Strength and Microstructural Characteristics of Fly Ash–Waste Glass Powder Ternary Blended Concrete
Abstract
1. Introduction
2. Materials and Methods
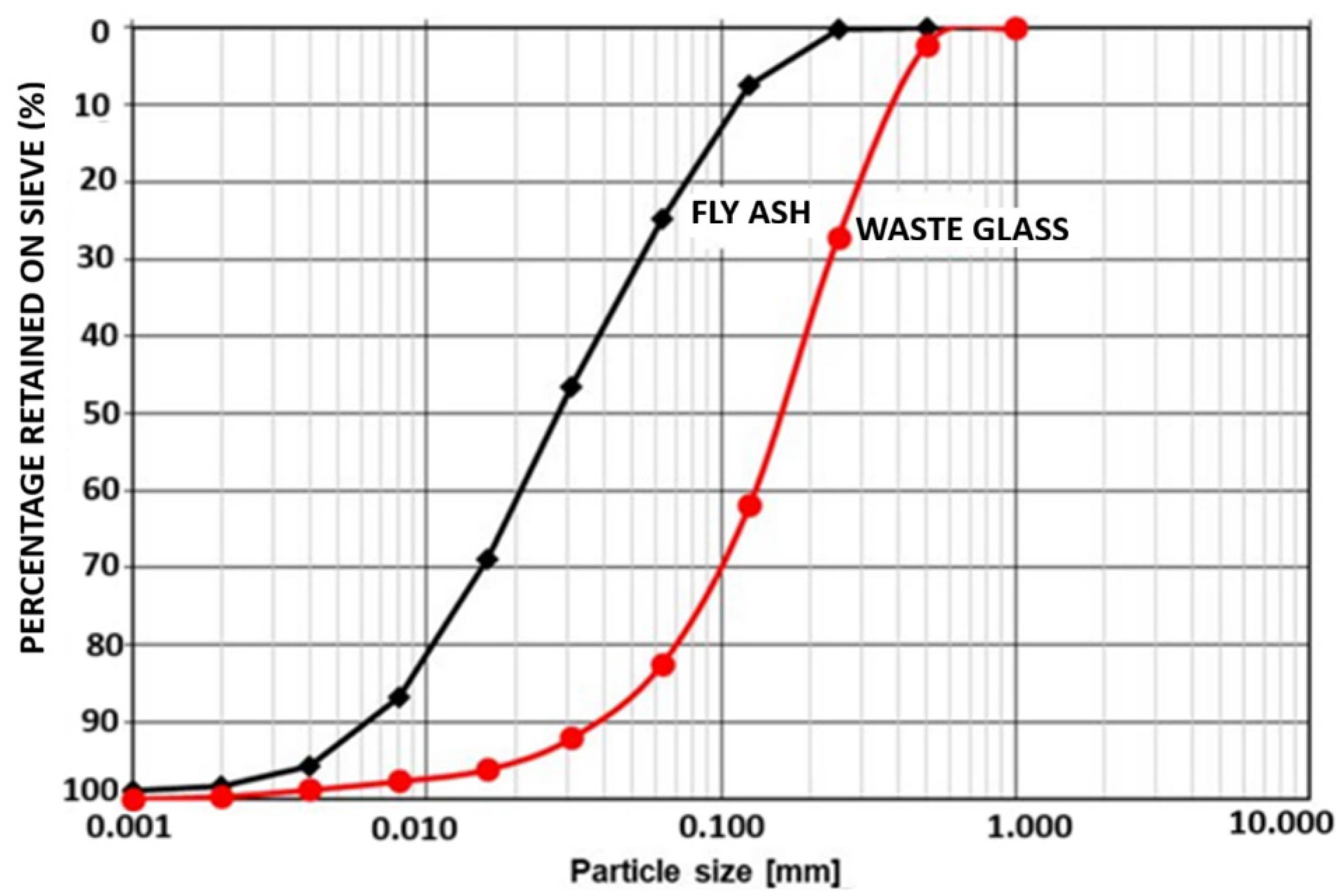
2.1. Binder Raw Materials
2.2. Aggregates
2.3. Superplasticizer
2.4. Mix Design and Sample Preparations
2.4.1. Mix Design
2.4.2. Sample Preparations
2.5. Sample Designation
2.6. Experimental Testing Method
2.6.1. Workability
2.6.2. Water Absorption
2.6.3. Compressive Strength Test
2.6.4. Characterization and Morphology of the Specimens
3. Discussion of Results
3.1. Workability of Glass–Fly Ash Ternary Blended Concrete
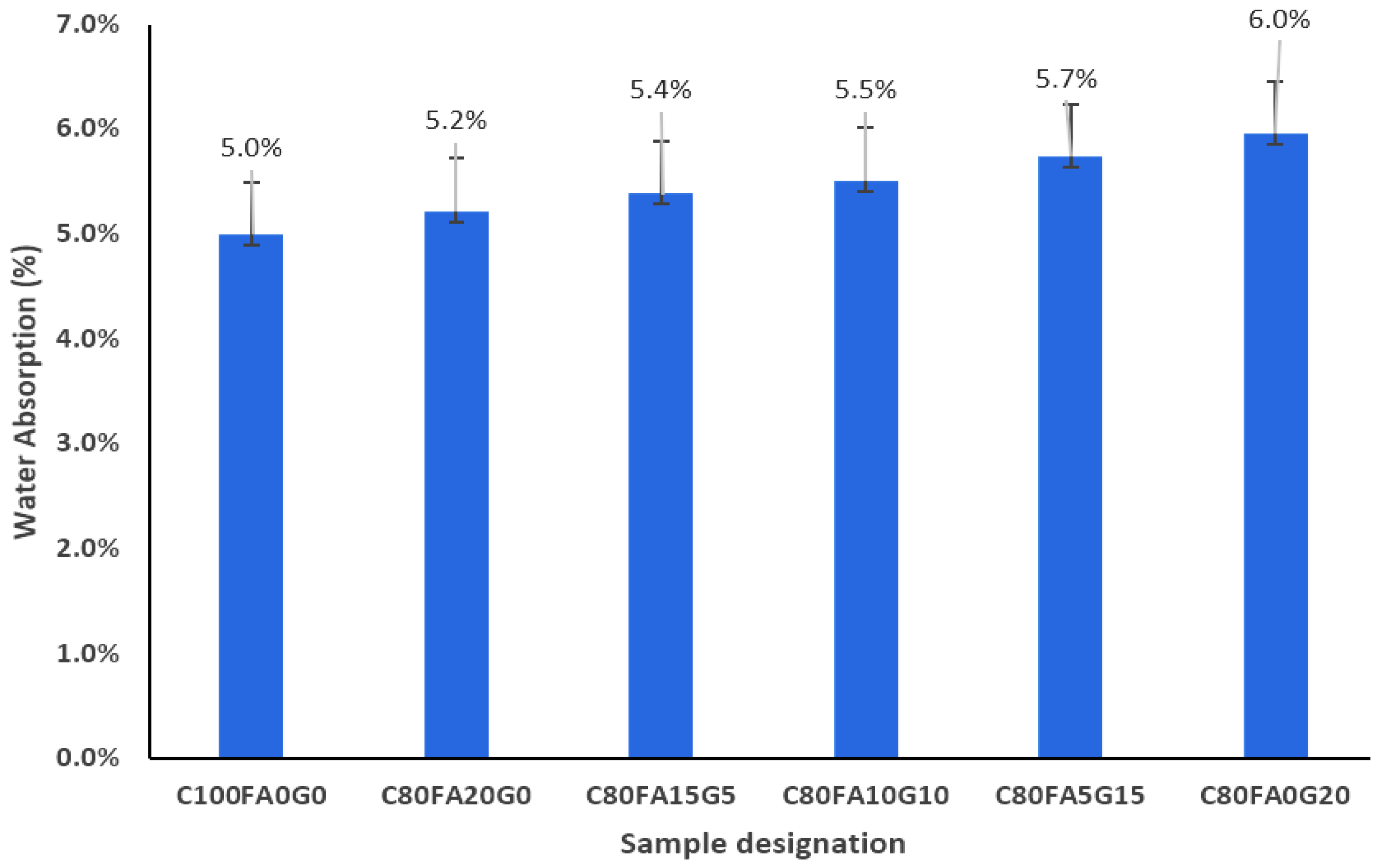
3.2. Absorption of Fly Ash–Waste Glass Ternary Blended Concrete
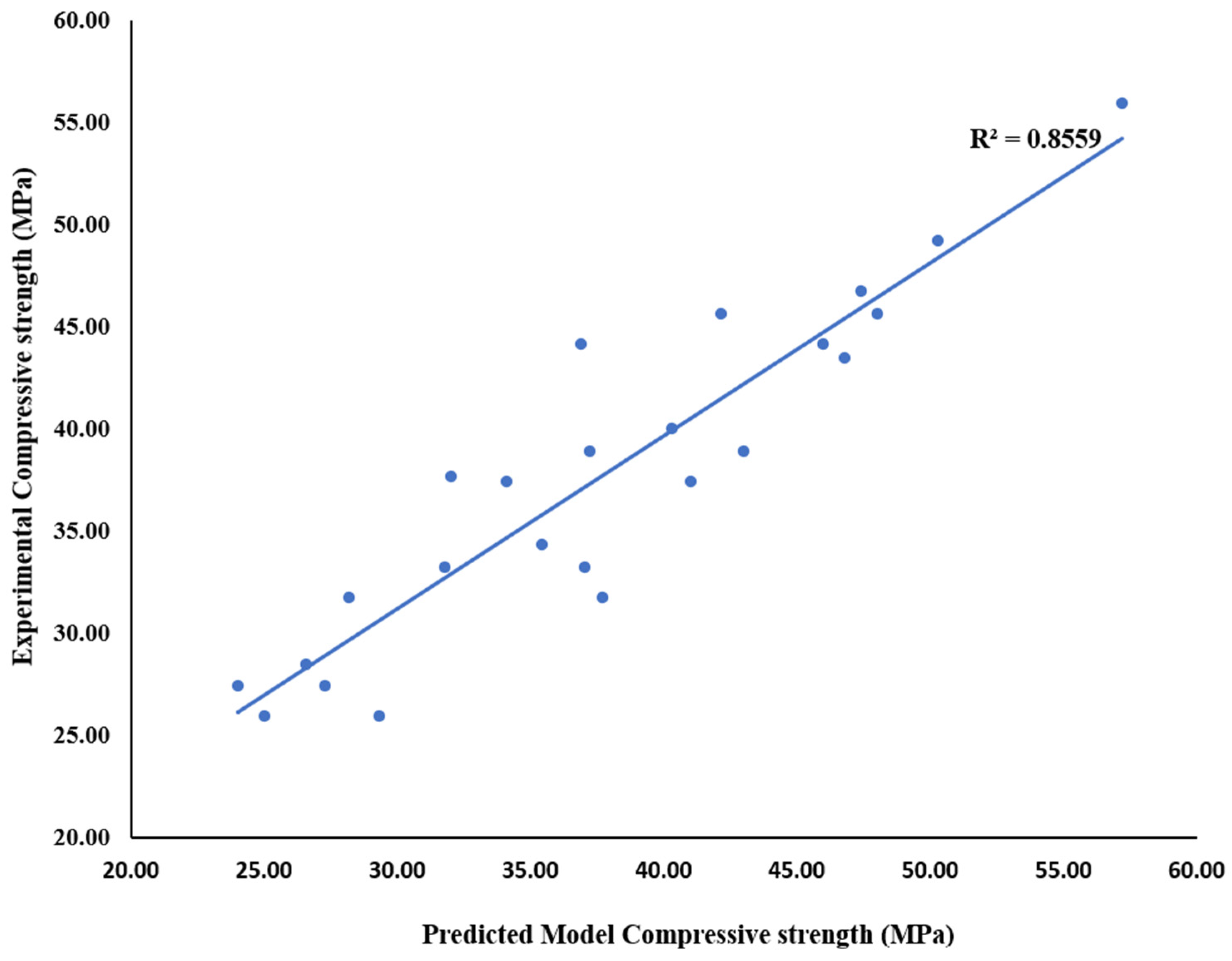
3.3. Compressive Strength of Glass–Fly Ash Ternary Blended Concrete
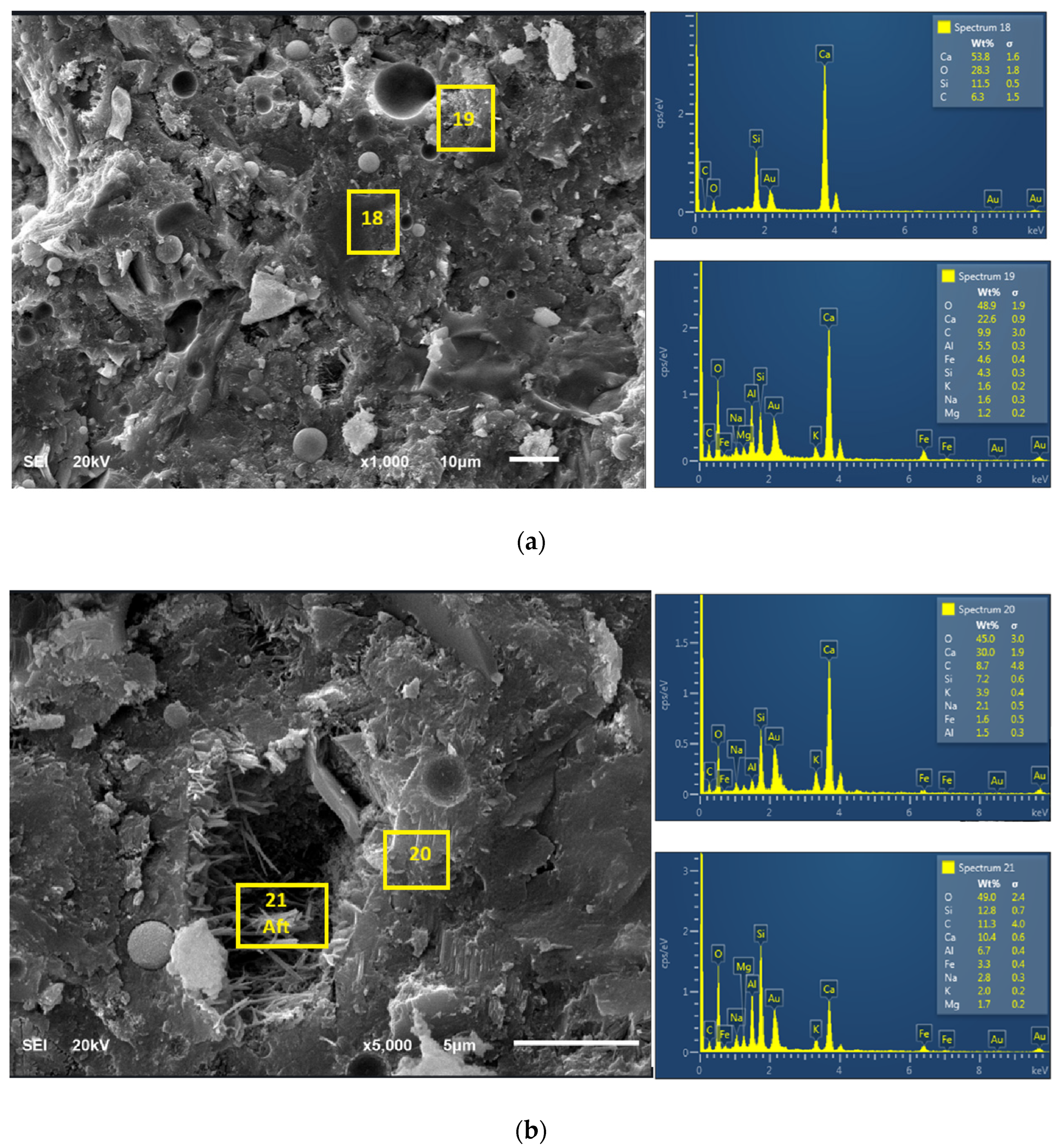
3.4. Microstructural Characteristics and Elemental Analyses of the Products
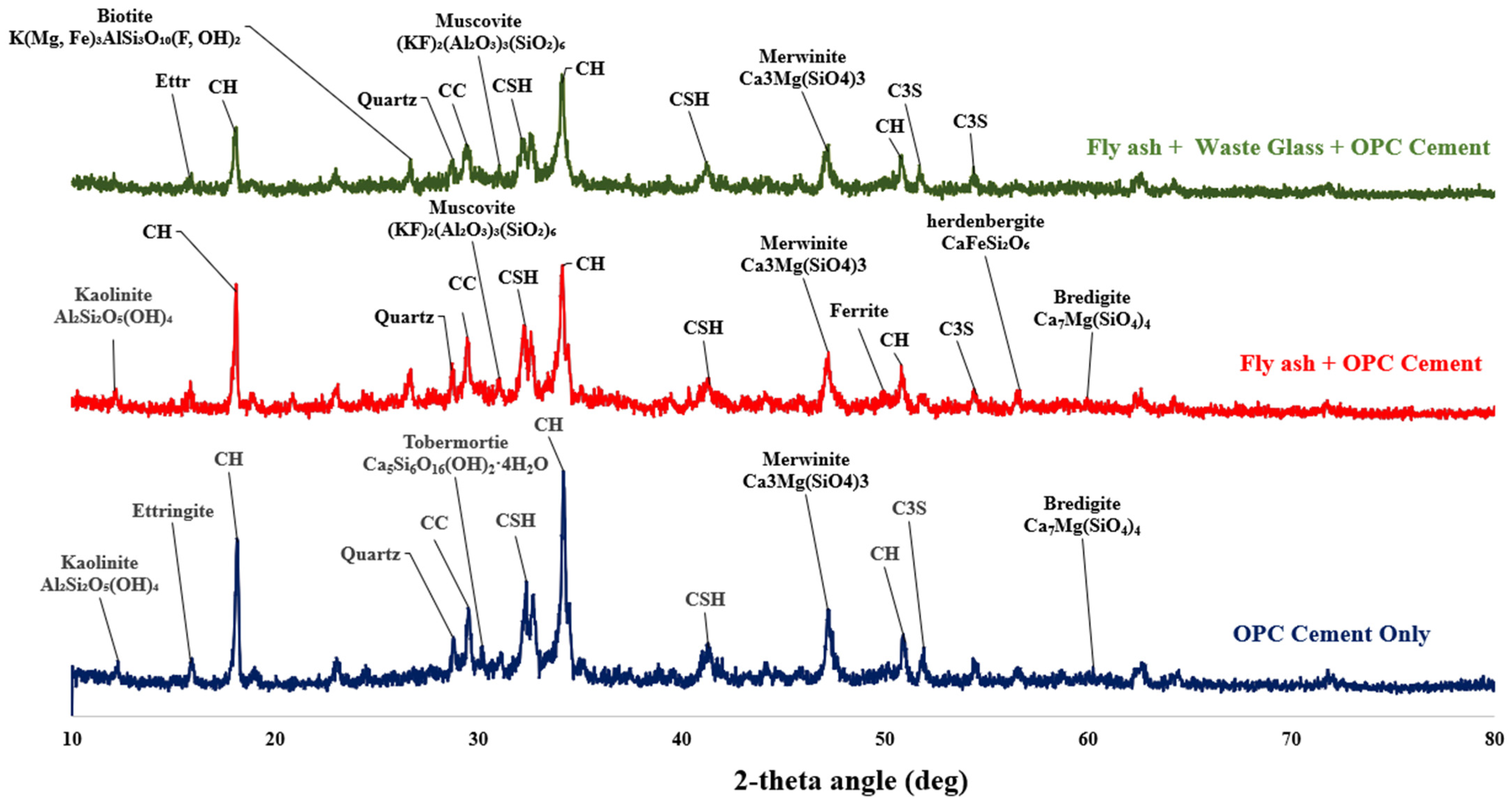
3.5. X-Ray Diffraction of the Fly Ash–Glass Ternary Blended Binder
3.6. Bond Characteristics of Binary (FA-OPC) and Ternary Blended (WG/FAP/OPC) Binders
4. Conclusions
- The impact of WG on FA-blended concrete in affecting the workability performance of the ternary blended concrete could vary depending on the WG/FA ratios in the mixture.
- When WG preponderates FA in the ternary blending, porosity and absorption improved due to differences in the particle size and oxide composition.
- Keeping FA and WG compositions within 20% will produce a structural concrete whose optimum value was 48 MPa at WG and FA contents of 15% and 5%, respectively.
- C80FA10WG10 had better morphological characteristics and microstructural density than C80FA20WG0 due to pore-filling effect.
- Energy-dispersive spectroscopy (EDS) indicates that C80FA10WG10 has higher Si/Al, Si/Ca, and Ca/Al ratios than C80FA20G0.
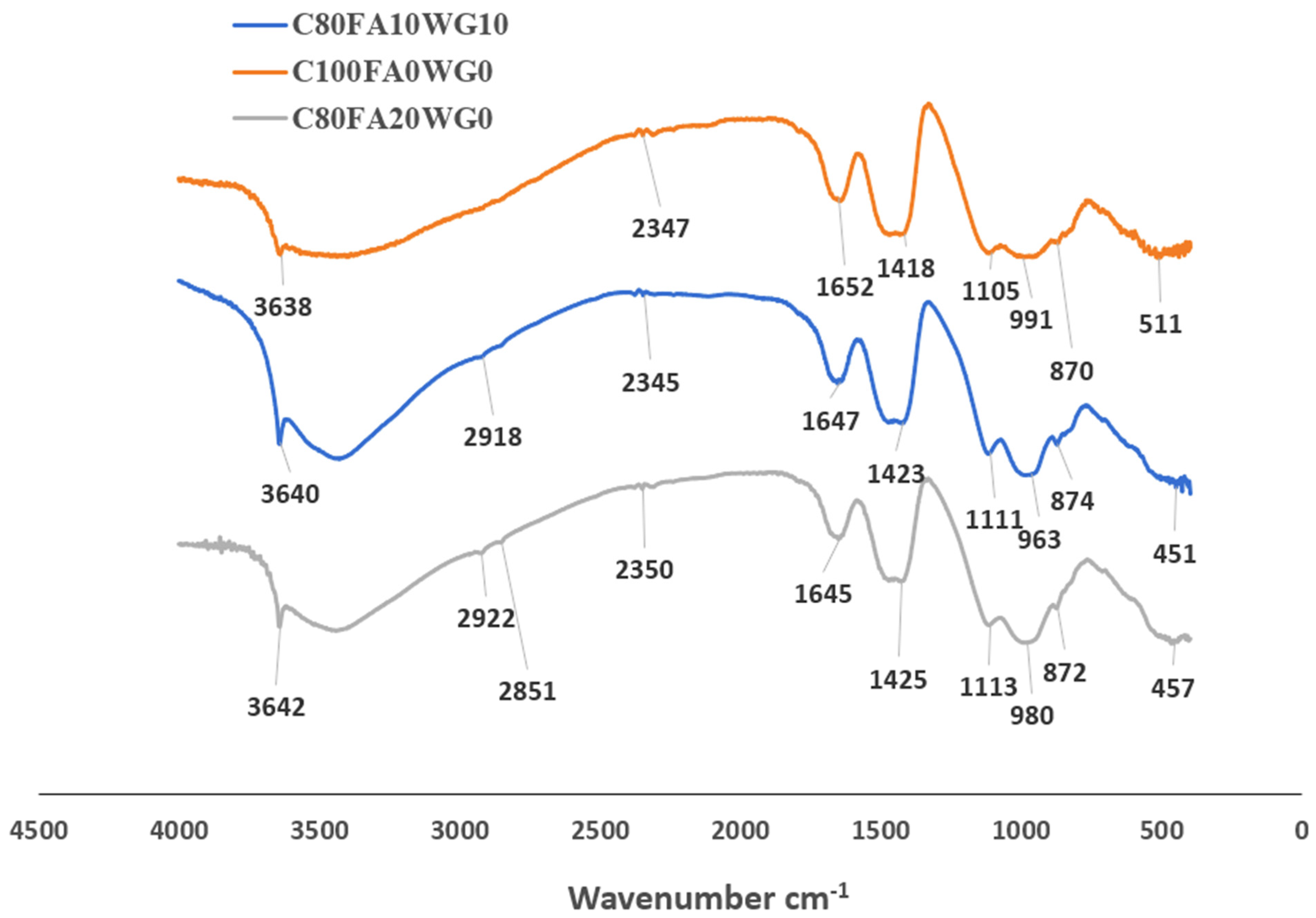
- Fourier transform infrared spectroscopy (FTIR) reveals that the compressive strength is directly related to Si-O asymmetric stretching vibrations. C100FA0WG0 has the highest frequencies of Si-O-Al bending vibration at wavenumber 511 cm−1 and capillary adsorbed water (H-O-H) asymmetric stretching vibration at 1652 cm−1 in comparison with binary (C80FA20WG0) and ternary (C80FA10WG10) blended systems.
- An X-ray diffractogram (XRD) indicates that WG contributed to the amorphous content of the ternary binder due to the disappearance of the hedenbergite phase (CaFeSi2O6) in C80FA10G10, which was found in C80FA20G0.
- There was a phase transformation when WG and FA were incorporated into the OPC system due to the disappearance of the tobermorite (C-S-H) phase and the formation of amorphous C-A-S-H.
- Finally, the use of these solid-waste additives (WG and FA) promotes a reduction in greenhouse gas (CO2) due to the partial replacement of OPC, thereby enhancing the reduction in landfills, valorization of solid waste, and economic concrete production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parande, A. Role of ingredients for high strength and high performance concrete—A review. Adv. Concr. Constr. 2013, 1, 151–162. [Google Scholar] [CrossRef]
- Chore, H.; Joshi, M. Strength evaluation of concrete with fly ash and GGBFS as cement replacing materials. Adv. Concr. Constr. 2015, 3, 223–236. [Google Scholar] [CrossRef]
- Clazer, B.; Garber, C.; Roose, C.; Syrett, P.; Youssef, C. Fly Ash in Concrete; The Perkin & Will Team: Chicago, IL, USA, 2011. [Google Scholar]
- Muhit, I.B.; Raihan, M.T. Nuruzzaman Determination of mortar strength using stone dust as a partially replaced material for cement and sand. Adv. Concr. Constr. 2014, 2, 249–259. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, H.; Li, A.; Li, G. Performance of waste glass powder as a pozzolanic material in blended cement mortar. Constr. Build. Mater. 2022, 324, 126531. [Google Scholar] [CrossRef]
- Tamanna, N.; Tuladhar, R. Sustainable Use of Recycled Glass Powder as Cement Replacement in Concrete. Open Waste Manag. J. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Cao, J.; Lu, J.; Jiang, L.; Wang, Z. Sinterability, microstructure and compressive strength of porous glass-ceramics from metallurgical silicon slag and waste glass. Ceram. Int. 2016, 42, 10079–10084. [Google Scholar] [CrossRef]
- Singh, B.; Jain, R. Use of waste glass in concrete: A review. J. Pharmacogn. Phytochem. 2018, 5, 96–99. [Google Scholar]
- Tan, K.H.; Du, H. Use of waste glass as sand in mortar: Part I—Fresh, mechanical and durability properties. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Zhao, H.; Poon, C.S.; Ling, T.C. Utilizing recycled cathode ray tube funnel glass sand as river sand replacement in the high-density concrete. J. Clean. Prod. 2013, 51, 184–190. [Google Scholar] [CrossRef]
- Islam, G.S.; Rahman, M.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Lu, J.-X.; Poon, C.S. Use of waste glass in alkali activated cement mortar. Constr. Build. Mater. 2018, 160, 399–407. [Google Scholar] [CrossRef]
- Rahma, A.; El Naber, N.; Ismail, S.I. Strength and the workability of concrete Effect of glass powder on the compression strength and the workability of concrete. Cogent Eng. 2017, 4, 1373415. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. An investigation into the hydration and microstructure of cement pastes modified with glass powders. Constr. Build. Mater. 2016, 112, 915–924. [Google Scholar] [CrossRef]
- Carsana, M.; Frassoni, M.; Bertolini, L. Comparison of ground waste glass with other supplementary cementitious materials. Cem. Concr. Compos. 2014, 45, 39–45. [Google Scholar] [CrossRef]
- Kawalu, N.; Naghizadeh, A.; Mahachi, J. The effect of glass waste as an aggregate on the compressive strength and durability of fly ash-based geopolymer mortar. MATEC Web Conf. 2022, 361, 05007. [Google Scholar] [CrossRef]
- Althoey, F.; Zaid, O.; Majdi, A.; Alsharari, F.; Alsulamy, S.; Arbili, M.M. RETRACTED: Effect of fly ash and waste glass powder as a fractional substitute on the performance of natural fibers reinforced concrete. Ain Shams Eng. J. 2023, 14, 102247. [Google Scholar] [CrossRef]
- Nadarajah, A.; Nasir, N.A.M.; Abu Bakar, N.; Safiee, N.A. Fly ash-GGBS blended geopolymer mortar for early engineering characteristic at ambient temperature. Ain Shams Eng. J. 2024, 15, 102821. [Google Scholar] [CrossRef]
- Hwang, K.; Noguchi, T.; Tomosawa, F. Prediction model of compressive strength development of fly-ash concrete. Cem. Concr. Res. 2004, 34, 2269–2276. [Google Scholar] [CrossRef]
- Sunil, B.; Manjunatha, L.; Ravi, L.; Yaragal, S.C. Potential use of mine tailings and fly ash in concrete. Adv. Concr. Constr. 2015, 3, 55–69. [Google Scholar] [CrossRef]
- Gökşen, Y.; Durak, U.; İlkentapar, S.; Atabey, İ.İ.; Kaya, M.; Karahan, O.; Duran Atiş, C. Synergistic effect of waste glass powder and fly ash on some properties of mortar and notably suppressing alkali-silica reaction. Rev. De La Constr. 2023, 22, 419–430. [Google Scholar] [CrossRef]
- Jurczak, R.; Szmatuła, F. Evaluation of the Possibility of Replacing Fly Ash with Glass Powder in Lower-Strength Concrete Mixes. Appl. Sci. 2021, 11, 396. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Bücker, M.; Bossert, J.; Marszalek, K. Glass matrix composites from coal flyash and waste glass. Waste Manag. 1997, 17, 39–45. [Google Scholar] [CrossRef]
- Erol, M.M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. The Recycling of the Coal Fly Ash in Glass Production. J. Environ. Sci. Heal. Part A 2006, 41, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Sunarsih, E.S.; Patanti, G.W.; Agustin, R.S.; Rahmawati, K. Utilization of Waste Glass and Fly Ash as a Replacement of Material Concrete. J. Phys. Conf. Ser. 2021, 1808, 012004. [Google Scholar] [CrossRef]
- Diamond, S.; Roy, C.D.M. Utilization of Flyash; Elsevier: Amsterdam, The Netherlands, 1984; pp. 455–462. [Google Scholar]
- Meyer, C.; Baxter, S. Use of Recycled Glass and Flyash for Precast Concrete. J. Mater. Civ. Eng. 1997, 11, 1388–1397. [Google Scholar]
- Škvára, F.; Kopecký, L.; Nemecek, J.; Bittnar, Z.D.E.N.I.K. Microstructure of geopolymer materials based on flyash. Ceramics-Silikaty 2006, 50, 208–215. [Google Scholar]
- Golewski, G.L. Assessing of water absorption on concrete composites containing fly ash up to 30% in regards to structures completely immersed in water. Case Stud. Constr. Mater. 2023, 19, e02337. [Google Scholar] [CrossRef]
- Guo, P.; Meng, W.; Nassif, H.; Gou, H.; Bao, Y. New perspectives on recycling waste glass in manufacturing concrete for sustainable civil infrastructure. Constr. Build. Mater. 2020, 257, 119579. [Google Scholar] [CrossRef]
- Wang, X.-Y. Effect of fly ash on properties evolution of cement based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- ASTM C150/C150M-19; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C33/C33M-18; Standard Specification for Concrete Aggregates. ASTM International: West Conshohocken, PA, USA, 2018.
- AlAteah, A.H.; Al-Sodani, K.A.A.; Yusuf, M.O.; Adewumi, A.A.; Al-Tholaia, M.M.H.; Bakare, A.O.; Momohjimoh, I.; Usman, A.K. Modelling of strength characteristics of silica fume/glass ternary blended concrete using destructive and non-destructive testing methods. Mater. Res. Technol. 2023, 22, 997–1013. [Google Scholar] [CrossRef]
- ASTM C143/C143M-12; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- BS EN 12390-3: 2019; Testing Hardened Concrete: Compressive Strength of Test Specimens. British Standards Institution: London, UK, 2019.
- Gatta, G.D.; Hålenius, U.; Bosi, F.; Cañadillas-Delgado, L.; Fernandez-Diaz, M.T. Minerals in cement chemistry: A single-crystal neutron diffraction study of ettringite, Ca6Al2(SO4)3(OH)12·27H2O. Am. Miner. 2019, 104, 73–78. [Google Scholar] [CrossRef]
- Richardson, I. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F. Alkali sorption by C-S-H and C-A-S-H gels. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Al-Sodani, K.A.A.; AlAteah, A.H.; Al-Tholaia, M.M.H.; Adewumi, A.A.; Bakare, A.O.; Usman, A.K.; Momohjimoh, I. Performances of the Synergy of Silica Fume and Waste Glass Powder in Ternary Blended Concrete. Appl. Sci. 2022, 12, 6637. [Google Scholar] [CrossRef]





| Oxide Composition | Glass | Fly Ash | OPC |
|---|---|---|---|
| SiO2 | 68.10 | 60.34 | 19.01 |
| Al2O3 | 0.90 | 28.11 | 4.68 |
| Fe2O3 | 0.60 | 3.71 | 3.20 |
| CaO | 14.50 | 1.34 | 66.89 |
| MgO | 1.80 | - | 0.81 |
| Na2O | 12.20 | 0.55 | 0.09 |
| TiO2 | 0.00 | - | 0.22 |
| K2O | 0.80 | 1.00 | 1.17 |
| P2O5 | - | - | 0.08 |
| SO3 | 0.40 | 0.80 | 3.66 |
| MnO2 | - | - | 0.19 |
| SiO2 + Al2O3 + Fe2O3 | 69.60 | 92.16 | 26.89 |
| SG | 2.48 | 2.38 | 3.14 |
| LOI (%) | 0.80 | 0.50 | 2.80 |
| Surface area (m2/g) | 0.223 | 420 | 0.33 |
| D10 (microns) | 40 | 6 | - |
| D50 (microns) | 160 | 27 | - |
| D90 (microns) | 300 | 120 | - |
| Coarse Aggregate Size (mm) | Percentage Composition |
|---|---|
| 10 | 30 |
| 12 | 20 |
| 14 | 30 |
| 20 | 20 |
| Materials | Specific Gravity Values |
|---|---|
| Cement | 3.14 |
| Glass | 2.48 |
| Fly ash | 2.38 |
| Sand | 2.71 |
| Coarse | 2.54 |
| Mixes | Fly Ash (%FAs) | Waste Glass (%WG) | OPC (%) | w/b Ratio | OPC (kg/m3) | Fly Ash (kg/m3) | WG (kg/m3) | Fine Agg. (kg/m3) | Coarse Agg. (kg/m3) | Water SSD | SP | Total Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C100FA0WG0 | 0 | 0 | 100 | 0.4 | 350 | 0.0 | 0.0 | 753 | 1120 | 175 | 1.80 | 2400 |
| C80FA20WG0 | 20 | 0 | 80 | 0.4 | 280 | 70.0 | 0.0 | 760 | 1120 | 168 | 1.80 | 2400 |
| C80FA15 WG5 | 15 | 5 | 80 | 0.4 | 280 | 52.5 | 17.5 | 760 | 1120 | 168 | 1.80 | 2400 |
| C80FA10 WG10 | 10 | 10 | 80 | 0.4 | 280 | 35.0 | 35.0 | 760 | 1120 | 168 | 1.80 | 2400 |
| C80FA5WG15 | 5 | 15 | 80 | 0.4 | 280 | 17.5 | 52.5 | 760 | 1120 | 168 | 1.80 | 2400 |
| C80FA0WG20 | 0 | 20 | 80 | 0.4 | 280 | 0.0 | 70 | 760 | 1120 | 168 | 1.80 | 2400 |
| Elemental Ratio | Fly Ash–OPC Paste C80FA20WG0 | Fly Ash–Glass–OPC Paste C80FA10WG10 |
|---|---|---|
| Ca/Si | 3.82 | 3.39 |
| Ca/Al | 11.46 | 12.52 |
| Si/Al | 3.00 | 3.69 |
| S/N | C80FA10WG10 | C100FA0WG0 | C80FA20WG0 | Functional Group Assignment |
|---|---|---|---|---|
| 1 | 3640 | 3638 | 3642 | O–H stretching (hydroxyl groups, adsorbed water) |
| 2 | 2918 | - | 2922 | C–H stretching (aliphatic compounds) |
| 3 | - | - | 2851 | C–H symmetric stretching |
| 4 | 2345 | 2347 | 2350 | CO2 asymmetric stretching (atmospheric CO2) |
| 5 | 1647 | 1652 | 1645 | H–O–H bending (water) |
| 6 | 1423 | 1418 | 1425 | C–O stretching/carbonate group (CO32−) |
| 7 | 1111 | 1105 | 1113 | Si–O–Si asymmetric stretching |
| 8 | 963 | 991 | 980 | Al–O or Si–O vibrations (aluminosilicates) |
| 9 | 874 | 870 | 872 | C–O bending (carbonates) |
| 10 | 451 | 511 | 457 | Si–O–Si bending vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusuf, M.O.; Al-Sodani, K.A.A.; Adewumi, A.A.; Abdulkareem, M.; Alateah, A.H. Strength and Microstructural Characteristics of Fly Ash–Waste Glass Powder Ternary Blended Concrete. Materials 2025, 18, 4483. https://doi.org/10.3390/ma18194483
Yusuf MO, Al-Sodani KAA, Adewumi AA, Abdulkareem M, Alateah AH. Strength and Microstructural Characteristics of Fly Ash–Waste Glass Powder Ternary Blended Concrete. Materials. 2025; 18(19):4483. https://doi.org/10.3390/ma18194483
Chicago/Turabian StyleYusuf, Moruf O., Khaled A. Alawi Al-Sodani, Adeshina A. Adewumi, Muyideen Abdulkareem, and Ali H. Alateah. 2025. "Strength and Microstructural Characteristics of Fly Ash–Waste Glass Powder Ternary Blended Concrete" Materials 18, no. 19: 4483. https://doi.org/10.3390/ma18194483
APA StyleYusuf, M. O., Al-Sodani, K. A. A., Adewumi, A. A., Abdulkareem, M., & Alateah, A. H. (2025). Strength and Microstructural Characteristics of Fly Ash–Waste Glass Powder Ternary Blended Concrete. Materials, 18(19), 4483. https://doi.org/10.3390/ma18194483









