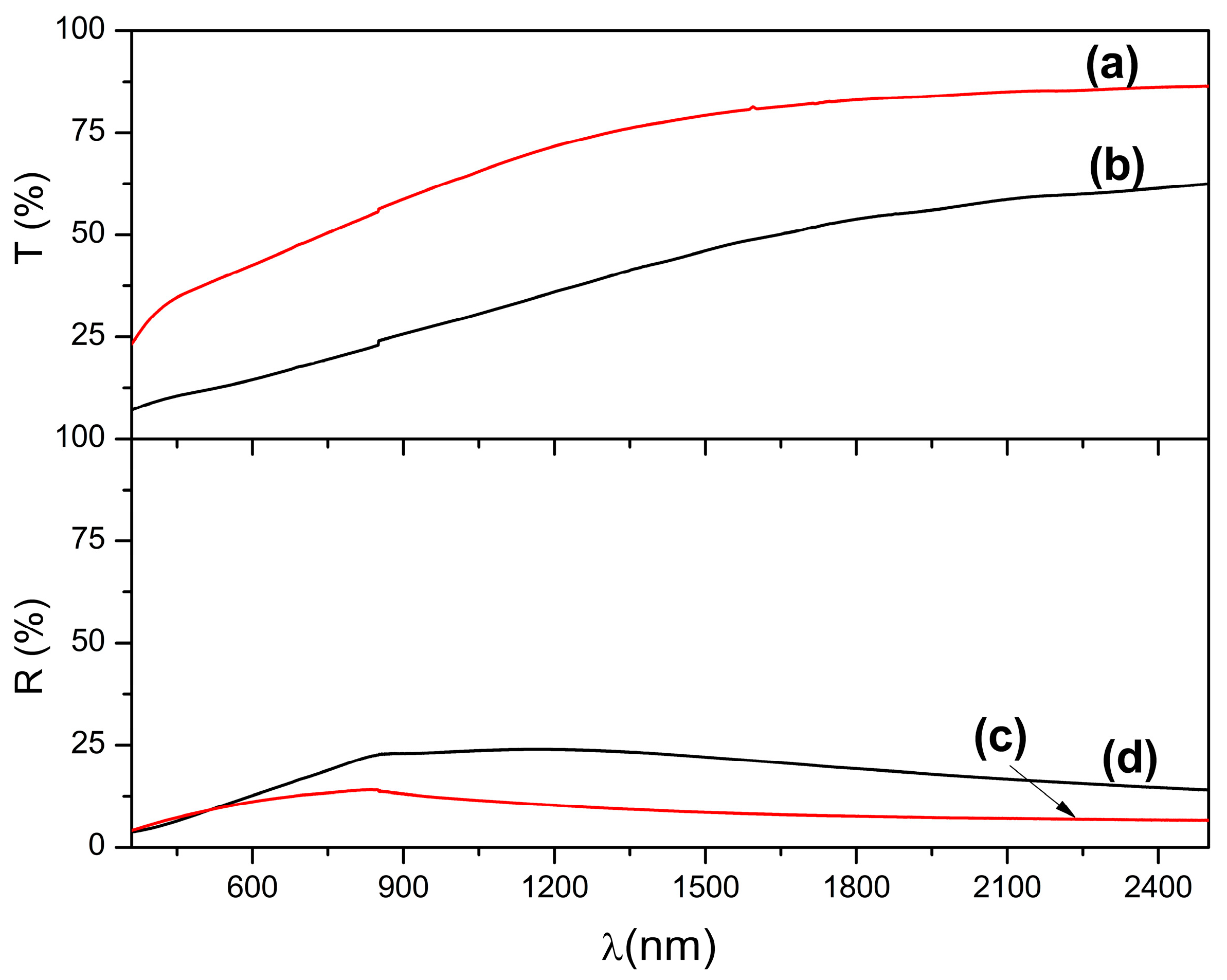
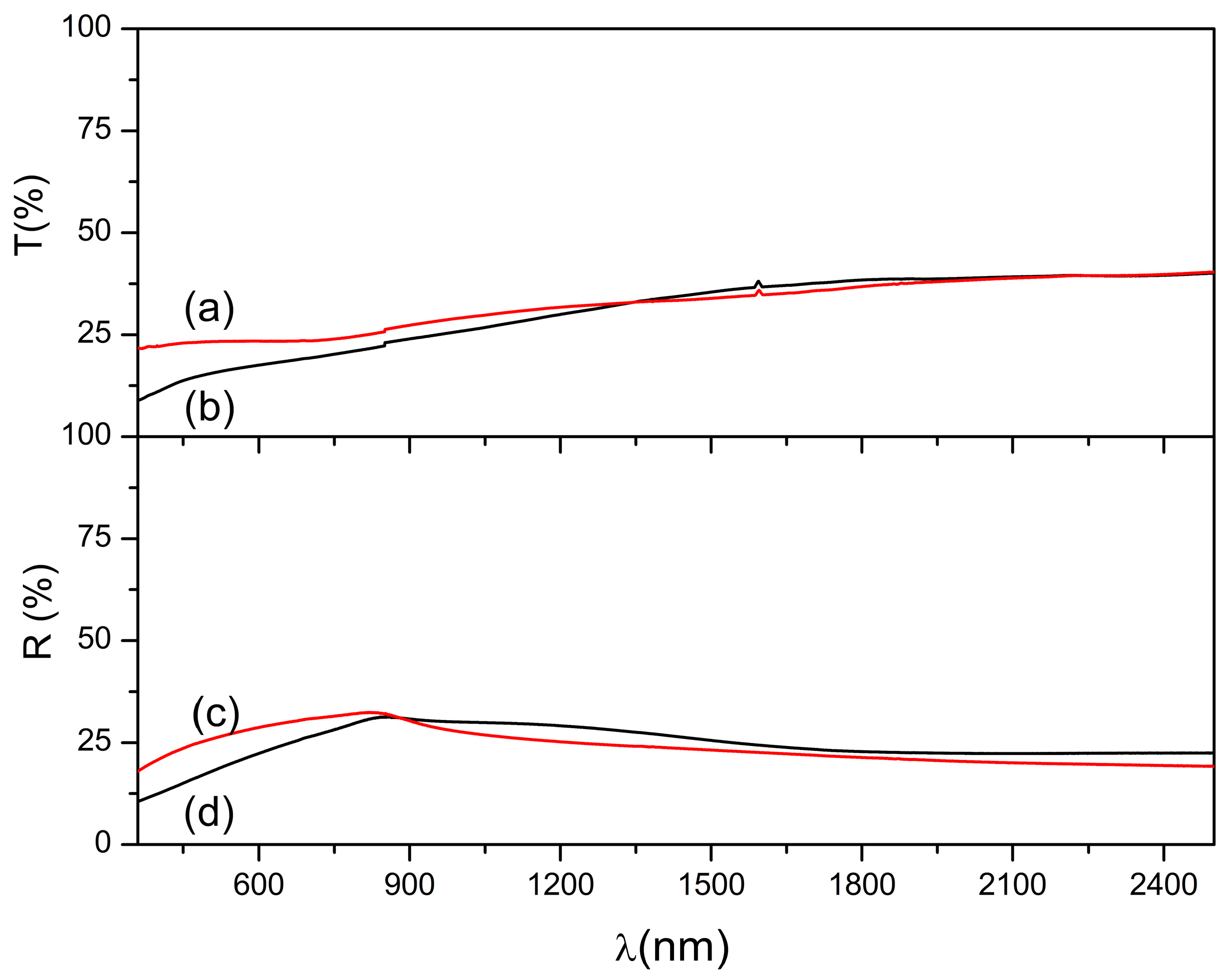
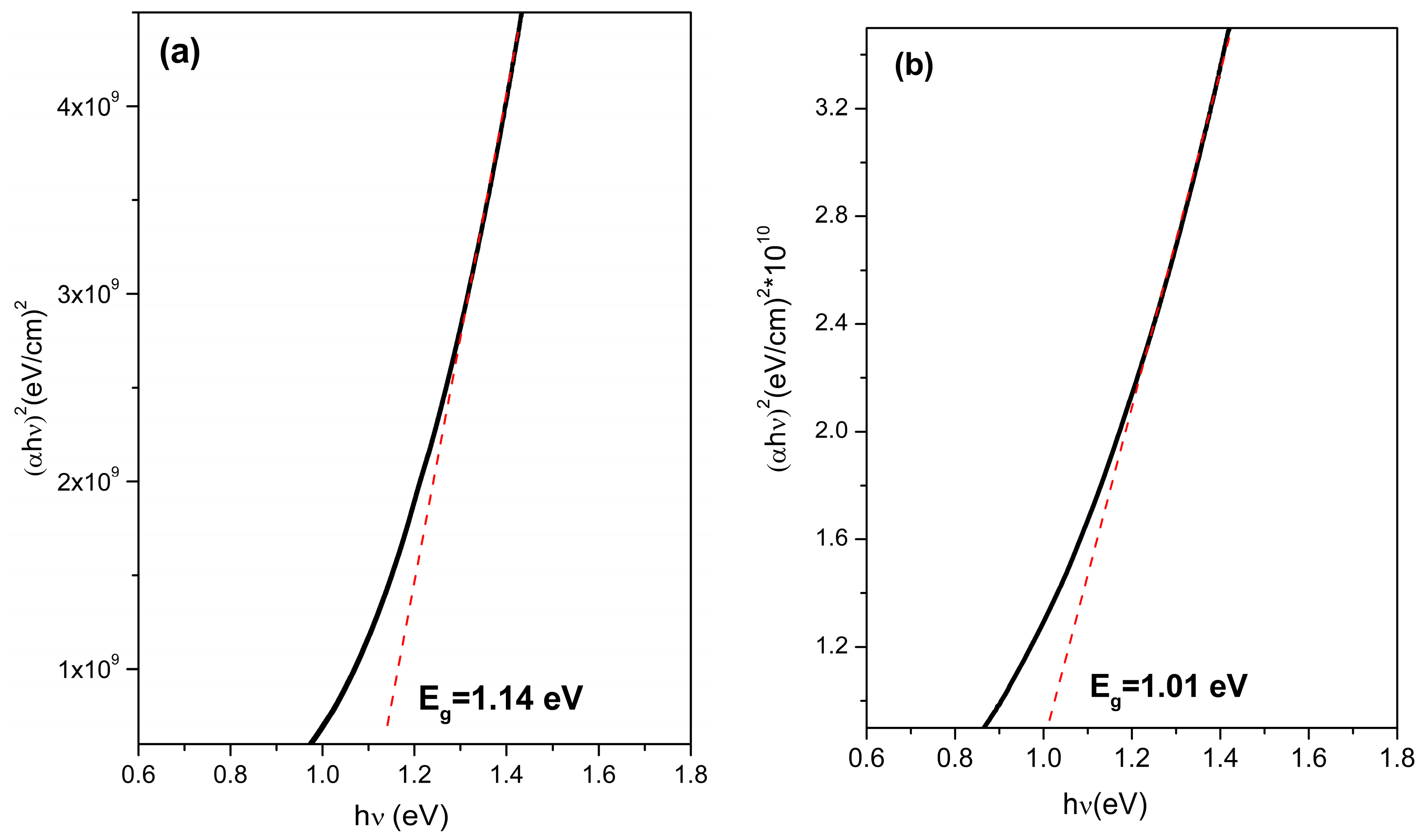
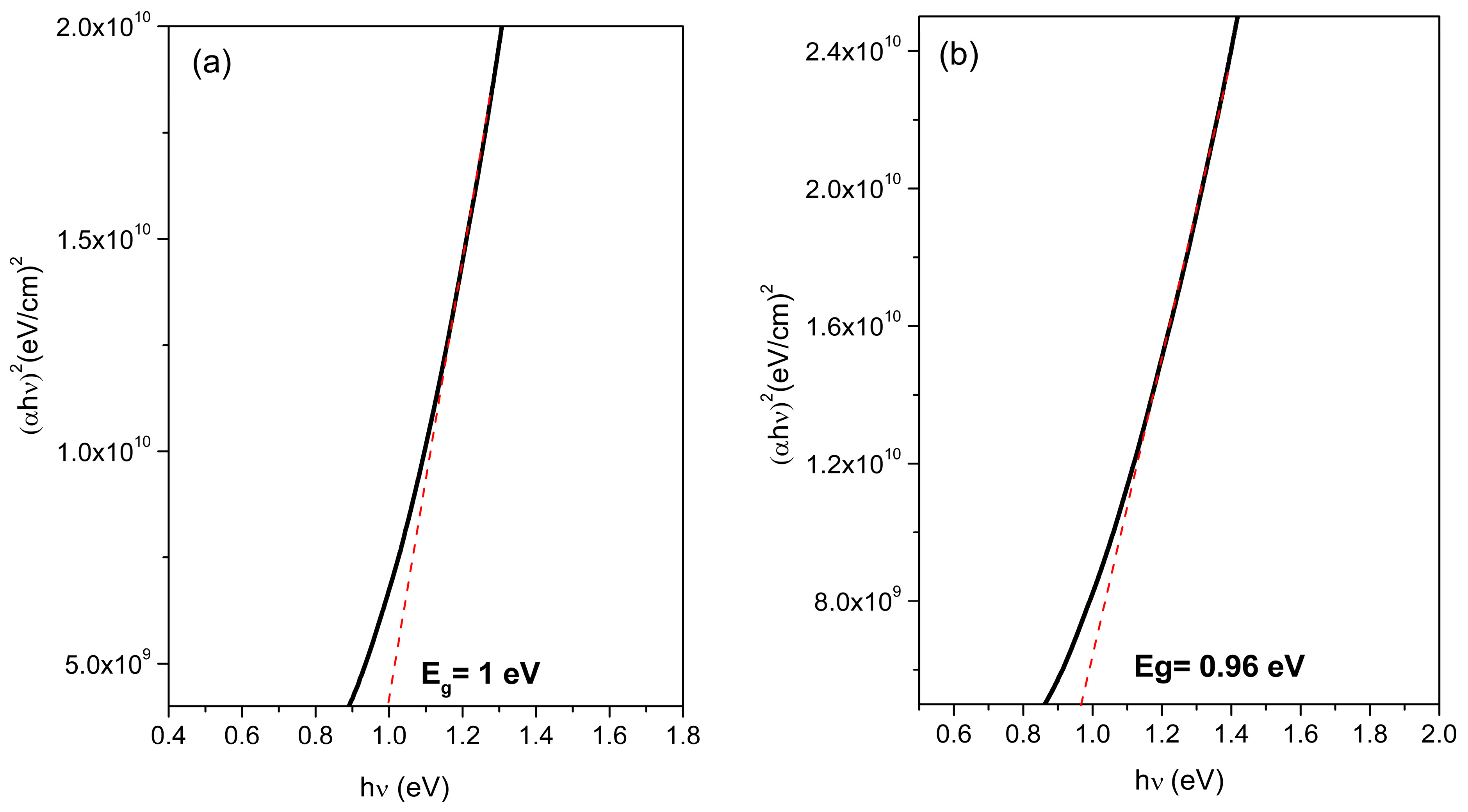
3.4. Optical Properties and Bandgap Calculation
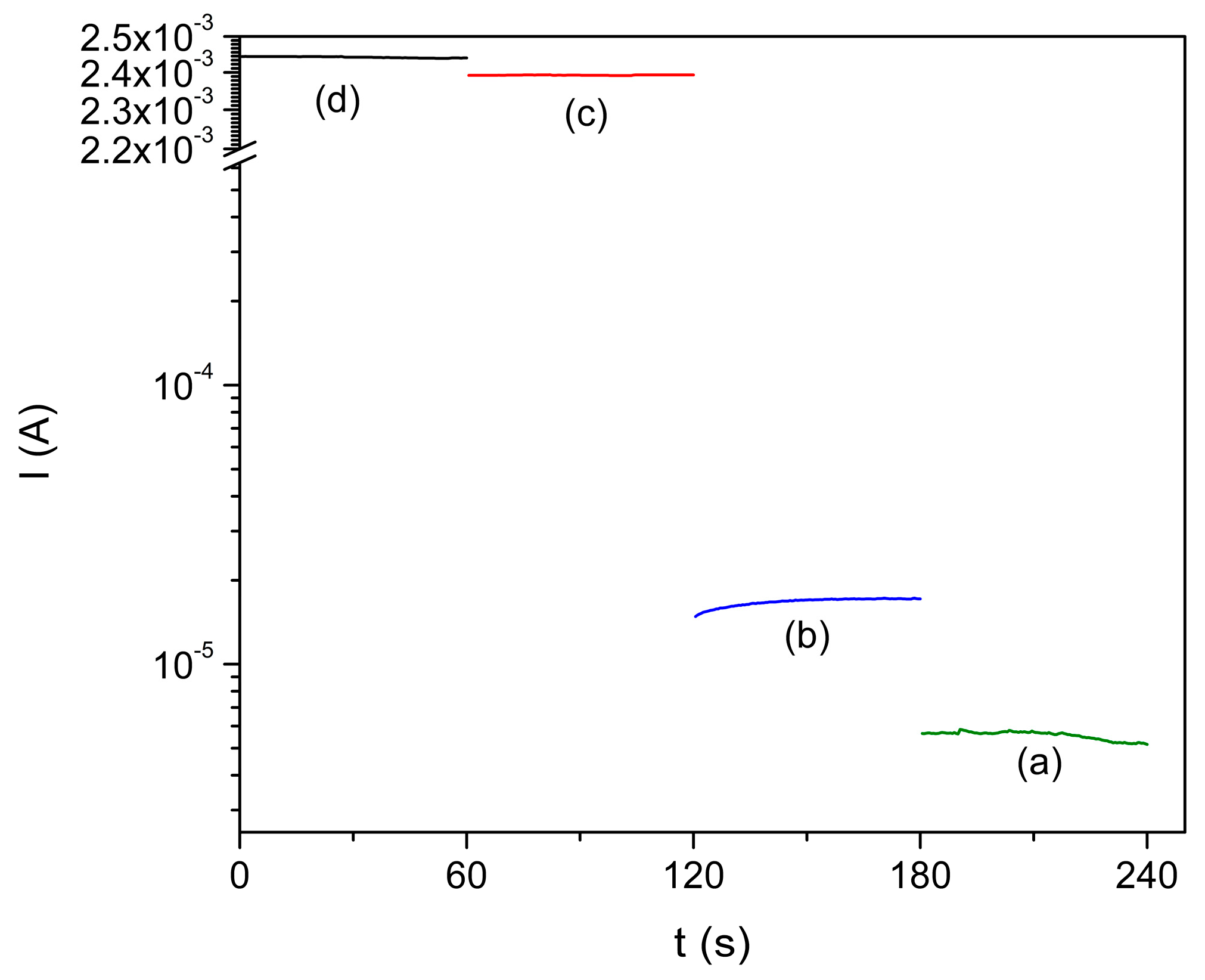
3.5. Electrical Measurement
3.6. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Bai, J.; Liu, J.; Zhou, X. Synthesis of Flower-Like FeS2 and NiS2 Microspheres Based on Nanoflakes and Nanoparticles: Electronic Structures, Magnetic and Optical Properties. Phys. Status Solidi B 2023, 260, 2300288. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, G.-M.; Shi, F.-N.; Wang, X.-L.; Li, S.-T. The synthesis and excellent peroxidase-like activity for the colorimetric detection of H2O2 of core-shell Fe/FeS2@C Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2022, 642, 128612. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Lin, B. Empirical analysis on energy rebound effect from the perspective of technological progress—A case study of China’s transport sector. J. Clean. Prod. 2018, 205, 1082–1093. [Google Scholar] [CrossRef]
- Hossain, J.; Mondal, B.K.; Mostaque, S.K. Design of a highly efficient FeS2-based dual-heterojunction thin film solar cell. Int. J. Green Energy 2022, 19, 1531–1542. [Google Scholar] [CrossRef]
- Sai, R.; Abumousa, R.A. Impact of iron pyrite nanoparticles sizes in photovoltaic performance. Coatings 2023, 13, 167, Erratum in Coatings 2024, 14, 531. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, J.; Wang, X.; Yang, W.; Jing, Y.; Yang, Y. Effect of vacancy defects on the properties of CoS2 and FeS2. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2024, 39, 627–638. [Google Scholar] [CrossRef]
- Das, B.; Voigt, B.; Moore, W.; Lee, Y.; Maiti, M.; Chaturvedi, V.; Haugstad, G.; Manno, M.; Aydil, E.; Leighton, C. Electronic transport across the insulator-metal transition in Co-doped pyrite FeS2 single crystals. Phys. Rev. Mater. 2025, 9, 054601. [Google Scholar] [CrossRef]
- Shi, Z.; Jayatissa, A.H. Preparation and characterization of cobalt-doped iron pyrite (FeS2) thin films. Prog. Nat. Sci. Mater. Int. 2020, 30, 352–359. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, L.W.C.; Yang, S.; Liu, Y.; Zhao, Y.; Cui, D.; Zhang, S.; Dou, S.; Du, H.; Lin, L. The surface binding and energy issues in rational design of the separation membrane of Li||S batteries. arXiv 2025, arXiv:2502.16135. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Xiong, S. Insights into the optimization of catalytic active sites in lithium–sulfur batteries. Acc. Chem. Res. 2024, 57, 2093–2104. [Google Scholar] [CrossRef]
- Faramawy, A.; Elsayed, H.; Scian, C.; Mattei, G. Structural, Optical, Magnetic and Electrical Properties of Sputtered ZnO and ZnO:Fe Thin Films: The Role of Deposition Power. Ceramics 2022, 5, 1128–1153. [Google Scholar] [CrossRef]
- Voigt, B.; Moore, W.; Maiti, M.; Walter, J.; Das, B.; Manno, M.; Leighton, C.; Aydil, E.S. Observation of an internal p–n junction in pyrite FeS2 single crystals: Potential origin of the low open circuit voltage in pyrite solar cells. ACS Mater. Lett. 2020, 2, 861–868. [Google Scholar] [CrossRef]
- Soonmin, H. Deposition of metal sulphide thin films by chemical bath deposition technique: Review. Int. J. Thin Films Sci. Technol. 2021, 10, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jahan Tamanna, N.; Sahadat Hossain, M.; Mohammed Bahadur, N.; Ahmed, S. Green synthesis of Ag2O & facile synthesis of ZnO and characterization using FTIR, bandgap energy & XRD (Scherrer Equation, Williamson-Hall, size-train Plot, Monshi-Scherrer model). Results Chem. 2024, 7, 101313. [Google Scholar]
- Prabukanthan, P.; Thamaraiselvi, S.; Harichandran, G. Single step electrochemical deposition of p-type undoped and Co2+ doped FeS2 thin films and performance in heterojunction solid solar cells. J. Electrochem. Soc. 2017, 164, D581. [Google Scholar] [CrossRef]
- Debmalya, R.; Voigt, B.; Leighton, C.; Manno, M.; Eray, A.; Gagliardi, L. Sulfur Vacancy Clustering and Its Impact on Electronic Properties in Pyrite FeS2. Chem. Mater. 2020, 32, 4820–4831. [Google Scholar] [CrossRef]
- Behera, G.C.; Rani, S.; Khatun, N.; Rath, J.K.; Roy, S.C. WS2 nanosheets functionalized Fe2O3 nanorod arrays as a type II heterojunction for photoelectrochemical water splitting. Appl. Surf. Sci. Adv. 2022, 11, 100293. [Google Scholar] [CrossRef]
- Catalano, P.; Chaudhary, R.; Desimone, M.; Santo Orihuela, P. A survey on analytical methods for the characterization of green synthesized nanomaterials. Curr. Pharm. Biotechnol. 2021, 22, 813–837. [Google Scholar] [CrossRef]
- Zaka, A.; Alhassan, S.M.; Nayfeh, A. Iron Pyrite in Photovoltaics: A Review on Recent Trends and Challenges. ACS Appl. Electron. Mater. 2022, 4, 4173–4211. [Google Scholar] [CrossRef]
- Moon, M.M.A.; Ali, M.H.; Rahman, M.F.; Hossain, J.; Ismail, A.B.M. Design and simulation of FeSi2-based novel heterojunction solar cells for harnessing visible and near-infrared light. Phys. Status Solidi A 2020, 217, 1900921. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohshita, Y.; Shibata, H.; Tampo, H.; Nagai, T. Overview and Perspective for High-Efficiency Single-Junction Solar Cells. Solar RRL 2023, 7, 2300308. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Li, H.; Sun, J.; Li, H.; He, Y.; Liu, Z. Iron-chalcogenide-based electrode materials for electrochemical energy storage. J. Mater. Chem. A 2022, 10, 7517–7556. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Yue, H.; Cheng, G.; Xue, X. Enhanced photo-fenton catalytic activity by spherical FeS2 nanoparticles and photoelectric property of hybrid FeS2/rGO. Vacuum 2021, 192, 110433. [Google Scholar] [CrossRef]
- Kokilavani, S.; Syed, A.; Raju, L.L.; Al-Rashed, S.; Elgorban, A.M.; Thomas, A.M.; Khan, S. Synthesis of novel heterostructured FeS2/Ag2MoO4 nanocomposite: Characterization, efficient antibacterial and enhanced visible light driven photocatalytic activity. Surf. Interfaces 2021, 23, 101003. [Google Scholar] [CrossRef]
- Nandihalli, N. Thermoelectric films and periodic structures and spin Seebeck effect systems: Facets of performance optimization. Mater. Today Energy 2022, 25, 100965. [Google Scholar] [CrossRef]
- Day-Roberts, E.; Birol, T.; Fernandes, R.M. Contrasting ferromagnetism in pyrite FeS2 induced by chemical doping versus electrostatic gating. Phys. Rev. Mater. 2020, 4, 054405. [Google Scholar] [CrossRef]
- Guo, S.; Young, D.P.; Macaluso, R.T.; Browne, D.A.; Henderson, N.L.; Chan, J.Y.; Henry, L.; DiTusa, J.F. Magnetic and thermodynamic properties of cobalt-doped iron pyrite: Griffiths phase in a magnetic semiconductor. Am. Phys. Soc. Phys. Rev. B 2010, 81, 144423. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeño Morales, E.; Peña Méndez, Y.; Gamboa-Sánchez, S.A.; Ildusovich Kharissov, B.; Hernández García, T.C.; Garza-Navarro, M.A. Tunable Bandgap in Cobalt-Doped FeS2 Thin Films for Enhanced Solar Cell Performance. Materials 2025, 18, 4546. https://doi.org/10.3390/ma18194546
Cedeño Morales E, Peña Méndez Y, Gamboa-Sánchez SA, Ildusovich Kharissov B, Hernández García TC, Garza-Navarro MA. Tunable Bandgap in Cobalt-Doped FeS2 Thin Films for Enhanced Solar Cell Performance. Materials. 2025; 18(19):4546. https://doi.org/10.3390/ma18194546
Chicago/Turabian StyleCedeño Morales, Eder, Yolanda Peña Méndez, Sergio A. Gamboa-Sánchez, Boris Ildusovich Kharissov, Tomás C. Hernández García, and Marco A. Garza-Navarro. 2025. "Tunable Bandgap in Cobalt-Doped FeS2 Thin Films for Enhanced Solar Cell Performance" Materials 18, no. 19: 4546. https://doi.org/10.3390/ma18194546
APA StyleCedeño Morales, E., Peña Méndez, Y., Gamboa-Sánchez, S. A., Ildusovich Kharissov, B., Hernández García, T. C., & Garza-Navarro, M. A. (2025). Tunable Bandgap in Cobalt-Doped FeS2 Thin Films for Enhanced Solar Cell Performance. Materials, 18(19), 4546. https://doi.org/10.3390/ma18194546







