Nanoscopic Insight into Water Adsorption and Desorption in Commercial Activated Alumina by Positron Annihilation Lifetime Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
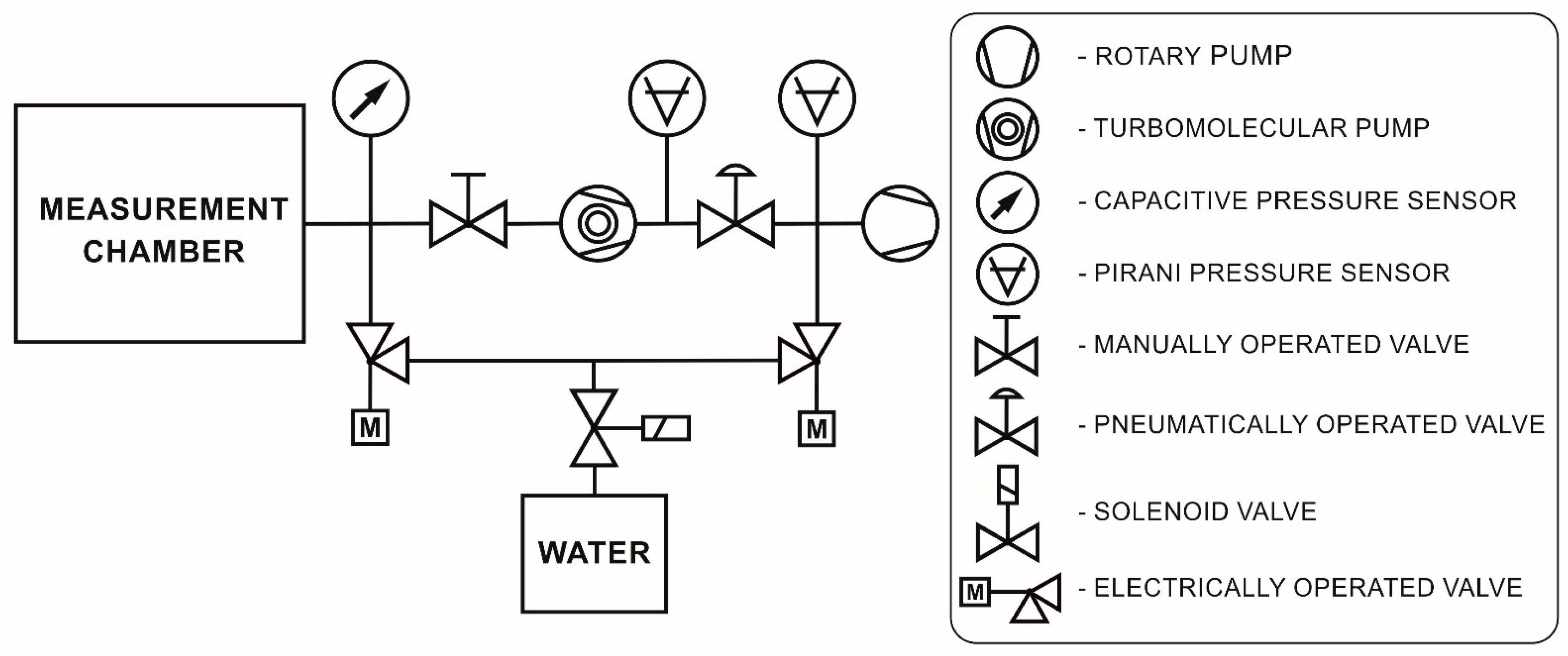
2.2. Methods
2.3. Water Adsorption and Desorption Procedure
3. Results and Discussion
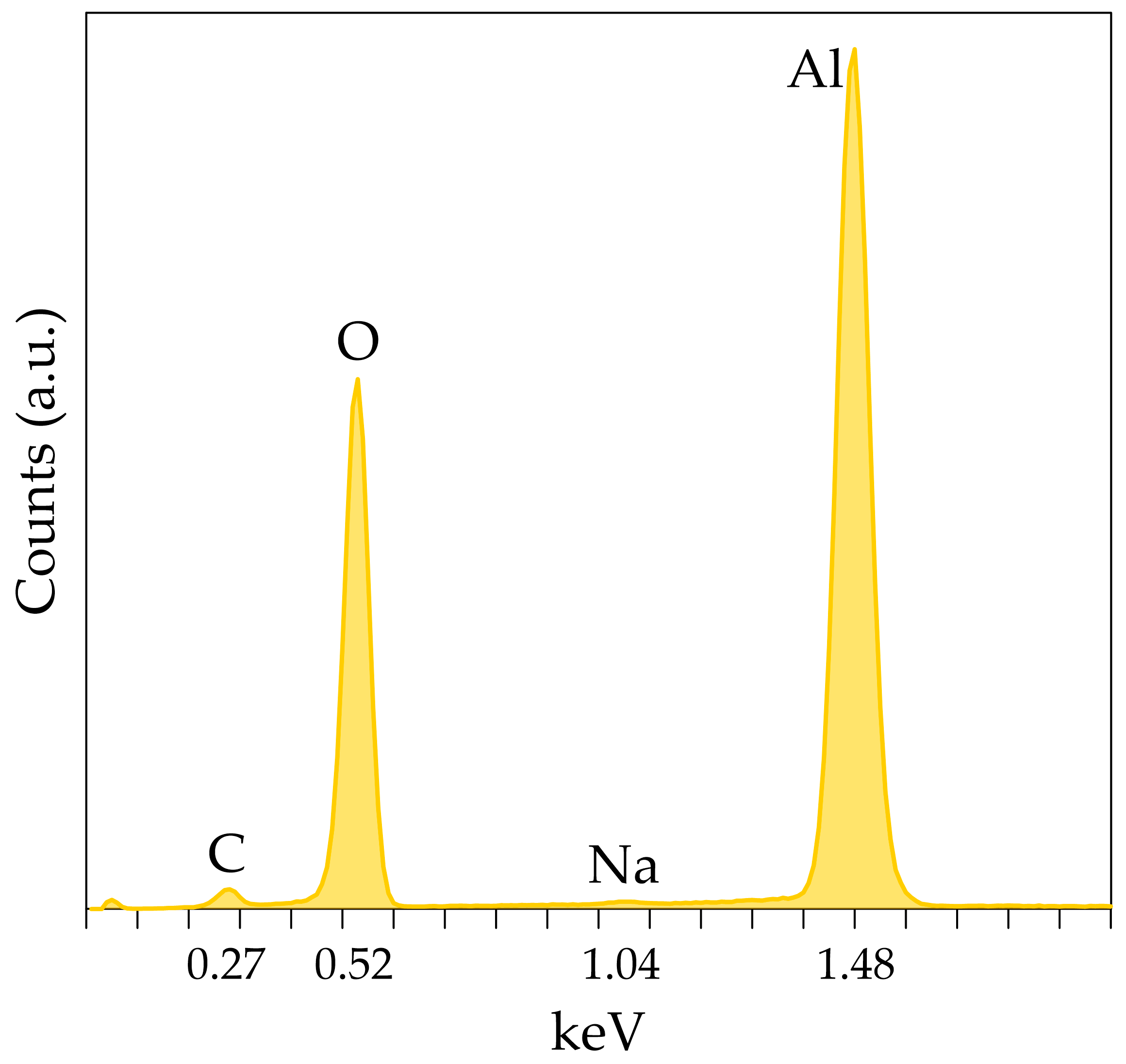
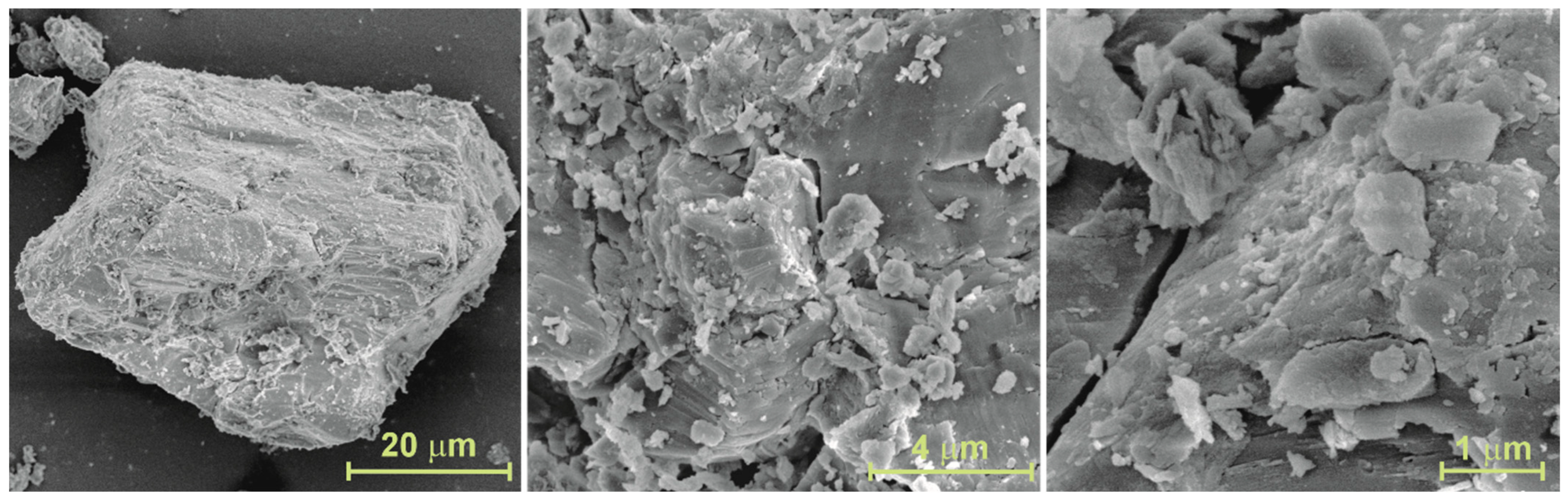
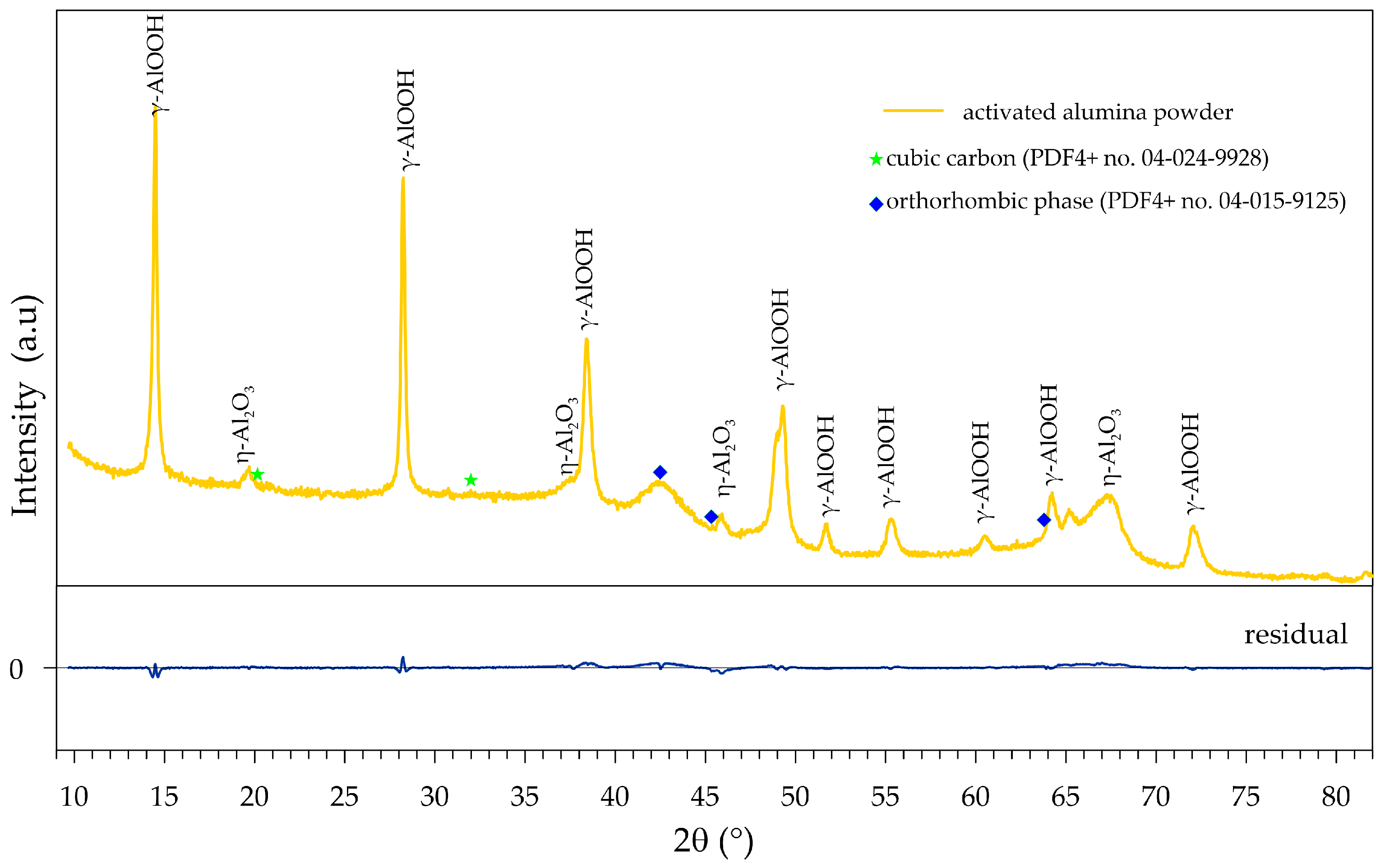
3.1. Characterization
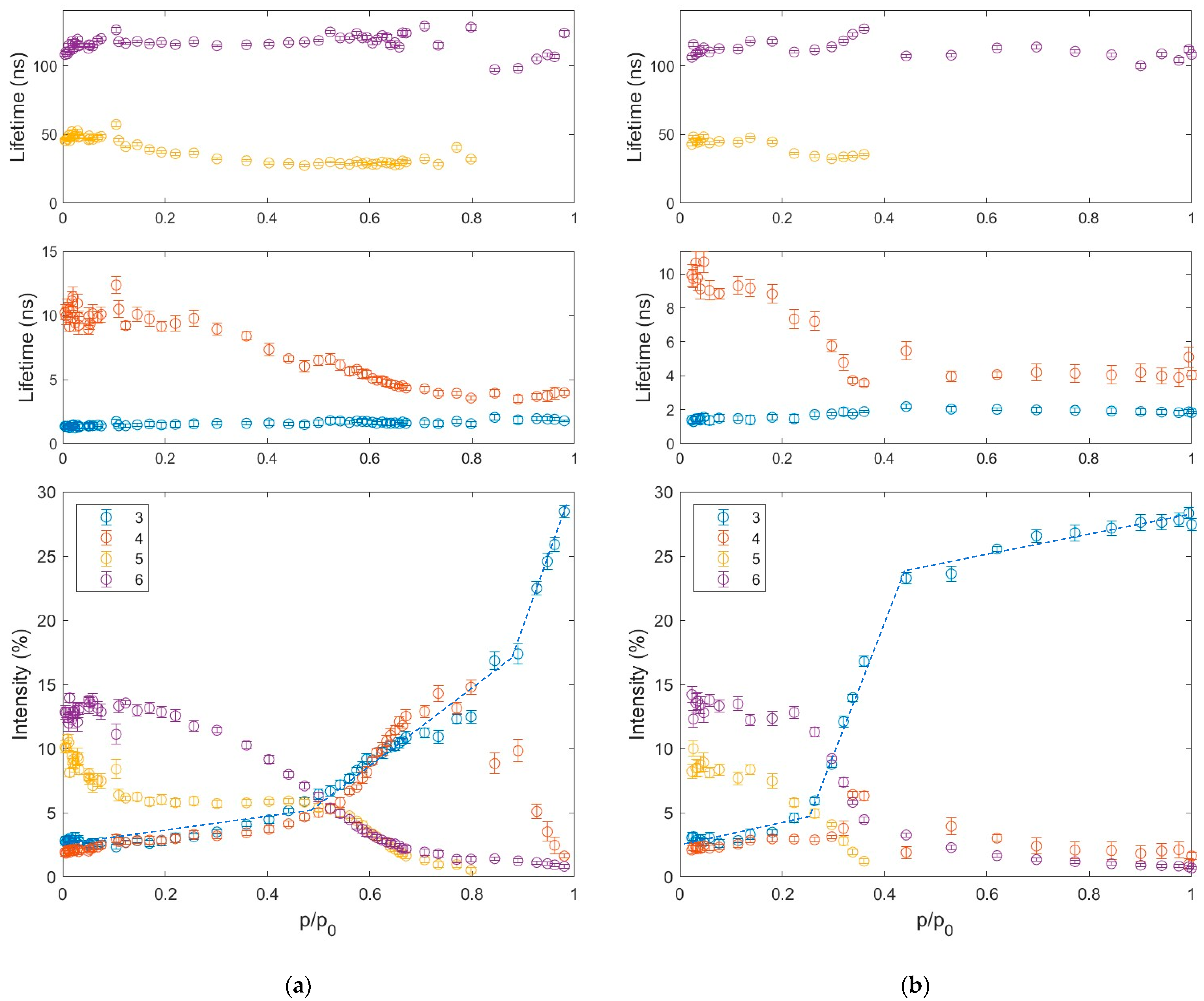
3.2. Water Adsorption and Desorption
- dry (p/p0 = 0),
- approximately in the middle of mesopore filling (p/p0 = 0.50),
- pores almost filled (p/p0 = 1.00),
- empty mesopores start to appear (p/p0 = 0.44),
- approximately in the middle of mesopore emptying (p/p0 = 0.30),
- pores almost completely emptied (p/p0 = 0.02).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer−Emmett−Teller equation |
| DFT | Density Functional Theory |
| EDS | energy-dispersive X-ray spectrometer |
| MOFs | metal-organic frameworks |
| PALS | positron annihilation lifetime spectroscopy |
| PSD | pore size distribution |
| SEM | scanning electron microscope |
| XRD | X-ray diffraction |
Appendix A
References
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; p. 424. [Google Scholar]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Use of DFT to achieve a rational understanding of acid–basic properties of γ-alumina surfaces. J. Catal. 2004, 226, 54–68. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-T.; Lim, S.-J.; Kim, J.H.; Lee, C.-H. Adsorption Equilibria of Water Vapor on an Alumina/Zeolite 13X Composite and Silica Gel. J. Chem. Eng. Data 2017, 62, 804–811. [Google Scholar] [CrossRef]
- Reshetnikov, S.; Kurzina, I. Investigation of adsorption of water vapor on porous aluminium oxide material. IOP Conf. Ser. Mater. Sci. Eng. 2019, 597, 012011. [Google Scholar] [CrossRef]
- Hong, S.-H.; Jin, S.; Ho, K.; Hur, E.; Lee, C.-H. Adsorption Equilibria of Water Vapor on Surface-Modified Activated Carbons and Alumina. J. Chem. Eng. Data 2019, 64, 4834–4843. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Characteristics of Ammonia Adsorption on Activated Alumina. J. Chem. Eng. Data 2010, 55, 5587–5593. [Google Scholar] [CrossRef]
- Srivastav, A.; Srivastava, V.C. Adsorptive desulfurization by activated alumina. J. Hazard. Mater. 2009, 170, 1133–1140. [Google Scholar] [CrossRef]
- De Paula, N.; Maraschin, M.; Knani, S.; De Oliveira, J.T.; Agustini, C.B.; Féris, L.A.; Claussen, L.E.; De Souza, D.M.; Oliveira, M.L.S.; Silva, L.F.O.; et al. Ozone-treated activated alumina as an alternative adsorbent to remove fluoride from water: Conventional and Bayesian approaches to evaluate the isotherms, kinetics, and thermodynamics. J. Environ. Chem. Eng. 2023, 11, 111403. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electrocoagulation. J. Colloid Interface Sci. 2005, 283, 472–476. [Google Scholar] [CrossRef]
- Zamorategui Molina, A.; Ramírez Ramírez, N.; Martínez Rosales, J.M.; Serafín, M.A.H. Síntesis y caracterización de gamma alúmina y su comparación con un carbón activado para remover el fluoruro presente en agua potable de pozos. Acta Univ. 2016, 26, 30–35. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Di Luca, C.; Mori, F.; Pintar, A.; Žerjav, G.; Valiente, M.; Palet, C. Synthesis and adsorption behavior of mesoporous alumina and Fe-doped alumina for the removal of dominant arsenic species in contaminated waters. J. Environ. Chem. Eng. 2019, 7, 102901. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Fernandes, E.P.; Silva, T.S.; Carvalho, C.M.; Selvasembian, R.; Chaukura, N.; Oliveira, L.M.T.M.; Meneghetti, S.M.P.; Meili, L. Efficient adsorption of dyes by γ-alumina synthesized from aluminum wastes: Kinetics, isotherms, thermodynamics and toxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106198. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef]
- Vityuk, A. Adsorbents for Heat Reactivated Compressed Air Dryers. Available online: https://www.airbestpractices.com/system-assessments/air-treatmentn2/adsorbents-heat-reactivated-compressed-air-dryers (accessed on 30 July 2025).
- BASF. Adsorbents Solutions for Compressed Air Drying. Available online: https://basf-catalystsmetals.com/multimedia/literature-library/adsorbents/adsorbents-for-air-drying (accessed on 30 July 2025).
- Sakurai, M.; Shima, A.; Hirai, K.; Yamazaki, C.; Futamura, S.; Matsumoto, S.; Saruwatari, H. Preliminary Study of Moisture Absorption and Desorption in CO2 Removal System. In Proceedings of the 52nd International Conference on Environmental Systems, Calgary, AB, Canada, 16–20 July 2023. [Google Scholar]
- Ahn, H. Equilibrium theory analysis of thermal regeneration of a humid adsorption column: Selection of optimal hot purge gas temperature. Chem. Eng. Res. Des. 2019, 151, 91–99. [Google Scholar] [CrossRef]
- Viisanen, Y.; Lbadaoui-Darvas, M.; Alvarez Piedehierro, A.; Welti, A.; Nenes, A.; Laaksonen, A. Water Vapor Adsorption–Desorption Hysteresis Due to Clustering of Water on Nonporous Surfaces. Langmuir 2024, 40, 20311–20321. [Google Scholar] [CrossRef]
- Ionescu, A.; Allouche, A.; Aycard, J.-P.; Rajzmann, M.; Hutschka, F. Study of γ-Alumina Surface Reactivity: Adsorption of Water and Hydrogen Sulfide on Octahedral Aluminum Sites. J. Phys. Chem. B 2002, 106, 9359–9366. [Google Scholar] [CrossRef]
- Serbezov, A. Adsorption Equilibrium of Water Vapor on F-200 Activated Alumina. J. Chem. Eng. Data 2003, 48, 421–425. [Google Scholar] [CrossRef]
- Borello, E. Surface rehydration of variously dehydrated eta-alumina. J. Catal. 1974, 35, 1–10. [Google Scholar] [CrossRef]
- Altarawneh, M.; Assaf, N.W.; Hussain, H.M.; Dlugogorski, B.Z. Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs). Nanotechnol. Rev. 2023, 12, 20220536. [Google Scholar] [CrossRef]
- Kozerozhets, I.V.; Semenov, E.A.; Avdeeva, V.V.; Ivakin, Y.D.; Kupreenko, S.Y.; Egorov, A.V.; Kholodkova, A.A.; Vasil’ev, M.G.; Kozlova, L.O.; Panasyuk, G.P. State and forms of water in dispersed aluminum oxides and hydroxides. Ceram. Int. 2023, 49, 30381–30394. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A.; Grassian, V.H. FT-IR Study of Water Adsorption on Aluminum Oxide Surfaces. Langmuir 2003, 19, 341–347. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, C.; Cai, J.; Wang, J.; Liu, K.; Cheng, X. Synthesis and characterization of activated alumina with high thermal stability by a low-heat solid-phase precursor method. Microporous Mesoporous Mater. 2022, 337, 111921. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Hung, I.; Mentink-Vigier, F.; Wang, X.; Qi, G.; Wang, X.; Gan, Z.; Xu, J.; Deng, F. Mapping the oxygen structure of γ-Al2O3 by high-field solid-state NMR spectroscopy. Nat. Commun. 2020, 11, 3620. [Google Scholar] [CrossRef]
- Rui, Z.; Yan, Z.; Kai, H.; Zhen-ping, J.; Gong-zhen, C. NMR revealed activated alumina-water interaction. Wuhan Univ. J. Nat. Sci. 2005, 10, 572–576. [Google Scholar] [CrossRef]
- Roussenova, M.; Alam, M.A.; Townrow, S.; Kilburn, D.; Sokol, P.E.; Guillet-Nicolas, R.; Kleitz, F. A nano-scale free volume perspective on the glass transition of supercooled water in confinement. New J. Phys. 2014, 16, 103030. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Cooke, D.; Crivelli, P.; Pérez-Ramírez, J. Impact of Pore Connectivity on the Design of Long-Lived Zeolite Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Warringham, R.; Gerchow, L.; Zubiaga, A.; Cooke, D.; Crivelli, P.; Mitchell, S.; Pérez-Ramírez, J. Insights into the Mechanism of Zeolite Detemplation by Positron Annihilation Lifetime Spectroscopy. J. Phys. Chem. C 2016, 120, 25451–25461. [Google Scholar] [CrossRef]
- Zubiaga, A.; Warringham, R.; Mitchell, S.; Gerchow, L.; Cooke, D.; Crivelli, P.; Pérez-Ramírez, J. Pore Topology Effects in Positron Annihilation Spectroscopy of Zeolites. ChemPhysChem 2017, 18, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Urban-Klaehn, J.; Shikhaliyev, K.; Gaffney, A.; Martinez, A.; Zaleski, R.; Lauterbach, J.; Katz, A.; Jaeshke, A.; Li, X. Zeolite-Based Catalysts for Conversion of Oxygenated Polymer Waste by Positron Annihilation. ChemCatChem 2024, 16, e202301282. [Google Scholar] [CrossRef]
- Stassin, T.; Verbeke, R.; Cruz, A.J.; Rodríguez-Hermida, S.; Stassen, I.; Marreiros, J.; Krishtab, M.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; et al. Porosimetry for Thin Films of Metal–Organic Frameworks: A Comparison of Positron Annihilation Lifetime Spectroscopy and Adsorption-Based Methods. Adv. Mater. 2021, 33, 2006993. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaleski, R.; Gorgol, M. Investigation of porous structure polymeric materials based on 1-vinyl-2-pyrrolidone. Polym. Adv. Technol. 2018, 29, 2042–2049. [Google Scholar] [CrossRef]
- Zaleski, R.; Goworek, J.; Maciejewska, M. Positronium lifetime in porous VP–DVB copolymer. Phys. Status Solidi C 2009, 6, 2445–2447. [Google Scholar] [CrossRef]
- Zaleski, R.; Błażewicz, A.; Kierys, A. Ortho-positronium migration in mesopores of MCM-41, MSF and SBA-3. Nukleonika 2013, 58, 233–238. [Google Scholar]
- Uhlig, H.; Adouane, G.; Bluhm, C.; Zieger, S.; Krause-Rehberg, R.; Enke, D. Positron-Annihilation-Lifetime-Spectroscopy (PALS) for the characterization of bimodal silica-gel synthesized by pseudomorphic transformation. J. Porous Mat. 2016, 23, 139–144. [Google Scholar] [CrossRef]
- Zaleski, R.; Stefaniak, W.; Maciejewska, M.; Goworek, J. Porosity evolution of VP-DVB/MCM-41 nanocomposite. J. Colloid Interface Sci. 2010, 343, 134–140. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Zhang, C.; Gong, L.; Cao, X.; Wang, B.; Yu, R.; Zhou, W. Pore structure of the polyethyleneimine/SBA-15 nanocomposites studied by positron annihilation lifetime spectroscopy. Microporous Mesoporous Mater. 2022, 334, 111761. [Google Scholar] [CrossRef]
- Zaleski, R.; Maciejewska, M.; Puzio, M. Mechanical Stability of Porous Copolymers by Positron Annihilation Lifetime Spectroscopy. J. Phys. Chem. C 2015, 119, 11636–11645. [Google Scholar] [CrossRef]
- Zaleski, R.; Krasucka, P.; Skrzypiec, K.; Goworek, J. Macro- and Nanoscopic Studies of Porous Polymer Swelling. Macromolecules 2017, 50, 5080–5089. [Google Scholar] [CrossRef]
- Lupa, J.; Gorgol, M.; Greluk, M.; Rotko, M.; Słowik, G.; Zaleski, R.; Kierys, A. Toward Understanding the Reduction Process of Nickel Oxide and Ceria-Based Nickel Catalyst via Positron Annihilation Lifetime Spectroscopy. J. Phys. Chem. C 2025, 129, 11355–11364. [Google Scholar] [CrossRef]
- Attallah, A.G.; Bon, V.; Hirschmann, E.; Butterling, M.; Wagner, A.; Zaleski, R.; Kaskel, S. Uncovering the Dynamic CO2 Gas Uptake Behavior of CALF-20 (Zn) under Varying Conditions via Positronium Lifetime Analysis. Small 2025, 21, 2500544. [Google Scholar] [CrossRef]
- Maheshwari, P.; Pujari, P.K.; Sharma, S.K.; Dutta, D.; Sudarshan, K.; Mithu, V.S.; Madhu, P.K.; Deshpande, S.K.; Patil, P.N.; Raje, N. Phase Transition of Nanoconfined Water in Clay: Positron Annihilation, Nuclear Magnetic Resonance, and Dielectric Relaxation Studies. J. Phys. Chem. C 2013, 117, 14313–14324. [Google Scholar] [CrossRef]
- Šauša, O.; Mat’ko, I.; Illeková, E.; Macová, E.; Berek, D. Confined water in controlled pore glass CPG-10-120 studied by positron annihilation lifetime spectroscopy and differential scanning calorimetry. J. Phys. Conf. Ser. 2015, 618, 012041. [Google Scholar] [CrossRef]
- Klym, H.; Ingram, A.; Shpotyuk, O.; Hadzaman, I.; Solntsev, V. Water-Vapor Sorption Processes in Nanoporous MgO-Al2O3 Ceramics: The PAL Spectroscopy Study. Nanoscale Res. Lett. 2016, 11, 133. [Google Scholar] [CrossRef]
- Maheshwari, P.; Dutta, D.; Muthulakshmi, T.; Chakraborty, B.; Raje, N.; Pujari, P.K. Desorption of water from hydrophilic MCM-41 mesopores: Positron annihilation, FTIR and MD simulation studies. J. Phys. Condens. Matter 2016, 29, 055003. [Google Scholar] [CrossRef]
- Maheshwari, P.; Gorgol, M.; Kierys, A.; Zaleski, R. Positron Probing of Liquid-free Volume To Investigate Adsorption–Desorption Behavior of Water in Two-Dimensional Mesoporous SBA-3. J. Phys. Chem. C 2017, 121, 17251–17262. [Google Scholar] [CrossRef]
- Ito, K.; Yoshimoto, S.; O’Rourke, B.E.; Oshima, N.; Kumagai, K. Subnanopore filling during water vapor adsorption on microporous silica thin films as seen by low-energy positron annihilation. Appl. Phys. Lett. 2018, 112, 083701. [Google Scholar] [CrossRef]
- Thangswamy, M.; Dutta, D.; Maheshwari, P.; Sen, D.; Pujari, P.K. Energetics of ice nucleation in mesoporous titania using positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 6033–6041. [Google Scholar] [CrossRef] [PubMed]
- Attallah, A.G.; Bon, V.; Maity, K.; Hirschmann, E.; Butterling, M.; Wagner, A.; Kaskel, S. Unravelling the Water Adsorption Mechanism in Hierarchical MOFs: Insights from In Situ Positron Annihilation Lifetime Studies. ACS Appl. Mater. Interfaces 2023, 15, 48264–48276. [Google Scholar] [CrossRef]
- Stuart, N.M.; Sohlberg, K. The Microstructure of γ-Alumina. Energies 2021, 14, 6472. [Google Scholar] [CrossRef]
- Compalox® Activated Aluminum Oxides. Available online: https://chembase-st.com/wp-content/uploads/Compalox-Activated-Aluminum-Oxides-for-Sustainability.pdf (accessed on 12 June 2025).
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Vorobiev, J.K.; Badaev, B.N.; Ljubushko, G.I.; Levitsky, E.A.; Boreskov, G.K.; Andrushkevich, M.M.; Baum, B.A.; Pakhomov, N.A.; Khomyakova, L.G.; Khramov, A.E.; et al. Method of Preparing Granulated Activated Alumina. U.S. Patent 4,166,100, 26 May 1978. [Google Scholar]
- Loarer, J.-L.L.; Nussbaum, H.; Bortzmeyer, D. Alumina Extrudates, Methods for Preparing and Use as Catalysts Supports. U.S. Patent 6,656,875 B1, 10 June 1998. [Google Scholar]
- Chopin, T.; Fourre, P.; Jaeger, P.; Taxil, B. Crush Resistant Adsorptive Agglomerates of Activated Alumina. U.S. Patent 5,637,547, 22 September 1995. [Google Scholar]
- Zhou, R.-S.; Snyder, R.L. Structures and transformation mechanisms of the [eta], [gamma] and [theta] transition aluminas. Acta Crystallogr. Sect. B 1991, 47, 617–630. [Google Scholar] [CrossRef]
- van Gog, H. First-principles study of dehydration interfaces between diaspore and corundum, gibbsite and boehmite, and boehmite and γ-Al2O3: Energetic stability, interface charge effects, and dehydration defects. Appl. Surf. Sci. 2021, 541, 148501. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Gorgol, M.; Tydda, M.; Kierys, A.; Zaleski, R. Composition of pore surface investigated by positron annihilation lifetime spectroscopy. Microporous Mesoporous Mater. 2012, 163, 276–281. [Google Scholar] [CrossRef]
- Thraenert, S.; Hassan, E.M.; Enke, D.; Fuerst, D.; Krause-Rehberg, R. Verifying the RTE model: Ortho-positronium lifetime measurement on controlled pore glasses. Phys. Status Solidi C 2007, 4, 3819–3822. [Google Scholar] [CrossRef]
- Consolati, G.; Quasso, F. Positronium dynamics in aqueous solutions of ionic surfactants. Chem. Phys. 1996, 213, 449–457. [Google Scholar] [CrossRef]
- Murakami, H.; Endo, T.; Matsuda, I. Positron lifetime in voids induced in quenched aluminum. Phys. Rev. B Condens. Matter Mater. Phys. 1991, 44, 2504–2506. [Google Scholar] [CrossRef]
- Eldrup, M.; Mogensen, O.; Trumpy, G. Positron Lifetimes in Pure and Doped Ice and in Water. J. Chem. Phys. 1972, 57, 495–504. [Google Scholar] [CrossRef]
- Stepanov, S.V.; Byakov, V.M.; Duplâtre, G.; Zvezhinskiy, D.S.; Lomachuk, Y.V. Positronium formation in a liquid phase: Influence of intratrack reactions and temperature. Phys. Status Solidi C 2009, 6, 2476–2481. [Google Scholar] [CrossRef]
- Stepanov, S.V.; Byakov, V.M.; Hirade, T. To the theory of Ps formation. New interpretation of the e+ lifetime spectrum in water. Radiat. Phys. Chem. 2007, 76, 90–95. [Google Scholar] [CrossRef]
- Zaleski, R.; Gorgol, M.; Kierys, A.; Maheshwari, P.; Pietrow, M.; Pujari, P.K.; Zgardzińska, B. Unraveling the Phase Behavior of Water Confined in Nanochannels through Positron Annihilation. J. Phys. Chem. C 2022, 126, 5916–5926. [Google Scholar] [CrossRef]
- Ferrell, R.A. Long Lifetime of Positronium in Liquid Helium. Phys. Rev. 1957, 108, 167–168. [Google Scholar] [CrossRef]
- Zaleski, R. Measurement and Analysis of the Positron Annihilation Lifetime Spectra for Mesoporous Silica. Acta Phys. Pol. A 2006, 110, 729–738. [Google Scholar] [CrossRef]
- Zaleski, R.; Gorgol, M.; Kierys, A.; Goworek, J. Positron porosimetry study of mesoporous polymer–silica composites. Adsorption 2016, 22, 745–754. [Google Scholar] [CrossRef]
- Kierys, A.; Zaleski, R.; Gorgol, M.; Goworek, J. N-Heptane adsorption in periodic mesoporous silica by in situ positron annihilation lifetime spectroscopy. Microporous Mesoporous Mater. 2013, 179, 104–110. [Google Scholar] [CrossRef]
- Gorgol, M.; Krasucka, P.; Goworek, J.; Zaleski, R. Controlled Porosity of MCM-41 Obtained by Partial Blocking of Pores by Silicon Oil. Acta Phys. Pol. A 2017, 132, 1559–1563. [Google Scholar] [CrossRef]
- Kierys, A.; Zaleski, R.; Tydda, M.; Goworek, J. What can positronium tell us about adsorption? Adsorption 2013, 19, 529–535. [Google Scholar] [CrossRef]
- Zaleski, R.; Kierys, A.; Pietrow, M.; Zgardzińska, B.; Błażewicz, A. Influence of different confining matrices on negative pressure in liquid n-heptane investigated using positronium bubbles as a probe. J. Colloid Interface Sci. 2020, 558, 259–268. [Google Scholar] [CrossRef]
- Becvár, F. Methodology of positron lifetime spectroscopy: Present status and perspectives. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 261, 871–874. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. Sect. A 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Shukla, A.; Peter, M.; Hoffmann, L. Analysis of positron lifetime spectra using quantified maximum entropy and a general linear filter. Nucl. Instrum. Methods Phys. Res. Sect. A 1993, 335, 310–317. [Google Scholar] [CrossRef]
- Zaleski, R. Principles of positron porosimetry. Nukleonika 2015, 60, 795–800. [Google Scholar] [CrossRef]
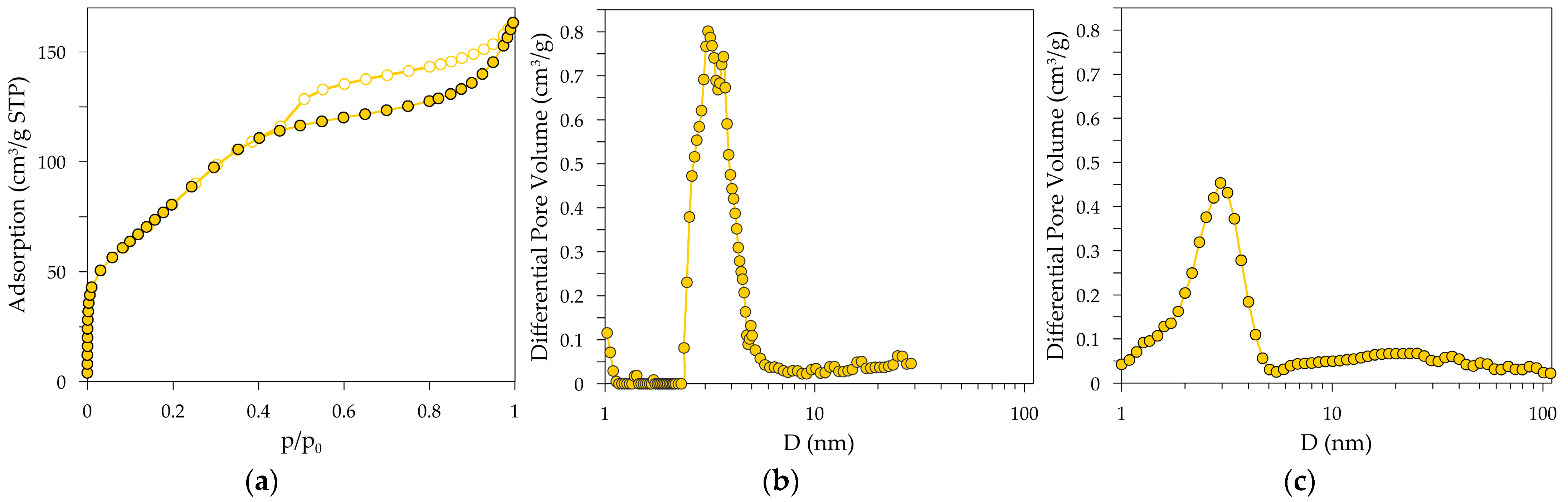


| Sample Name | SBET (m2/g) | Vp (cm3/g) | Dp1 (nm) | Dp2 (nm) |
|---|---|---|---|---|
| activated alumina | 320 | 0.25 | 2.9 | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, W.; Kochel, M.; Kierys, A.; Gorgol, M.; Drewniak, M.; Zaleski, R. Nanoscopic Insight into Water Adsorption and Desorption in Commercial Activated Alumina by Positron Annihilation Lifetime Spectroscopy. Materials 2025, 18, 3876. https://doi.org/10.3390/ma18163876
Kowalski W, Kochel M, Kierys A, Gorgol M, Drewniak M, Zaleski R. Nanoscopic Insight into Water Adsorption and Desorption in Commercial Activated Alumina by Positron Annihilation Lifetime Spectroscopy. Materials. 2025; 18(16):3876. https://doi.org/10.3390/ma18163876
Chicago/Turabian StyleKowalski, Wojciech, Mateusz Kochel, Agnieszka Kierys, Marek Gorgol, Marek Drewniak, and Radosław Zaleski. 2025. "Nanoscopic Insight into Water Adsorption and Desorption in Commercial Activated Alumina by Positron Annihilation Lifetime Spectroscopy" Materials 18, no. 16: 3876. https://doi.org/10.3390/ma18163876
APA StyleKowalski, W., Kochel, M., Kierys, A., Gorgol, M., Drewniak, M., & Zaleski, R. (2025). Nanoscopic Insight into Water Adsorption and Desorption in Commercial Activated Alumina by Positron Annihilation Lifetime Spectroscopy. Materials, 18(16), 3876. https://doi.org/10.3390/ma18163876








