Mechanisms for Migration of Alkali in Dolomitic Limestones
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Migration Characterization of Na+ Ions in Rocks
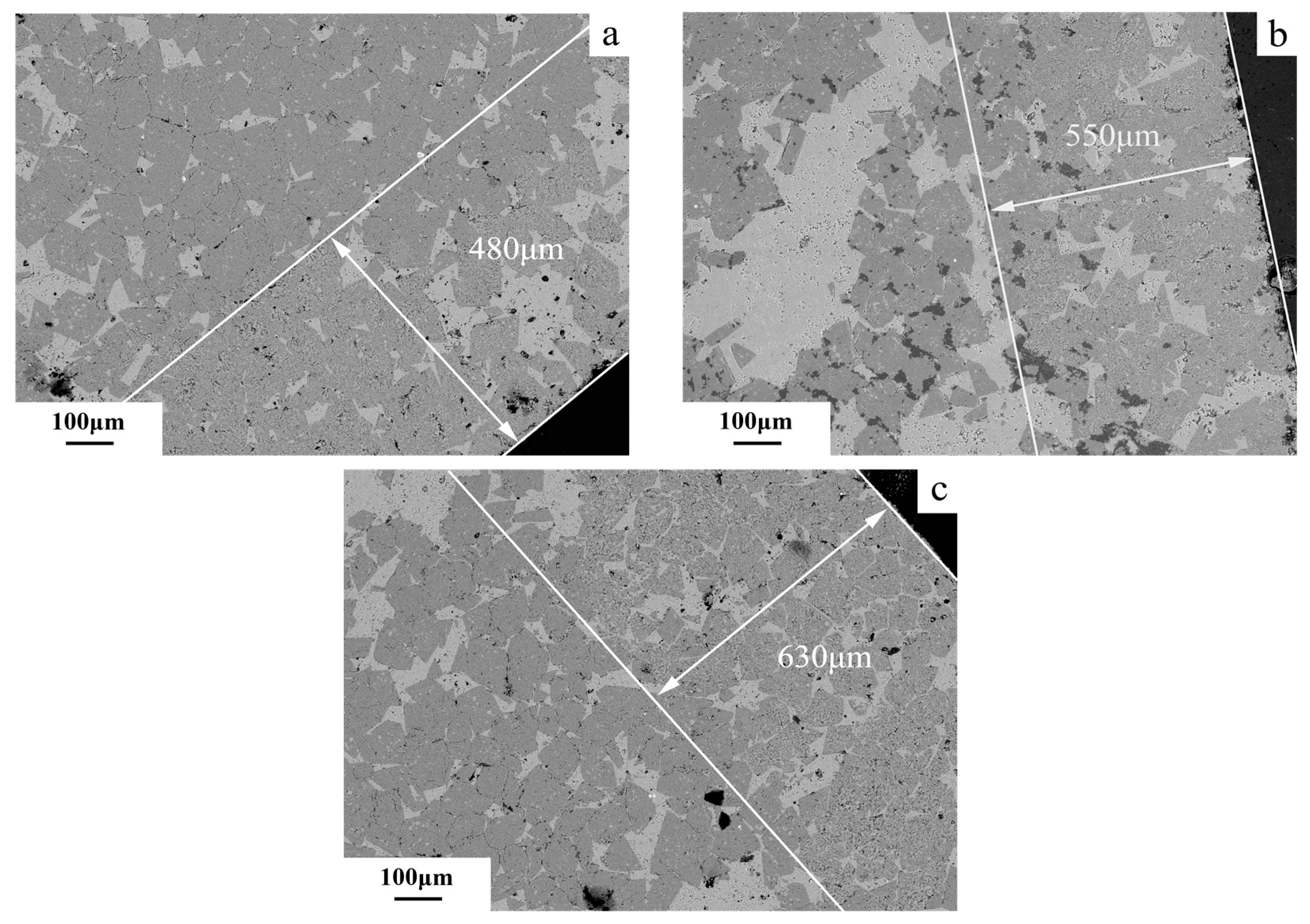
2.2.2. Determination of Degree of Dedolomitization
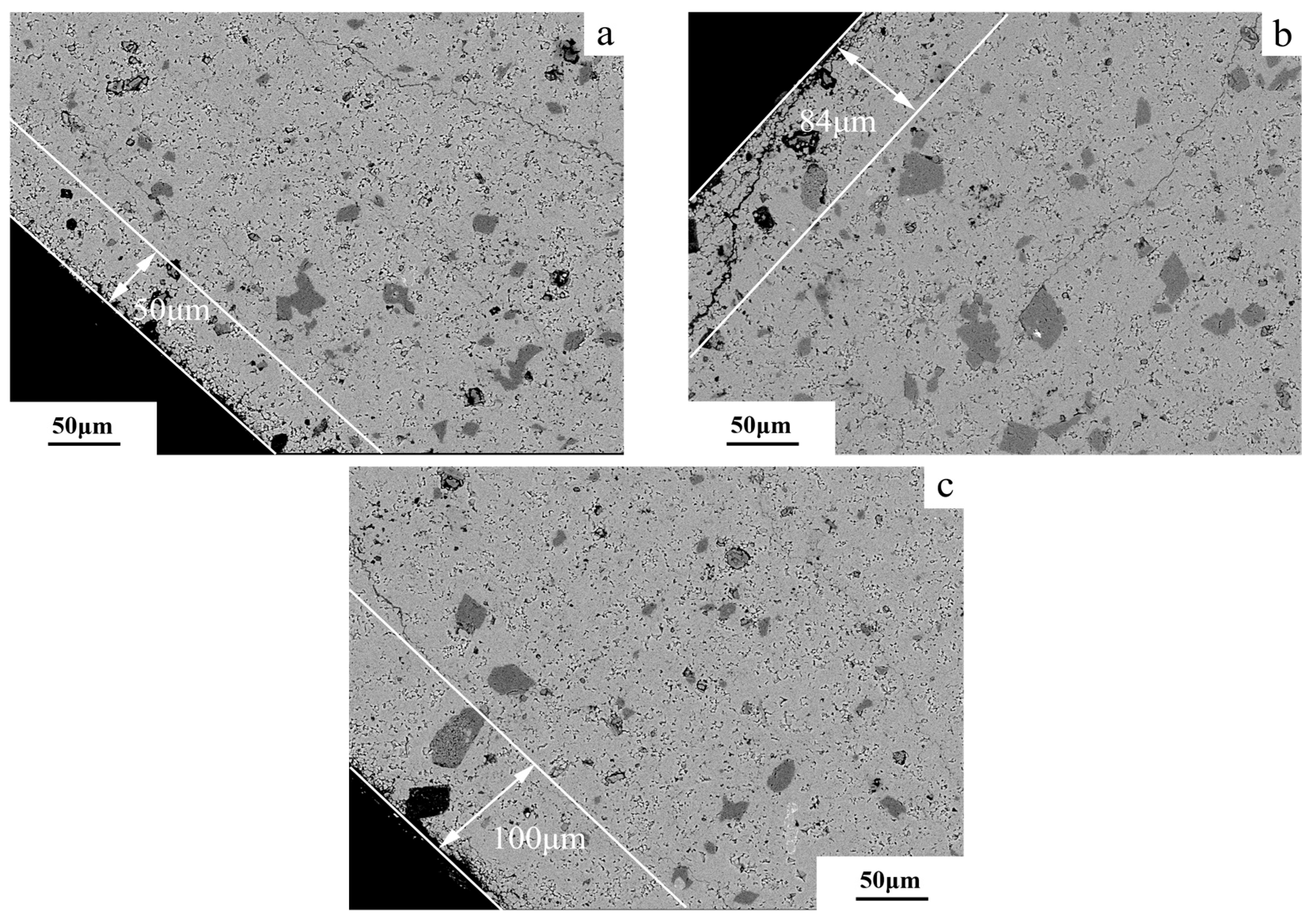
2.2.3. Determination of the Pore Structure of Rocks
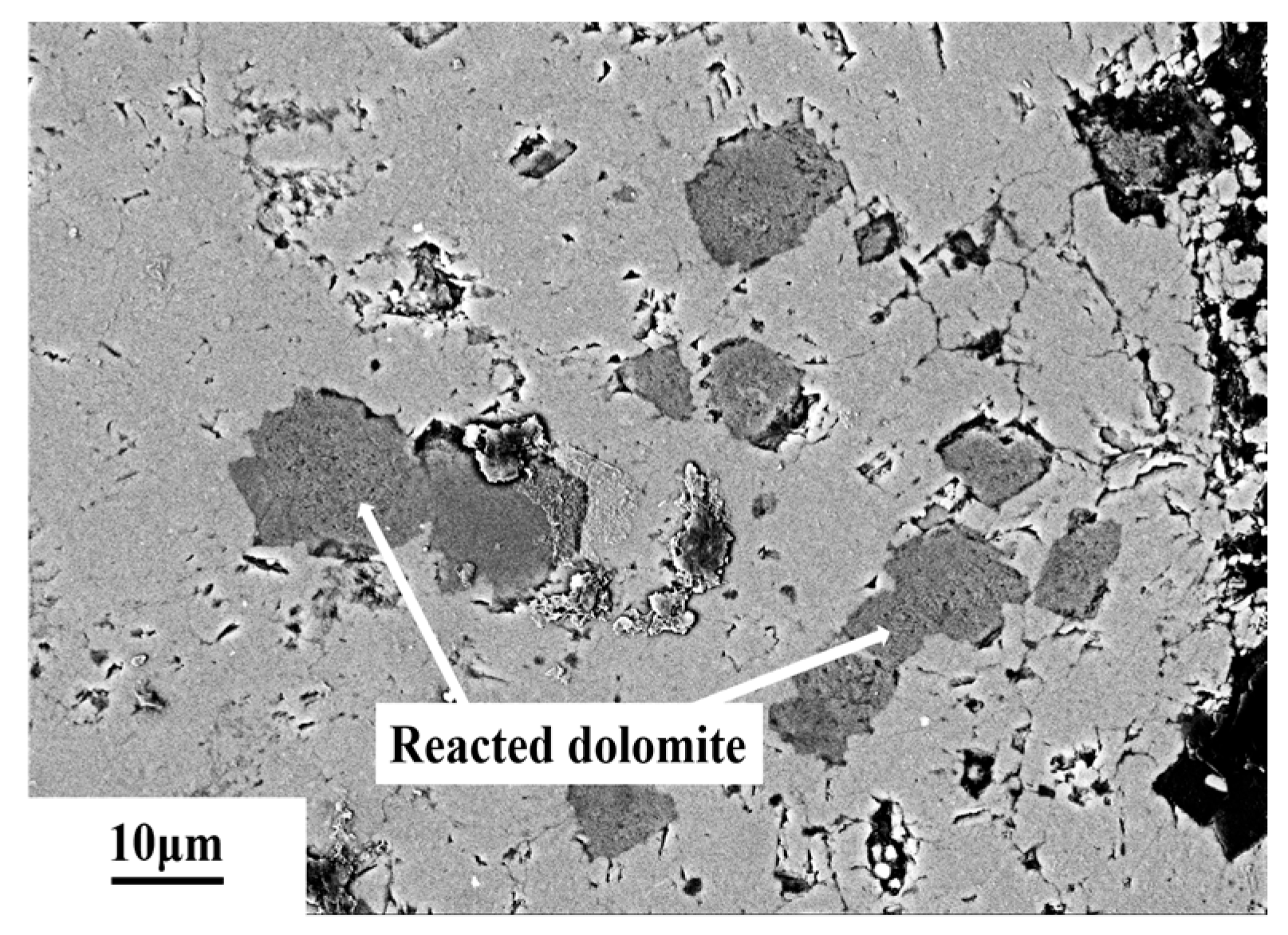
2.2.4. Microstructural Analysis
3. Results
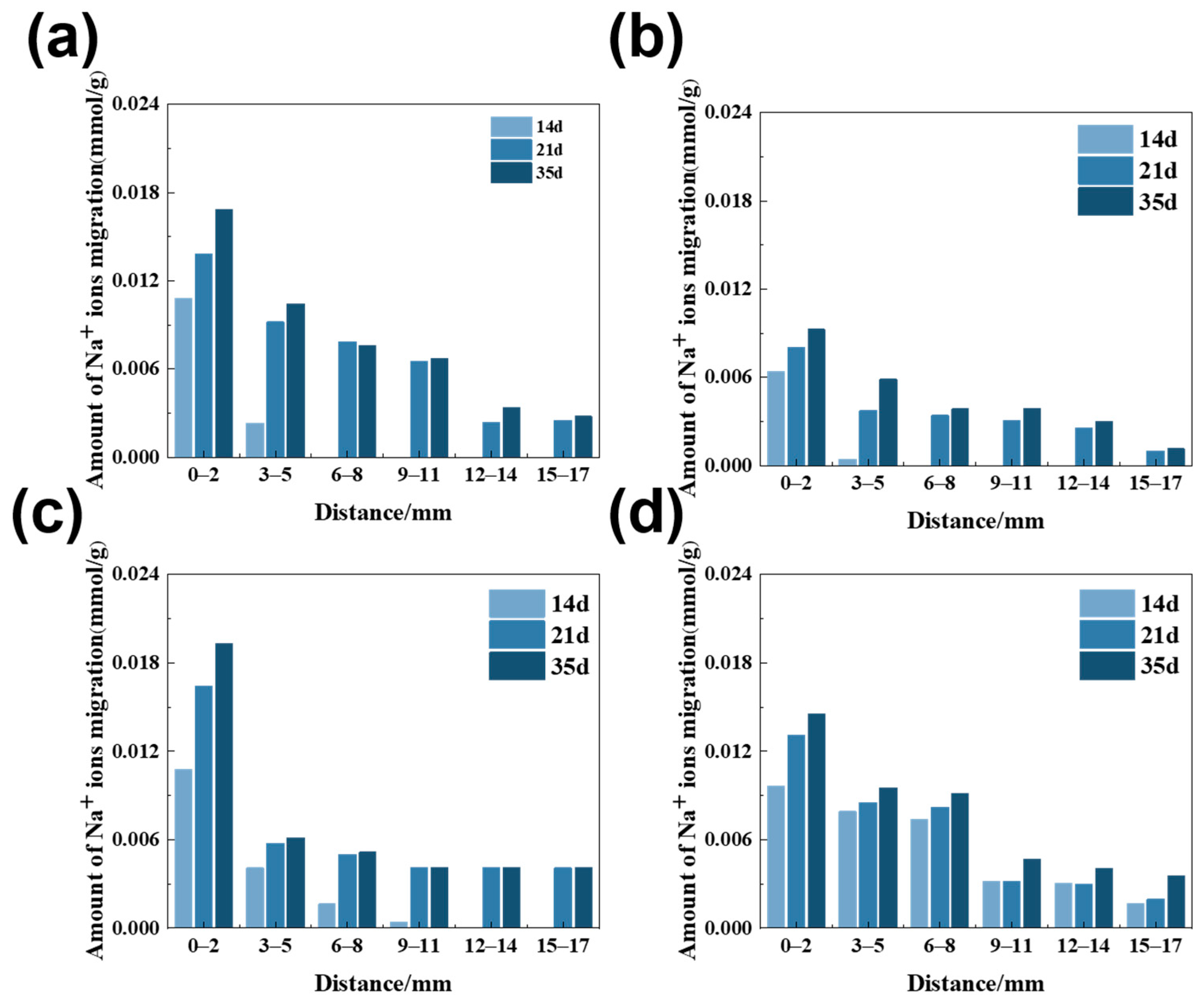
3.1. The Amount of Na+ Ion Migration in Rock Columns
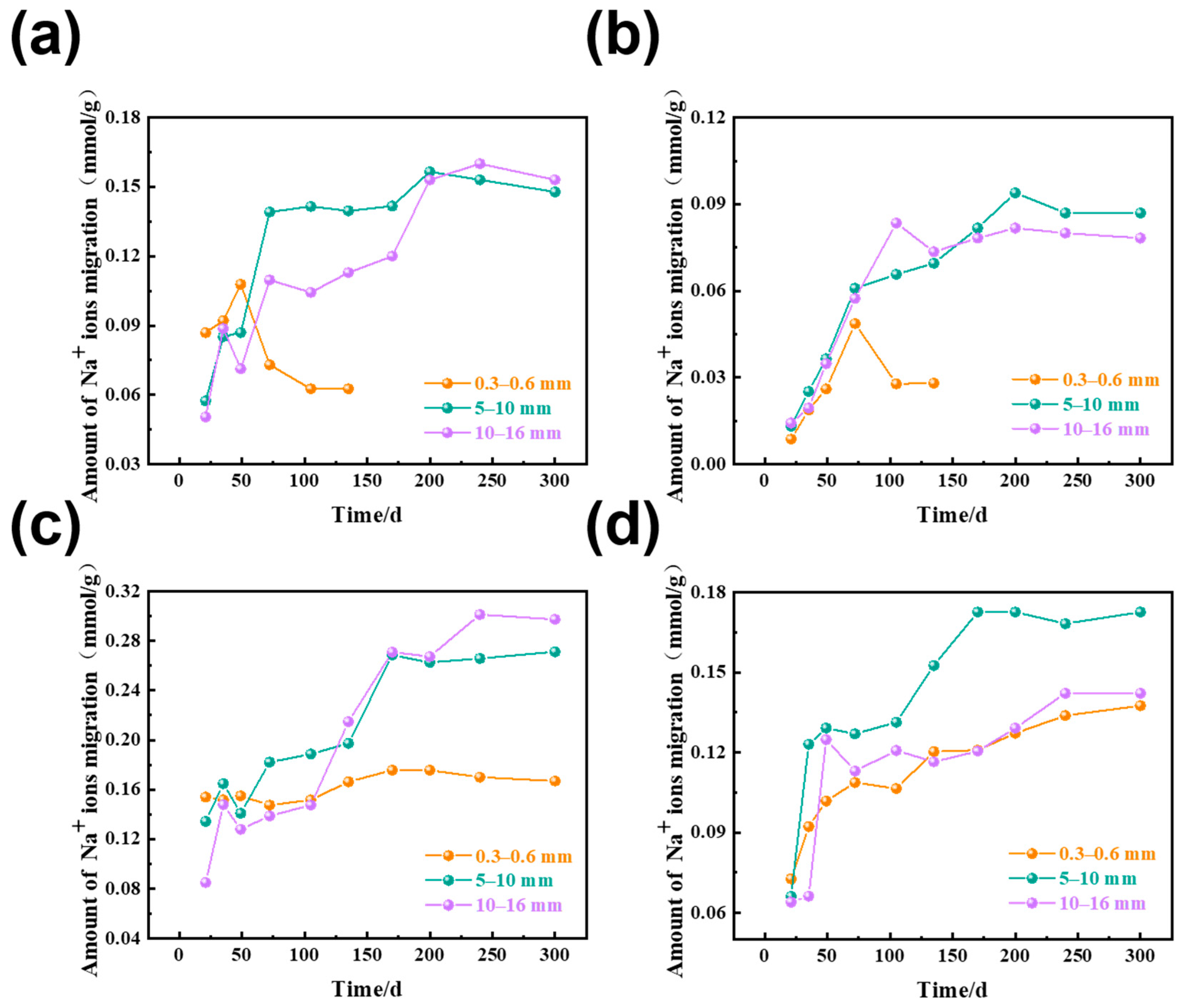
3.2. The Amount of Na+ Ion Migration in Rocks of Different Sizes
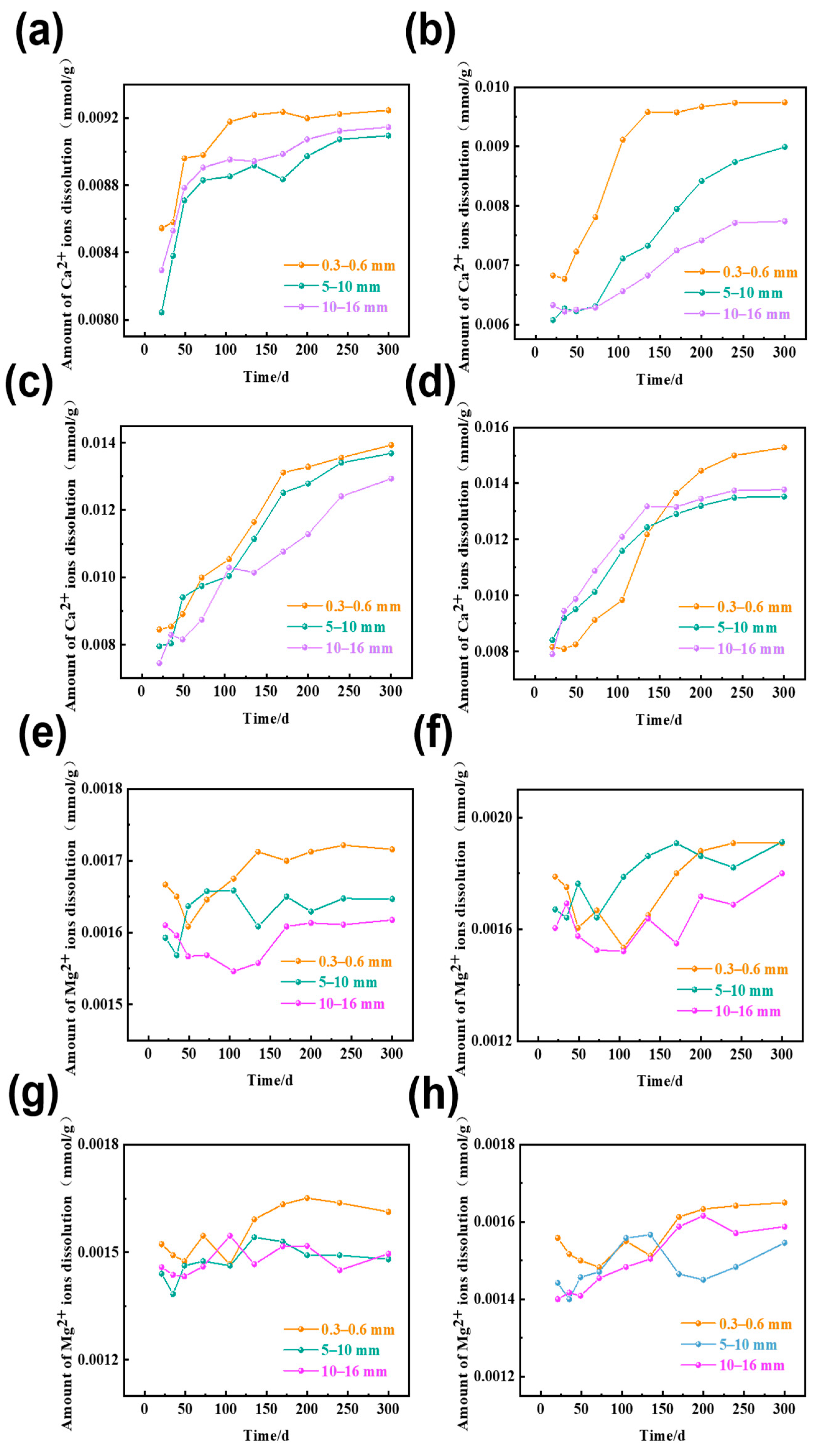
3.3. The Amount of Ca2+ and Mg2+ Ions Dissolution in Rocks of Different Sizes
3.4. Degree of Dedolomitization
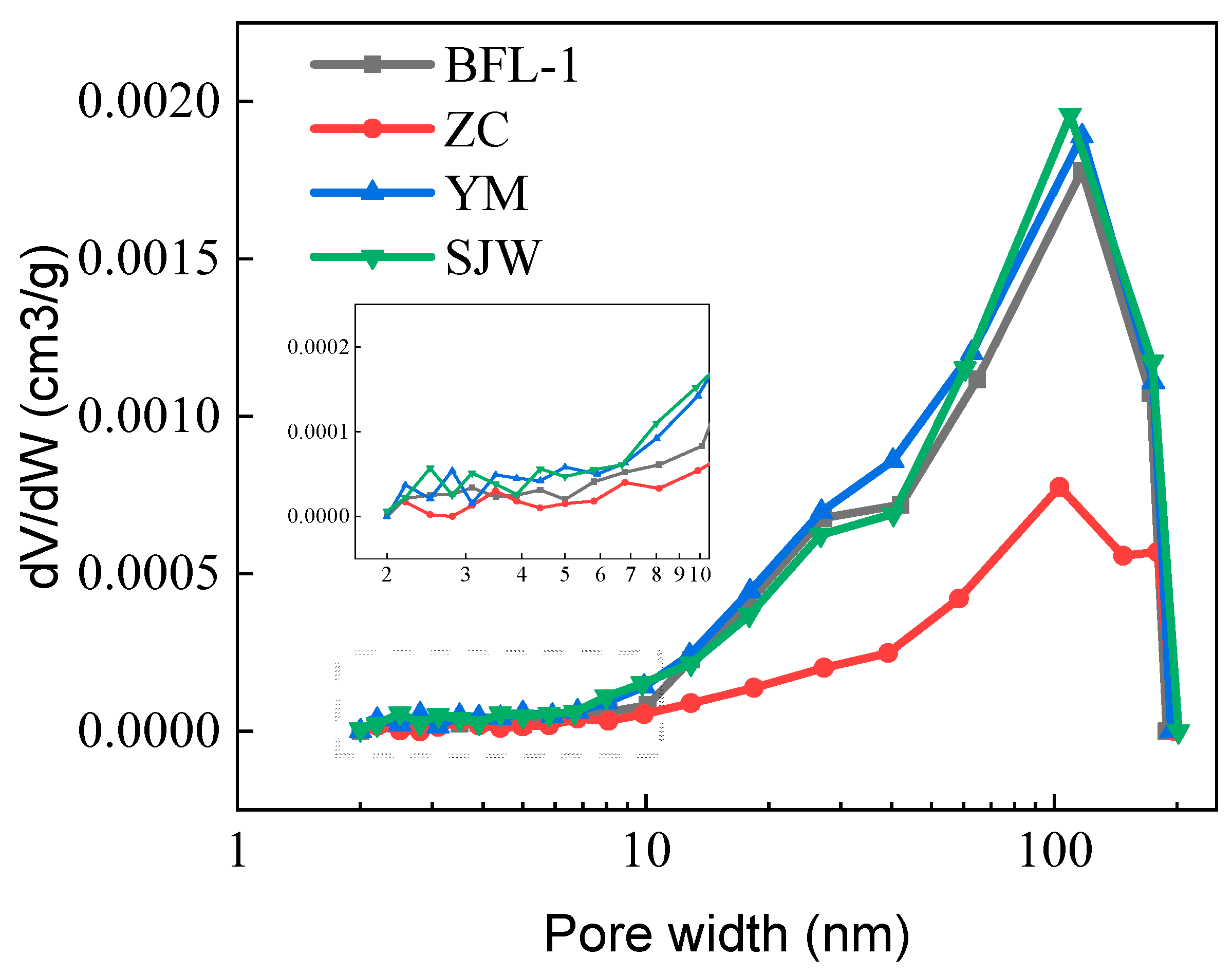
3.5. Pore Structure
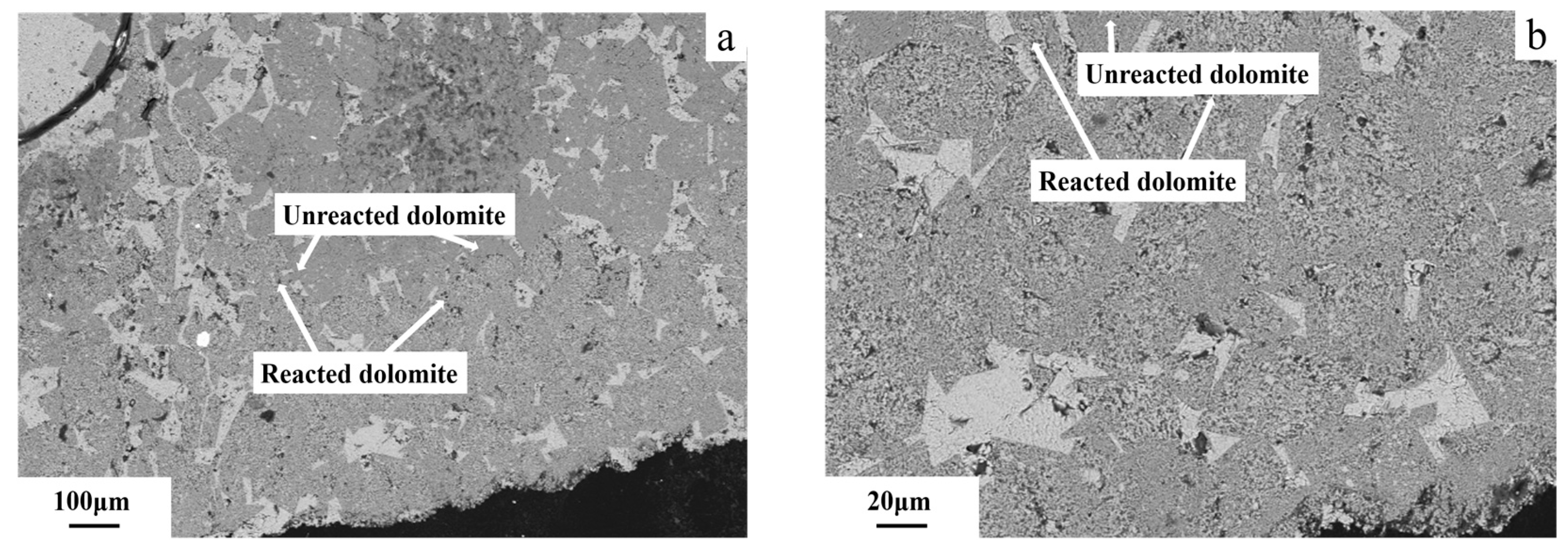
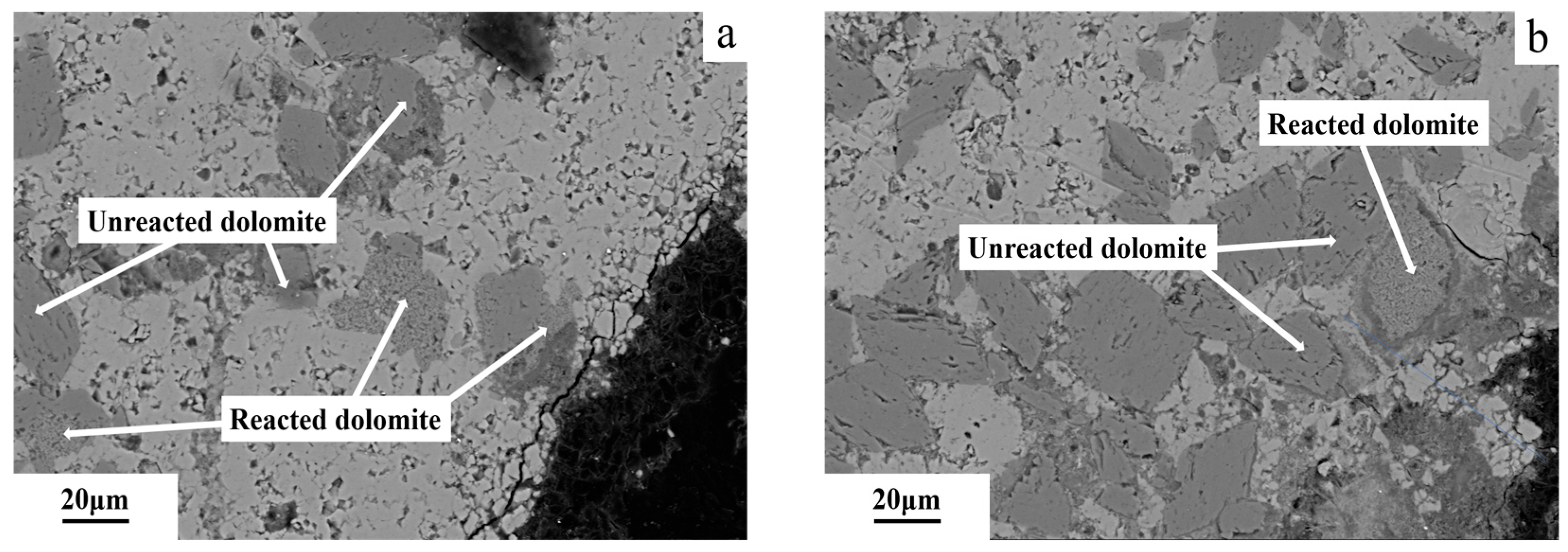
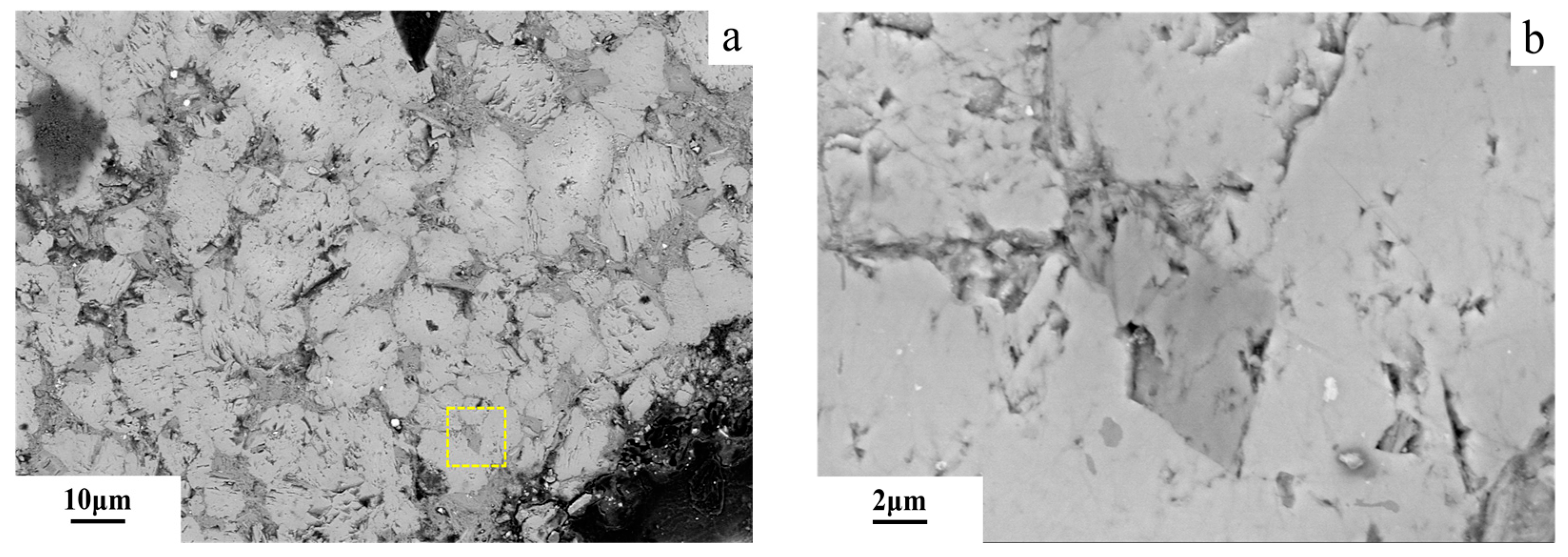
3.6. Microstructures of Dedolomitization
4. Discussion
4.1. The Influence of Ca2+ and Mg2+ Ions Dissolution on Na+ Ion Migration
4.2. The Influence of Particle Size
4.3. The Influence of Rock Pore Structure on Ion Migration
4.4. Alkali Ion Migration After Dedolomitization
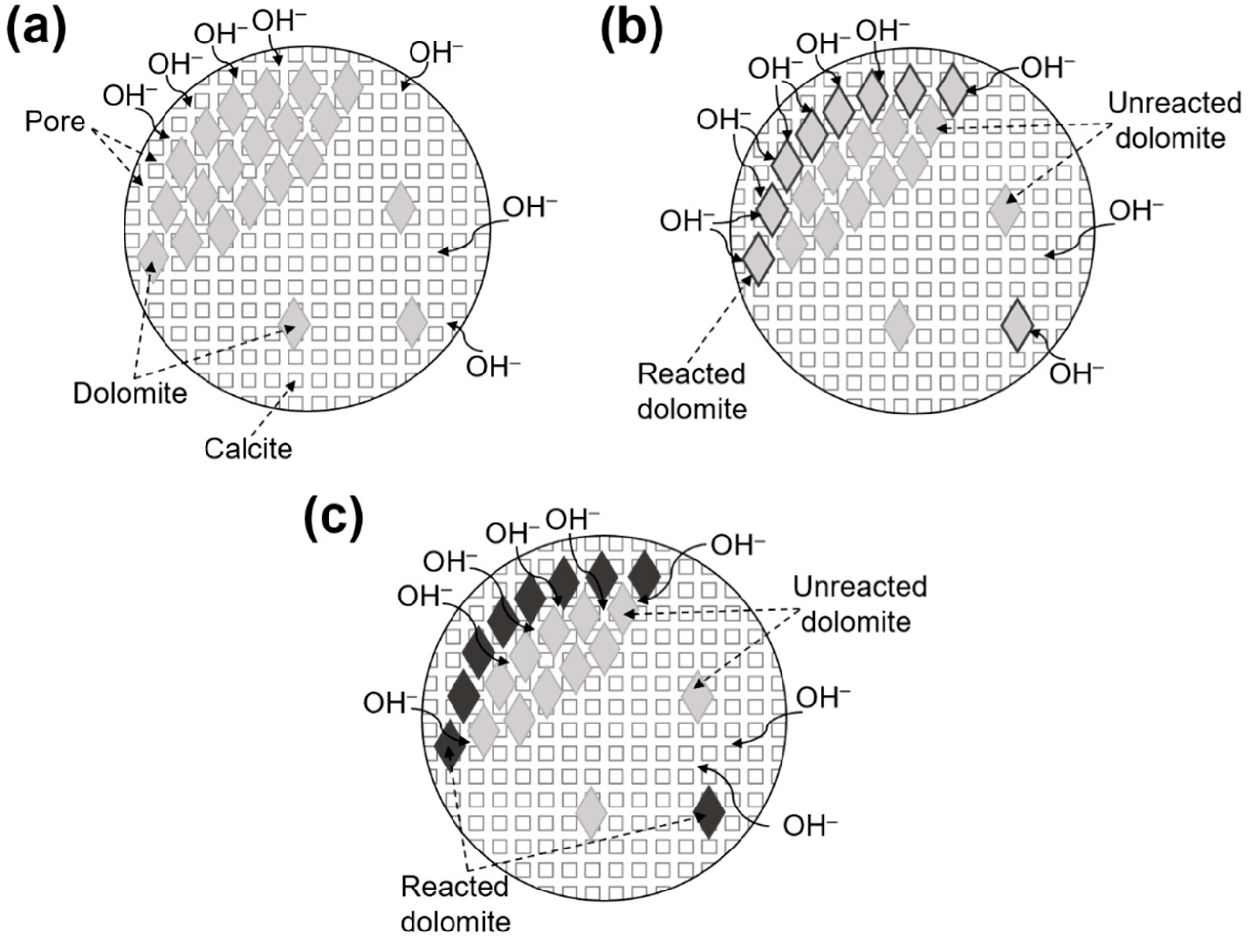
4.5. The Migration Mechanism of Alkali Ions
5. Conclusions
- The pores between crystals are the physical pathways for the migration of alkali cations, whereas the concentration gradient established upon dedolomitization is a driving force for alkali ions migration. The interactions between pores and concentration gradient dictate the migration efficacy of alkali ions.
- Clay minerals such as illite often have small crystal sizes and between these there is a large number of micropores (1–10 nm). These micropores provide efficient pathways for the migration of alkali ions.
- The particle size has two effects on alkali ion migration: (a) Dedolomitization facilitates alkali ion migration. The smaller the size, the higher the degree of dedolomitization, but less dolomite content means less alkali ion will migrate after dedolomitization; and (b) The matrix has impediment effect on ion migration. The larger the size of the particle, the stronger the impediment effect.
- The process of the migration of alkali ions in dolomitic limestone can be represented as: (a) alkali ions migrate in the pores between crystals and contact with dolomite crystals; (b) OH− ions react with dolomite crystals and dedolomitization consumes OH− ions and creates a concentration gradient; (c) alkali ions migrate to the dolomite reaction zone due to the concentration gradient; and (d) after dolomite crystals have reacted completely, alkali ions continue to migrate deeper into the rock.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Deng, M.; Li, W.; Lan, X.; Tang, M. Whether so-called Mg-Si gels are produced under dolomite-quartz system under alkaline conditions. Cem. Concr. Res. 2024, 186, 107701. [Google Scholar] [CrossRef]
- Leemann, A.; Münch, B.; Lothenbach, B.; Bernard, E.; Trottier, C.; Winnefeld, F.; Sanchez, L. Alkali-carbonate reaction in concrete—Microstructural consequences and mechanism of expansion. Cem. Concr. Res. 2025, 195, 107903. [Google Scholar] [CrossRef]
- Salim, M.U.; Mosaberpanah, M.A. The mechanism of alkali-aggregate reaction in concrete/mortar and its mitigation by using geopolymer materials and mineral admixtures: A comprehensive review. Eur. J. Environ. Civ. Eng. 2022, 26, 6766–6806. [Google Scholar] [CrossRef]
- Swenson, E.G. A Reactive aggregate undetected by ASTM tests. ASTM Bull. 1957, 226, 48–51. [Google Scholar]
- Yang, H.; Li, P.; Rao, M. Long term investigation and inhibition on alkali-aggregates reaction of three gorges dam concrete. Constr. Build. Mater. 2017, 151, 673–681. [Google Scholar] [CrossRef]
- Hadley, D.W. Alkali reactivity of dolomitic carbonate rocks. Highw. Res. Rec. 1945, 25, 147–168. [Google Scholar]
- Sims, I.; Poole, A.B. Alkali-Aggregate Reaction in Concrete: A World Review; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Deng, M.; Xu, L.; Lan, X.; Tang, M. Microstructures and Alkali-Reactivity of Permian Emeishan Group Basaltic Rocks. In Proceedings of the 13th International Conference on Alkali Aggregate Reaction in Concrete, Trondheim, Norway, 16–20 June 2008. [Google Scholar]
- Deng, M.; Han, S.; Lu, Y.; Lan, X.; Hu, Y.; Tang, M. Deterioration of concrete structures due to alkali-dolomite reaction in China. Cem. Concr. Res. 1993, 23, 1040–1046. [Google Scholar] [CrossRef]
- Stukovnik, P.; Bosiljkov, V.B.; Marinsek, M. Acr progress in concretes with dolomite aggregate and blast furnace slag cem iii binder. Ceram. Silik. 2024, 68, 67–79. [Google Scholar] [CrossRef]
- Zahedi, A.; Akpinar, P. A Case Study for exploring the alkali-aggregate reactivity of Cyprus aggregates. Case Stud. Constr. Mater. 2022, 16, e01000. [Google Scholar] [CrossRef]
- Tong, L.; Tang, M. Expansion mechanism of alkali-dolomite and alkali-magnesite reaction. Cem. Concr. Compos. 1999, 21, 361–373. [Google Scholar] [CrossRef]
- Tang, M.S.; Deng, M.; Lan, X.H.; Han, S. Studies on alkali-carbonate reaction. Aci Mater. J. 1994, 91, 26–29. [Google Scholar]
- Deng, M.; Tang, M.S. Measures to inhibit alkali-dolomite reaction. Cem. Concr. Res. 1993, 23, 1115–1120. [Google Scholar] [CrossRef]
- Tang, M. Classification of alkali aggregate reaction. In Proceedings of the 9th International Conference on Alkali Aggregate Reaction in Concrete, London, UK, 27–31 July 1992; pp. 6348–6353. [Google Scholar]
- Katayama, T. The so-called alkali-carbonate reaction (ACR)—Its mineralogical and geochemical details, with special reference to ASR. Cem. Concr. Res. 2010, 40, 643–675. [Google Scholar] [CrossRef]
- Katayama, T.; Jensen, V.; Rogers, C.A. The enigma of the ‘so-called’alkali–carbonate reaction. Proc. Inst. Civ. Eng. Constr. Mater. 2016, 169, 223–232. [Google Scholar] [CrossRef]
- Grattan-Bellew, P.E. Alkali-silica reaction—Canadian experience. In The Alkali-Silica Reaction in Concrete; CRC Press: Boca Raton, FL, USA, 1991; pp. 223–248. [Google Scholar]
- Grattan-Bellew, P.E.; Mitchell, L.D.; Margeson, J.; Min, D. Is alkali–carbonate reaction just a variant of alkali–silica reaction ACR = ASR? Cem. Concr. Res. 2010, 40, 556–562. [Google Scholar] [CrossRef]
- Chen, B.; Deng, M.; Huang, X.; Mo, L.; Huang, B.; Lan, X. Siliceous and dolomitic-bearing aggregates reaction in Tetramethylammonium hydroxide. Constr. Build. Mater. 2021, 299, 123948. [Google Scholar] [CrossRef]
- Milanesi, C.A.; Marfil, S.A.; Locati, F. Expansive behavior of an alkali-carbonate reactive dolostone from Argentina: Proposal of an osmotic theory-based model to explain the expansion caused by the alkali attack. Cem. Concr. Res. 2020, 138, 106239. [Google Scholar] [CrossRef]
- Ren, X.; Li, W.; Mao, Z.Y.; Deng, M. Inhibition of the alkali-carbonate reaction using fly ash and the underlying mechanism. Crystals 2020, 10, 484. [Google Scholar] [CrossRef]
- Garcia, E.; Alfonso, P.; Tauler, E. Mineralogical Characterization of Dolomitic Aggregate Concrete: The Camarasa Dam (Catalonia, Spain). Minerals 2020, 10, 117. [Google Scholar] [CrossRef]
- Garcıa, E.; Alfonso, P.; Tauler, E.; Galı, S. Surface alteration of dolomite in dedolomitization reaction in alkaline media. Cem. Concr. Res. 2003, 33, 1449–1456. [Google Scholar] [CrossRef]
- Stukovnik, P.; Bosiljkov, V.B.; Marinsek, M. ACR in dolomitic aggregate concrete for an autogenous self-healing process. Mater. Tehnol. 2020, 54, 379–384. [Google Scholar] [CrossRef]
- Milla, J.; Rupnow, T.; Saunders, W. Effect of Clay Content on Alkali-Carbonate Reactive (ACR) Dolomitic Limestone; Louisiana Transportation Research Center: Baton Rouge, LA, USA, 2021. [Google Scholar]
- Dantas, S.R.A.; Bergmann, A.C.; Sanchez, L.F.M. Exploring the Effectiveness of TiO2 Treatments to Mitigate Alkali-Aggregate Reaction (AAR). In Proceedings of the International Conference on Alkali-Aggregate Reaction in Concrete, Ottawa, ON, Canada, 18–24 May 2024; Springer Nature: Cham, Switzerland, 2024; pp. 515–522. [Google Scholar]
- Leemann, A.; Münch, B.; Trottier, C.; Sanchez, L. Microstructural Consequences of Alkali-Carbonate Reaction. In Proceedings of the International Conference on Alkali-Aggregate Reaction in Concrete, Ottawa, ON, Canada, 18–24 May 2024; Springer Nature: Cham, Switzerland, 2024; pp. 87–94. [Google Scholar]
- Li, W.; Deng, M.; Mo, L.; Panesar, D.K.; Mao, Z. Alkali carbonate reaction (ACR): Investigations on mechanism of dedolomitization of dolomite in dolostones. Constr. Build. Mater. 2022, 351, 128942. [Google Scholar] [CrossRef]
- Duke, E.F. Reactions between dolomite and cement paste in deteriorated concrete. In Proceedings of the USA-Australia Workshop on High Performance Concrete, Sydney, Australia, 20–23 August 1997; pp. 20–23. [Google Scholar]
- Pękala, A.; Vilcekova, S. Secondary mineralization processes in the assessment of alkaline reactivity of aggregates and durability of concrete. Case Stud. Constr. Mater. 2024, 21, e04113. [Google Scholar] [CrossRef]
- Liu, X.; Mao, Z.; Yi, L.; Fan, Z.; Zhang, T.; Huang, X.; Deng, M.; Tang, M. Experimental Method for Evaluating the Reactivity of Alkali-Carbonate Reaction Activity. Materials 2022, 15, 2853. [Google Scholar] [CrossRef]
- JY/T 0587-2020; General Rules for X-Ray Polycrystalline Diffractometry. Ministry of Education of PRC: Beijing, China, 2020.
- ASTM D1976-20; Standard Test Method for Elements in Water by Inductively-Coupled Plasma Atomic Emission Spectroscopy. ASTM International: West Conshohocken, PA, USA, 2020.
- Konno, H.; Nanri, Y.; Kitamura, M. Crystallization of aragonite in the causticizing reaction. Powder Technol. 2002, 123, 33–39. [Google Scholar] [CrossRef]
- Simoni, M.; Hanein, T.; Woo, C.L.; Tyrer, M.; Nyberg, M.; Martinez, J.-C.; Quintero-Mora, N.I.; Provis, J.L.; Kinoshita, H. Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: Effect of residence time and mixing rates. Phys. Chem. Chem. Phys. 2022, 24, 16125–16138. [Google Scholar] [CrossRef]
- Weiss, B.D.; Harasek, M. Solubility Data of Potential Salts in the MgO-CaO-SO2-H2O-O2 System for Process Modeling. Processes 2021, 9, 50. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Baudet, B.A. Evolution of nano-pores in illite-dominant clay during consolidation. Acta Geotech. 2024, 19, 71–83. [Google Scholar] [CrossRef]
- Gomez, L.; Perales, F.; Multon, S.; Socié, A.; Fournier, B.; Argouges, M. Numerical investigation of mechanisms affecting alkali-silica reaction advancement by reactive transport simulations. Cem. Concr. Res. 2025, 190, 107791. [Google Scholar] [CrossRef]

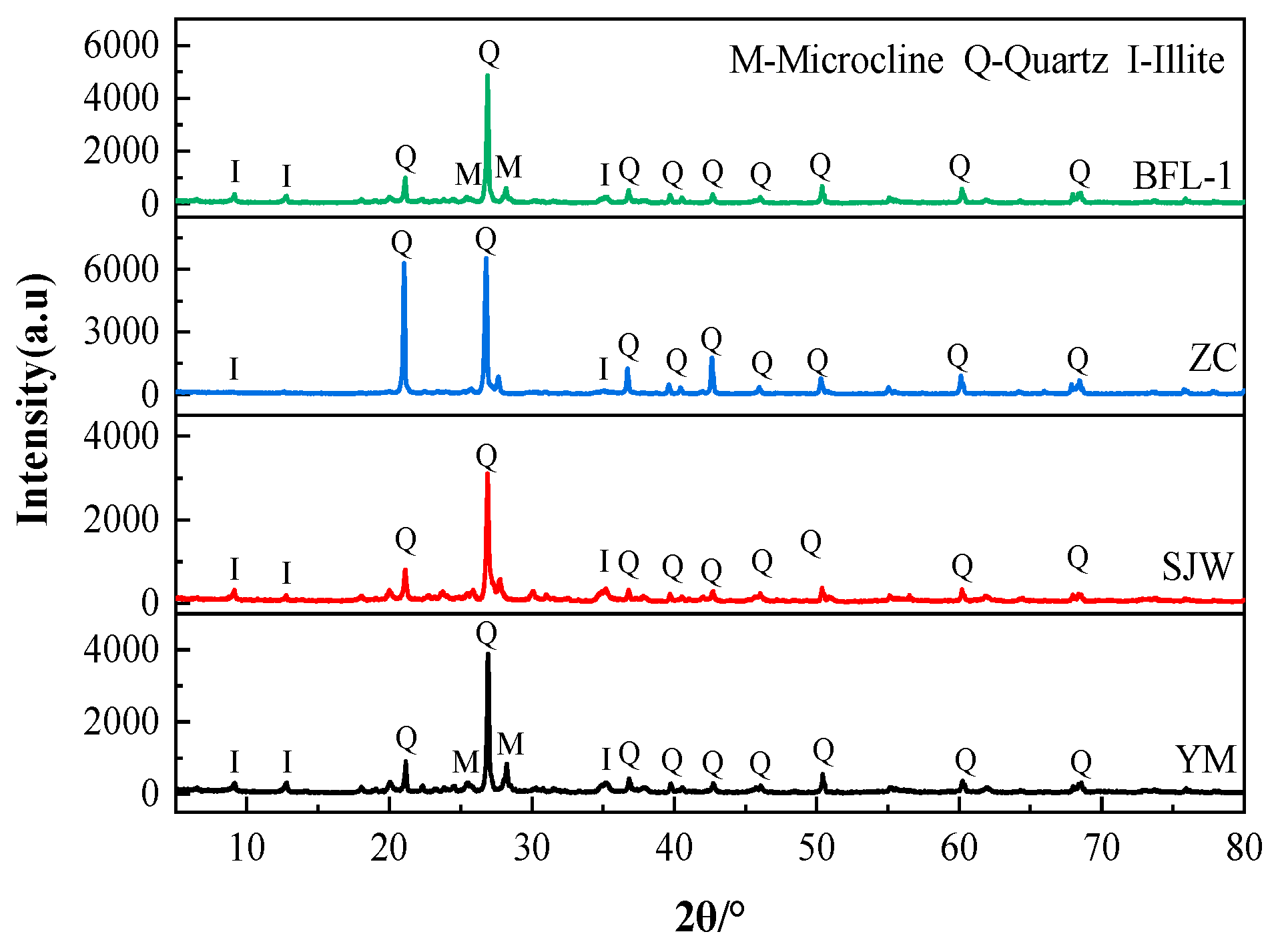
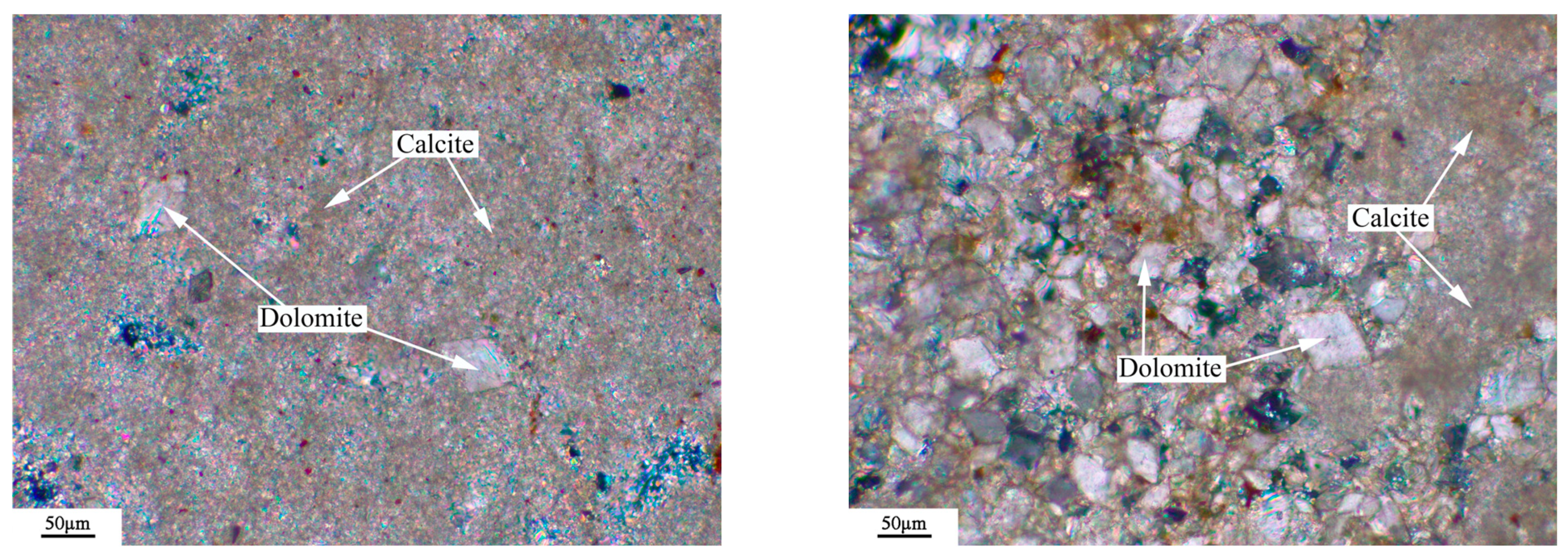

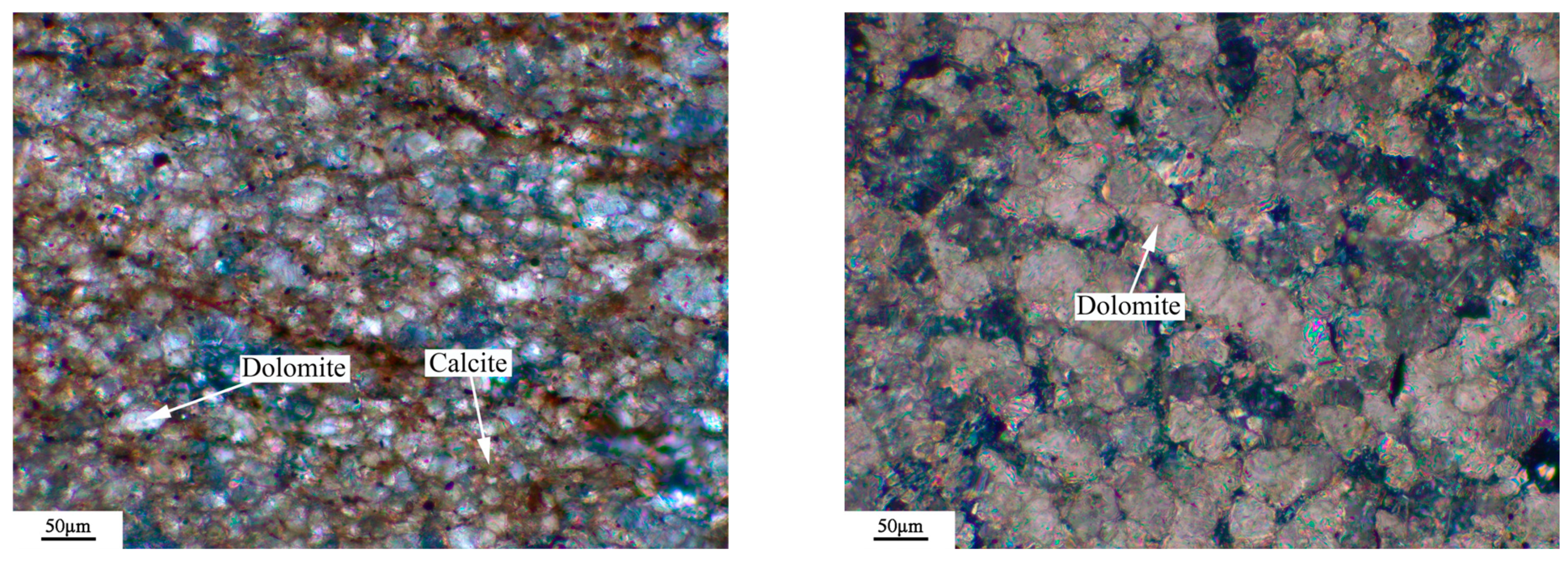
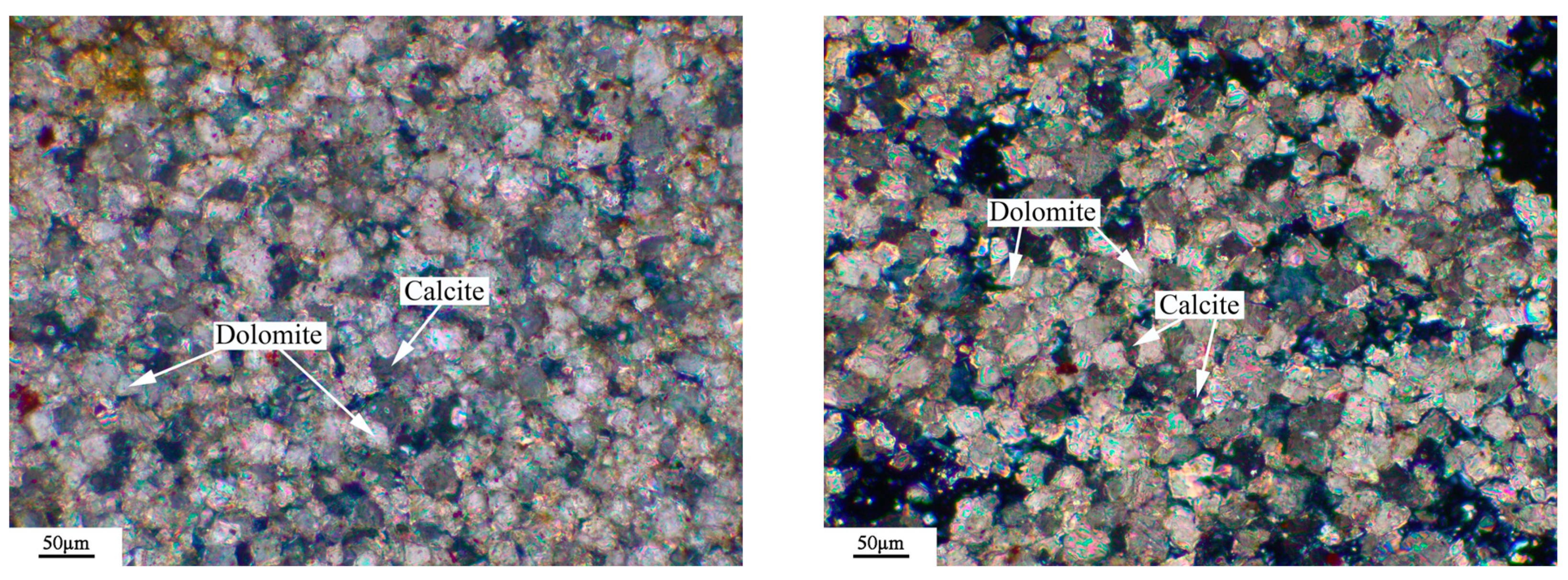

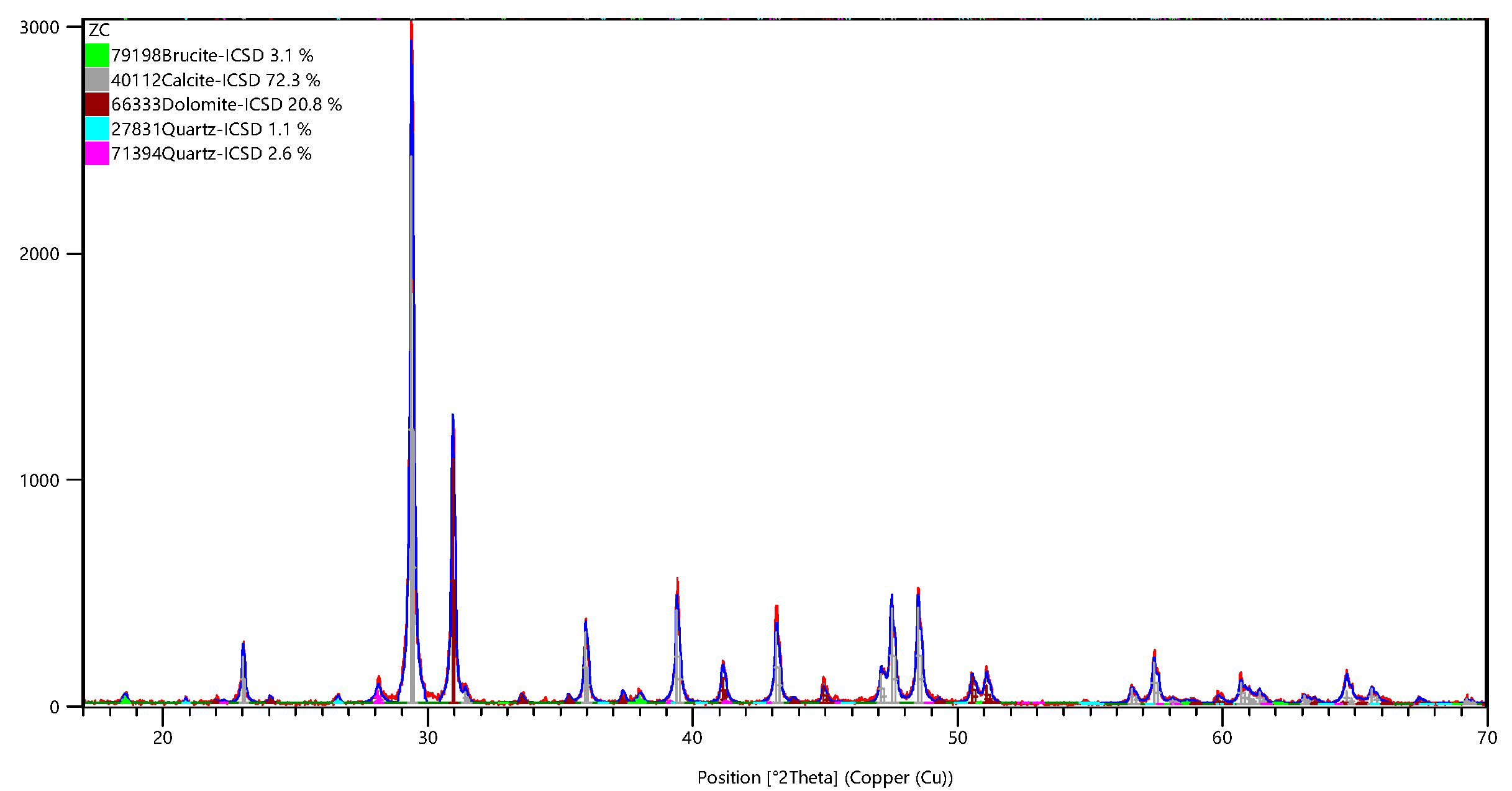



| Rock | Chemical Composition/wt% | Loss | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | MgO | Al2O3 | Fe2O3 | K2O | Na2O | |||
| BFL-1 | 3.93 | 52.34 | 1.19 | 0.70 | 0.25 | 0.16 | 0.15 | 40.57 | 99.35 |
| ZC | 2.01 | 47.07 | 6.65 | 0.85 | 0.25 | 0.13 | 0.12 | 41.43 | 98.66 |
| YM | 11.18 | 42.26 | 2.48 | 2.80 | 1.03 | 0.82 | 0.55 | 37.19 | 96.94 |
| SJW | 5.99 | 45.68 | 2.55 | 1.49 | 0.26 | 0.63 | 0.20 | 40.58 | 96.55 |
| Rocks | Mineral Content/wt% | AIR/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| The Mineral Content in the Acid Insoluble | The Mineral Content in Raw Materials | ||||||||
| Albite | Illite | Quartz | Microcline | Albite | Illite | Quartz | Microcline | ||
| BFL-1 | 0.00 | 22.75 | 67.30 | 7.35 | 0.00 | 0.96 | 2.84 | 0.31 | 4.22 |
| ZC | 0.00 | 13.14 | 58.29 | 0.00 | 0.00 | 0.46 | 2.04 | 0.00 | 3.50 |
| YM | 13.81 | 44.64 | 33.29 | 8.16 | 3.00 | 9.71 | 7.24 | 1.77 | 21.75 |
| SJW | 0.00 | 56.28 | 36.61 | 0.00 | 0.00 | 5.38 | 3.50 | 0.00 | 9.56 |
| No | Sample | Clay Minerals Contents/% | Total Pore Volume/(mm3/g) | Micropore (1–10 nm) Volume/(mm3/g) | Mesopore (10–100 nm) Volume/(mm3/g) | Macropore (100–250 nm) Volume/(mm3/g) |
|---|---|---|---|---|---|---|
| 1 | BFL-1 | 0.96 | 6.4 | 0.4 | 4.9 | 1.1 |
| 2 | ZC | 0.46 | 3.2 | 0.3 | 1.8 | 1.1 |
| 3 | YM | 10.04 | 7.1 | 0.7 | 5.3 | 1.1 |
| 4 | SJW | 5.42 | 6.9 | 0.7 | 5.0 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, W.; Huang, X.; Wang, Z.; Deng, M. Mechanisms for Migration of Alkali in Dolomitic Limestones. Materials 2025, 18, 4404. https://doi.org/10.3390/ma18184404
Zhang X, Li W, Huang X, Wang Z, Deng M. Mechanisms for Migration of Alkali in Dolomitic Limestones. Materials. 2025; 18(18):4404. https://doi.org/10.3390/ma18184404
Chicago/Turabian StyleZhang, Xinyu, Wei Li, Xiaojun Huang, Zhixin Wang, and Min Deng. 2025. "Mechanisms for Migration of Alkali in Dolomitic Limestones" Materials 18, no. 18: 4404. https://doi.org/10.3390/ma18184404
APA StyleZhang, X., Li, W., Huang, X., Wang, Z., & Deng, M. (2025). Mechanisms for Migration of Alkali in Dolomitic Limestones. Materials, 18(18), 4404. https://doi.org/10.3390/ma18184404







