Effect of Heat Treatment on In Vitro Cytotoxicity of Ti-Nb-Zr Gum Metal Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
2.2. Surface Characterization
2.3. Cell Viability Assessment
2.4. Statistical Analysis
3. Results and Discussion
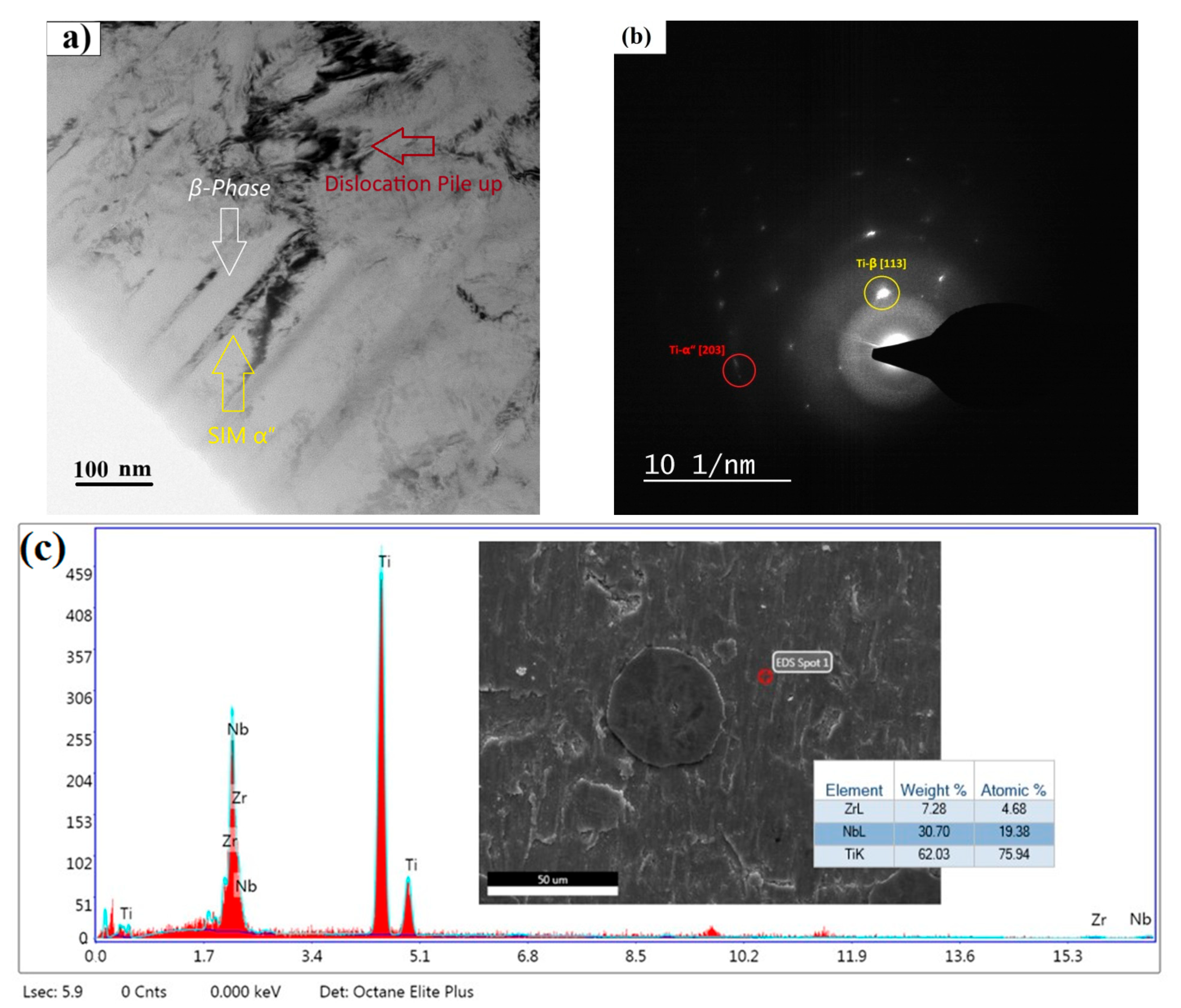
3.1. Morphological Features and Phase Analysis
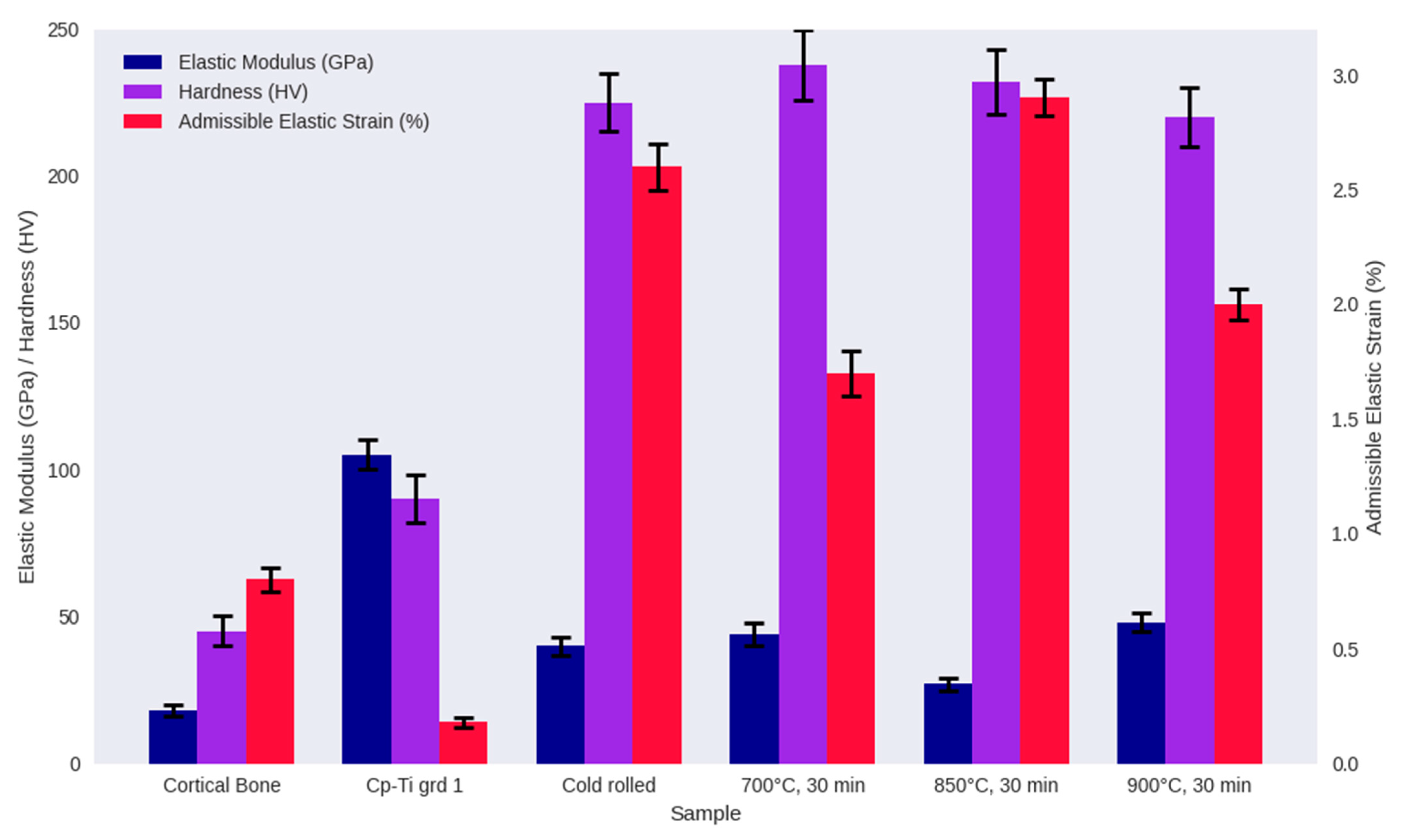
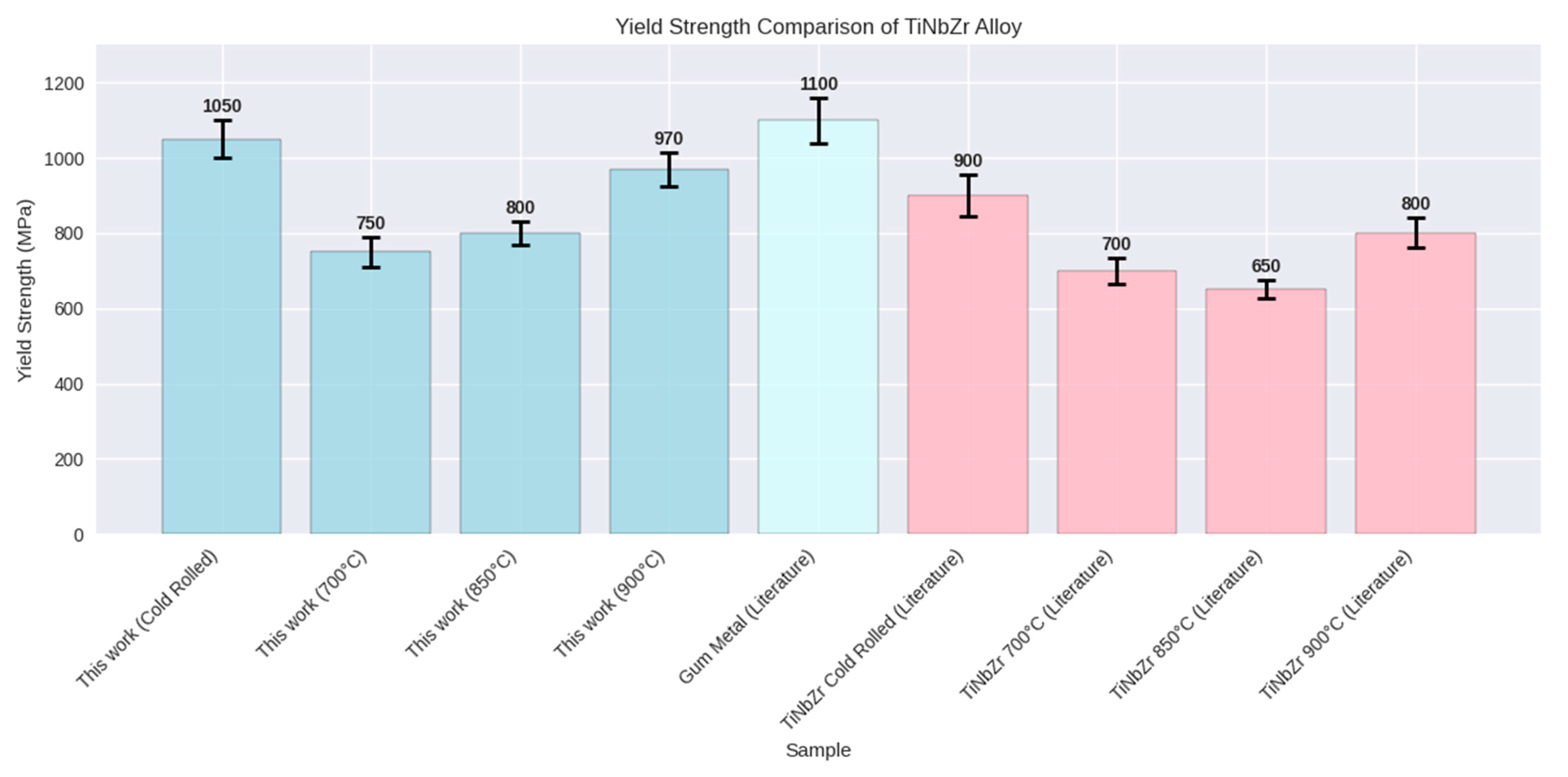
3.2. Mechanical Properties
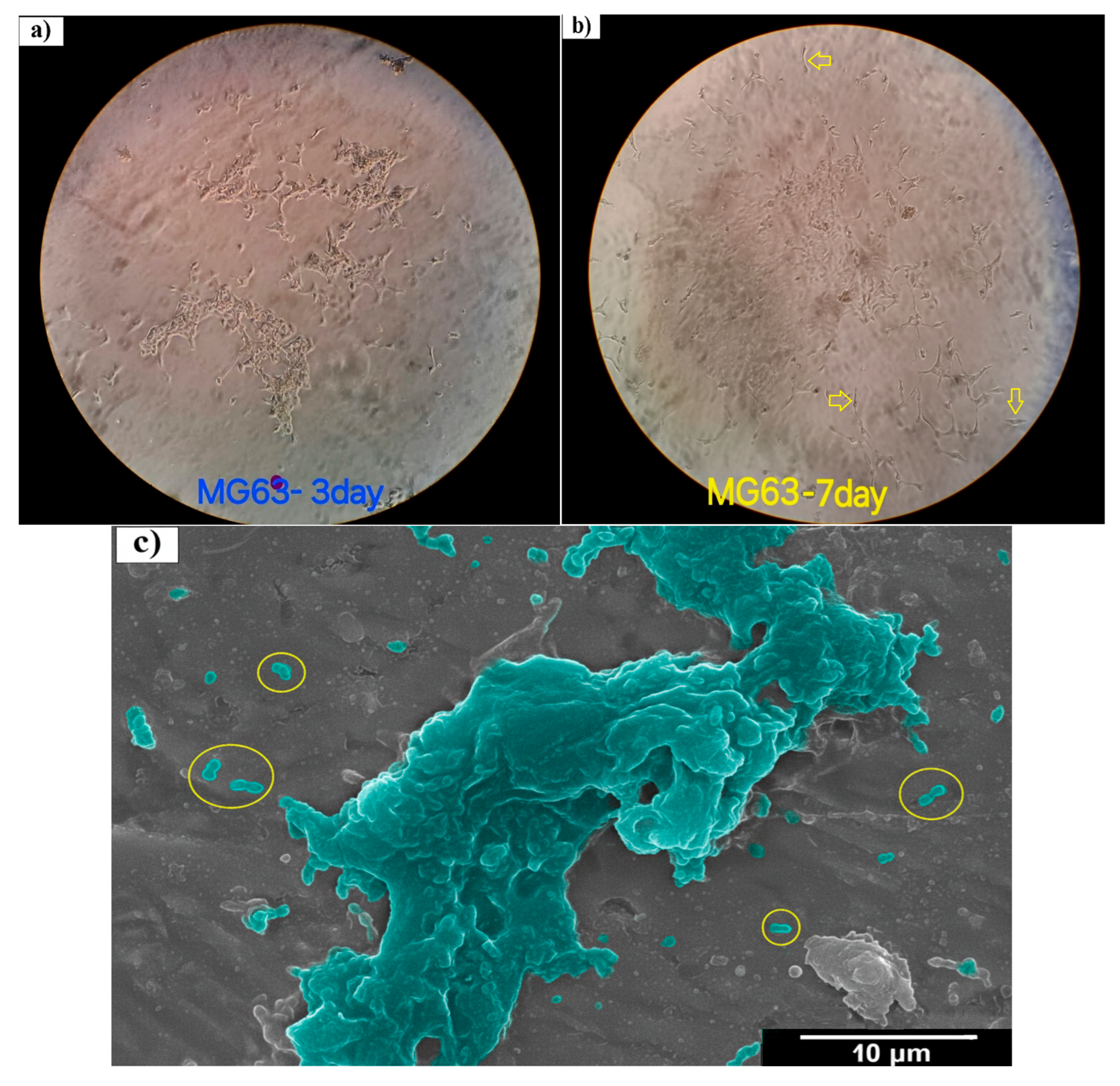
3.3. Cell Viability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Kanapaakala, G.; Subramani, V. A comprehensive review of Gum metal’s potential as a biomedical material. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2024, 238, 1200–1225. [Google Scholar] [CrossRef]
- Gordin, D.M.; Ion, R.; Vasilescu, C.; Drob, S.I.; Cimpean, A.; Gloriant, T. Potentiality of the “Gum Metal” titanium-based alloy for biomedical applications. Mater. Sci. Eng. C 2014, 44, 362–370. [Google Scholar] [CrossRef]
- Golasiński, K.M.; Detsch, R.; Szklarska, M.; Łosiewicz, B.; Zubko, M.; Mackiewicz, S.; Pieczyska, E.A.; Boccaccini, A.R. Evaluation of mechanical properties, in vitro corrosion resistance and biocompatibility of Gum Metal in the context of implant applications. J. Mech. Behav. Biomed. Mater. 2021, 115, 104289. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lopes Dias, N.F.; Pramanik, S.; Stangier, D.; Hoyer, K.-P.; Tillmann, W.; Schaper, M. Heat Treatments of Metastable β Titanium Alloy Ti-24Nb-4Zr-8Sn Processed by Laser Powder Bed Fusion. Materials 2022, 15, 3774. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Luo, H.; Wu, B.; Liu, W.; Rong, Y.; Chen, D.; Qin, Y.; Zhang, N.; Hao, F.; Deng, H.; et al. Effect of Heat Treatment on the Microstructure and Property of Metastable β Titanium Alloy. Materials 2024, 17, 6294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.N.; Muneshwar, P.; Singh, S.K.; Jha, A.K.; Pant, B. Thermo Mechanical Working and Heat Treatment Studies on Meta-Stable Beta Titanium Alloy (Ti15V3Al3Sn3Cr) Plates. Mater. Sci. Forum 2015, 830–831, 151–155. [Google Scholar] [CrossRef]
- Kopova, I.; Stráský, J.; Harcuba, P.; Landa, M.; Janeček, M.; Bačákova, L. Newly developed Ti–Nb–Zr–Ta–Si–Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Hara, M.; Shimizu, Y.; Yano, T.; Takesue, N.; Furuta, T.; Kuramoto, S. Mechanical anisotropy and ideal strength in a multifunctional Ti–Nb–Ta–Zr–O alloy (Gum Metal). Int. J. Mater. Res. 2009, 100, 345–348. [Google Scholar] [CrossRef]
- Liu, J.-P.; Wang, Y.-D.; Hao, Y.-L.; Wang, Y.; Nie, Z.-H.; Wang, D.; Ren, Y.; Lu, Z.-P.; Wang, J.; Wang, H.; et al. New intrinsic mechanism on gum-like superelasticity of multifunctional alloys. Sci. Rep. 2013, 3, 2156. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Plaine, A.H.; Pinotti, V.E.; Mazzer, E.M.; Bolfarini, C. A review of Gum Metal: Developments over the years and new perspectives. J. Mater. Res. 2023, 38, 96–111. [Google Scholar] [CrossRef]
- Furuta, T.; Kuramoto, S.; Hwang, J.; Nishino, K.; Saito, T. Elastic Deformation Behavior of Multi-Functional Ti–Nb–Ta–Zr–O Alloys. Mater. Trans. 2005, 46, 3001–3007. [Google Scholar] [CrossRef]
- Ozerov, M.; Sokolovsky, V.; Gazizova, M.; Povolyaeva, E.; Tagirov, D.; Yapryntsev, M.; Yunusov, F.; Nadezhdin, S. Biocompatibility, Corrosion Resistance, and Wear Resistance of TiNbZr-Based Composites Reinforced with Borides. Metals 2025, 15, 240. [Google Scholar] [CrossRef]
- Naganawa, T.; Ishihara, Y.; Iwata, T.; Koide, M.; Ohguchi, M.; Ohguchi, Y.; Murase, Y.; Kamei, H.; Sato, N.; Mizuno, M.; et al. In Vitro Biocompatibility of a New Titanium-29Niobium-13Tantalum-4.6Zirconium Alloy With Osteoblast-Like MG63 Cells. J. Periodontol. 2004, 75, 1701–1707. [Google Scholar] [CrossRef]
- García-Hernández, C.; García-Cabezón, C.; González-Diez, F.; Ampudia, M.; Juanes-Gusano, D.; Rodriguez-Cabello, J.C.; Martín-Pedrosa, F. Effect of processing on microstructure, mechanical properties, corrosion and biocompatibility of additive manufacturing Ti-6Al-4V orthopaedic implants. Sci. Rep. 2025, 15, 14087. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Yamaguchi, S.; Kizuki, T.; Matsushita, T.; Niinomi, M.; Kokubo, T.; et al. Bone bonding bioactivity of Ti metal and Ti–Zr–Nb–Ta alloys with Ca ions incorporated on their surfaces by simple chemical and heat treatments. Acta Biomater. 2011, 7, 1379–1386. [Google Scholar] [CrossRef]
- Divakarla, S.K.; Yamaguchi, S.; Kokubo, T.; Han, D.-W.; Lee, J.H.; Chrzanowski, W. Improved bioactivity of GUMMETAL®, Ti59Nb36Ta2Zr3O0.3, via formation of nanostructured surfaces. J. Tissue Eng. 2018, 9, 2041731418774178. [Google Scholar] [CrossRef]
- King, B.M. Analysis of Variance. In International Encyclopedia of Education, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 32–36. [Google Scholar] [CrossRef]
- Bozzolo, N.; Dewobroto, N.; Wenk, H.R.; Wagner, F. Microstructure and microtexture of highly cold-rolled commercially pure titanium. J. Mater. Sci. 2007, 42, 2405–2416. [Google Scholar] [CrossRef]
- Ogawa, T.; Dannoshita, H.; Maruoka, K.; Ushioda, K. Microstructural Evolution During Cold Rolling and Subsequent Annealing in Low-Carbon Steel with Different Initial Microstructures. J. Mater. Eng. Perform. 2017, 26, 3821–3830. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Xie, Z.; Kong, C.; Yu, H. Grain Growth Mechanism of Lamellar-Structure High-Purity Nickel via Cold Rolling and Cryorolling during Annealing. Materials 2021, 14, 4025. [Google Scholar] [CrossRef]
- He, F.; Yang, S.; Cao, J. Effect of Cold Rolling and Aging on the Microstructure and Mechanical Properties of Ti-Nb-Zr Alloy. J. Mater. Eng. Perform. 2020, 29, 3411–3419. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Li, Y.; Zhang, Y.; Munir, K.; Jiang, H.; Wen, C. Deformation mechanism and mechanical properties of a thermomechanically processed β Ti–28Nb–35.4Zr alloy. J. Mech. Behav. Biomed. Mater. 2018, 78, 224–234. [Google Scholar] [CrossRef]
- Tabei, A.; Startt, J.; Hoffman, R.T.; Yavari, E.; Deo, C.; Garmestani, H. Microstructural Evolution in Hot and Cold-Rolled Ti-Nb Alloy. J. Mater. Eng. Perform. 2017, 26, 61–68. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. Intra-granular alpha precipitation in Ti–Nb–Zr–Ta biomedical alloys. J. Mater. Sci. 2009, 44, 808–815. [Google Scholar] [CrossRef]
- Dan, A.; Angelescu, M.L.; Serban, N.; Cojocaru, E.M.; Zarnescu-Ivan, N.; Cojocaru, V.D.; Galbinasu, B.M. Evolution of Microstructural and Mechanical Properties during Cold-Rolling Deformation of a Biocompatible Ti-Nb-Zr-Ta Alloy. Materials 2022, 15, 3580. [Google Scholar] [CrossRef]
- Dai, Y.; Song, M. Microstructural Evolution and Phase Transformation of a Ti-5Nb-5Al Alloy During Annealing Treatment. Mater. Res. 2019, 22, e20190507. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Azim, F.; Khan, S.J. Effect of Nb on β → α″ Martensitic Phase Transformation and Characterization of New Biomedical Ti-xNb-3Fe-9Zr Alloys. Scanning 2021, 2021, 8173425. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gao, Y.; Jiang, W.; Xiao, W.; Zhao, X.; Ma, C. A Review of Deformation Mechanisms, Compositional Design, and Development of Titanium Alloys with Transformation-Induced Plasticity and Twinning-Induced Plasticity Effects. Metals 2024, 14, 97. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, F.; Chen, Z.; Yang, Y.; Shen, B.; Li, J.; Prima, F. Strong and ductile beta Ti–18Zr–13Mo alloy with multimodal twinning. Mater. Res. Lett. 2019, 7, 251–257. [Google Scholar] [CrossRef]
- Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Dybała, B.; Poradowski, D.; Janeczek, M. Assessment of Mechanical, Chemical, and Biological Properties of Ti-Nb-Zr Alloy for Medical Applications. Materials 2020, 14, 126. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Lin, J.; Munir, K.; Ozan, S.; Li, Y.; Wen, C. An investigation of the mechanical and microstructural evolution of a TiNbZr alloy with varied ageing time. Sci. Rep. 2018, 8, 5737. [Google Scholar] [CrossRef]
- Cao, M.Z.; He, B.B. A review on deformation mechanisms of metastable β titanium alloys. J. Mater. Sci. 2024, 59, 14981–15016. [Google Scholar] [CrossRef]
- Yao, T.; Du, K.; Wang, H.; Huang, Z.; Li, C.; Li, L.; Hao, Y.; Yang, R.; Ye, H. In situ scanning and transmission electron microscopy investigation on plastic deformation in a metastable β titanium alloy. Acta Mater. 2017, 133, 21–29. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Zhao, X.; Wei, Q.; Xu, H. Design and fabrication of a metastable β-type titanium alloy with ultralow elastic modulus and high strength. Sci. Rep. 2015, 5, 14688. [Google Scholar] [CrossRef] [PubMed]
- Forderhase, A.G.; Ligons, L.A.; Norwood, E.; McCarty, G.S.; Sombers, L.A. Optimized Fabrication of Carbon-Fiber Microbiosensors for Codetection of Glucose and Dopamine in Brain Tissue. ACS Sens. 2024, 9, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Salloom, R.; Mantri, S.A.; Banerjee, R.; Srinivasan, S.G. First principles computation of composition dependent elastic constants of omega in titanium alloys: Implications on mechanical behavior. Sci. Rep. 2021, 11, 12005. [Google Scholar] [CrossRef]
- Ballor, J.; Li, T.; Prima, F.; Boehlert, C.J.; Devaraj, A. A review of the metastable omega phase in beta titanium alloys: The phase transformation mechanisms and its effect on mechanical properties. Int. Mater. Rev. 2023, 68, 26–45. [Google Scholar] [CrossRef]
- Guo, A.X.Y.; Geng, C.; Lin, Z.; Cao, S.C. A Review of Research Progress on ω-phase in Titanium Alloys. J. Mater. Sci. Eng. 2022, 11, 620. [Google Scholar]
- Guo, Y.; Wei, S.; Yang, S.; Ke, Y.; Zhang, X.; Zhou, K. Precipitation Behavior of ω Phase and ω→α Transformation in Near β Ti-5Al-5Mo-5V-1Cr-1Fe Alloy during Aging Process. Metals 2021, 11, 273. [Google Scholar] [CrossRef]
- Yang, C.; Yang, S.J.; Chen, L.Q.; Dong, H.K.; Duan, X.B.; Yu, C.Y.; Zhang, W.W.; Liu, L.H. Low modulus Ti-rich biocompatible TiNbZrTaHf concentrated alloys with exceptional plasticity. Mater. Res. Lett. 2023, 11, 604–612. [Google Scholar] [CrossRef]
- Nocivin, A.; Cinca, I.; Raducanu, D.; Cojocaru, V.D.; Popovici, I.A. Mechanical properties of a Gum-type Ti–Nb–Zr–Fe–O alloy. Int. J. Miner. Metall. Mater. 2017, 24, 909–917. [Google Scholar] [CrossRef]
- Dal Bó, M.R.; Salvador, C.A.F.; Mello, M.G.; Lima, D.D.; Faria, G.A.; Ramirez, A.J.; Caram, R. The effect of Zr and Sn additions on the microstructure of Ti-Nb-Fe gum metals with high elastic admissible strain. Mater. Des. 2018, 160, 1186–1195. [Google Scholar] [CrossRef]





| Test | Specimen Shape | Dimensions (mm) |
|---|---|---|
| Optical microscopy (OM) | Plate | 10 × 10 × 0.25 |
| Vickers micro hardness | Flat surface from sheet | 10 × 10 × 0.25 |
| X-ray diffraction (XRD) | Flat-cut plate | 10 × 10 × 0.25 |
| Transmission electron microscopy (TEM) | Thin disc | Diameter: 3 |
| Thickness < 0.1 | ||
| Cell viability assessment | Plate | 5 × 5 × 0.25 |
| Field emission scanning electron microscopy (FE-SEM) | Rectangular plate | 5 × 5 × 0.25 |
| Uniaxial tensile test | Dog-bone/rectangular | Gauge length: 15 |
| Width: 3 | ||
| Thickness: 0.25 | ||
| Total length: 45 | ||
| Fillet radius: 3 | ||
| Width of ends: 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etemad, A.; Hasani, S.; Mashreghi, A.; Heidari, F.; Salehikahrizsangi, P.; Schwarz, S.; Bloch, K.; Nabialek, M. Effect of Heat Treatment on In Vitro Cytotoxicity of Ti-Nb-Zr Gum Metal Alloy. Materials 2025, 18, 4473. https://doi.org/10.3390/ma18194473
Etemad A, Hasani S, Mashreghi A, Heidari F, Salehikahrizsangi P, Schwarz S, Bloch K, Nabialek M. Effect of Heat Treatment on In Vitro Cytotoxicity of Ti-Nb-Zr Gum Metal Alloy. Materials. 2025; 18(19):4473. https://doi.org/10.3390/ma18194473
Chicago/Turabian StyleEtemad, Arash, Saeed Hasani, Alireza Mashreghi, Fariba Heidari, Parinaz Salehikahrizsangi, Sabine Schwarz, Katarzyna Bloch, and Marcin Nabialek. 2025. "Effect of Heat Treatment on In Vitro Cytotoxicity of Ti-Nb-Zr Gum Metal Alloy" Materials 18, no. 19: 4473. https://doi.org/10.3390/ma18194473
APA StyleEtemad, A., Hasani, S., Mashreghi, A., Heidari, F., Salehikahrizsangi, P., Schwarz, S., Bloch, K., & Nabialek, M. (2025). Effect of Heat Treatment on In Vitro Cytotoxicity of Ti-Nb-Zr Gum Metal Alloy. Materials, 18(19), 4473. https://doi.org/10.3390/ma18194473









