Bacterial Tolerance and Bioleaching in the Presence of Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Growth Conditions
2.2. NaCl Tolerance
2.3. Bioleaching of Pyrite and Chalcopyrite
3. Results and Discussion
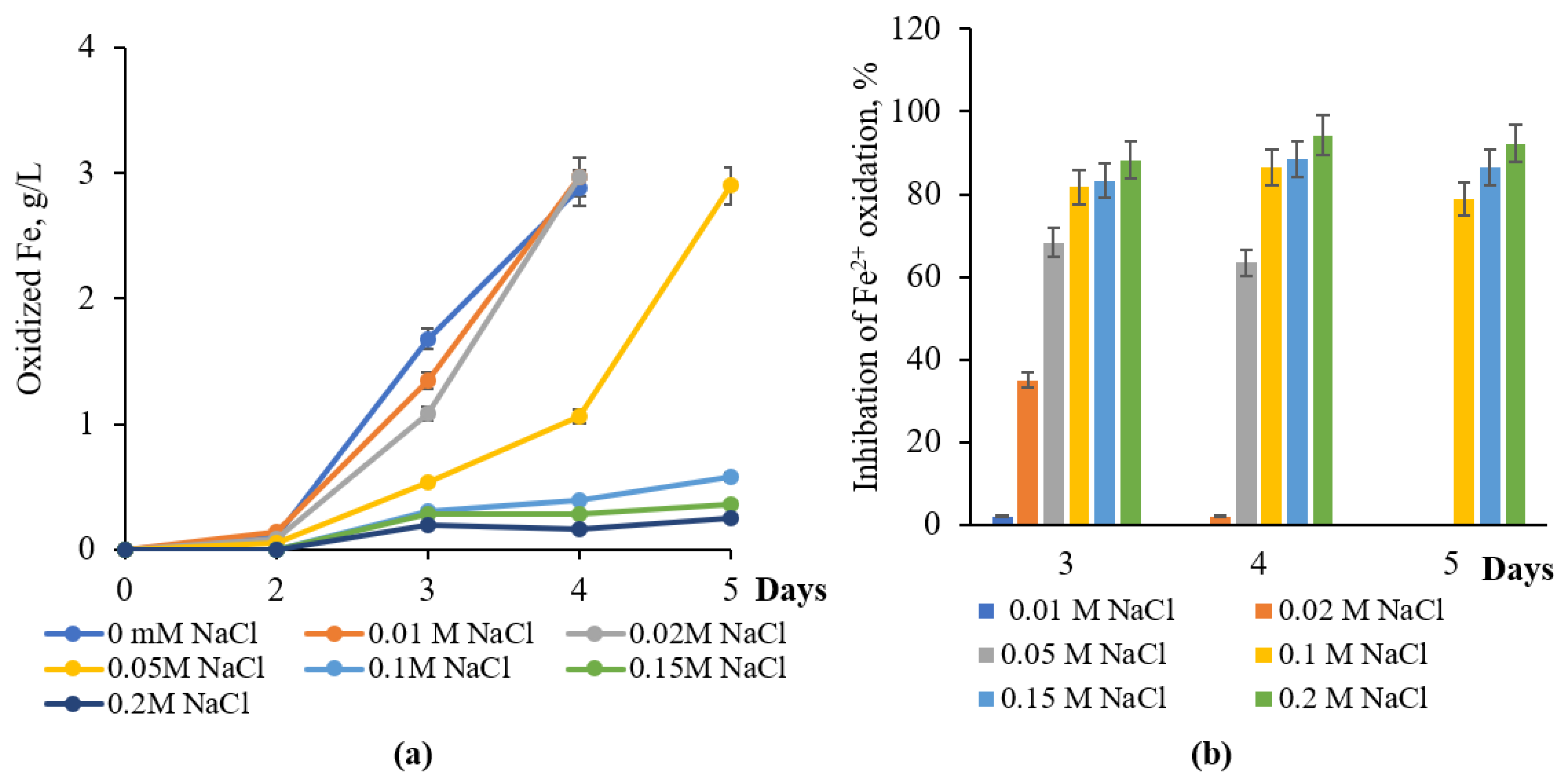
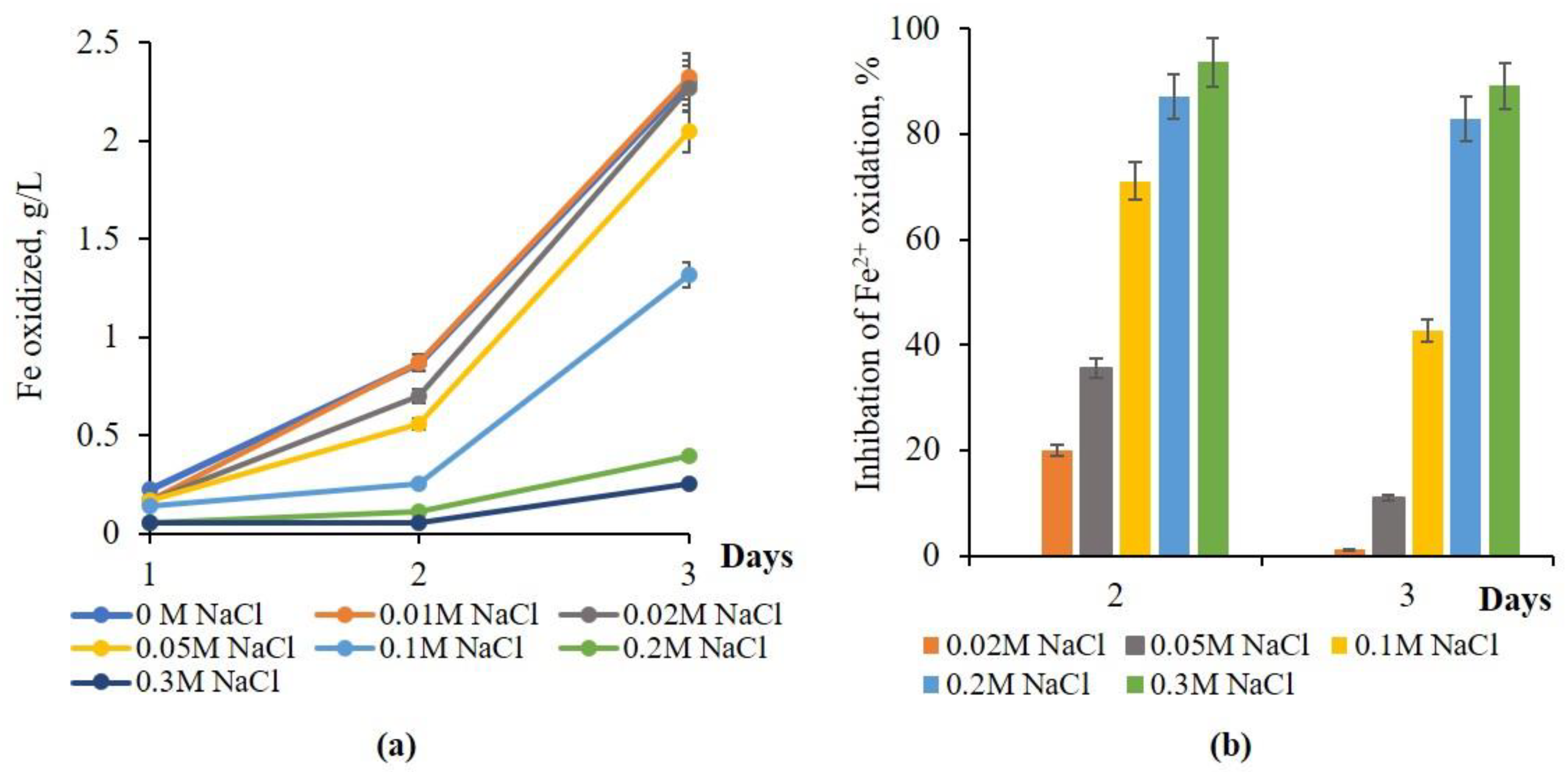
3.1. Effect of NaCl on Iron Oxidation
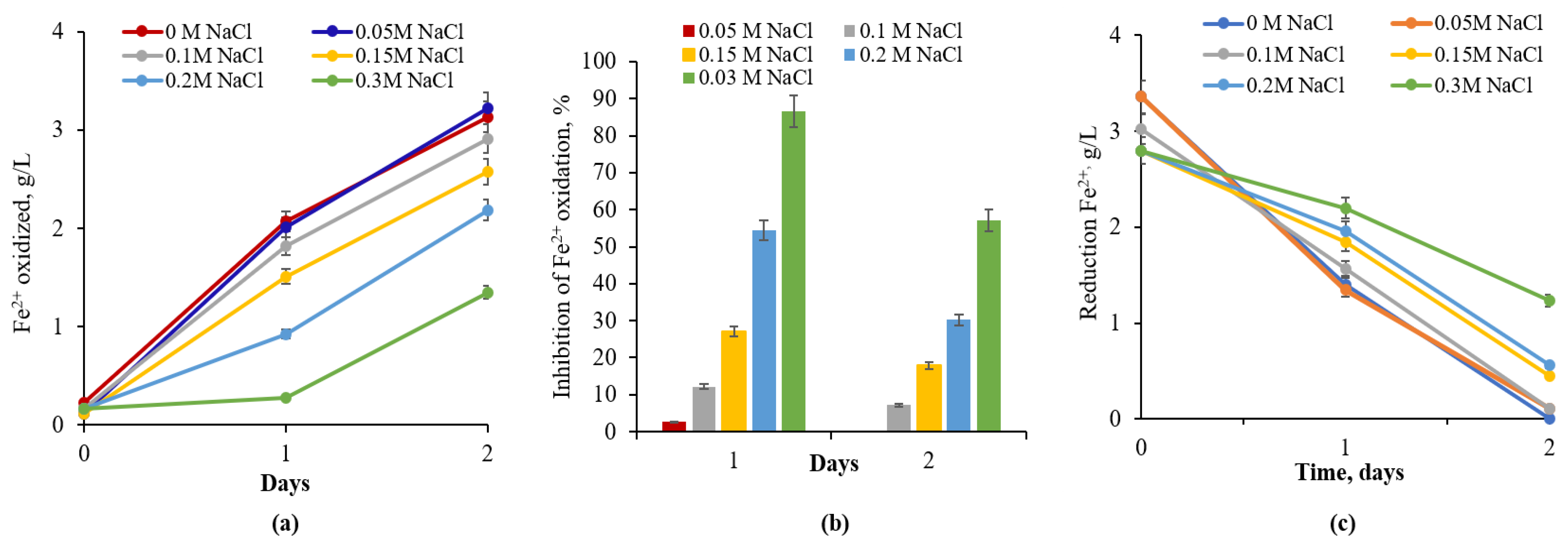
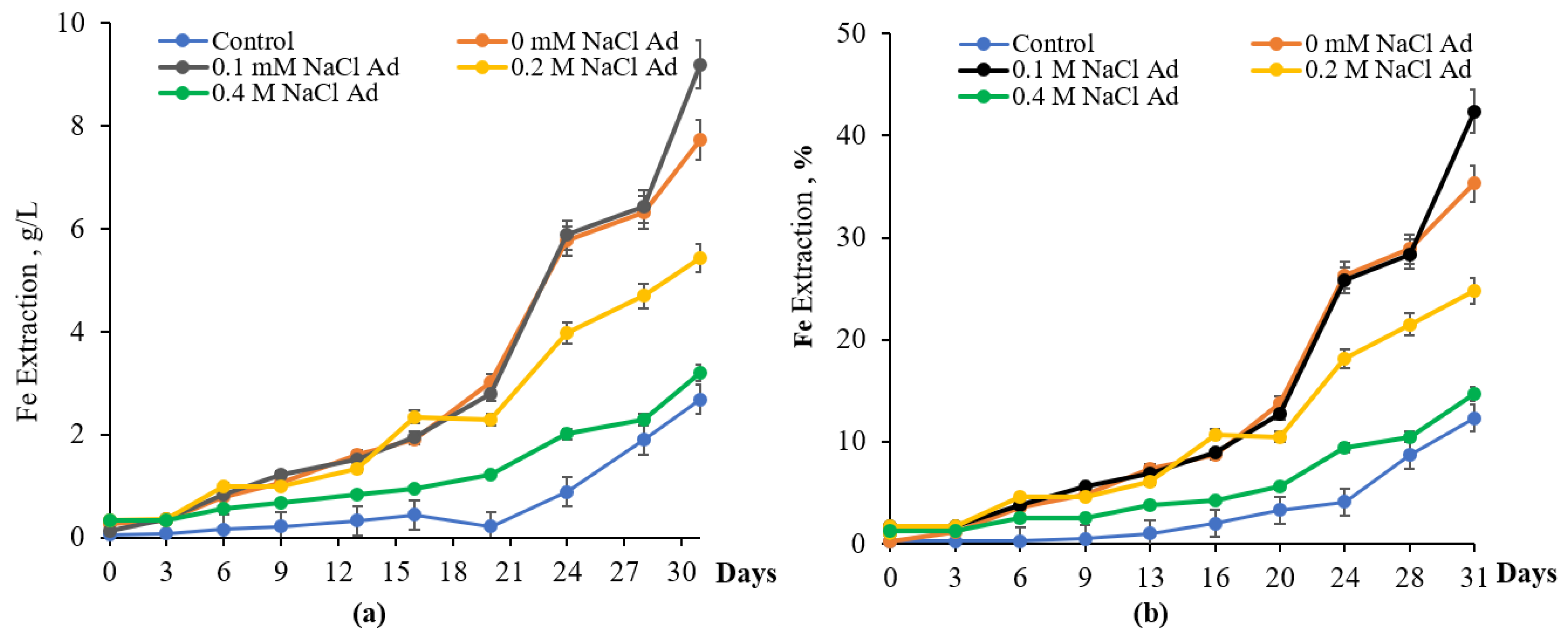
3.2. Iron Oxidation Bacteria Adapted to NaCl
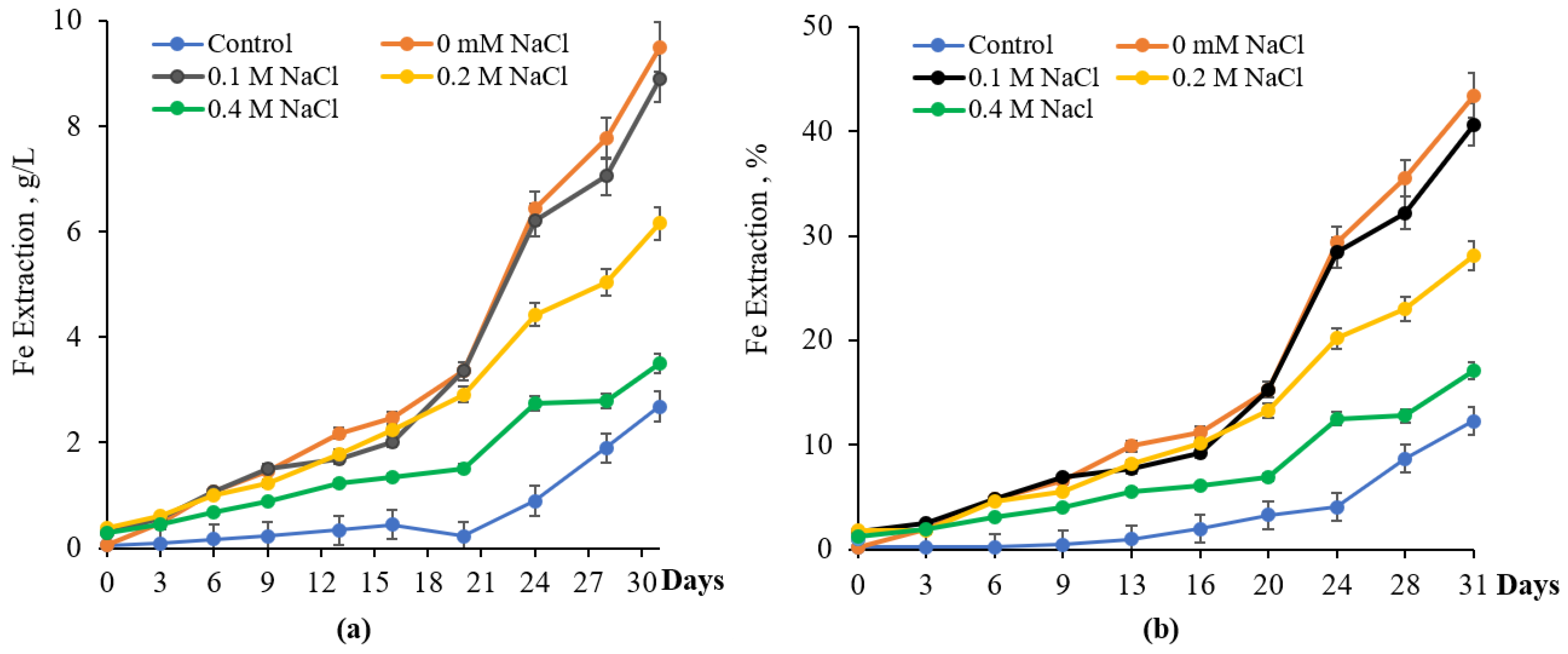
3.3. Bioleaching of Pyrite with and Without NaCl
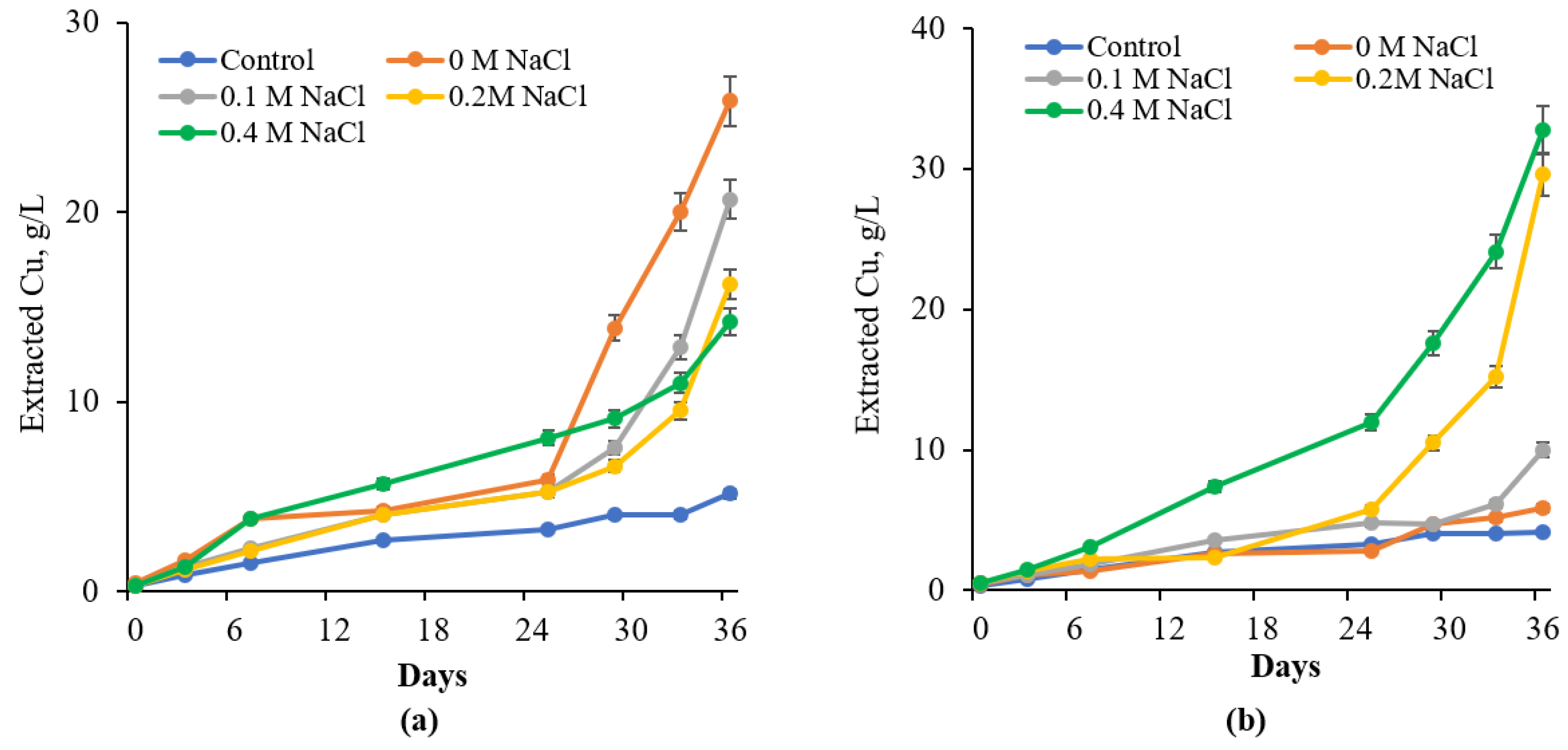
3.4. Effect of NaCl on Chalcopyrite Bioleaching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | Minimal inhibitory concentration |
| IC50 | Half maximal inhibitory concentration |
| AMD | Acid mine drainage |
| RISCs | Reduced inorganic sulfur compounds |
| MAC | Mackintosh medium |
| EDTA | Ethylenediaminetetraacetic acid |
| AAS | Atomic absorption spectrometer |
References
- Mishra, D.; Kim, D.J.; Ahn, J.G.; Rhee, Y.H. Bioleaching: A microbial process of metal recovery. A review. Met. Mater. Int. 2005, 11, 249–256. [Google Scholar] [CrossRef]
- Kumar, P.S.; Yaashikaa, P.R. Chapter 20—Recent trends and challenges in bioleaching technologies. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–388. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Luo, X.; Teng, T.; Jiang, C.; Drewniak, L.; Yang, Z.; Yin, H. An integrated insight into bioleaching performance of chalcopyrite mediated by microbial factors: Functional types and biodiversity. Bioresour. Technol. 2021, 319, 124219. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Naomi, J.B.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.U.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterization. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Gomez, E.; Ballester, A.; Blazquez, M.L.; Gonzalez, F. Silver catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy 1999, 51, 37–46. [Google Scholar] [CrossRef]
- Xia, J.L.; Song, J.J.; Liu, H.C.; Nie, Z.Y.; Shen, L.; Yuan, P.; Ma, C.Y.; Zheng, L.; Zhao, Y.D. Study on catalytic mechanism of silver ions in bioleaching of chalcopyrite by SR-XRD and XANES. Hydrometallurgy 2018, 180, 26–35. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, X.D.; Chen, A.J.; Liu, H.W.; Yin, H.Q.; Qiu, G.Z.; Hao, X.D.; Liang, Y.L. Comparative study on chalcopyrite bioleaching with assistance of different carbon materials by mixed moderate thermophiles. Trans. Nonferrous Met. Soc. China 2019, 29, 1294–1303. [Google Scholar] [CrossRef]
- Vakylabad, B.A.; Nazari, S.; Darezereshki, E. Bioleaching of copper from chalcopyrite ore at higher NaCl concentrations. Miner. Eng. 2022, 175, 107281. [Google Scholar] [CrossRef]
- Ghadiri, M.; Harrison, S.T.L.; Fagan-Enders, M.A. Effect of surfactant on the growth and activity of microorganisms in a heap bioleaching system. Miner. Eng. 2019, 138, 43–51. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Ma, C.; Zheng, L.; Hong, C.; Zhao, Y.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Hong, M.; Huang, X.; Gan, X.; Qiu, G.; Wang, J. The use of pyrite to control redox potential to enhance chalcopyrite bioleaching in the presence of Leptospirillum ferriphilum. Miner. Eng. 2021, 172, 107145. [Google Scholar] [CrossRef]
- Gu, C.Y.; Zhang, R.Y.; Xia, J.L.; Liu, H.C.; Sand, W.; Wang, Y.R.; Chen, L.; Nie, Z.Y.; Zhang, Y.S.; Wang, J. Chalcopyrite bioleaching by an enriched microbial community in acidic artificial seawater. J. Cent. South Univ. 2025, 32, 1802–1821. [Google Scholar] [CrossRef]
- Shiers, D.W.; Blight, K.R.; Ralph, D.E. Sodium sulphate and sodium chloride effects on batch culture of iron oxidising bacteria. Hydrometallurgy 2005, 80, 75–82. [Google Scholar] [CrossRef]
- Zammit, C.M.; Mangold, S.; rao Jonna, V.; Mutch, L.A.; Watling, H.R.; Dopson, M.; Watkin, E.L. Bioleaching in brackish waters—Effect of chloride ions on the acidophile population and proteomes of model species. Appl. Microbiol. Biotechnol. 2012, 93, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zammit, C.M.; Mutch, L.A.; Watling, H.; Watkin, E. The characterization of salt tolerance in biomining microorganisms and the search for novel salt tolerant strains. Adv. Mater. Res. 2009, 71–73, 283–286. [Google Scholar] [CrossRef]
- Rea, S.M.; McSweeney, N.J.; Degens, B.P.; Morris, C.; Siebert, H.M.; Kaksonen, A.H. Salt-tolerant microorganisms potentially useful for bioleaching operations where fresh water is scarce. Miner. Eng. 2015, 75, 126–132. [Google Scholar] [CrossRef]
- Khaleque, H.L.; González, C.; Shafique, R.; Kaksonen, A.H.; Holmes, A.H.; Watkin, E.L.J. Uncovering the Mechanisms of Halotolerance in the Extremely Acidophilic Members of the Acidihalobacter Genus Through Comparative Genome Analysis. Front. Microbiol. 2019, 10, 155. [Google Scholar] [CrossRef]
- Rivera-Araya, J.; Schlömann, M.; Levicán, G.J. Comparative study of NaCl-tolerance mechanisms in acidophilic iron-oxidizing bacteria and archaea. Solid State Phenom. 2017, 262, 385–388. [Google Scholar] [CrossRef]
- Gahan, C.S.; Sundkvist, J.E.; Dopson, M.; Sandström, Å. Effect chloride ferrous iron oxidation Leptospirillum ferriphilum dominated chemostat culture. Biotechnol. Bioeng. 2010, 106, 422–431. [Google Scholar] [CrossRef]
- Wang, Y.; Su, L.; Zhang, L.; Zeng, W.; Wu, J.; Wan, L.; Qiu, G.; Chen, X.; Zhou, H. Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium. Bioresour. Technol. 2012, 121, 348–354. [Google Scholar] [CrossRef]
- Suzuki, I.; Lee, D.; Mackay, B.; Harahuc, L.; Oh, J.K. Effect of various ions, pH, and osmotic pressure on oxidation of elemental sulfur by Thiobacillus thiooxidans. Appl. Environ. Microbiol. 1999, 65, 5163–5168. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–79. [Google Scholar] [CrossRef]
- Rivera-Araya, J.; Pollender, A.; Huynh, D.; Schlömann, M.; Chávez, R.; Levicán, G.J. Osmotic imbalance, cytoplasm acidification, and oxidative stress induction support the high toxicity of chloride in acidophilic bacteria. Front. Microbiol. 2019, 10, 2455. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Araya, J.; Huynh, N.D.; Kaszuba, M.; Chávez, R.; Schlömann, M.; Levicán, G. Mechanisms of NaCl-tolerance in acidophilic iron-oxidizing bacteria and archaea: Comparative genomic predictions and insights. Hydrometallurgy 2020, 194, 105334. [Google Scholar] [CrossRef]
- Huber, H.; Stetter, K.O. Thiobacillus prosperus sp. nov., represents a new group of halotolerant metal-mobilizing bacteria isolated from a marine geothermal field. Arch. Microbiol. 1989, 151, 479–485. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Martins, F.L.; Leão, V.A. Chalcopyrite bioleaching in chloride media: A mini-review. Hydrometallurgy 2023, 216, 105995. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A.; Van Rooyen, J.V. Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture. Int. J. Miner. Process 2001, 62, 243–255. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Lahti, H.; Suegama, P.H.; Garcia, O., Jr.; Benedetti, A.V.; Puhakka, J.A.; Tuovinen, P.H. Effect of Na–chloride on the bioleaching of a chalcopyrite concentrate in shake flasks and stirred tank bioreactors. Hydrometallurgy 2013, 138, 1–13. [Google Scholar] [CrossRef]
- Bobadilla-Fazzini, R.A.; Perez, A.; Gautier, V.; Jordan, H.; Parada, P. Primary copper sulfides bioleaching vs. chloride leaching: Advantages and drawbacks. Hydrometallurgy 2017, 168, 26–31. [Google Scholar] [CrossRef]
- Bobadilla-Fazzini, R.A.; Cortés, M.P.; Maass, A.; Parada, P. Sulfobacillus thermosulfidooxidans strain Cutipay enhances chalcopyrite bioleaching under moderate thermophilic conditions in the presence of chloride ion. AMB Express 2014, 4, 84. [Google Scholar] [CrossRef]
- Bakhshoude, M.; Darezereshki, E.; Bakhtiari, F. Thermoacidophilic bioleaching of copper sulfide concentrate in the presence of chloride ions. J. Cent. South Univer. 2023, 30, 749–762. [Google Scholar] [CrossRef]
- Martins, F.L.; Patto, G.B.; Leão, V.A. Chalcopyrite bioleaching in the presence of high chloride concentrations. J. Chem. Technol. Biotechnol. 2019, 94, 2333–2344. [Google Scholar] [CrossRef]
- Martins, F.L.; Leão, V.A. Bioleaching of two different chalcopyrite ores in chloride media. Braz. J. Chem. Eng. 2024, 41, 475–485. [Google Scholar] [CrossRef]
- Noguchi, H.; Okibe, N. The role of bioleaching microorganisms in saline water leaching of chalcopyrite concentrate. Hydrometallurgy 2020, 195, 105397. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The role of microorganisms in gold processing and recovery- a review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Huynh, D.; Giebner, F.; Kaschabek, S.R.; Rivera-Araya, J.; Levicán, G.J.; Sand, W.; Schlömann, M. Effect of sodium chloride on Leptospirillum ferriphilum DSM 14647T and Sulfobacillus thermosulfidooxidans DSM 9293T: Growth, iron oxidation activity and bioleaching of sulfidic metal ores. Miner. Eng. 2019, 138, 52–59. [Google Scholar] [CrossRef]
- Huynh, D.; Norambuena, J.; Boldt, C.; Kaschabek, S.R.; Levicán, G.; Schlömann, M. Effect of Sodium Chloride on Pyrite Bioleaching and Initial Attachment by Sulfobacillus thermosulfidooxidans. Front. Microbiol. 2020, 11, 2102. [Google Scholar] [CrossRef]
- Gahan, C.S.; Sundkvist, J.E.; Sandström, Å. A study on the toxic effects of chloride on the biooxidation efficiency of pyrite. J. Hazard. Mater. 2009, 172, 1273–1281. [Google Scholar] [CrossRef]
- Toro, N.; Moraga, C.; Torres, D.; Saldaña, M.; Pérez, K.; Gálvez, E. Leaching Chalcocite in Chloride Media—A Review. Minerals 2021, 11, 1197. [Google Scholar] [CrossRef]
- Huynh, D.; Kaschabek, S.R.; Sand, W.; Schlömann, M. Microorganisms oxidize iron (II) ions in the presence of high concentrations of sodium chloride—Potentially useful for bioleaching. Solid State Phenom. 2017, 262, 364–367. [Google Scholar] [CrossRef]
- Khaleque, H.N.; Kaksonen, A.H.; Boxall, N.J.; Watkin, E.L.J. Chloride ion tolerance and pyrite bioleaching capabilities of pure and mixed halotolerant, acidophilic iron- and sulfur-oxidizing cultures. Miner. Eng. 2018, 120, 87–93. [Google Scholar] [CrossRef]
- Norris, P.R.; Davis-Belmar, C.S.; Calvo-Bado, L.A.; Ogden, T.J. Salt-tolerant Acidihalobacter and Acidithiobacillus species from Vulcano (Italy) and Milos (Greece). Extremophiles 2020, 24, 593–602. [Google Scholar] [CrossRef]
- Mackintosh, M.E. Nitrogen Fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215. [Google Scholar] [CrossRef]
- Lucchesi, C.A.; Hirn, C.F. EDTA Titration of total Iron in Iron(II) and Iron(III) mixtures. Application to Iron driers. Anal Chem. 1960, 32, 1191–1193. [Google Scholar] [CrossRef]
- Kamimura, K.; Higashino, E.; Kanao, T.; Sugio, T. Effects of inhibitors and NaCl on the oxidation of reduced inorganic sulfur compounds by a marine acidophilic, sulfur-oxidizing bacterium, Acidithiobacillus thiooxidans strain SH. Extremophiles 2005, 9, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.R.; Nicolle, J.L.C.; Calvo-Bado, L.; Angelatou, V. Pyrite oxidation by halotolerant, thermotolerant bacteria. Adv. Mater. Res. 2009, 71–73, 75–78. [Google Scholar] [CrossRef]
- Romero, J.; Yanez, C.; Vasquez, M.; Moore, R.R.; Espejo, R.T. Characterization and identification of an iron-oxidizing, Leptospirillum- like bacterium, present in the high sulfate leaching solution of a commercial bioleaching plant. Res. Microbiol. 2003, 154, 353–359. [Google Scholar] [CrossRef]
- Kanaev, Z.K.; Bulaev, A.G.; Kanaev, A.T.; Kondrat’eva, T.F. Physiological properties of Acidithiobacillus ferrooxidans strains isolated from sulfide ore deposits in Kazakhstan. Microbiology 2015, 84, 370–376. [Google Scholar] [CrossRef]
- Torres, C.M.; Ghorbani, Y.; Hernández, P.C.; Justel, F.J.; Aravena, M.I.; Herreros, O.O. Cupric and Chloride Ions: Leaching of Chalcopyrite Concentrate with Low Chloride Concentration Media. Minerals 2019, 9, 639. [Google Scholar] [CrossRef]


| Bacterial Culture | NaCl, Concentration, M | ||||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.4 | ||
| Extracted Cu by native culture S. thermosulfidooxidans subsp. asporogenes 41 | g/L | 25.9 | 20.7 | 16.2 | 14.3 |
| % | 64 | 51.2 | 40.0 | 35.0 | |
| Extracted Cu by adapted culture S. thermosulfidooxidans subsp. asporogenes 41 | g/L | 5.83 | 10.0 | 29.58 | 32.8 |
| % | 17.3 | 24.7 | 73.2 | 97.3 | |
| Parameter | A. ferrooxidans | L. ferriphilum | S. thermosulfidooxidans | Reference |
|---|---|---|---|---|
| Maximum NaCl tolerance (native strain) | 0.1 M (6 g/L) | 0.15–0.225 M (9–13 g/L) | 0.05–0.1 M (2.9–5.8 g/L) | [20], this study (Figure 3) |
| NaCl MIC (inhibition threshold) | 0.005 M (41% Fe2+ oxid. inhibition) | 0.05 M (63–68% inhibition) | 0.05 M (35% inhibition) | [19], this study (Figure 1, Figure 2 and Figure 3) |
| Adapted strain tolerance | 0.05 M (10× improvement) | - | 0.1 M (2× improvement) | This study (Figure 4 and Figure 5) |
| Fe2+ oxidation inhibition at 0.1 M NaCl | 93% (native), 36% (adapted) | 86.5% (native) | 42.7% (native), 30% (adapted) | [14], this study (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5) |
| Pyrite bioleaching with NaCl | Inhibited at >0.05 M | Inhibited at >0.1 M | 26% inhibition at 0.4 M (native), 20.6% (adapted) | [40], this study (Figure 6 and Figure 7) |
| Chalcopyrite bioleaching with NaCl | Improved at 0.1 M [30] | - | 100% Cu recovery at 0.4 M (adapted) | [31], this study (Table 1) |
| Proposed tolerance mechanism | Osmotic imbalance, cytoplasmic acidification | K+ uptake, ectoine syntheses (inferred) | Compatible solutes (e.g., trehalose), membrane adaptation | [15,25] |
| Industrial relevance | Limited to low-salinity systems | Potential for brackish water | Best candidate for seawater bioleaching (0.4 M NaCl tolerance) | [21], this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardanyan, N.; Khachatryan, A.; Melkonyan, Z.; Abrahamyan, N.; Barseghyan, S.; Zhang, R.; Vardanyan, A. Bacterial Tolerance and Bioleaching in the Presence of Chloride. Materials 2025, 18, 4407. https://doi.org/10.3390/ma18184407
Vardanyan N, Khachatryan A, Melkonyan Z, Abrahamyan N, Barseghyan S, Zhang R, Vardanyan A. Bacterial Tolerance and Bioleaching in the Presence of Chloride. Materials. 2025; 18(18):4407. https://doi.org/10.3390/ma18184407
Chicago/Turabian StyleVardanyan, Narine, Anna Khachatryan, Zaruhi Melkonyan, Nelli Abrahamyan, Sona Barseghyan, Ruiyong Zhang, and Arevik Vardanyan. 2025. "Bacterial Tolerance and Bioleaching in the Presence of Chloride" Materials 18, no. 18: 4407. https://doi.org/10.3390/ma18184407
APA StyleVardanyan, N., Khachatryan, A., Melkonyan, Z., Abrahamyan, N., Barseghyan, S., Zhang, R., & Vardanyan, A. (2025). Bacterial Tolerance and Bioleaching in the Presence of Chloride. Materials, 18(18), 4407. https://doi.org/10.3390/ma18184407










