Synergistic Corrosion Inhibition of Mild Steel in Acidic Media by a Benzimidazole–Thiophene Ligand and Its Metal Complexes: A Multi-Technique Electrochemical Approach
Abstract
1. Introduction
2. Materials and Methods
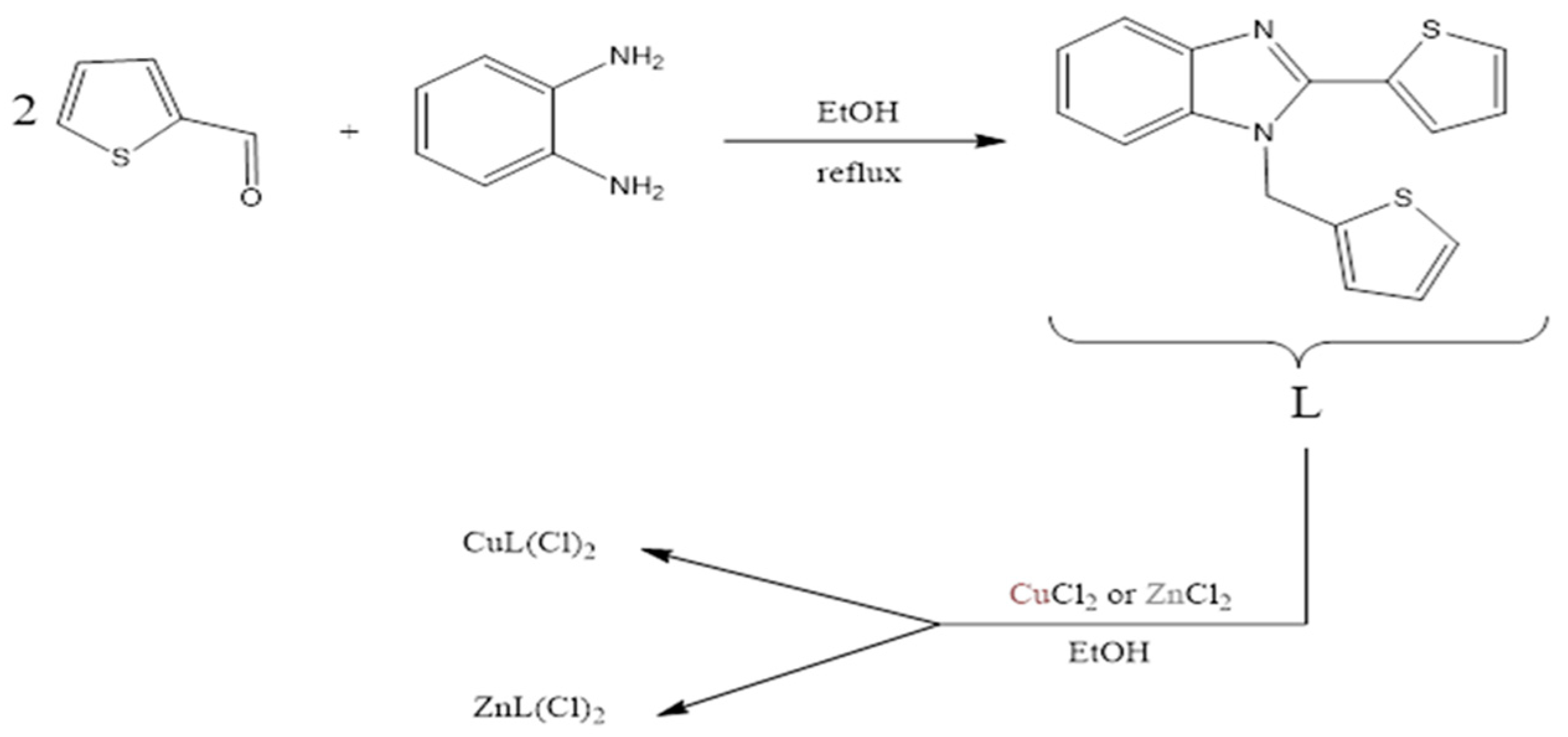
2.1. Materials
2.2. Electrochemical Measurements
2.3. Morphology and Surface Analysis
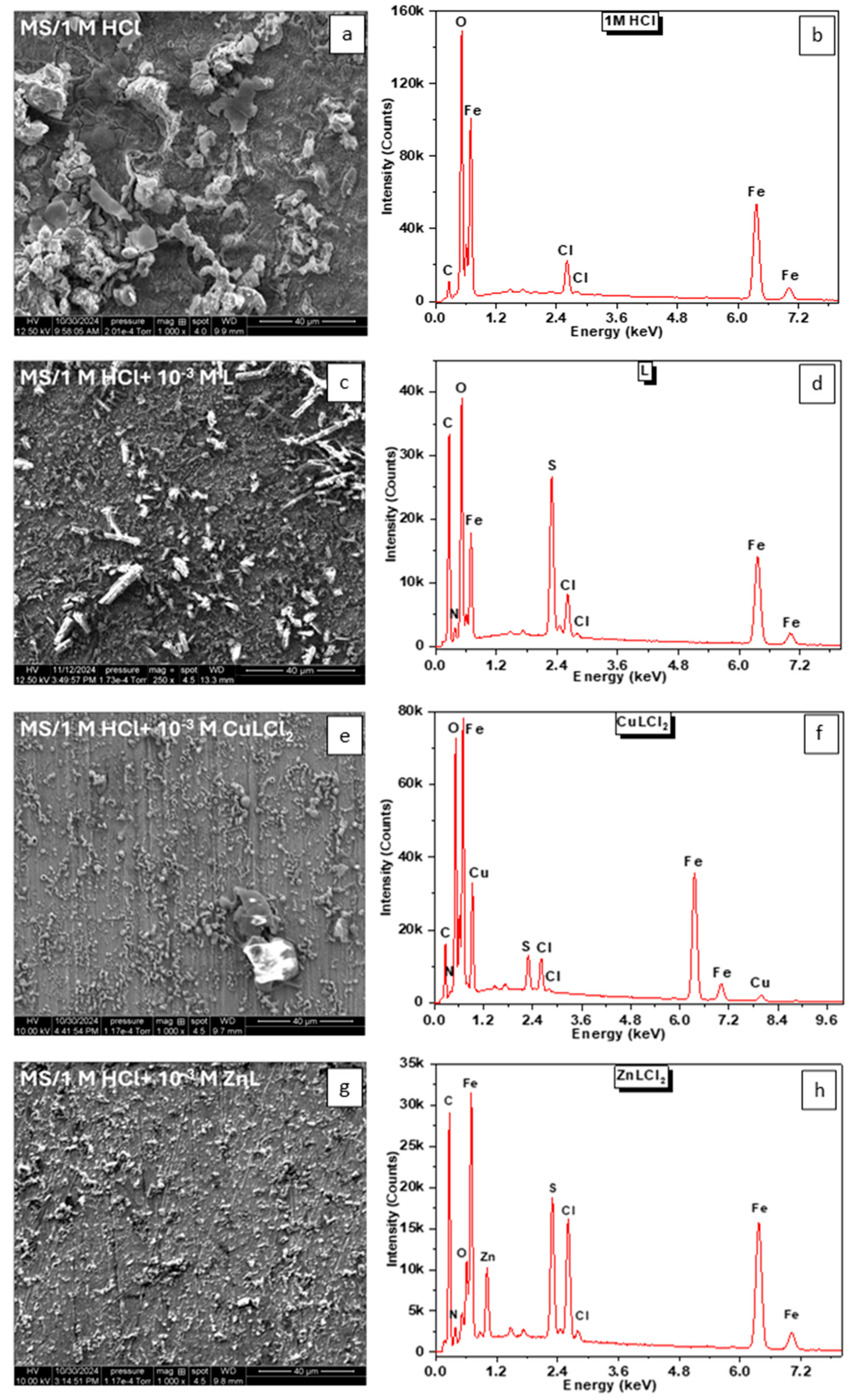
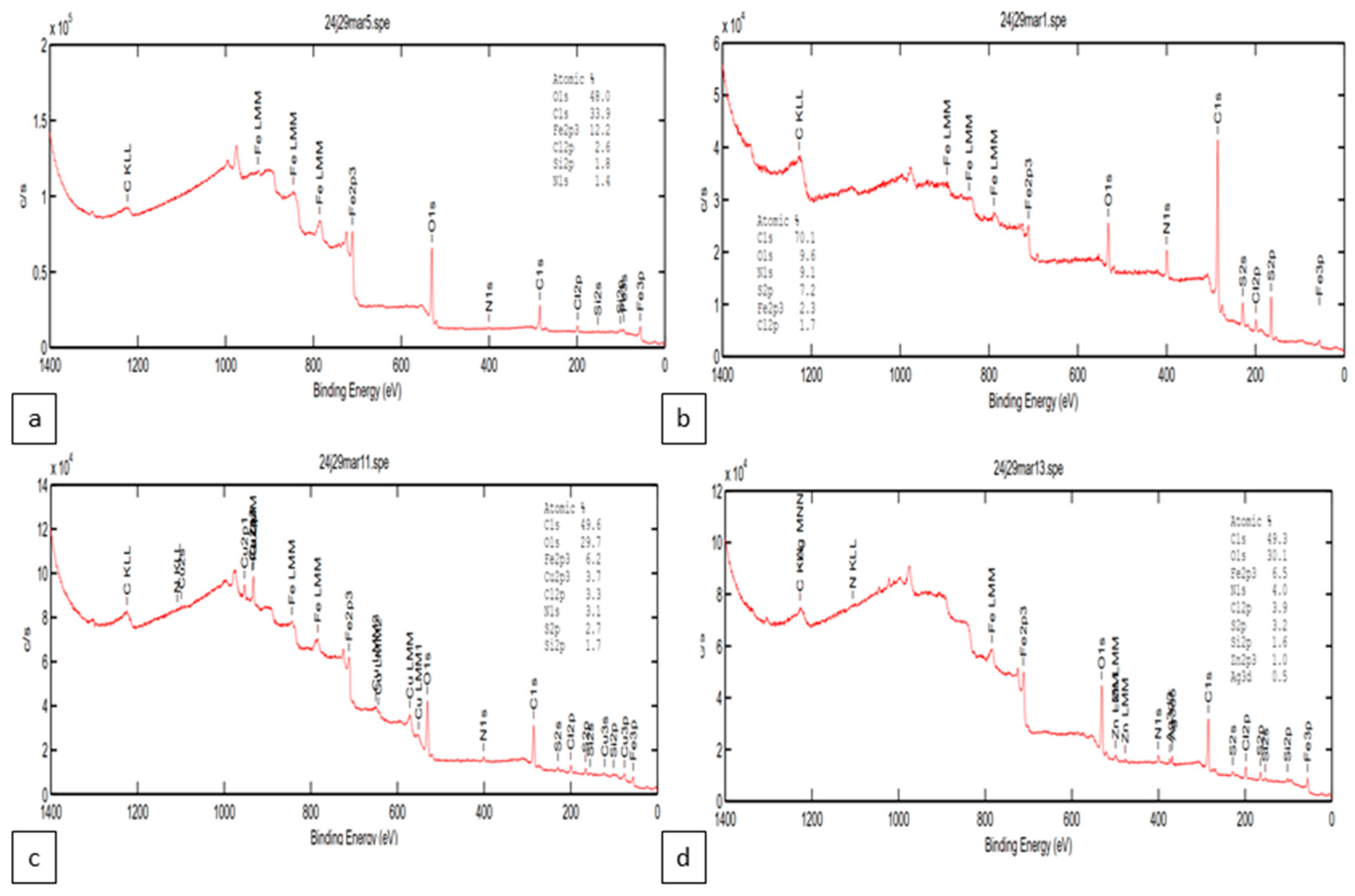
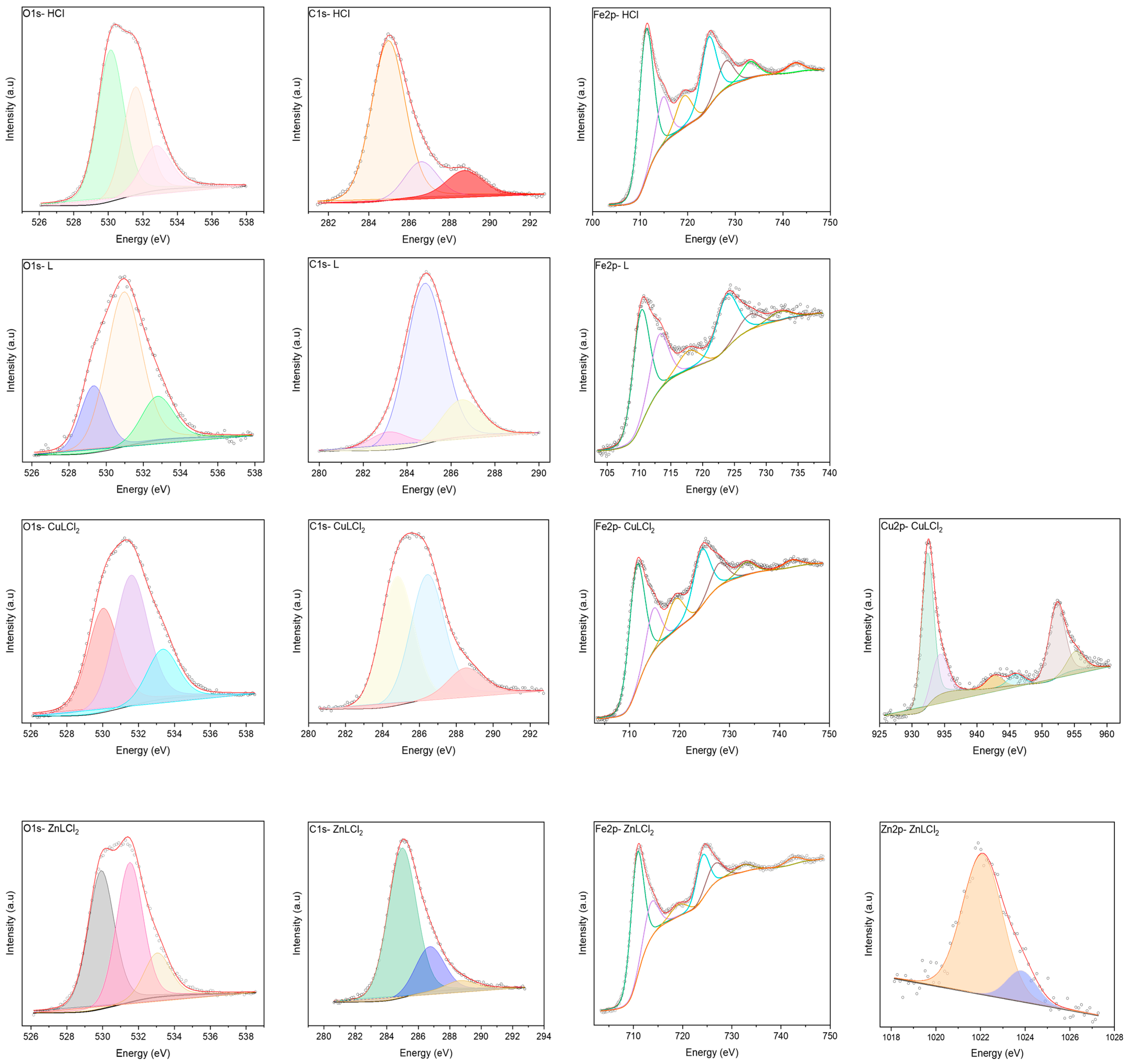
3. Results and Discussion
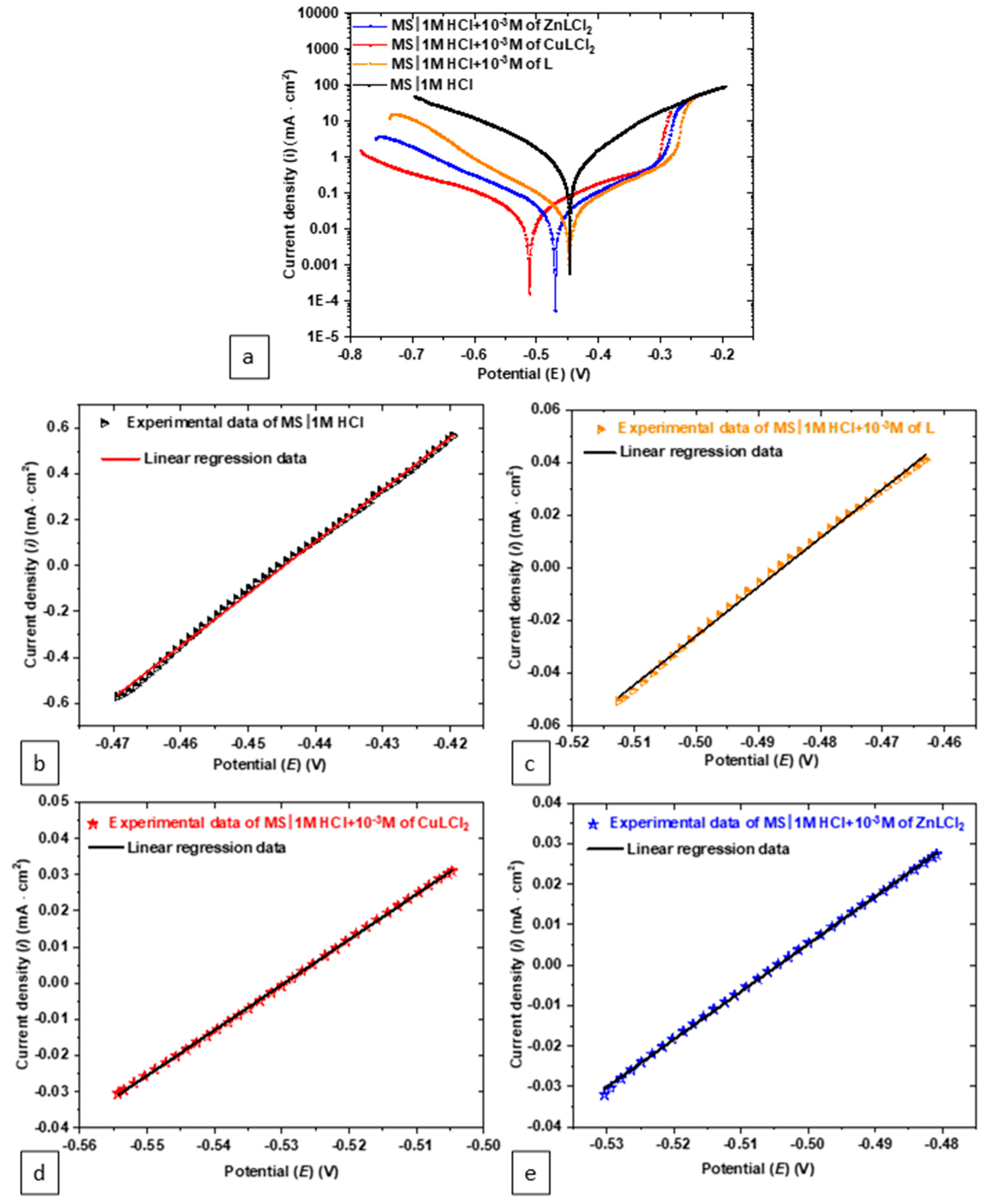
3.1. Electrochemical Evaluation
3.2. Analysis of Corrosion Products and Protective Layer
3.3. Corrosion Inhibition Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iannuzzi, M.; Frankel, G.S. The carbon footprint of steel corrosion. npj Mater. Degrad. 2022, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; Report No. OAPUS310GKOCH; NACE Int.: Houston, TX, USA, 2016; p. 216. [Google Scholar]
- World GDP (USD). Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD (accessed on 1 August 2025).
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies; World Steel Association: Brussels, Belgium, 2016. [Google Scholar]
- Alamiery, A.A. Comprehensive evaluation of 5-imino-1,2,4-dithiazolidine-3-thione as a corrosion inhibitor for mild steel in hydrochloric acid solution. Sci. Rep. 2025, 15, 10349. [Google Scholar] [CrossRef]
- Gapsari, F.; Darmadi, D.B.; Setyarini, P.H.; Wijaya, H.; Madurani, K.A.; Juliano, H.; Sulaiman, A.M.; Hidayatullah, S.; Tanji, A.; Hermawan, H. Analysis of corrosion inhibition of Kleinhovia hospita plant extract aided by quantification of hydrogen evolution using a GLCM/SVM method. Int. J. Hydrog. Energy 2023, 48, 15392–15405. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution. Metals 2017, 7, 44. [Google Scholar] [CrossRef]
- Ren, X.; Xu, S.; Chen, S.; Chen, N.; Zhang, S. Experimental and theoretical studies of triisopropanolamine as an inhibitor for aluminum alloy in 3% NaCl solution. RSC Adv. 2015, 5, 101693–101700. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Abdel-Rahman, L.H.; Sañudo, E.C.; Aliaga-Alcalde, N. Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J. Electroanal. Chem. 2015, 743, 120–133. [Google Scholar] [CrossRef]
- Ansari, K.; Quraishi, M.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Gharbi, O.; Tran, M.T.; Turmine, M.; Vivier, V. The ionic liquid route for the development of environmentally friendly corrosion inhibitors: The inhibition mechanism of ammonium and amino-acid based ionic liquids for high strength Al alloys. In Proceedings of the EUROCORR 2021, Virtual Conference, 20–24 September 2021. [Google Scholar]
- Benbouzid, A.Z.; Gharbi, O.; Sidi-Yakoub, N.; Tran, M.T.; Turmine, M.; Vivier, V. Ionic liquid route for the corrosion inhibition of Al alloys: The effect of butylammonium nitrate on the corrosion of AA2024-T6. Corros. Commun. 2023, 9, 57–64. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Z.; Xu, F.; Zou, X. Adsorption and corrosion inhibitive properties of gemini surfactants in the series of hexanediyl-1,6-bis-(diethyl alkyl ammonium bromide) on aluminium in hydrochloric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 191–200. [Google Scholar] [CrossRef]
- Alamry, K.A.; Khan, A.; Aslam, J.; Hussein, M.A.; Aslam, R. Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug. Sci. Rep. 2023, 13, 6724. [Google Scholar] [CrossRef]
- Akouibaa, M.; Kadiri, M.; Driouch, M.; Tanji, K.; Ouarsal, R.; Rakib, S.; Sfaira, M.; Morley, N.; Lachkar, M.; El Bali, B.; et al. Synthesis, catalytic activity, magnetic study and anticorrosive activity of mild steel in HCl 1 M medium of (H3dien)[Cu(NO3)(C2O4)2].2H2O. A redetermination at 100 K. Mater. Chem. Phys. 2023, 307, 128130. [Google Scholar] [CrossRef]
- Idboumlik, M.; Kadiri, M.; Hamdi, N.; Driouch, M.; Ngopoh, A.F.I.; Lakkab, I.; Bendeif, E.E.; Sfaira, M.; El Bali, B.; Lachkar, M.; et al. Synthesis of novel hybrid decavanadate material (NH4)2(H2en)2{V10O28}.4H2O: Characterization, anticorrosion and biological activities. Mater. Chem. Phys. 2022, 287, 126211. [Google Scholar] [CrossRef]
- Obot, I.; Macdonald, D.; Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Achnine, N.; Driouch, M.; Elhaloui, A.; Lakbaibi, Z.; El Assiri, E.H.; Kadiri, M.; Sfaira, M.; Dagdag, O.; Berisha, A.; El Ibrahimi, B. A critical view of computational chemistry methods in understanding corrosion inhibition of 6-chloro-2-phenylimidazo [1, 2-b] pyridazine in 1 M and 5 M HCl. Int. J. Corros. Scale Inhib. 2023, 12, 888–912. [Google Scholar]
- El Adnani, Z.; McHarfi, M.; Sfaira, M.; Benzakour, M.; Benjelloun, A.T.; Ebn Touhami, M. DFT theoretical study of 7-R-3methylquinoxalin-2(1H)-thiones (RH.; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci. 2013, 68, 223–230. [Google Scholar] [CrossRef]
- Stevanović, N.L.; Aleksic, I.; Kljun, J.; Skaro Bogojevic, S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B. Copper(II) and Zinc(II) Complexes with the Clinically Used Fluconazole: Comparison of Antifungal Activity and Therapeutic Potential. Pharmaceuticals 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Ahmad Khan, R.; Al-Tamimi, J.; Ebaid, H.; Mabood Husain, F.; Alhazza, I.M. Comparative efficacy of ternary Cu (II) complex and Zn (II)-complex in amelioration of carbon tetrachloride-induced hepatotoxicity in vivo. J. King Saud Univ. Sci. 2023, 35, 102420. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, X.; Zhang, X.; Legut, D.; Zhang, D. Towards rational design of organic copper corrosion inhibitors: High-throughput computational evaluation of standard adsorption Gibbs energy. Corros. Sci. 2024, 227, 111783. [Google Scholar] [CrossRef]
- Gan, Y.; Tan, B.; Hu, Q.; Zhang, S.; Li, W. Bi-functional inhibitors of zinc corrosion and dendrite formation in aqueous electrolyte: Insights from experiments and theoretical calculations. Corros. Sci. 2022, 208, 110619. [Google Scholar] [CrossRef]
- Elaaraj, I.; Raouan, S.E.R.; Nakkabi, A.; Es-sounni, B.; Koraichi, I.; El moualij, N.; Fahim, M. Synthesis, characterization and antioxidant, antibacterial activity Zn2+, Cu2+, Ni2+ and Co2+, complexes of ligand [2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H-benzo[d]imidazole]. J. Indian Chem. Soc. 2022, 99, 100404. [Google Scholar] [CrossRef]
- Zielinski, A.; Cieslik, M.; Sobaszek, M.; Bogdanowicz, R.; Darowicki, K.; Ryl, J. Multifrequency nanoscale impedance microscopy (m-NIM): A novel approach towards detection of selective and subtle modifications on the surface of polycrystalline boron-doped diamond electrodes. Ultramicroscopy 2019, 199, 34–45. [Google Scholar] [CrossRef]
- Application Note 37-CASP: A New Method for the Determination of Corrosion Parameters (CASP Rp Determination). Available online: https://www.biologic.net/documents/casp-rp-determination-corrosion-application-note-37/ (accessed on 1 August 2025).
- Désilets, C.; Lasia, A. Dynamic impedance study of ethanol and acetaldehyde oxidation at platinum in acid solutions. Electrochim. Acta 2012, 78, 286–293. [Google Scholar] [CrossRef]
- Orazem, M.E. Measurement model for analysis of electrochemical impedance data. J. Solid State Electrochem. 2024, 28, 1273–1289. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Kadiri, M.; Bourhia, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Chedadi, M.; Sfaira, M. Promising antioxidant and anticorrosion activities of mild steel in 1.0 M hydrochloric acid solution by Withania frutescens L. essential oil. Front. Chem. 2021, 9, 739273. [Google Scholar] [CrossRef] [PubMed]
- Vivier, V.; Orazem, M.E. Impedance Analysis of Electrochemical Systems. Chem. Rev. 2022, 122, 11131–11168. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Lgaz, H.; Bhat, K.S.; Salghi, R.; Jodeh, S.; Algarra, M.; Hammouti, B.; Ali, I.H.; Essamri, A. Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J. Mol. Liq. 2017, 238, 71–83. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A.; Obot, I.B. Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: Experimental and theoretical studies. J. Phys. Chem. C 2016, 120, 11598–11611. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Esan, T.; Oyeneyin, O.; Olanipekun, A.; Ipinloju, N. Corrosion inhibitive potentials of some amino acid derivatives of 1, 4-naphthoquinone–DFT calculations. Adv. J. Chem. Sect. A 2022, 5, 263. [Google Scholar]
- Zhang, Y.; Qiang, Y.; Zhang, W.; Ramakrishna, S.; Farhadian, A.; Jin, Y. Formation of a green protective layer by symmetric imidazolium-based ionic liquids for metal corrosion inhibition: Interfacial adsorption and mechanistic insights. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137299. [Google Scholar] [CrossRef]
- Aslam, R.; Wang, Q.; Sun, Y.; Yan, Z. Fabrication, characterization and corrosion inhibition performance of sustainable metal-organic framework nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2025, 720, 137095. [Google Scholar] [CrossRef]
- Elkhadim, M.; Driouch, M.; Kadiri, M.; Dagdag, O.; Kim, H.; Hsissou, R.; Serrar, H.; Berdimurodov, E.; Berisha, A.; Kaya, S.; et al. Formulation of thiosemicarbazide based epoxy resin as inhibitor for corrosion of mild steel in 1M HCl: Electrochemcial and theoretical studies. Can. Metall. Q. 2025, 1–23. [Google Scholar] [CrossRef]
- Rafik, M.; Dagdag, O.; Hsissou, R.; Haidara, H.; Safi, Z.S.; Kadiri, M.; Kim, H.; Berisha, A.; Wazzan, N.A.; Al-Qurashi, O.S.; et al. New triglycidyl ether triazine as a protective agent for metal: Comprehensive analysis. Mater. Chem. Phys. 2024, 327, 129907. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Wagner, C.; Traud, W. The interpretation of corrosion phenomena by super-imposition of electrochemical partial reactions and the formation of potentials of mixed electrodes. Z. Elektrochem. 1938, 44, 391–402. [Google Scholar]
- Bonhoeffer, K.; Jena, W. Über das elektromotorische Verhalten von Eisen. Z. Elektrochem. Angew. Phys. Chem. 1951, 55, 151–154. [Google Scholar] [CrossRef]
- Mészáros, L.; Mészáros, G.; Lengyel, B. Application of harmonic analysis in the measuring technique of corrosion. J. Electrochem. Soc. 1994, 141, 2068. [Google Scholar] [CrossRef]
- Diard, J.P.; Le Gorrec, B.; Montella, C. Comment on “Application of Harmonic Analysis in the Measuring Technique of Corrosion” [J. Electrochem. Soc., 141, 2068]. J. Electrochem. Soc. 1995, 142, 3612. [Google Scholar] [CrossRef]
- Montella, C.; Diard, J.-P.; Le Gorrec, B. Exercices de Cinétique Électrochimique; Hermann: Paris, France, 2000. [Google Scholar]
- Slepski, P.; Szocinski, M.; Lentka, G.; Darowicki, K. Novel fast non-linear electrochemical impedance method for corrosion investigations. Measurement 2021, 173, 108667. [Google Scholar] [CrossRef]
- Setti, N.; Barrahi, A.; Maatallah, M.; Kaddouri, Y.; Hadda, T.B.; Outada, H.; Thakur, A.; Touzani, R.; Karrouchi, K.; Abuelizz, H.A.; et al. Experimental and computational approach on the corrosion inhibition properties of two newly pyrazole derivatives on carbon steel in acid medium. Sci. Rep. 2025, 15, 3631. [Google Scholar] [CrossRef]
- Hu, N.; Zhu, J.; Song, G.; Song, C.; Xiong, J. Synthesis of the vanillin-modified lauryl imidazoline derivative and its corrosion inhibition properties. Corros. Eng. Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Rhodes, K. Application Note: Analysis of Iron Oxidation States by XPS. Available online: https://www.mccrone.com/mm/application-note-analysis-of-iron-oxidation-states-by-xps/ (accessed on 1 August 2025).
- XPS Database. Basic XPS Information Section: Iron (Fe). Available online: https://xpsdatabase.net/iron-fe-z26 (accessed on 1 August 2025).
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Walczak, M.S.; Morales-Gil, P.; Belashehr, T.; Kousar, K.; Arellanes Lozada, P.; Lindsay, R. Determining the Chemical Composition of Corrosion Inhibitor/Metal Interfaces with XPS: Minimizing Post Immersion Oxidation. J. Vis. Exp. JoVE 2017, 55163. [Google Scholar] [CrossRef]
- Kumar, V.; BV, A.R. Chemically modified biopolymer as an eco-friendly corrosion inhibitor for mild steel in a neutral chloride environment. New J. Chem. 2017, 41, 6278–6289. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Sommertune, J.; Örnek, C.; Ryl, J.; Kurilo, I.I.; Claesson, P.M.; Pan, J. Corrosion inhibition of aluminium alloy AA6063-T5 by vanadates: Local surface chemical events elucidated by confocal Raman micro-spectroscopy. Corros. Sci. 2019, 148, 237–250. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Tan, B.; Qiang, Y.; Li, W.; Chen, S.; Guo, L. Investigation of Losartan Potassium as an eco-friendly corrosion inhibitor for copper in 0.5 M H2SO4. J. Mol. Liq. 2020, 305, 112789. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Chaouiki, A.; Salghi, R.; Thakur, S.; Pani, B. A comprehensive study about anti-corrosion behaviour of pyrazine carbohydrazide: Gravimetric, electrochemical, surface and theoretical study. J. Mol. Liq. 2020, 299, 112160. [Google Scholar] [CrossRef]
- Khamaysa, O.M.A.; Selatnia, I.; Zeghache, H.; Lgaz, H.; Sid, A.; Chung, I.-M.; Benahmed, M.; Gherraf, N.; Mosset, P. Enhanced corrosion inhibition of carbon steel in HCl solution by a newly synthesized hydrazone derivative: Mechanism exploration from electrochemical, XPS, and computational studies. J. Mol. Liq. 2020, 315, 113805. [Google Scholar] [CrossRef]
- Prabakaran, M.; Venkatesh, M.; Ramesh, S.; Periasamy, V. Corrosion inhibition behavior of propyl phosphonic acid–Zn2+ system for carbon steel in aqueous solution. Appl. Surf. Sci. 2013, 276, 592–603. [Google Scholar] [CrossRef]
- XPS Database. Basic XPS Information Section: Copper (Cu). Available online: https://xpsdatabase.net/copper-cu-z29-chemicals (accessed on 1 August 2025).
- Torres-Ochoa, J.A.; Cabrera-German, D.; Cortazar-Martinez, O.; Bravo-Sanchez, M.; Gomez-Sosa, G.; Herrera-Gomez, A. Peak-fitting of Cu 2p photoemission spectra in Cu0, Cu1+, and Cu2+ oxides: A method for discriminating Cu0 from Cu1+. Appl. Surf. Sci. 2023, 622, 156960. [Google Scholar] [CrossRef]
- Soldemo, M.; Garcia-Martinez, F.; Goodwin, C.M.; Lömker, P.; Shipilin, M.; Nilsson, A.; Amann, P.; Kaya, S.; Weissenrieder, J. Using Auger transitions as a route to determine the oxidation state of copper in high-pressure electron spectroscopy. Surf. Sci. 2024, 749, 122565. [Google Scholar] [CrossRef]
- XPS Database. Basic XPS Information Section: Zinc (Zn). Available online: https://xpsdatabase.net/zinc-zn-z30-chemicals (accessed on 1 August 2025).
- Champagne, S.; Mostaed, E.; Safizadeh, F.; Ghali, E.; Vedani, M.; Hermawan, H. In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine. Materials 2019, 12, 295. [Google Scholar] [CrossRef]
- Rao, B.V.A.; Rao, M.V.; Srinivasa Rao, S.; Sreedhar, B. Surface analysis of carbon steel protected from corrosion by a new ternary inhibitor formulation containing phosphonated glycine, Zn2+ and citrate. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 28–42. [Google Scholar]
- Kargar, H.; Ashfaq, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Tahir, M.N. Synthesis, crystal structure, spectral characterization, theoretical and computational studies of Ni(II), Cu(II) and Zn(II) complexes incorporating Schiff base ligand derived from 4-(diethylamino)salicylaldehyde. Inorganica Chim. Acta 2022, 536, 120878. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; El-Essawy, F.; Nassar, A.A.; El-Samanody, E.-S.A. Synthesis, spectral, thermal and antimicrobial studies on cobalt(II), nickel(II), copper(II), zinc(II) and palladium(II) complexes containing thiosemicarbazone ligand. J. Mol. Struct. 2018, 1157, 381–394. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, K.; Singh, A.K.; Singh, V.P. Synthesis, structural and corrosion inhibition studies on Mn(II), Cu(II) and Zn(II) complexes with a Schiff base derived from 2-hydroxypropiophenone. Polyhedron 2014, 77, 57–65. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, K.; Mourya, P.; Singh, M.M.; Singh, V.P. Synthesis, characterization and corrosion inhibition property of nickel(II) and copper(II) complexes with some acylhydrazine Schiff bases. Polyhedron 2015, 89, 29–38. [Google Scholar] [CrossRef]
- Benabid, W.; Ouari, K.; Bendia, S.; Bourzami, R.; Ait Ali, M. Crystal structure, spectroscopic studies, DFT calculations, cyclic voltammetry and biological activity of a copper (II) Schiff base complex. J. Mol. Struct. 2020, 1203, 127313. [Google Scholar] [CrossRef]
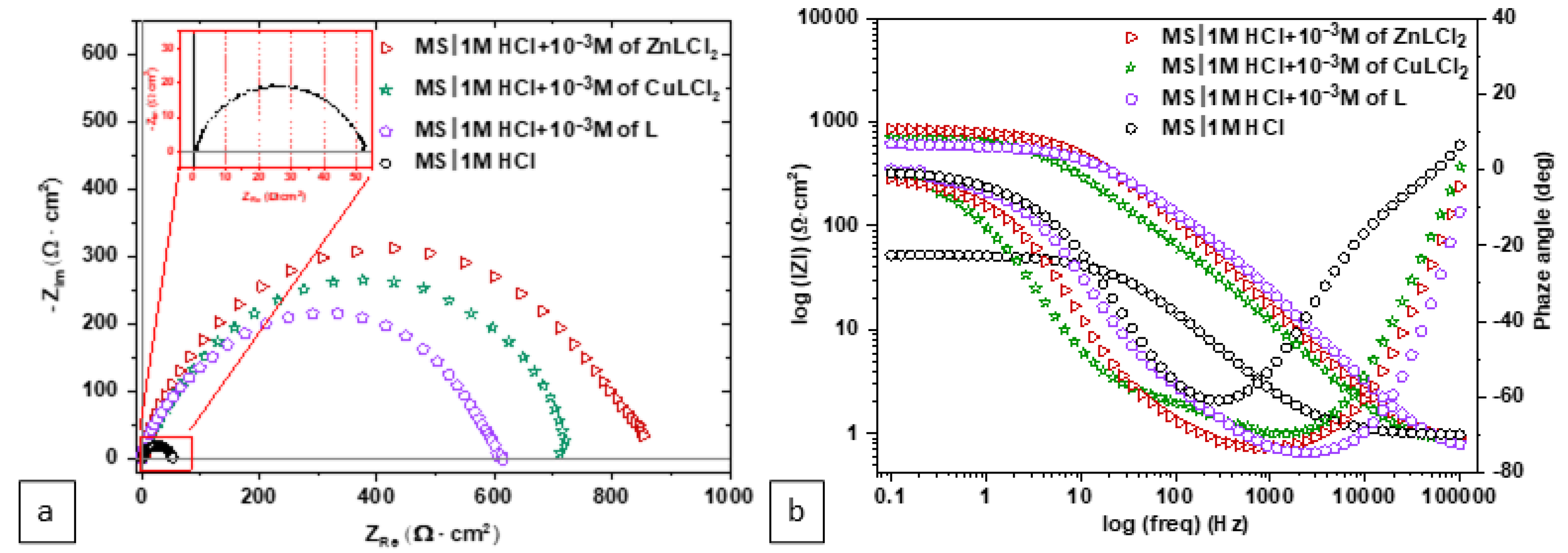
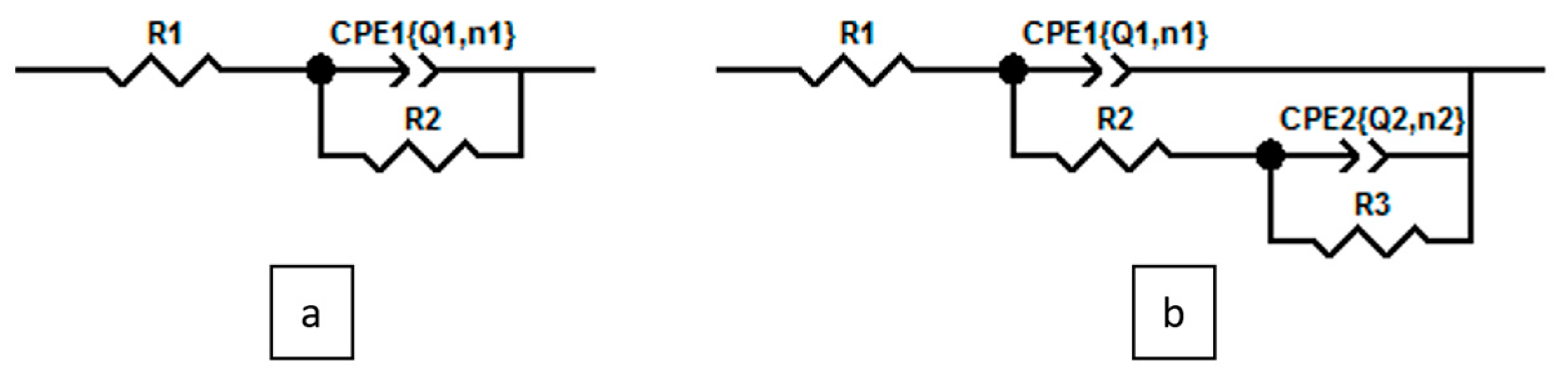

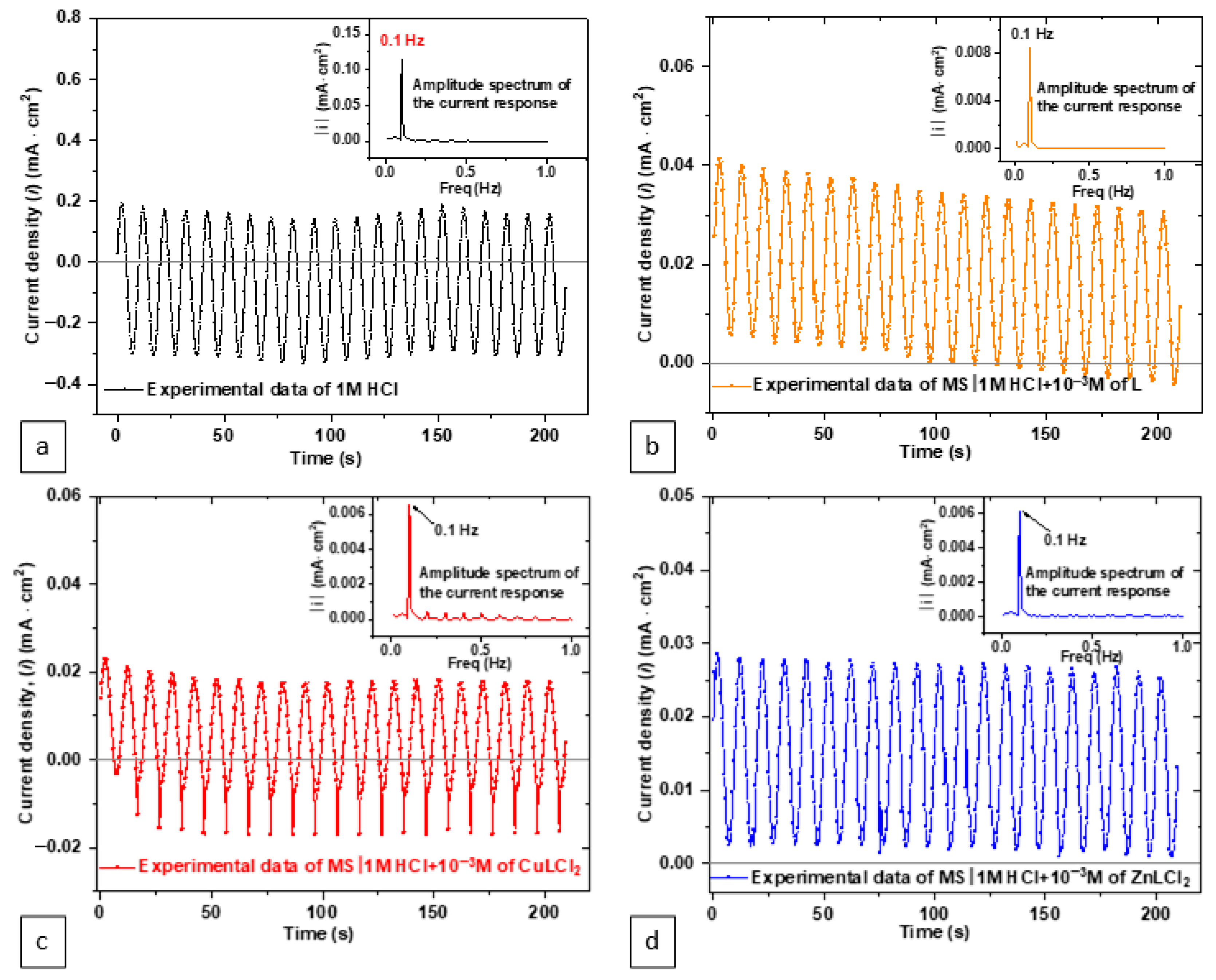
| MS/1.0 M HCl + Inhibitor System | 1.0 M HCl | L | CuLCl2 | ZnLCl2 |
|---|---|---|---|---|
| RS (Ω cm2) | 0.98 | 0.79 | 0.86 | 0.88 |
| Q1 (μF sn−1 cm−2) | 271.6 | 6.55 | 22.74 | 20.76 |
| n1 | 0.844 | 0.98 | 0.93 | 0.90 |
| R1 (Ω cm2) | 47.62 | 50.59 | 55.61 | 169.4 |
| Q2 (μF sn−1 cm−2) | 17670 | 59.3 | 95.46 | 49.91 |
| n2 | 0.71 | 0.67 | 0.72 | 0.67 |
| R2 (Ω cm2) | 4.41 | 564 | 686.9 | 681.3 |
| Rp (Ω cm2) | 52.03 | 614.59 | 742.51 | 845.70 |
| ηEIS% | - | 91.5 | 92.9 | 93.8 |
| χ2/|Z| | 0.058 | 0.094 | 0.119 | 0.106 |
| Specimen | CASP | PDP—Tafel Fit | LPR—Rp Fit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| icorr | βa | |βc| | Rp-calc | Ecorr | icorr | βa | |βc| | Rp- calc | Ecorr | Rp | icorr- calc | R2 | |
| HCl 1.0 M | 404.5 | 78.5 | 77.9 | 42 | −447.3 | 1059.4 | 120.5 | 147.5 | 27.18 | −444.6 | 44 | 386.2 | 0.99 |
| L | 30.3 | 70.9 | 76.3 | 526.2 | −453.6 | 33.8 | 99.6 | 107.9 | 665.75 | −482.8 | 552 | 28.9 | 0.99 |
| CuLCl2 | 37.7 | 108.7 | 109.4 | 628.3 | −506.6 | 60 | 218.3 | 191.8 | 738.99 | −512.2 | 615 | 38.5 | 0.99 |
| ZnLCl2 | 21.8 | 80.2 | 78.8 | 790.9 | −468.5 | 33.22 | 129.2 | 134.2 | 860.41 | −504.4 | 850 | 18.8 | 0.99 |
| Specimen | Element | Binding Energy (eV) |
|---|---|---|
| 1.0 M HCl | O | 530.13–531.58–532.77 |
| C | 284.97–286.60–288.75 | |
| Fe | 711.21–714.77–719.18–724.38–727.98–733.10–742.52 | |
| L | O | 529.32–530.96–532.77 |
| C | 283.16–284.81–286.48 | |
| Fe | 710.29–713.17–717.82–723.79–727.49–732.13 | |
| CuLCl2 | O | 530.03–531.58–533.34 |
| C | 284.81–286.43–288.52 | |
| Fe | 711.42–714.78–719.18–724.38–727.89–733.31–742.45 | |
| Cu | 932.40–934.27–942.69–946.0–952.29–955.16 | |
| ZnLCl2 | O | 529.91–531.51–533.04 |
| C | 284.98–286.74–288.71 | |
| Fe | 710.74–713.61–719.18–724.05–726.46–732.32–742.45 | |
| Zn | 1022.08–1023.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadiri, M.; Driouch, M.; Elaaraj, I.; Tanji, A.; Elabbadi, A.; Fahim, M.; Sfaira, M.; Hermawan, H. Synergistic Corrosion Inhibition of Mild Steel in Acidic Media by a Benzimidazole–Thiophene Ligand and Its Metal Complexes: A Multi-Technique Electrochemical Approach. Materials 2025, 18, 4545. https://doi.org/10.3390/ma18194545
Kadiri M, Driouch M, Elaaraj I, Tanji A, Elabbadi A, Fahim M, Sfaira M, Hermawan H. Synergistic Corrosion Inhibition of Mild Steel in Acidic Media by a Benzimidazole–Thiophene Ligand and Its Metal Complexes: A Multi-Technique Electrochemical Approach. Materials. 2025; 18(19):4545. https://doi.org/10.3390/ma18194545
Chicago/Turabian StyleKadiri, Mariya, Majid Driouch, Ibissam Elaaraj, Ayoub Tanji, Afafe Elabbadi, Mohammed Fahim, Mouhcine Sfaira, and Hendra Hermawan. 2025. "Synergistic Corrosion Inhibition of Mild Steel in Acidic Media by a Benzimidazole–Thiophene Ligand and Its Metal Complexes: A Multi-Technique Electrochemical Approach" Materials 18, no. 19: 4545. https://doi.org/10.3390/ma18194545
APA StyleKadiri, M., Driouch, M., Elaaraj, I., Tanji, A., Elabbadi, A., Fahim, M., Sfaira, M., & Hermawan, H. (2025). Synergistic Corrosion Inhibition of Mild Steel in Acidic Media by a Benzimidazole–Thiophene Ligand and Its Metal Complexes: A Multi-Technique Electrochemical Approach. Materials, 18(19), 4545. https://doi.org/10.3390/ma18194545







