High Thermal Conductivity Diamond–Copper Composites Prepared via Hot Pressing with Tungsten–Coated Interfacial Layer Optimization
Highlights
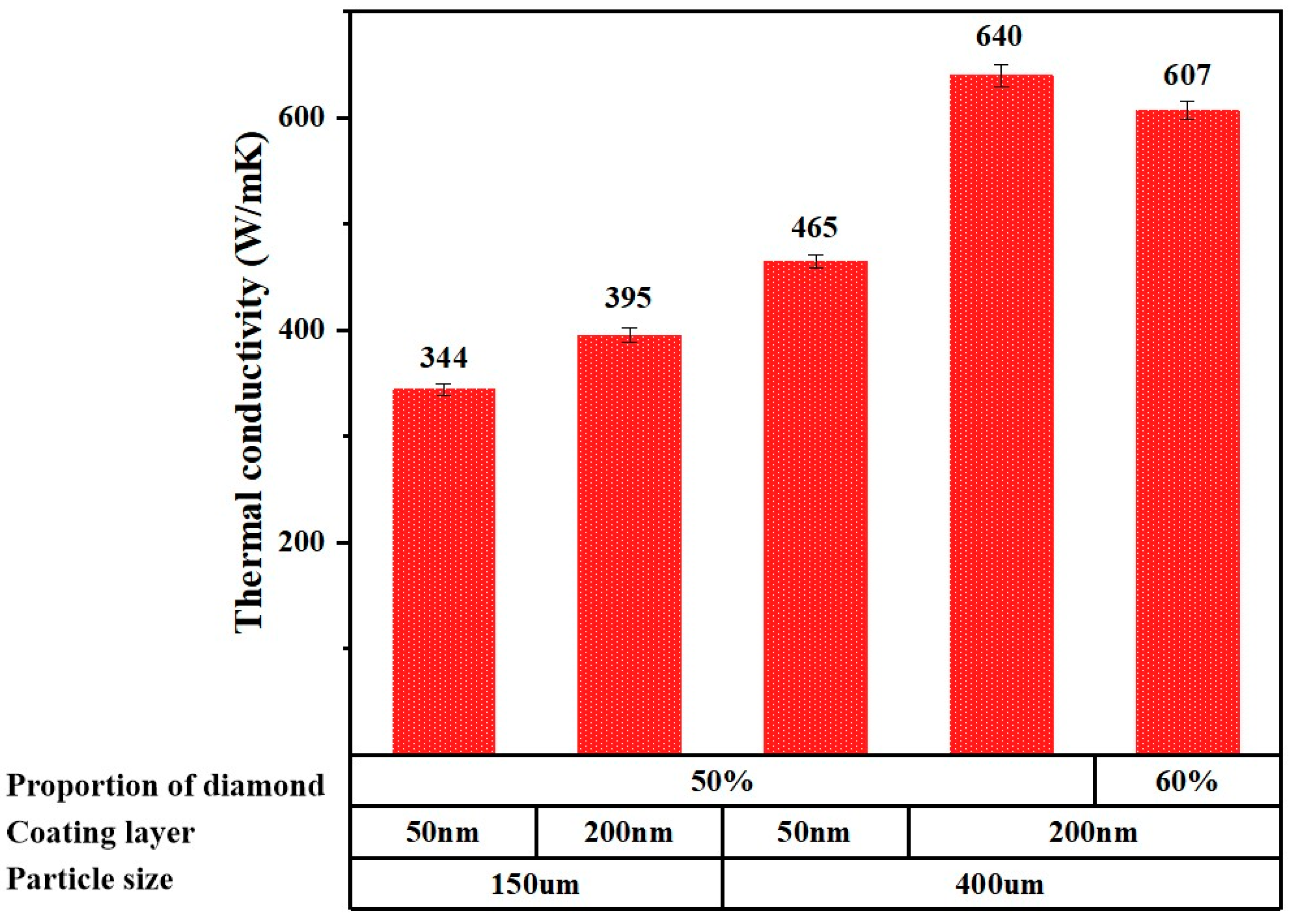
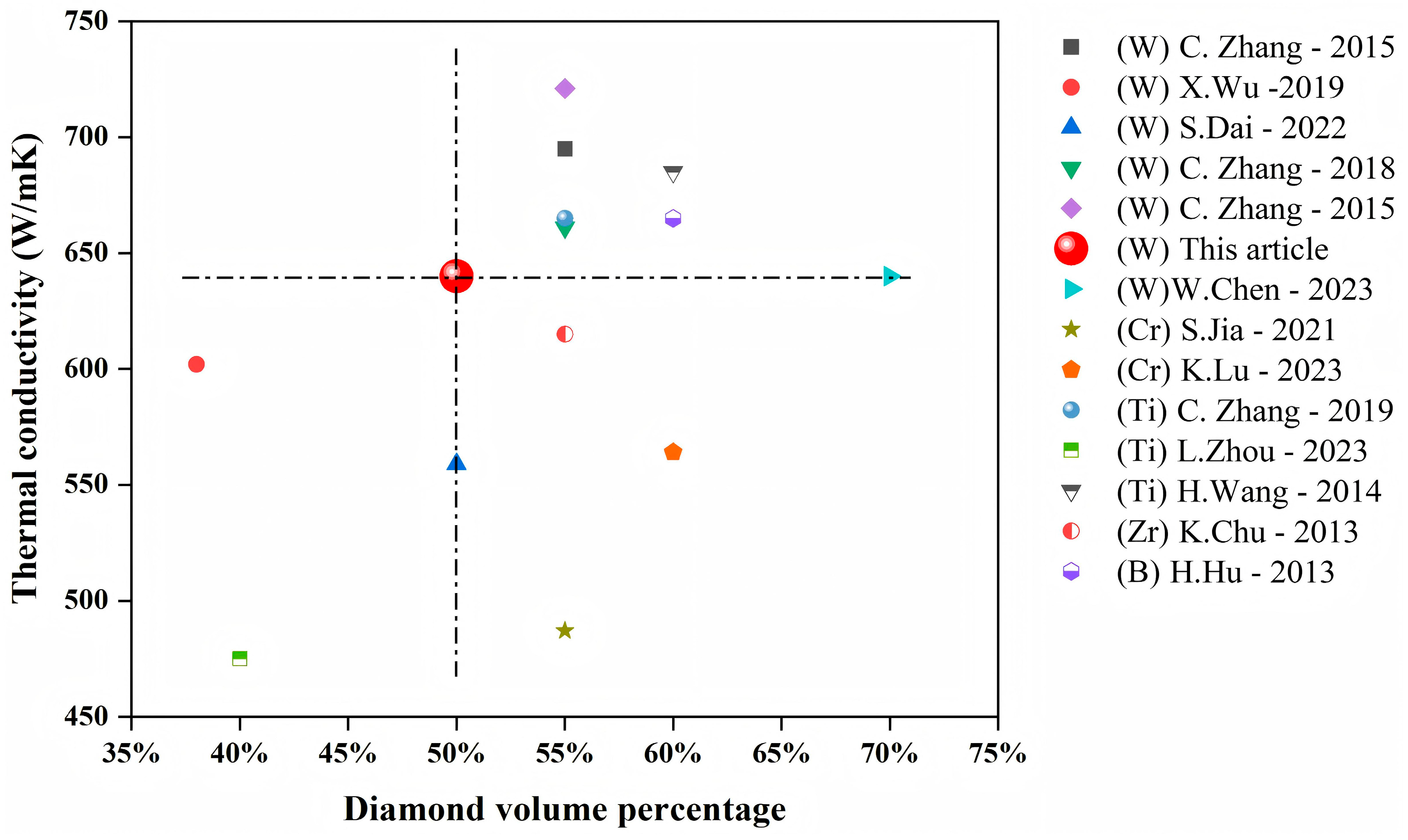
- Achieved outstanding thermal conductivity of 640 W/(m·K) in a pressure-sintered diamond-copper composite containing less than 50% diamond.
- Magnetron-sputtered tungsten coating significantly strengthened the interfacial bonding between diamond and copper.
- AMM and DMM thermal conductivity models validated the optimal diamond/W₂C/WC/W₂C/Cu interfacial structure.
Abstract
1. Introduction
2. Experiment
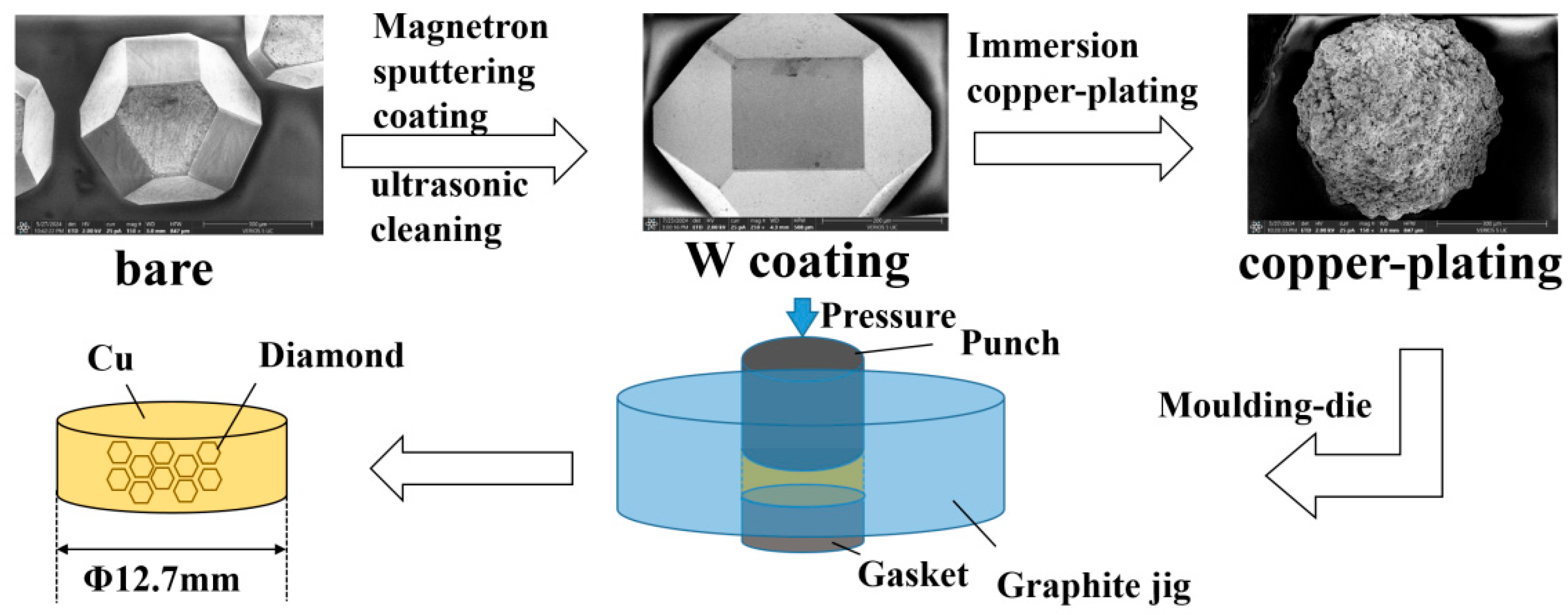
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
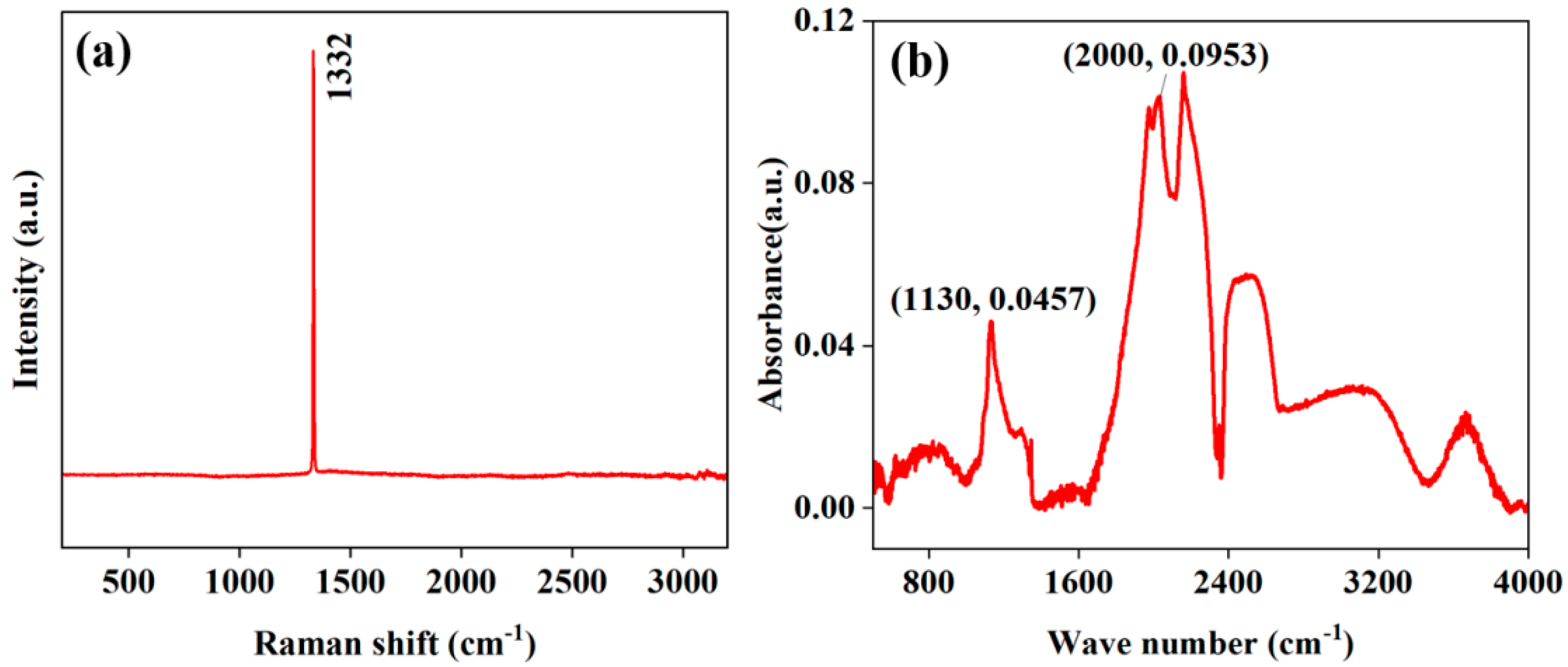
3.1. Diamond Characterization
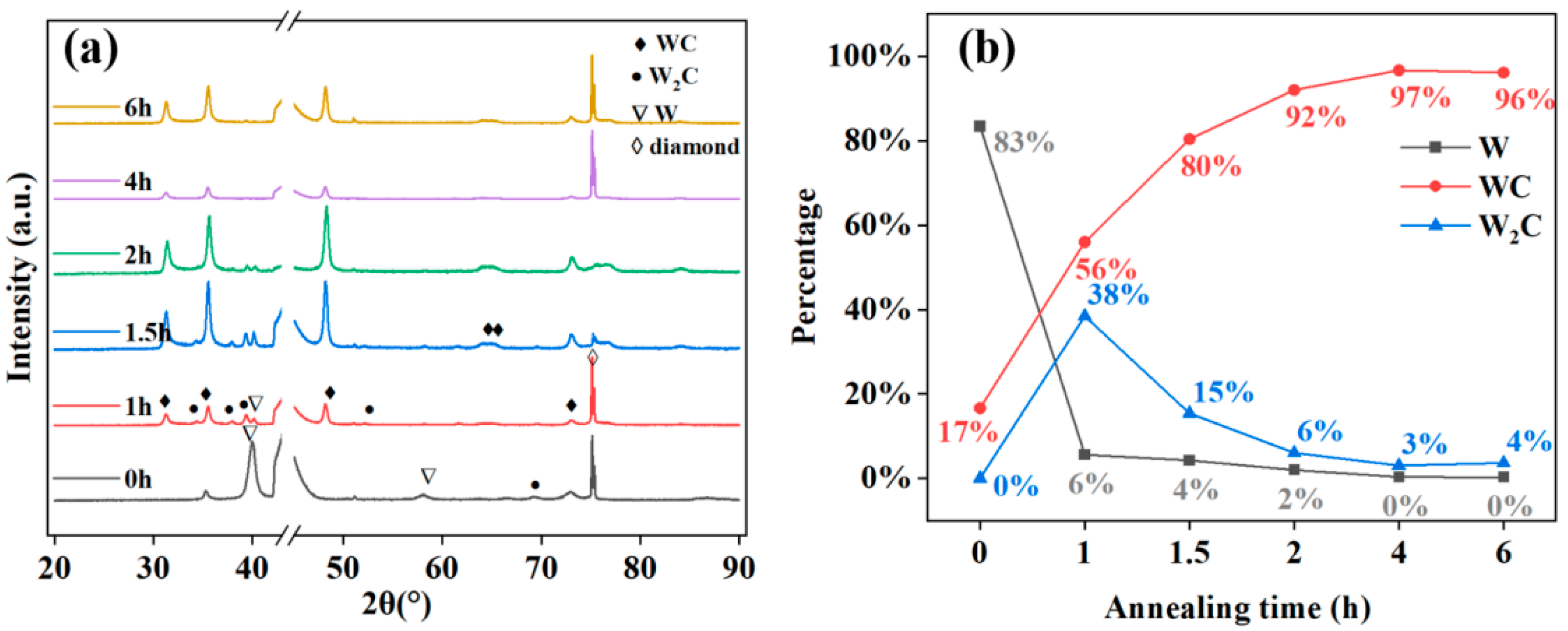
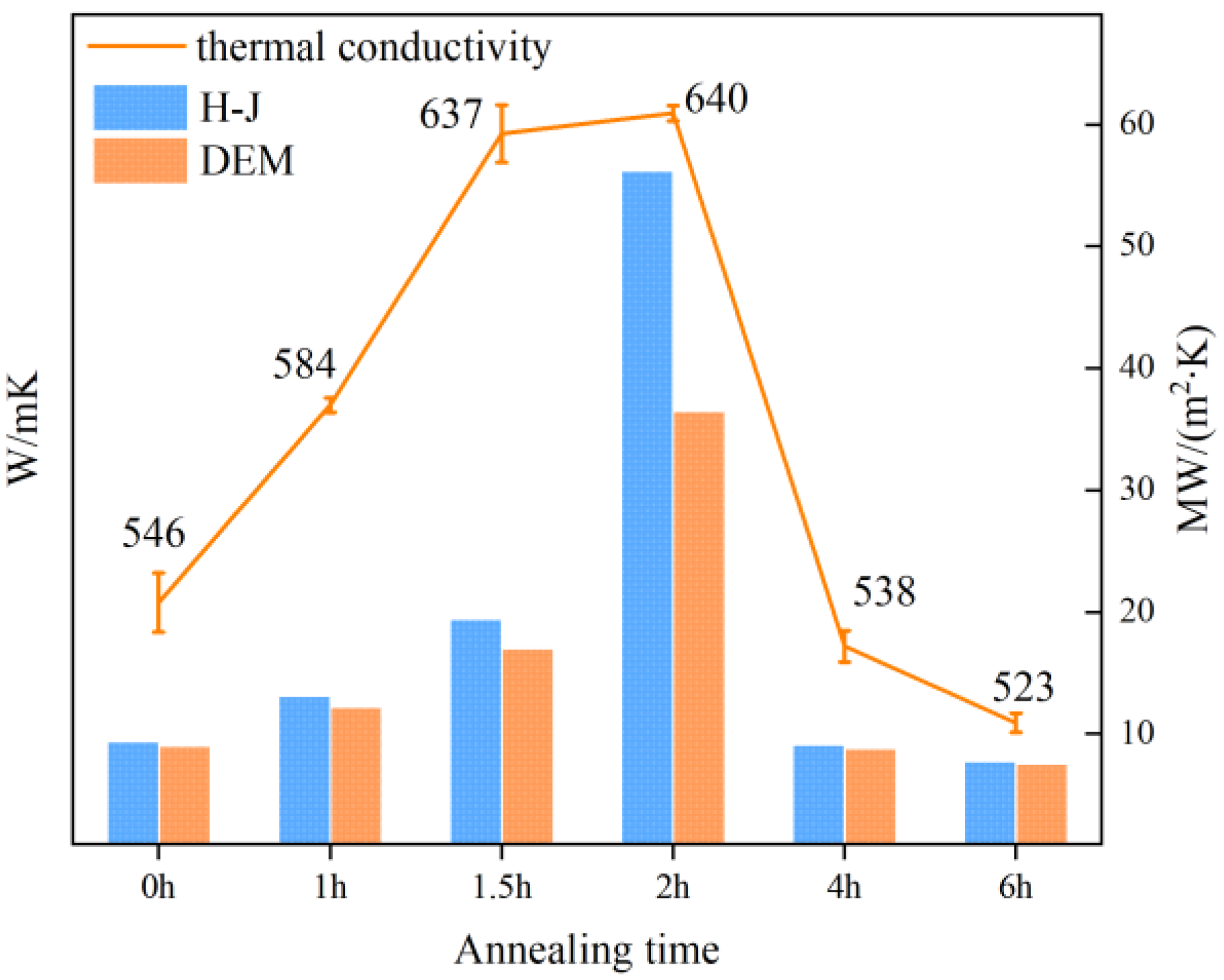
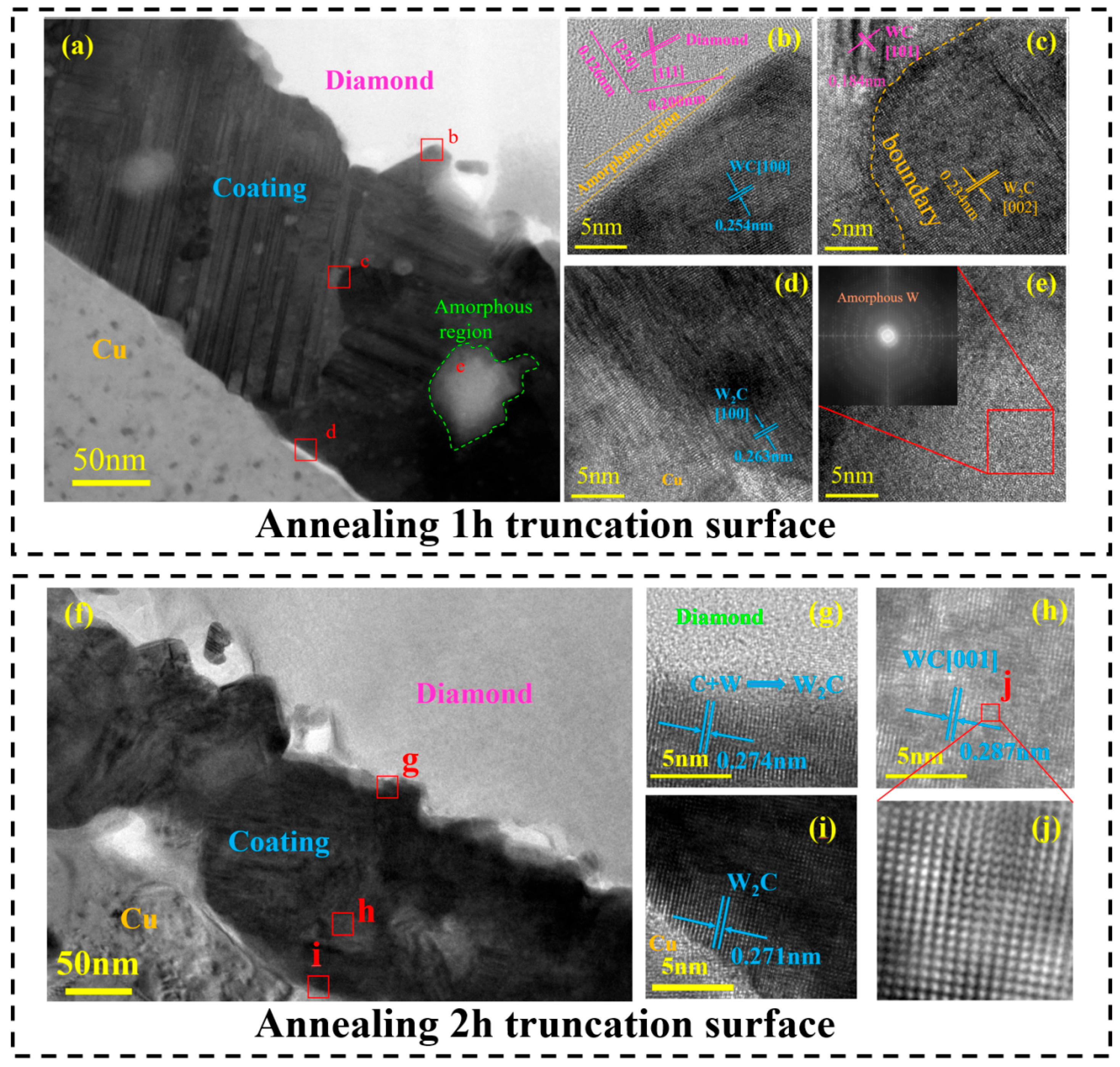
3.2. Influence of Annealing on Film Composition
3.3. Thermal Conductivity Performance of Composite Materials
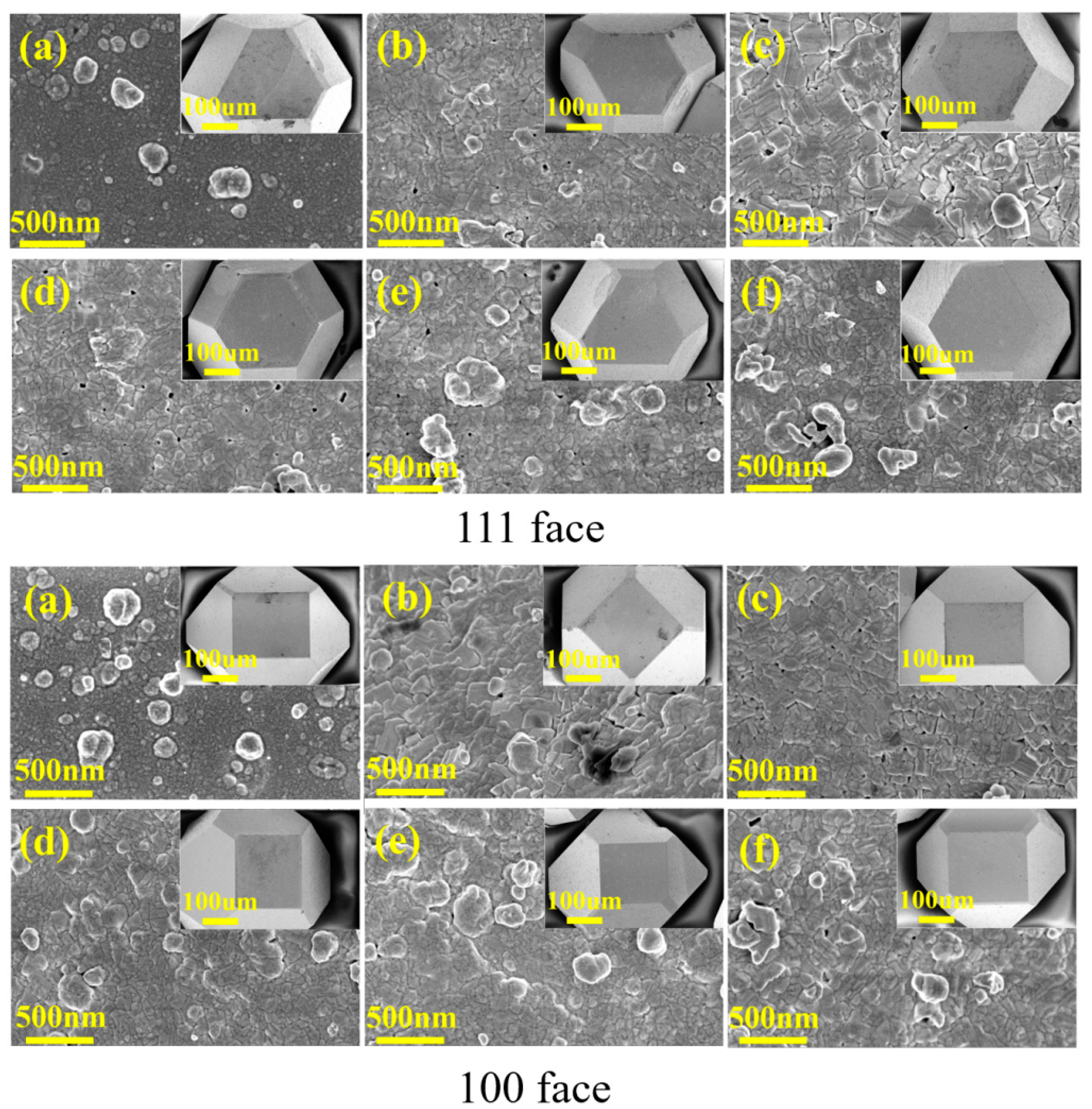
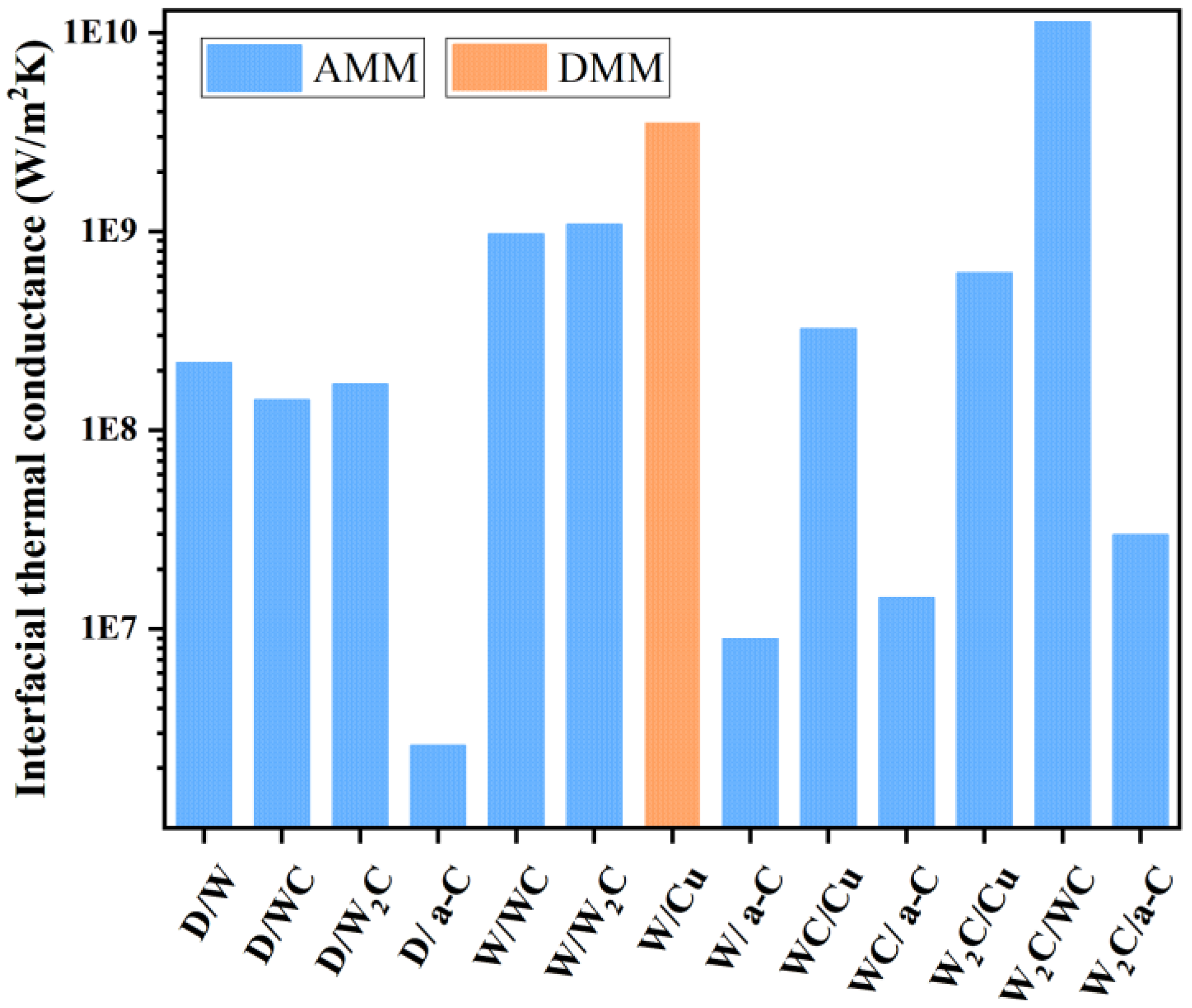
3.4. The Effect of Interface Structure on the Thermal Conductivity of Composites
| Annealing Time (h) | Thermal Diffusivity α (mm2/s) | Specific Heat Cp (J/g/K) | Density ρ (g/cm3) |
|---|---|---|---|
| 0 | 200.658 | 0.427 | 6.38 |
| 1 | 221.844 | 0.416 | 6.33 |
| 1.5 | 238.083 | 0.435 | 6.15 |
| 2 | 227.891 | 0.428 | 6.57 |
| 4 | 207.635 | 0.401 | 6.46 |
| 6 | 192.764 | 0.424 | 6.41 |
| AMM | DMM | |||||||
|---|---|---|---|---|---|---|---|---|
| λ W/(m·K) | ρ kg/m3 | Cp J/kg·K | ν m/s | γ J/m3·K2 | νF m/s | T K | Z | |
| diamond | 1717 | 3520 | 512 | 13,430 | ||||
| W | 173 | 1930 | 134 | 5200 | 136 | 5.00 × 105 | 298 | 2.03 × 1010 |
| WC | 121 | 15,700 | 132 | 4706 | ||||
| W2C | 1.139 | 17,200 | 325 | 3554 | ||||
| Cu | 385 | 8960 | 386 | 2801 | 98 | 1.60 × 106 | 298 | 4.67 × 1010 |
| a-C | 2000 | 710 | 2072 | |||||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Leng, X.; Zhao, R.; Kang, Y.; Chen, H. Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications. Crystals 2023, 13, 906. [Google Scholar] [CrossRef]
- Yuan, M.; Tan, Z.; Fan, G.; Xiong, D.B.; Guo, Q.; Guo, C.; Li, Z.; Zhang, D. Theoretical modelling for interface design and thermal conductivity prediction in diamond/Cu composites. Diam. Relat. Mater. 2018, 81, 38–44. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Y.; Jia, H.; Xu, X.; Yang, J.; He, Z.; Deng, K. A tunable gradient impedance matching layer based on piezoelectric materials with external circuits. J. Appl. Phys. 2020, 128, 064502. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Liu, X.; Dai, J.; Wang, X.; Zhang, H. Reinforcement size effect on thermal conductivity in Cu-B/diamond composite. J. Mater. Sci. Technol. 2021, 91, 1–4. [Google Scholar] [CrossRef]
- Bai, G.-Z.; Zhang, Y.-J.; Liu, X.-Y.; Dai, J.-J.; Wang, X.-T.; Zhang, H.-L. High-Temperature Thermal Conductivity and Thermal Cycling Behavior of Cu–B/Diamond Composites. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 626–636. [Google Scholar] [CrossRef]
- Zhu, C.; Lang, J.; Ma, N. Preparation of Si–diamond–SiC composites by in-situ reactive sintering and their thermal properties. Ceram. Int. 2012, 38, 6131–6136. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Che, Z.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Combining Cr pre-coating and Cr alloying to improve the thermal conductivity of diamond particles reinforced Cu matrix composites. J. Alloys Compd. 2018, 749, 1098–1105. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, X.; Huang, S.; Xie, H.; Mi, X. Tailoring the thermal and mechanical properties of diamond/Cu composites by interface regulation of Cr alloying. Diam. Relat. Mater. 2021, 114, 108309. [Google Scholar] [CrossRef]
- Liu, X.; Sun, F.; Wang, L.; Wu, Z.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. The role of Cr interlayer in determining interfacial thermal conductance between Cu and diamond. Appl. Surf. Sci. 2020, 515, 146046. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Catalano, M.; Bai, G.; Li, N.; Dai, J.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Enhanced thermal conductivity in Cu/diamond composites by tailoring the thickness of interfacial TiC layer. Compos. Part A Appl. Sci. Manuf. 2018, 113, 76–82. [Google Scholar] [CrossRef]
- Lei, L.; Bolzoni, L.; Yang, F. High thermal conductivity and strong interface bonding of a hot-forged Cu/Ti-coated-diamond composite. Carbon 2020, 168, 553–563. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. Optimized thermal conductivity of diamond/Cu composite prepared with tungsten-copper-coated diamond particles by vacuum sintering technique. Vacuum 2018, 153, 74–81. [Google Scholar] [CrossRef]
- Sang, J.; Yuan, Y.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Exploring the underlying causes of optimizing thermal conductivity of copper/diamond composites by interface thickness. J. Alloys Compd. 2022, 891, 161777. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Z.; Tang, Y.; Wang, R.; Peng, C.; Feng, Y. Microstructure and thermal behavior of diamond/Cu composites: Effects of surface modification. Diam. Relat. Mater. 2018, 86, 98–108. [Google Scholar] [CrossRef]
- Dai, S.; Li, J.; Wang, C. Preparation and thermal conductivity of tungsten coated diamond/copper composites. Trans. Nonferrous Met. Soc. China 2022, 32, 2979–2992. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; Xu, Z.; Qu, X. Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite. Metals 2021, 11, 437. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, L.; Peng, K.; Zhu, J.; Wan, L. Effect of tungsten addition on thermal conductivity of graphite/copper composites. Compos. Part B Eng. 2013, 55, 1–4. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, L.; Deng, Z.; Li, H.; Wang, X.; Gang, Y.; Wang, Y.; Ma, L.; Zhou, K.; Wei, Q. Highly conductive diamond skeleton reinforced Cu-matrix composites for high-efficiency thermal management. Appl. Surf. Sci. 2024, 645, 158829. [Google Scholar] [CrossRef]
- Zhang, X. Enhanced thermal conductivity of diamond/copper composite fabricated through doping with rare-earth oxide Sc2O3. Diam. Relat. Mater. 2020, 104, 107755. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Z.; Jia, C.; Chen, H.; Liang, X.; Gao, W.; Tian, W.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloys Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Bai, G.; Wang, L.; Zhang, Y.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Tailoring interface structure and enhancing thermal conductivity of Cu/diamond composites by alloying boron to the Cu matrix. Mater. Charact. 2019, 152, 265–275. [Google Scholar] [CrossRef]
- Chen, W.; Qian, J.; Peng, S.; Fan, L.; Zheng, H.; Zhang, Z.; Zheng, P.; Zheng, L.; Zhang, Y. Thermal properties of tungsten/tungsten carbide-coated double-size diamond/copper composite. Diam. Relat. Mater. 2023, 135, 109818. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Cai, Z.; Peng, C.; Feng, Y.; Zhang, L. Effects of dual-layer coatings on microstructure and thermal conductivity of diamond/Cu composites prepared by vacuum hot pressing. Surf. Coat. Technol. 2015, 277, 299–307. [Google Scholar] [CrossRef]
- Jia, S.Q. Unveiling the interface characteristics and their influence on the heat transfer behavior of hot-forged Cu-Cr/Diamond composites. Carbon 2021, 172, 390–401. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kruszewski, M.J.; Ciupiński, Ł.; Mazurkiewicz, M.; Michalski, A.; Kurzydłowski, K.J. Diamond–tungsten based coating–copper composites with high thermal conductivity produced by Pulse Plasma Sintering. Mater. Des. 2015, 76, 97–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhao, L.; Wang, X. Optimisation of high thermal conductivity Al/diamond composites produced by gas pressure infiltration by controlling infiltration temperature and pressure. J. Mater. Sci. 2015, 50, 688–696. [Google Scholar] [CrossRef]
- Ren, S.; Shen, X.; Guo, C.; Liu, N.; Zang, J.; He, X.; Qu, X. Effect of coating on the microstructure and thermal conductivities of diamond–Cu composites prepared by powder metallurgy. Compos. Sci. Technol. 2011, 71, 1550–1555. [Google Scholar] [CrossRef]
- Sang, J.; Zhou, L.; Yang, W.; Zhu, J.; Fu, L.; Li, D. Enhanced thermal conductivity of copper/diamond composites by fine-regulating microstructure of interfacial tungsten buffer layer. J. Alloys Compd. 2021, 856, 157440. [Google Scholar] [CrossRef]
- Zaitsev, A.; Zaitsev, A.M. Optical Properties of Diamond: A Data Handbook; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Barcelona, Spain; Hongkong; London, UK; Milan, Italy; Paris, France; Singapore; Tokyo, Japan, 2001; pp. 25, 27, 63, 82, 90, 110–112. [Google Scholar]
- Alexander, L.; Klug, H.P. Basic Aspects of X-Ray Absorption in Quantitative Diffraction Analysis of Powder Mixtures. Anal. Chem. 1948, 20, 886–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, N.; Che, Z.; Liu, X.; Chang, G.; Hao, J.; Dai, J.; Wang, X.; Sun, F.; et al. Interfacial Thermal Conductance between Cu and Diamond with Interconnected W−W2 C Interlayer. ACS Appl. Mater. Interfaces 2022, 14, 35215–35228. [Google Scholar] [CrossRef]
- Archer, R.J.; Becher-Nienhaus, B.; Dunderdale, G.J.; Hozumi, A. Recent Progress and Future Directions of Multifunctional (Super)Wetting Smooth/Structured Surfaces and Coatings. Adv. Funct. Mater. 2020, 30, 1907772. [Google Scholar] [CrossRef]
- Dash, T.; Nayak, B.B. Preparation of WC–W2C composites by arc plasma melting and their characterisations. Ceram. Int. 2013, 39, 3279–3292. [Google Scholar] [CrossRef]
- Mrabet, S.E.; Abad, M.D.; López-Cartes, C.; Martínez-Martínez, D.; Sánchez-López, J.C. Thermal Evolution of WC/C Nanostructured Coatings by Raman and In Situ XRD Analysis. Plasma Process. Polym. 2009, 6, S444–S449. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Liu, Q.; Liu, T.; Qu, X. Effect of molybdenum carbide intermediate layers on thermal properties of copper–diamond composites. J. Alloys Compd. 2013, 576, 380–385. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, X.; Zhao, H.; Fa, W.; Yang, X.; Zheng, Z. Thermal Physical Properties of Al-coated Diamond/Cu Composites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 315–319. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Cai, Z.; Peng, C.; Wang, N. Low-temperature densification of diamond/Cu composite prepared from dual-layer coated diamond particles. J. Mater. Sci. Mater. Electron. 2015, 26, 185–190. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Zhang, W.; Song, M.; Yang, W.; Peng, K. Effect of surface roughening on the interfacial thermal conductance ofdiamond/copper composites. Diam. Relat. Mater. 2019, 98, 107467. [Google Scholar] [CrossRef]
- Lu, K. Study of the hot-pressing sintering process of diamond/copper composites and their thermal conductivity. J. Alloys Compd. 2023, 960, 170608. [Google Scholar] [CrossRef]
- Zhang, C. Influence of titanium coating on the microstructure and thermal behavior of Dia./Cu composites. Diam. Relat. Mater. 2019, 97, 107449. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Wu, C.; Yin, Z.; Liu, C.; Huang, Y.; Liu, J.; Shi, Z. The Interface and Fabrication Process of Diamond/Cu Composites with Nanocoated Diamond for Heat Sink Applications. Metals 2021, 11, 196. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J. Thermal conductivity enhancement in Cu/diamond composites with surface-roughened diamonds. Appl. Phys. A 2014, 116, 265–271. [Google Scholar] [CrossRef]
- Chu, K.; Jia, C.; Guo, H.; Li, W. On the thermal conductivity of Cu–Zr/diamond composites. Mater. Des. 2013, 45, 36–42. [Google Scholar] [CrossRef]
- Hu, H.; Kong, J. Improved Thermal Performance of Diamond-Copper Composites with Boron Carbide Coating. J. Mater. Eng. Perform. 2013, 23, 651–657. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.; Wang, P.; Qiao, J.; Hu, F.; Liu, S.; Zhang, Q.; Hussain, M.; Dong, R.; Wu, G. Enhanced thermal conductivity in Diamond/Aluminum composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. J. Alloys Compd. 2017, 726, 623–631. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Cui, W.; Qu, X. Preparation of copper–diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Fan, Q.; Hou, H.; Yang, J. Pressure induced structural, mechanical and thermodynamic properties of W2C. Int. J. Refract. Met. Hard Mater. 2024, 123, 106745. [Google Scholar] [CrossRef]
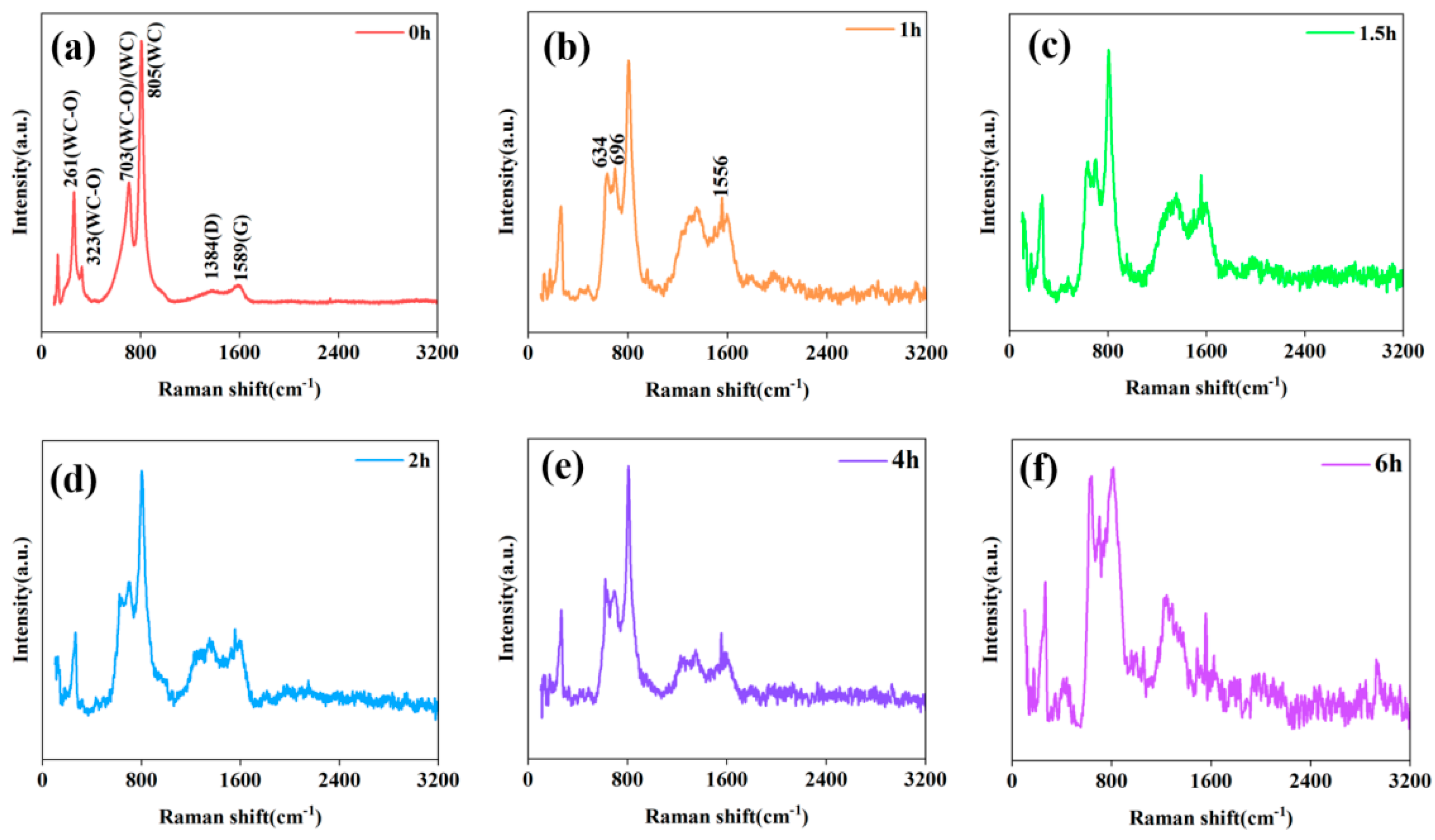
| Peak Assignment | Wave Number (cm−1) | Substance | Hybridization of C Atoms |
|---|---|---|---|
| WC–O | 261,323 | ||
| WC–O/WC | 703 | Diamond-like distorted graphite | Sp2 Sp2, Sp3 |
| WC | 805 | ||
| D | 1384 | ||
| D/G | 1556 | ||
| G | 1589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ye, Z.; Liu, L.; Bai, J.; Zhao, Y.; Hu, Q.; Liu, H.; Hu, L.; Guo, X.; Xiao, Y.; et al. High Thermal Conductivity Diamond–Copper Composites Prepared via Hot Pressing with Tungsten–Coated Interfacial Layer Optimization. Materials 2025, 18, 3882. https://doi.org/10.3390/ma18163882
Wang Q, Ye Z, Liu L, Bai J, Zhao Y, Hu Q, Liu H, Hu L, Guo X, Xiao Y, et al. High Thermal Conductivity Diamond–Copper Composites Prepared via Hot Pressing with Tungsten–Coated Interfacial Layer Optimization. Materials. 2025; 18(16):3882. https://doi.org/10.3390/ma18163882
Chicago/Turabian StyleWang, Qiang, Zhijie Ye, Lei Liu, Jie Bai, Yuning Zhao, Qiang Hu, Hong Liu, Lang Hu, Xiaodong Guo, Yongneng Xiao, and et al. 2025. "High Thermal Conductivity Diamond–Copper Composites Prepared via Hot Pressing with Tungsten–Coated Interfacial Layer Optimization" Materials 18, no. 16: 3882. https://doi.org/10.3390/ma18163882
APA StyleWang, Q., Ye, Z., Liu, L., Bai, J., Zhao, Y., Hu, Q., Liu, H., Hu, L., Guo, X., Xiao, Y., Cao, W., & Yang, Z. (2025). High Thermal Conductivity Diamond–Copper Composites Prepared via Hot Pressing with Tungsten–Coated Interfacial Layer Optimization. Materials, 18(16), 3882. https://doi.org/10.3390/ma18163882





