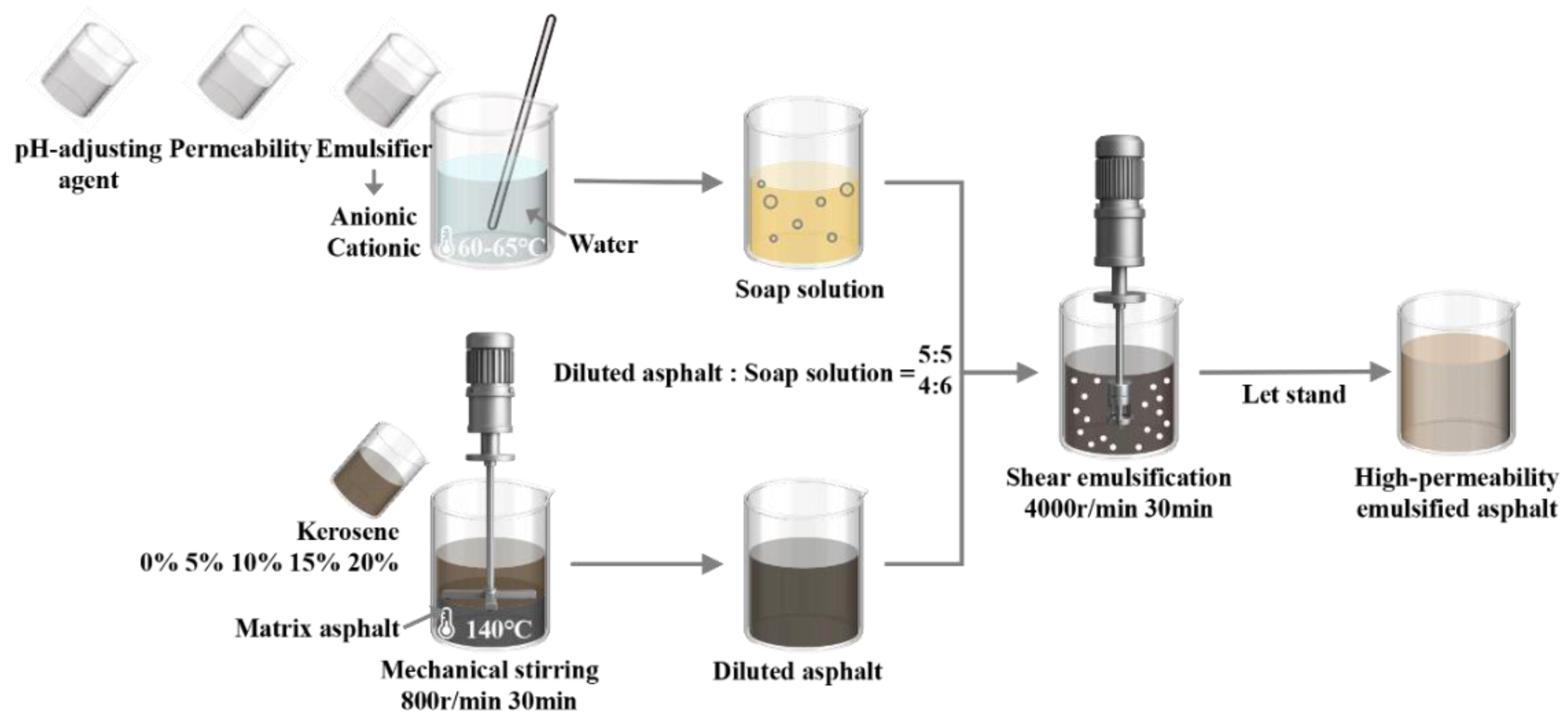
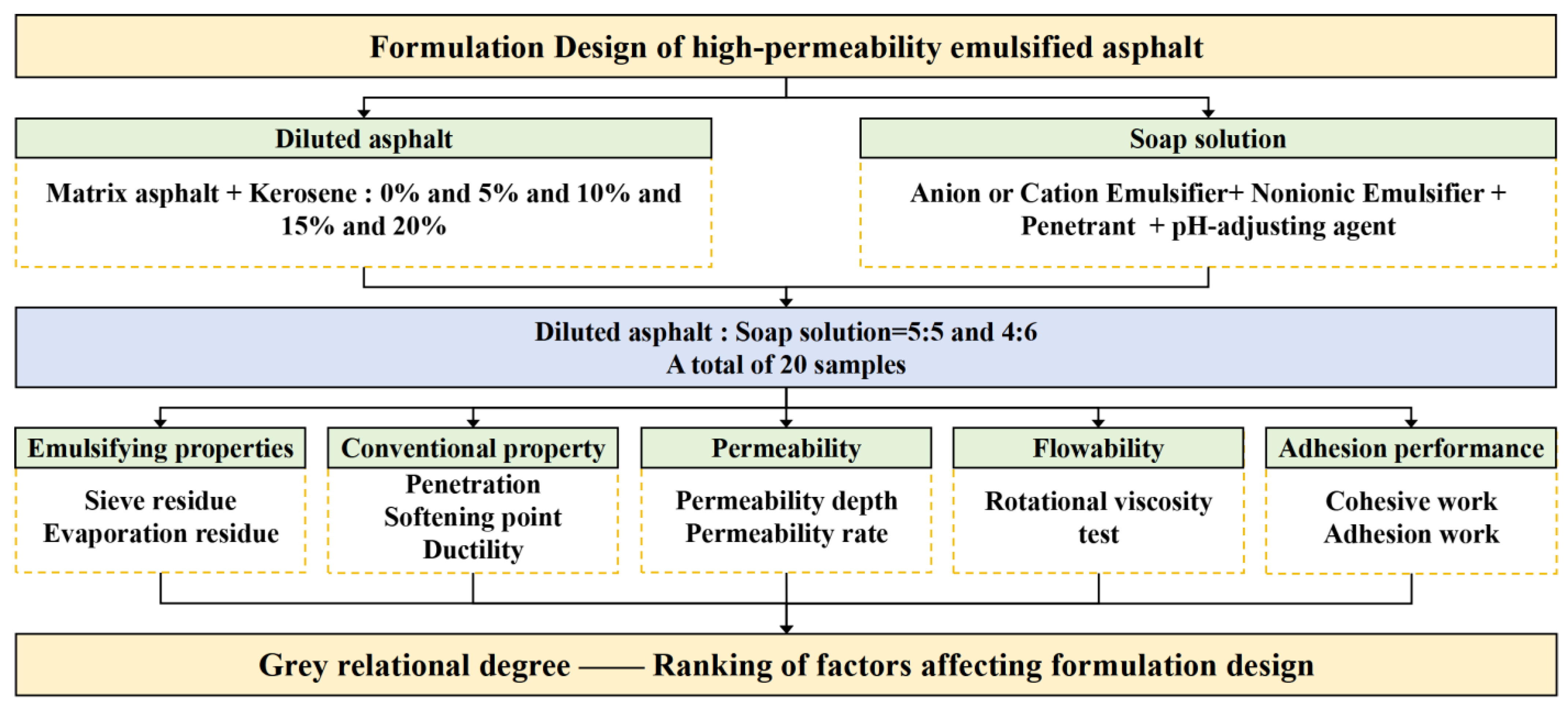
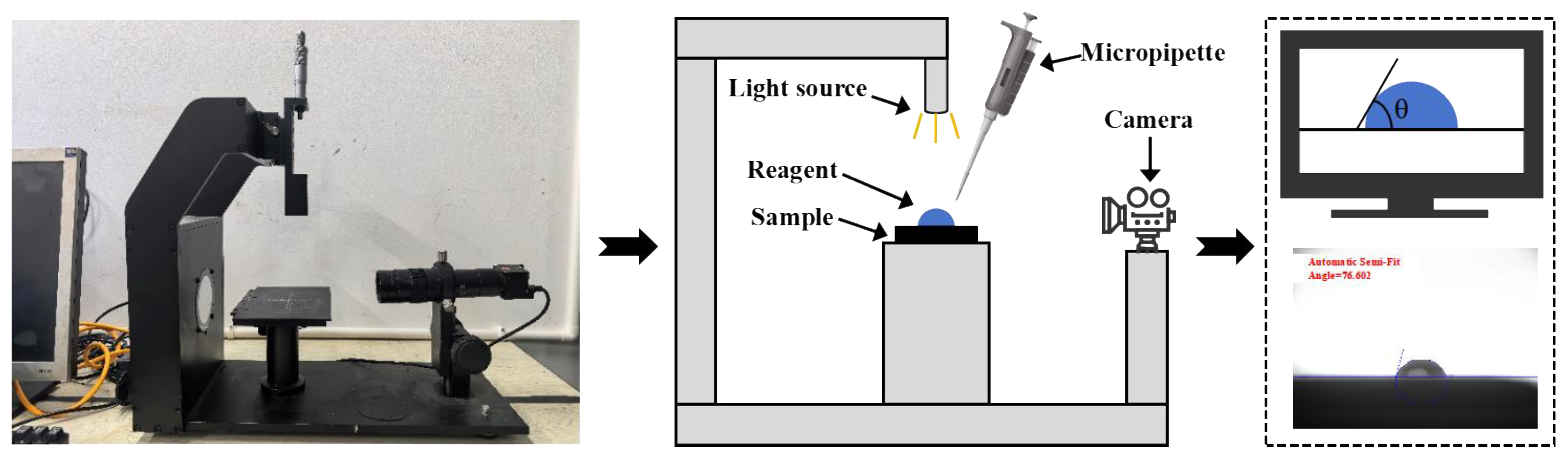
3.1. Fundamental Physical Performances
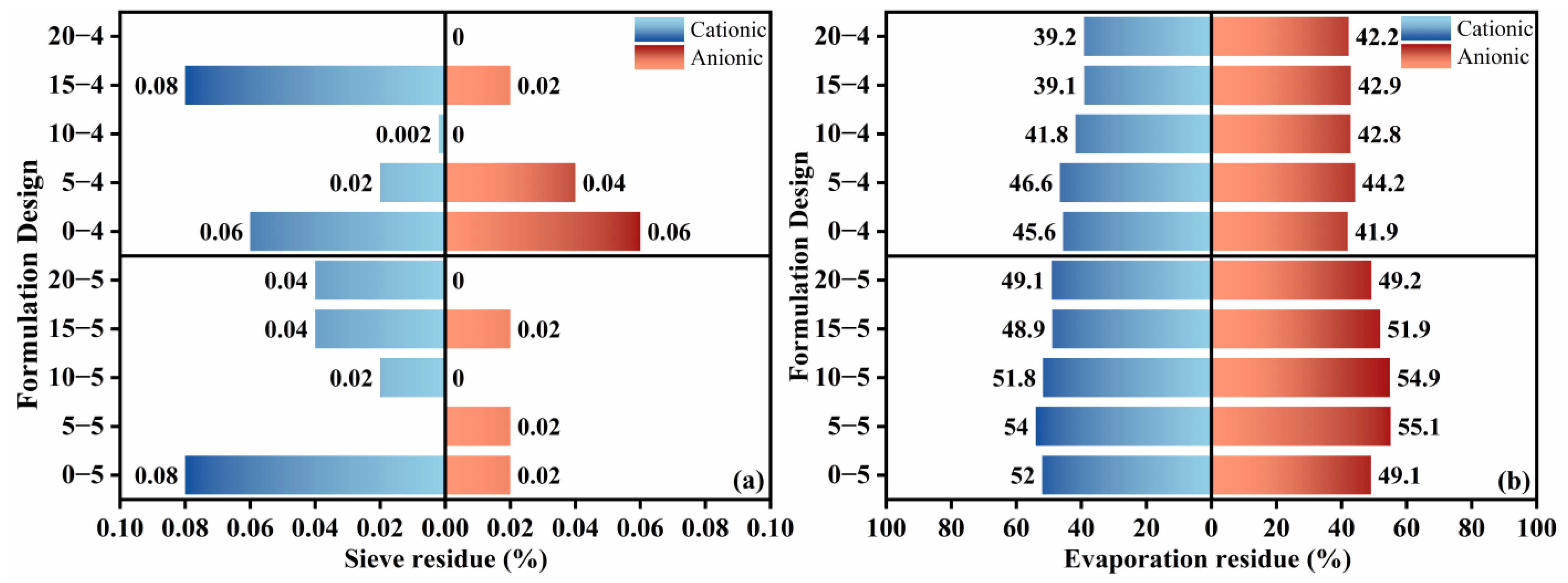
The fundamental physical performances of the high-permeability emulsified asphalt formulations were evaluated through residue on sieve, evaporation residue content, and the penetration, softening point, and ductility of the evaporation residue. The residue on the sieve test examines particle dispersion and uniformity within the emulsion, while the evaporation residue reflects the mass of usable asphalt, with the results presented in
Figure 6. In the sieve residue test, anionic emulsions exhibited markedly lower residue than their cationic counterparts, indicating that anionic emulsifiers promote more complete mixing and emulsification of asphalt with the soap solution. With increasing kerosene dosage, residue on the sieve generally declined, and higher diluted asphalt content yielded better emulsification quality. Notably, adding a small amount of kerosene increased the evaporation residue content, whereas further increases in kerosene led to a decrease in evaporation residue, consistent with previous findings that kerosene addition can reduce the evaporation residue of emulsified asphalt [
14]. Increasing the diluted asphalt content raised the evaporation residue content, in agreement with prior studies [
13], while the effect of emulsifier ionic type on evaporation residue was comparatively limited. Because the evaporation residue represents the effective material in emulsified asphalt, introducing a small kerosene dosage can reduce cost while maintaining effectiveness. To ensure construction quality and enhance emulsification, anionic emulsifiers are recommended as the first choice.
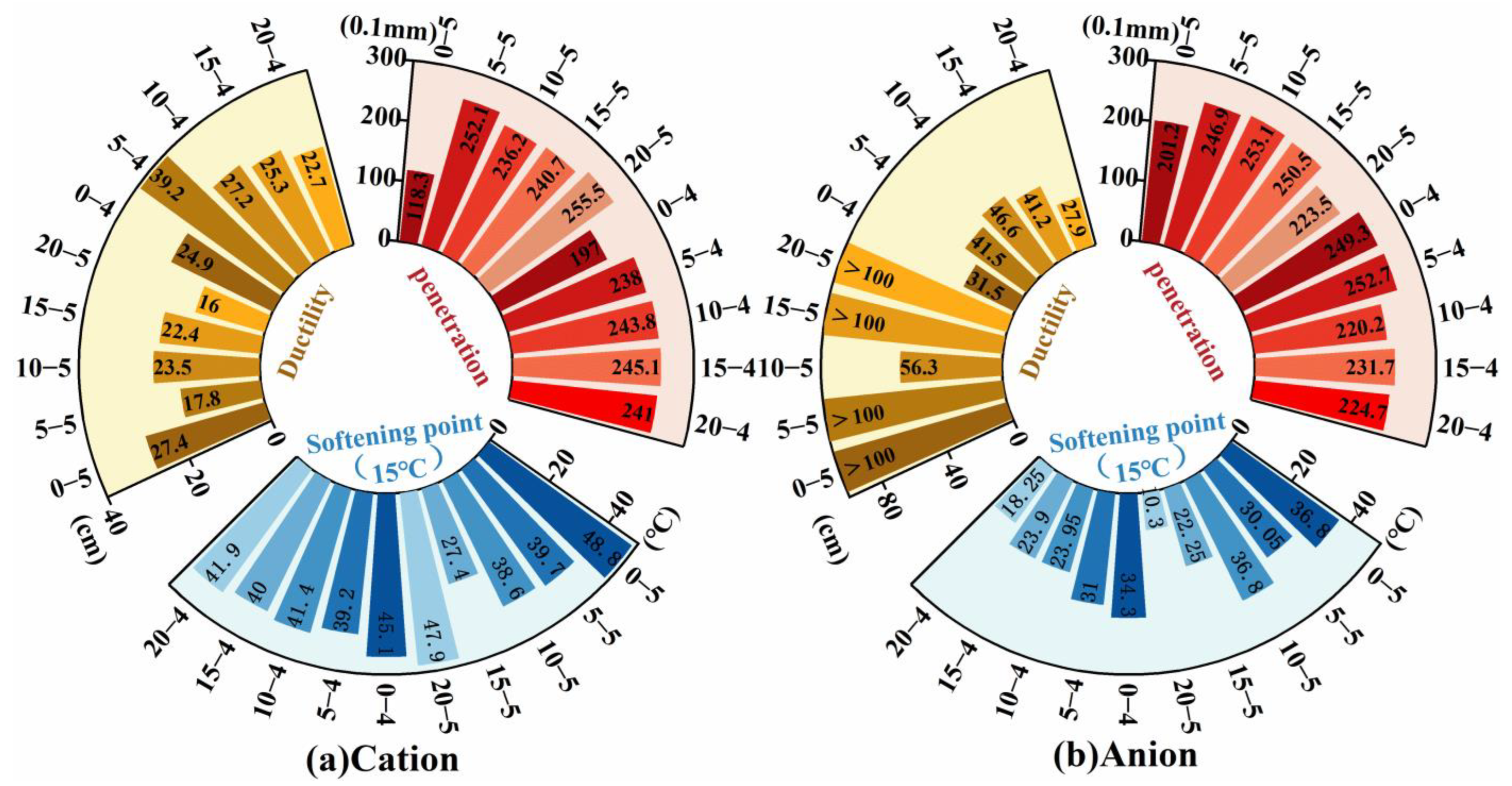
The penetration, softening point, and ductility of the evaporation residue were then assessed (
Figure 7). Overall, the softening point and ductility were strongly influenced by the emulsifier ionic type: cationic emulsions produced higher softening points, whereas anionic emulsions delivered greater ductility. Adding kerosene increased penetration, reduced the softening point, and caused ductility to first rise and then fall. Ductility decreased as the diluted asphalt content decreased. The results indicate that cationic emulsifiers can effectively improve high-temperature stability, while anionic emulsifiers substantially enhance low-temperature extensibility and crack resistance, in several anionic formulations, ductility exceeded 100 cm. Consistent with earlier reports [
1,
14], kerosene addition weakened deformation resistance and high-temperature stability by lowering asphalt viscosity and inter-particle interactions. Importantly, reducing the diluted asphalt content partially mitigated kerosene-induced reductions in deformation resistance. The influence of diluted asphalt content on low-temperature performance differed by ionic type: for cationic emulsions, plastic deformation capacity declined with decreasing diluted asphalt content, whereas the opposite trend was observed for anionic emulsions. The different low-temperature performance of cationic and anionic systems with varying liquefied bitumen contents suggests distinct demulsification mechanisms and residual emulsifier effects [
29,
30]. However, this interpretation requires validation through direct microstructural characterization using techniques such as SEM or AFM, which will be addressed in subsequent studies. These observations suggest a potential interaction between emulsifier ionic type and diluted asphalt content for the ductility index, which is further explored via grey relational analysis. Given that emulsifier ionic type, kerosene dosage, and diluted asphalt content can exert competing effects on deformation resistance, high-temperature stability, and low-temperature performance, selection should be tailored to service requirements. For enhanced low-temperature extensibility and crack resistance, an anionic emulsifier with higher diluted asphalt content and a modest kerosene dosage is preferred. Where high-temperature stability is critical, a cationic emulsifier should be prioritized, and kerosene addition avoided.
3.4. Adhesion Performance Analysis
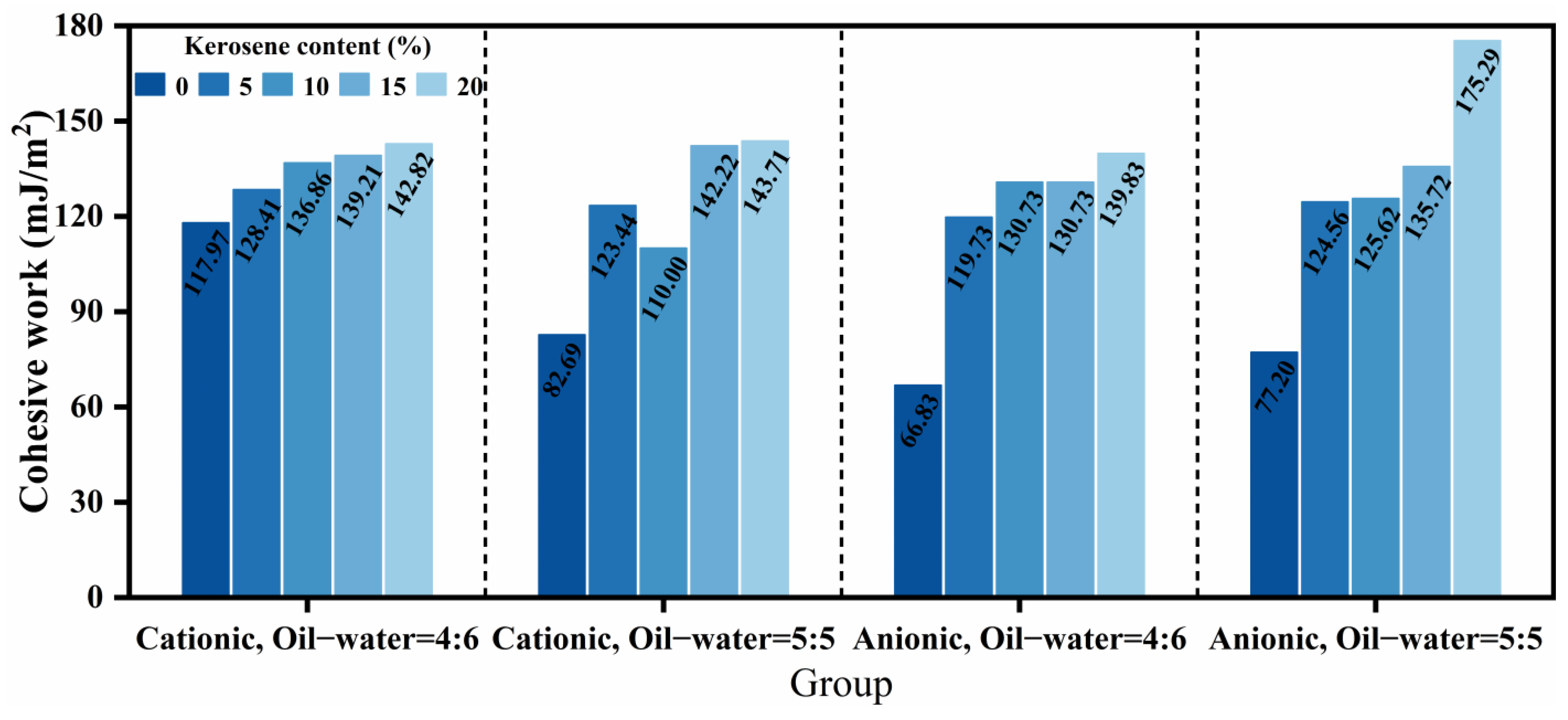
The cohesive work of asphalt serves as a critical indicator for assessing its intrinsic bonding capacity. A higher cohesive work value indicates reduced susceptibility to cohesive failure under stable conditions, conferring enhanced resistance to deformation and relative displacement caused by external forces [
27]. The cohesive work values were calculated from contact angle measurements of the twenty high-permeability emulsified asphalt formulations, as presented in
Figure 10. Overall, emulsifier ionic type and diluted asphalt content exhibited limited effects on cohesive work, while increasing kerosene dosage progressively enhanced it. This enhancement may stem from alterations in the surface composition of emulsified asphalt during demulsification and evaporation processes upon kerosene incorporation, ultimately manifested in the cohesive work values [
33]. Specifically, the addition of 20% kerosene increased the cohesive work by 21.06%, 73.79%, 109.23%, and 127.05% across the four respective formulations when compared with undiluted asphalt, demonstrating a more pronounced enhancement effect in anionic systems and formulations with higher diluted asphalt content. Furthermore, the variation trends in cohesive work across formulations correlated well with the permeability characteristics discussed in
Section 3.2, suggesting a potential fundamental relationship. In practical applications, kerosene dosage represents the primary adjustable parameter for modulating cohesive work to achieve optimal bonding performance.
Adhesion work directly characterizes the interfacial bonding performance between emulsified asphalt and aggregate, correlating closely with key macroscopic properties including moisture resistance, stripping resistance, and long-term durability [
16]. The adhesion work values for the twenty formulations were calculated based on surface free energy theory, with results presented in
Figure 11. In general, cationic emulsified asphalt demonstrated higher adhesion work, consistent with established mechanisms wherein cationic emulsifiers promote stronger interactions with negatively charged aggregate surfaces through combined electrostatic and polar effects, thereby enhancing interfacial bonding [
34]. Furthermore, increased diluted asphalt content reduced adhesion work, potentially resulting from the way in which higher soap solution content diminishes interfacial tension and enhances wettability, thereby promoting asphalt spreading across aggregate surfaces [
6,
35]. Additionally, the effect of kerosene dosage on adhesion work exhibited significant dependence on emulsifier ionic type. In cationic systems, adhesion work increased progressively with kerosene dosage, while an inverse relationship was observed in anionic systems. Notably, At the 4:6 oil-water ratio, variations in adhesion work with emulsifier type correlated inversely with permeability performance: higher adhesion work values generally corresponded to reduced permeability. This inverse correlation indicates that enhanced adhesion work corresponds to improved interfacial stability and strengthened asphalt-aggregate bonding, consistent with previous investigations [
26]. In engineering practice, emulsifier selection should be application-specific rather than generalized. Anionic systems are particularly suitable for applications requiring enhanced low-temperature crack resistance, while cationic systems are preferred for scenarios demanding superior interfacial bonding performance.
3.5. Grey Relational Analysis
Grey relational analysis (GRA) is well suited to systems characterized by limited samples and uncertain information, it can rank the relative importance of factors and quantify their effects on system performance with modest computational burden and minimal assumptions. Based on grey theory [
36,
37,
38], a GRA model was established using Python 3.7 software to relate composition variables, kerosene dosage (K), emulsifier ionic type (I), and diluted asphalt content (R), to performance metrics including residue on sieve (SR), evaporation residue (ER), penetration (P), softening point (SP), ductility (D), permeability depth (PD), viscosity (V), cohesive work (
), and adhesion work (
). A summary of the experimental results used for analysis is provided in
Table 7.
For a system characteristic series that includes multiple related factor series, the pointwise difference between each related factor curve and the system–characteristic curve can be expressed by Equation (10) [
38].
where
represents the relative difference between the kth relevant factor curve
and the system characteristic curve
, which can be interpreted as the grey relational coefficient of
relative to
at point k.
is the resolution coefficient, typically chosen between 0 and 1, with a common value of 0.5, while
is referred to as the minimum difference between the two levels and
represents “selecting the minimum value among k points.” The second level is “selecting the minimum among all i,” and
is the maximum difference between the two levels.
Although the foregoing steps yield the two-level minimum and maximum differences, they are not sufficient for the direct computation of the grey relational coefficient. This is because the series used in grey relational analysis must share commensurate dimensions, when the dimensions are inconsistent, the data must be standardized to avoid biasing the analysis. In this study, the min-max normalization method was employed to normalize the raw data, linearly mapping them to the [0,1] interval and thereby eliminating dimensional discrepancies that could affect the grey relational analysis, the transformation is given by Equation (11).
where
represents the standardized value, X is the original value, and
and
are the maximum and minimum values in the feature column, respectively.
In this study, for the emulsifier ionic type, the normalized values were retained as 0 and 1 to preserve the categorical information. After normalization, the geometric similarity among the series can be compared fairly, laying the foundation for subsequent grey relational analysis. The data were first normalized using Equation (11), and then the grey relational coefficients between the formulation design factors of high-permeability emulsified asphalt and each performance index were computed via Equation (10), yielding multiple coefficient sequences. Because the number of grey relational coefficients is large and their behavioral information is dispersed, direct visual comparison is inconvenient, therefore, the averaging method is commonly adopted to obtain the grey relational grades for cross-factor comparison, which are calculated by Equation (12).
where
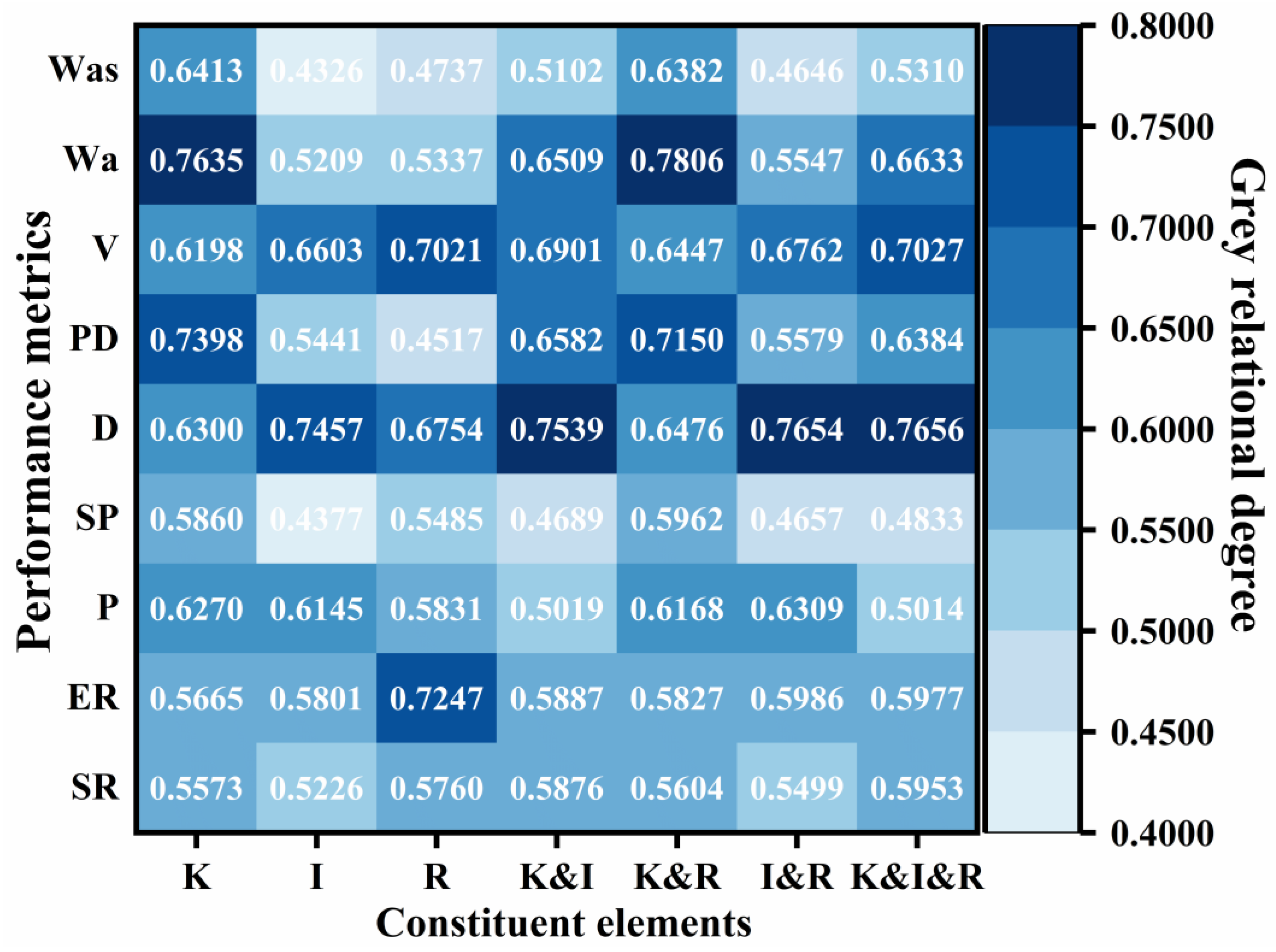
denotes the grey relational grade of each performance index with respect to the formulation design factors and N is the number of data points in a single series—in this study, N = 20. Accordingly, the grey relational grades of kerosene dosage, emulsifier ionic type, and diluted asphalt content (oil–water ratio) with respect to residue on sieve, evaporation residue, penetration, softening point, ductility, permeability depth, viscosity, cohesive work, and adhesion work are shown in
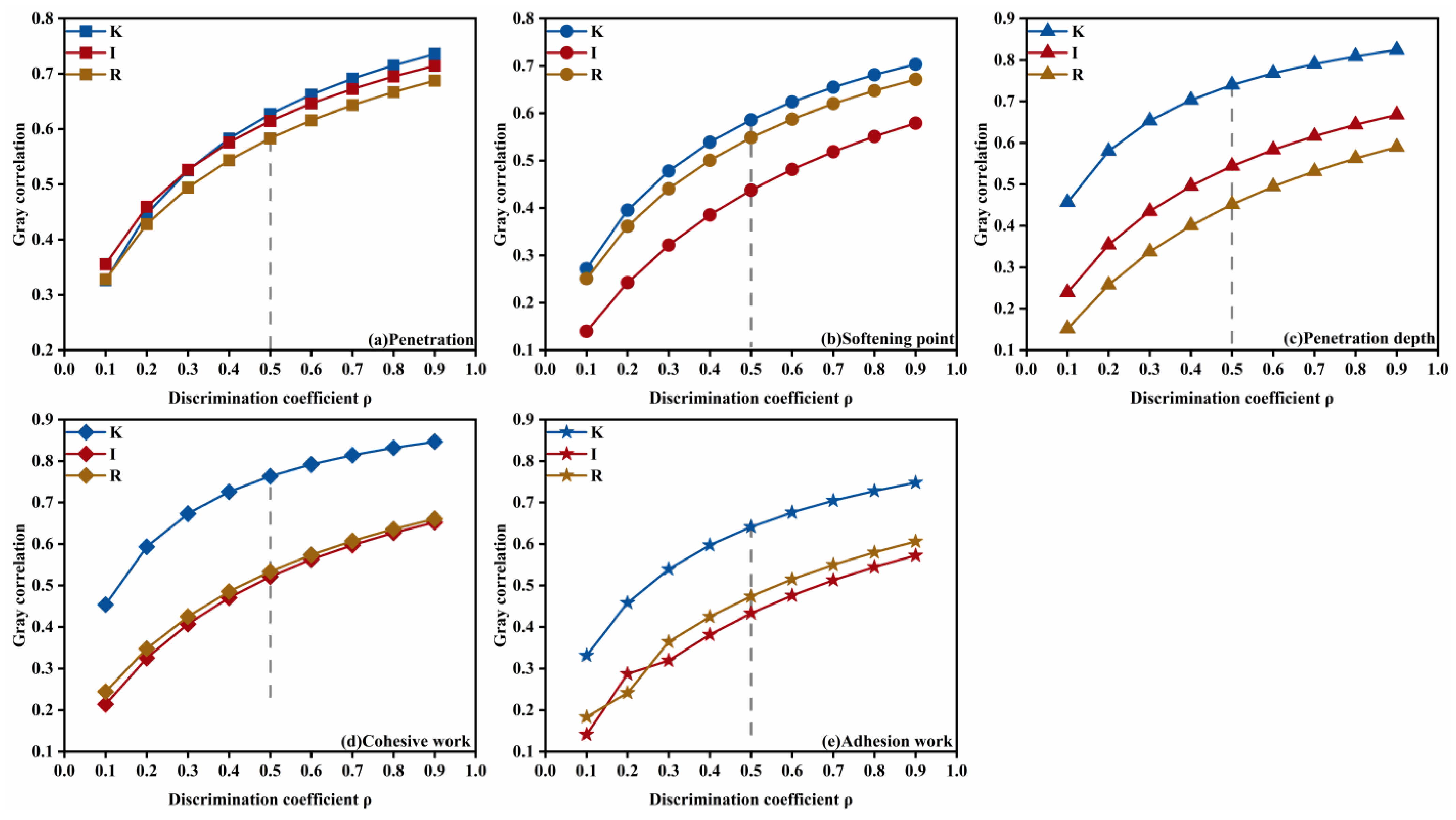
Figure 12. Among these, kerosene dosage exerted the most significant influence on penetration (grade = 0.6270), softening point (0.5860), permeability depth (0.7398), cohesive work (0.7635), and adhesion work (0.6413); emulsifier ionic type most strongly affected ductility (0.7457); and diluted asphalt content (oil–water ratio) dominated residue on sieve (0.6519), evaporation residue (0.7247), and viscosity (0.7021). These results indicate that, when optimizing the mix of high-permeability emulsified asphalt, kerosene dosage should be prioritized to tune deformation resistance, high-temperature stability, permeability, and adhesion; that improvement of low-temperature performance should focus on the selection of emulsifier ionic type, and emulsification quality; and that flowability should be controlled primarily by adjusting the diluted asphalt content (oil-water ratio) to achieve the desired outcome.
Meanwhile, because different choices of the resolution coefficient can influence the resulting grey relational grades, this study conducted sensitivity analyses for all performance indices with
ρ from 0.1 to 0.9 and grouped the indices according to the most significant governing factor in the formulation design. For the kerosene-dosage dominated indicators, the variation of the grey relational grade with
ρ is shown in
Figure 13. Kerosene dosage exerted the most pronounced influence on penetration, softening point, permeability depth, cohesive work, and adhesion work, and this prominence did not change as
ρ varied. However, for penetration, when
ρ < 0.5, the influence of the emulsifier ionic type exceeded that of kerosene dosage, with the two grades being close in magnitude, in line with a similar phenomenon that was observed for the softening point. These findings suggest that the high-temperature properties and thermal stability of high-permeability emulsified asphalt are likely governed by interacting factors.
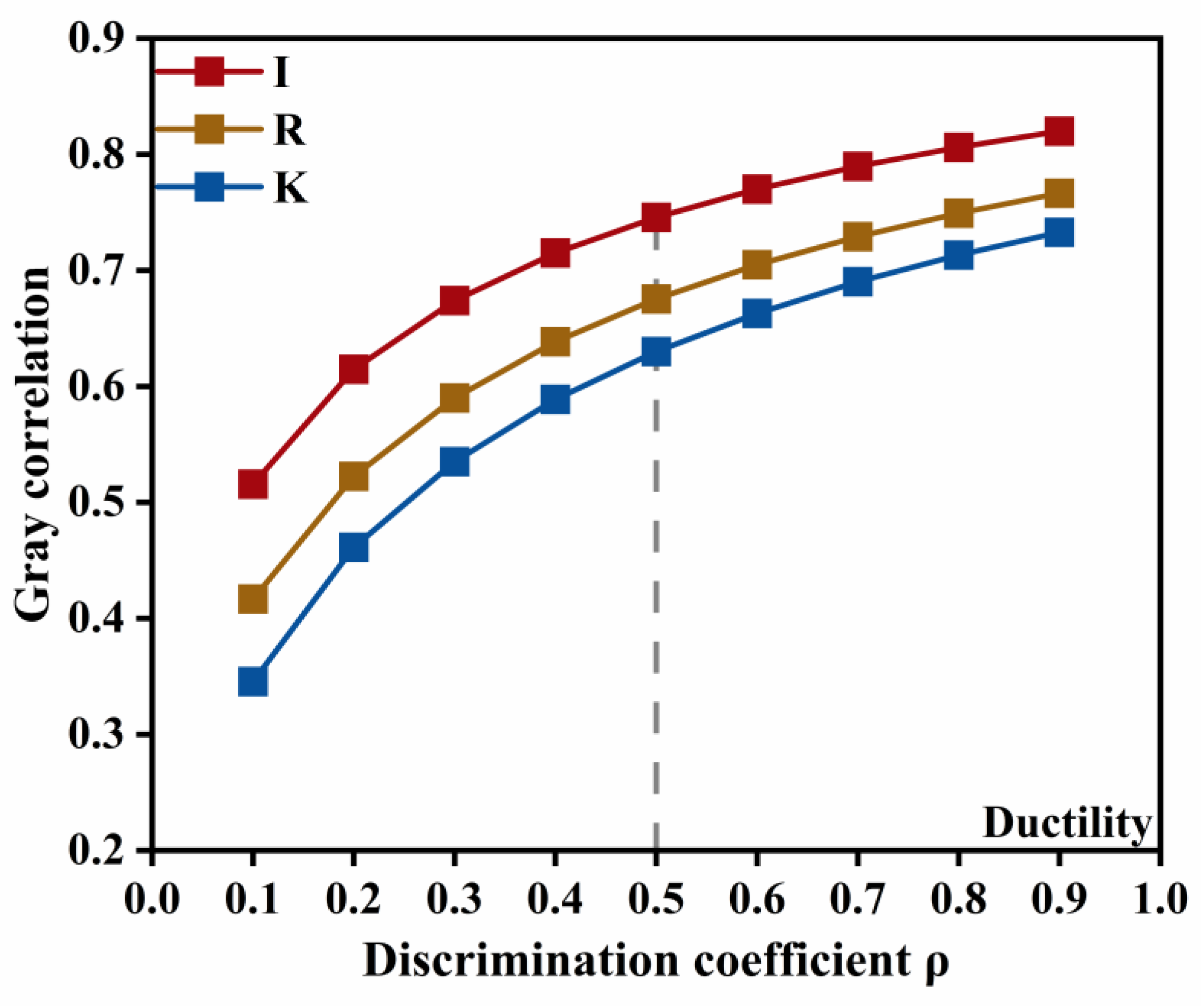
For the ionic type of dominated indicators, the variation of the grey relational grade with the resolution coefficient is shown in
Figure 14. The prominence of the emulsifier ionic type for ductility does not vary with changes in the resolution coefficient, and its influence on ductility is markedly stronger than that of the other two factors. This indicates that the emulsifier ionic type is the primary determinant of low-temperature performance, and that interaction effects may be neglected in this case.
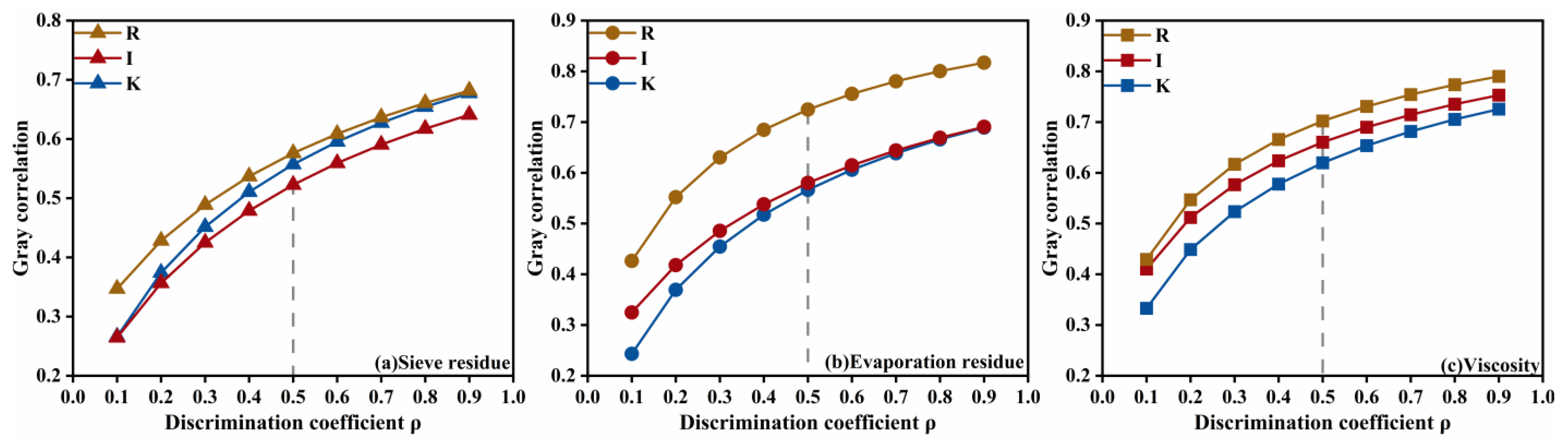
For the diluted asphalt content (oil-water ratio) dominated indicators, the variation of the grey relational grade with the resolution coefficient is shown in
Figure 15. The prominence of diluted asphalt content for residue on sieve, evaporation residue, and viscosity does not change with the resolution coefficient, and its influence on residue on sieve and evaporation residue is markedly stronger than that of the other two factors. This indicates that diluted asphalt content occupies a dominant role in governing the emulsification characteristics of high-permeability emulsified asphalt, whereas its grey relational grade for viscosity is not significantly higher than those of the other factors-implying that viscosity is likely subject to multi-factor interactions.
Considering the variation of grey relational grades with the resolution coefficient across the three factor-dominated groups, adopting ρ = 0.5 for the grey relational analysis of the effects of formulation design factors on the performance of high-permeability emulsified asphalt is appropriate, and the resulting ranking of influencing factors is credible. Moreover, because the grey relational grades of certain factors are close in magnitude, an analysis of interaction effects among factors is subsequently undertaken to further elucidate this phenomenon.
Additionally, prior studies have reported close relationships between the evaporation residue content and multiple properties of emulsified asphalt [
32,
39], though the specific associations remain unclear. Therefore, this study employed grey relational analysis to quantify the influence of evaporation residue content in high-permeability emulsified asphalt on its fundamental physical properties, permeability, and viscosity. The calculated order of influence was as follows: viscosity (grade = 0.7284) > ductility (0.6837) > residue on sieve (0.6664) > cohesive work (0.6389) > softening point (0.6187) > penetration (0.6132) > adhesion work > permeability depth (0.5725). These results indicate that evaporation residue content has a greater impact on flowability, low-temperature performance, and emulsification quality, and a comparatively smaller effect on deformation resistance, high-temperature stability, adhesion, and permeability, consistent with the conclusion in
Section 3 stating that the magnitude of evaporation residue content determines the amount of effective material and thereby governs overall performance.
Although single factor grey relational analysis clearly reveals the dominant influences and determinant parameters of different formulation design factors on the performance of high-permeability emulsified asphalt, it neglects nonlinear interaction effects among factors. In practice, the performance of high-permeability emulsified asphalt is not governed by a single factor mechanism. For example, within an emulsified asphalt system, kerosene can improve permeability by disrupting the long molecular chains in the asphalt components, while the emulsifier enhances permeability by reducing the interfacial tension at the asphalt/water boundary, moreover, different emulsifier ionic types yield distinct outcomes. Acting together, these factors jointly regulate permeability. Therefore, this study extends the grey relational model by introducing second order interaction terms—kerosene dosage and emulsifier ionic type (K&I), kerosene dosage and diluted asphalt content (K&R), and emulsifier ionic type and diluted asphalt content (I&R)—as well as a third order interaction term—kerosene dosage and emulsifier ionic type and diluted asphalt content (K&I&R), with the aim of quantifying the synergistic effects among formulation design factors and identifying critical thresholds in the formulation design. Specifically, the product method multiplies the normalized values of the two or three participating factors, when all factors are simultaneously at high/low levels, the product is markedly amplified, which facilitates discrimination of interaction effects. The constructions of these interaction terms are given by Equation (13).
where
is the value of the interaction term, and
and
are the normalized values of different factors. We substitute the calculated
into the above grey relational analysis steps to obtain the grey relational of the interaction term.
Building on the foregoing theory and equations, the interaction effects in multi-factor grey relational analysis were elucidated, and the computed results are shown in
Figure 16. The interaction between kerosene dosage and diluted asphalt content (K&R) exhibited pronounced influences on the softening point (grade = 0.5962), permeability depth (0.7150), cohesive work (0.7806), and adhesion work (0.6382). The interaction between emulsifier ionic type and diluted asphalt content (I&R) showed a significant cooperative effect on penetration (0.6309). The third order interaction (K&I&R) had strong effects on residue on sieve (0.5953), ductility (0.7656), and viscosity (0.7027). These findings indicate that high-temperature stability, permeability, and adhesion should be tuned with priority given to the K&R interaction; that deformation resistance should focus on the I&R interaction; and that emulsification characteristics, low-temperature performance, and flowability should account for multi-factor coordination. This clarifies the mechanisms by which formulation design adjustments improve performance, avoids the limitations and experimental brittleness of single-factor optimization, and provides a theoretical basis for subsequent performance-oriented formulation design optimization.