Cytotoxicity of Bulk-Fill Composites on Stem Cells from Human Exfoliated Deciduous Teeth—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Stem Cells from Human Exfoliated Deciduous Teeth
2.2. Cytotoxicity of Bulk-Fill Resin Composite Materials Assessment
- -
- Group 1—cells cultured in a medium in which SDR was immersed;
- -
- Group 2—cells cultured in a medium in which Tetric EvoCeram Bulk-Fill was immersed;
- -
- Group 3—cells cultured in a medium in which VisCalor Bulk was immersed;
- -
- Group 4—cells cultured in a medium in which Cention N was immersed;
- -
- Group 5—cells cultured in a medium in which Dyract XP was immersed;
- -
- Group 6 (Positive Control)—Cells Cultured in Dubbeco Modified Medium (DMEM) supplemented with 10% fetal bovine serum.
2.3. MTT Cell Proliferation Assay
2.4. Annexin V Apoptosis Assay
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SHED | Stem cells from human exfoliated deciduous teeth |
References
- Kirthiga, M.; Muthu, M.S.; Kayalvizhi, G.; Mathur, V.P.; Jayakumar, N.; Praveen, R. OXIS Contacts and Approximal Caries in Preschool Children: A Prospective Cohort Study. Caries Res. 2023, 57, 133–140. [Google Scholar] [CrossRef]
- Finucane, D. Restorative treatment of primary teeth: An evidence-based narrative review. Aust. Dent. J. 2019, 64 (Suppl. S1), S22–S36. [Google Scholar] [CrossRef] [PubMed]
- Dieguez-Perez, M.; Ticona-Flores, J.M. Three-Dimensional Analysis of the Pulp Chamber and Coronal Tooth of Primary Molars: An In Vitro Study. Int. J. Environ. Res. Public Health 2022, 19, 9279. [Google Scholar] [CrossRef]
- Santamaria, R.M.; Abudrya, M.H.; Gul, G.; Mourad, M.S.; Gomez, G.F.; Zandona, A.G.F. How to Intervene in the Caries Process: Dentin Caries in Primary Teeth. Caries Res. 2020, 54, 306–323. [Google Scholar] [CrossRef]
- Aiem, E.; Joseph, C.; Garcia, A.; Smail-Faugeron, V.; Muller-Bolla, M. Caries removal strategies for deep carious lesions in primary teeth: Systematic review. Int. J. Paediatr. Dent. 2020, 30, 392–404. [Google Scholar] [CrossRef]
- Rees, J.S.; Jagger, D.C.; Williams, D.R.; Brown, G.; Duguid, W. A reappraisal of the incremental packing technique for light cured composite resins. J. Oral. Rehabil. 2004, 31, 81–84. [Google Scholar] [CrossRef]
- Park, J.; Chang, J.; Ferracane, J.; Lee, I.B. How should composite be layered to reduce shrinkage stress: Incremental or bulk filling? Dent. Mater. 2008, 24, 1501–1505. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Rudrapati, L.; Badami, V.; Tummala, M. Incremental techniques in direct composite restoration. J. Conserv. Dent. 2017, 20, 386–391. [Google Scholar] [CrossRef]
- Baraka, M.; Ibrahim, Y.; Helmy, R. Adhesive strategies for restoring primary and young permanent dentition—A review. Alex. Dent. J. 2024, 49, 195–202. [Google Scholar] [CrossRef]
- Van Ende, A.; De Munck, J.; Lise, D.P.; Van Meerbeek, B. Bulk-Fill Composites: A Review of the Current Literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar] [CrossRef][Green Version]
- Arbildo-Vega, H.I.; Lapinska, B.; Panda, S.; Lamas-Lara, C.; Khan, A.S.; Lukomska-Szymanska, M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymers 2020, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Taubock, T.T.; Jager, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high- and low-viscosity dimethacrylate- and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef]
- El-Damanhoury, H.; Platt, J. Polymerization shrinkage stress kinetics and related properties of bulk-fill resin composites. Oper. Dent. 2014, 39, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Clozza, E.; Giannini, M.; Farrokhmanesh, E.; Janal, M.; Tovar, N.; Bonfante, E.A.; Coelho, P.G. Shrinkage assessment of low shrinkage composites using micro-computed tomography. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 798–806. [Google Scholar] [CrossRef]
- Paganini, A.; Attin, T.; Taubock, T.T. Margin Integrity of Bulk-Fill Composite Restorations in Primary Teeth. Materials 2020, 13, 3802. [Google Scholar] [CrossRef]
- Kireev, V.V.; Chistyakov, E.M.; Filatov, S.N.; Tupikov, A.S.; Panfilova, D.V.; Chetverikova, A.I. Polymeric dental composites modified with carboxy phosphazene methacrylates. Russ. J. Appl. Chem. 2015, 88, 866–870. [Google Scholar] [CrossRef]
- Chistyakov, E.M.; Kolpinskaya, N.; Posokhova, V.; Chuev, V. Dental Composition Modified with Aryloxyphosphazene Containing Carboxyl Groups. Polymers 2020, 12, 1176. [Google Scholar] [CrossRef]
- Yang, J.; Silikas, N.; Watts, D.C. Pre-heating effects on extrusion force, stickiness and packability of resin-based composite. Dent. Mater. 2019, 35, 1594–1602. [Google Scholar] [CrossRef]
- Dantagnan, C.A.; Babajko, S.; Nassif, A.; Houari, S.; Jedeon, K.; Francois, P.; Dursun, E.; Attal, J.P.; Bosco, J. Analysis of Resin-Based Dental Materials’ Composition Depending on Their Clinical Applications. Polymers 2024, 16, 1022. [Google Scholar] [CrossRef]
- Motevasselian, F.; Kermanshah, H.; Rasoulkhani, E.; Özcan, M. Comparison of microleakage of an alkasite restorative material, a composite resin and a resin-modified glass ionomer. Braz. J. Oral Sci. 2021, 20, e213981. [Google Scholar] [CrossRef]
- Hamza, B.; Zimmerman, M.; Attin, T.; Taubock, T.T. Marginal integrity of classical and bulk-fill composite restorations in permanent and primary molars. Sci. Rep. 2022, 12, 13670. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Najafpour, E.; Ebrahim Adhami, Z.; Sighari Deljavan, A.; Aminabadi, N.A.; Shirazi, S. Does the length of dental procedure influence children’s behavior during and after treatment? A systematic review and critical appraisal. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Aminabadi, N.A.; Oskouei, S.G.; Farahani, R.M. Dental treatment duration as an indicator of the behavior of 3-to 9-year-old pediatric patients in clinical dental settings. J. Contemp. Dent. Pract. 2009, 10, 23–32. [Google Scholar] [CrossRef]
- Ebrahimi Chaharom, M.E.; Bahari, M.; Safyari, L.; Safarvand, H.; Shafaei, H.; Jafari Navimipour, E.; Alizadeh Oskoee, P.; Ajami, A.A.; Abed Kahnamouei, M. Effect of preheating on the cytotoxicity of bulk-fill composite resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 19–25. [Google Scholar] [CrossRef]
- Pulgar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.M.; Olea, N. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar] [CrossRef]
- Barisic, M.L.; Sarajlija, H.; Klaric, E.; Knezevic, A.; Sabol, I.; Panduric, V. Detection of Leachable Components from Conventional and Dental Bulk-Fill Resin Composites (High and Low Viscosity) Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method. Polymers 2023, 15, 627. [Google Scholar] [CrossRef]
- Fonseca, A.S.; Labruna Moreira, A.D.; de Albuquerque, P.P.; de Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F. Effect of monomer type on the CC degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent. Mater. 2015, 31, 711–720. [Google Scholar] [CrossRef]
- Haugen, H.J.; Marovic, D.; Par, M.; Thieu, M.K.L.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]
- Bezgin, T.; Cimen, C.; Ozalp, N. Evaluation of Residual Monomers Eluted from Pediatric Dental Restorative Materials. Biomed. Res. Int. 2021, 2021, 6316171. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Mikulas, K.; Komlodi, T.; Foldes, A.; Svab, G.; Horvath, G.; Nagy, A.M.; Ambrus, A.; Gyulai-Gaal, S.; Gera, I.; Hermann, P.; et al. Bioenergetic Impairment of Triethylene Glycol Dimethacrylate- (TEGDMA-) Treated Dental Pulp Stem Cells (DPSCs) and Isolated Brain Mitochondria are Amended by Redox Compound Methylene Blue (dagger). Materials 2020, 13, 3472. [Google Scholar] [CrossRef]
- Kunert, M.; Rozpedek-Kaminska, W.; Galita, G.; Sauro, S.; Bourgi, R.; Hardan, L.; Majsterek, I.; Lukomska-Szymanska, M. The Cytotoxicity and Genotoxicity of Bioactive Dental Materials. Cells 2022, 11, 3238. [Google Scholar] [CrossRef]
- Rady, D.; Albar, N.; Khayat, W.; Khalil, M.; Raafat, S.; Ramadan, M.; Saber, S.; Shamel, M. Evaluation of dental pulp stem cells response to flowable nano-hybrid dental composites: A comparative analysis. PLoS ONE 2024, 19, e0303154. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55 (Suppl. S3), 497–511. [Google Scholar] [CrossRef]
- Alzahrani, B.; Alshabib, A.; Awliya, W. The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials. Materials 2023, 16, 6673. [Google Scholar] [CrossRef]
- Awad, M.; Alshehri, T.; Alqarni, A.; Magdy, N.; Alhalabi, F.; Alotaibi, D.; Alrahlah, A. Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material. Appl. Sci. 2020, 10, 6175. [Google Scholar] [CrossRef]
- Brackett, M.G.; Bouillaguet, S.; Lockwood, P.E.; Rotenberg, S.; Lewis, J.B.; Messer, R.L.; Wataha, J.C. In vitro cytotoxicity of dental composites based on new and traditional polymerization chemistries. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 397–402. [Google Scholar] [CrossRef]
- Meng, J.; Yang, H.; Cao, M.; Li, L.; Cai, Q. Correlating cytotoxicity to elution behaviors of composite resins in term of curing kinetic. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Cassese, A.; Miele, C.; Beguinot, F.; Garcia-Godoy, F.; Di Jeso, B.; Ulianich, L. Cytotoxicity of dental resin composites: An in vitro evaluation. J. Appl. Toxicol. 2013, 33, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Attik, N.; Hallay, F.; Bois, L.; Brioude, A.; Grosgogeat, B.; Colon, P. Mesoporous silica fillers and resin composition effect on dental composites cytocompatibility. Dent. Mater. 2017, 33, 166–174. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Simard, P.; Zhang, Z.; Zhu, X.X. Bile acids as constituents for dental composites: In vitro cytotoxicity of (meth)acrylate and other ester derivatives of bile acids. J. R. Soc. Interface 2007, 4, 1145–1150. [Google Scholar] [CrossRef]
- Franz, A.; Konig, F.; Lucas, T.; Watts, D.C.; Schedle, A. Cytotoxic effects of dental bonding substances as a function of degree of conversion. Dent. Mater. 2009, 25, 232–239. [Google Scholar] [CrossRef]
- Gilli, M.; Hollaert, T.G.; Setbon, H.M.; des Rieux, A.; Leprince, J.G. Quality of Cure in Depth of Commercially Available Bulk-fill Composites: A Layer-by-layer Mechanical and Biological Evaluation. Oper. Dent. 2022, 47, 437–448. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gomez-Lechon, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Kamalak, H.; Kamalak, A.; Taghizadehghalehjoughi, A. Cytotoxic effects of new-generation bulk-fill composites on human dental pulp stem cells. Cell. Mol. Biol. 2018, 64, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Mascarenhas, P.; Avelar, M.; Ribeiro, A.C.; Barahona, I. Biocompatibility of bulk-fill resins in vitro. Clin. Oral. Investig. 2023, 27, 7851–7858. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Yap, A.U.; Lim, S.Y. In Vitro Biocompatibility of Contemporary Bulk-fill Composites. Oper. Dent. 2015, 40, 644–652. [Google Scholar] [CrossRef]
- Wiertelak-Makala, K.; Szymczak-Pajor, I.; Bociong, K.; Sliwinska, A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2023, 25, 152. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.Y.; Kim, J.H.; Jun, S.K.; Kim, H.W.; Lee, J.H.; Lee, H.H. Depth-Dependent Cellular Response from Dental Bulk-Fill Resins in Human Dental Pulp Stem Cells. Stem Cells Int. 2019, 2019, 1251536. [Google Scholar] [CrossRef]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef]
- Rodriguez, A.; Yaman, P.; Dennison, J.; Garcia, D. Effect of Light-Curing Exposure Time, Shade, and Thickness on the Depth of Cure of Bulk Fill Composites. Oper. Dent. 2017, 42, 505–513. [Google Scholar] [CrossRef]
- Miletic, V.; Pongprueksa, P.; De Munck, J.; Brooks, N.R.; Van Meerbeek, B. Curing characteristics of flowable and sculptable bulk-fill composites. Clin. Oral Investig. 2017, 21, 1201–1212. [Google Scholar] [CrossRef]
- Dos Santos Sousa, G.; Guimaraes, G.F.; Marcelino, E.; Rodokas, J.E.P.; de Oliveira Junior, A.J.; Cesarino, I.; Leao, A.L.; Dos Santos Riccardi, C.; Arjmand, M.; Simoes, R.P. Shrinkage Stress and Temperature Variation in Resin Composites Cured via Different Photoactivation Methods: Insights for Standardisation of the Photopolymerisation. Polymers 2021, 13, 2065. [Google Scholar] [CrossRef]
- Carrillo-Cotto, R.; Etges, A.; Jardim, P.S.; Torre, E.; Kaizer, M.R.; Ferrua, C.P.; Nedel, F.; Cuevas-Suarez, C.E.; Moraes, R.R. Cytotoxicity of contemporary resin-based dental materials in contact with dentin. Eur. J. Oral Sci. 2020, 128, 436–443. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Sumikawa, D.A.; Marshall, G.W.; Gee, L.; Marshall, S.J. Microstructure of primary tooth dentin. Pediatr. Dent. 1999, 21, 439–444. [Google Scholar]

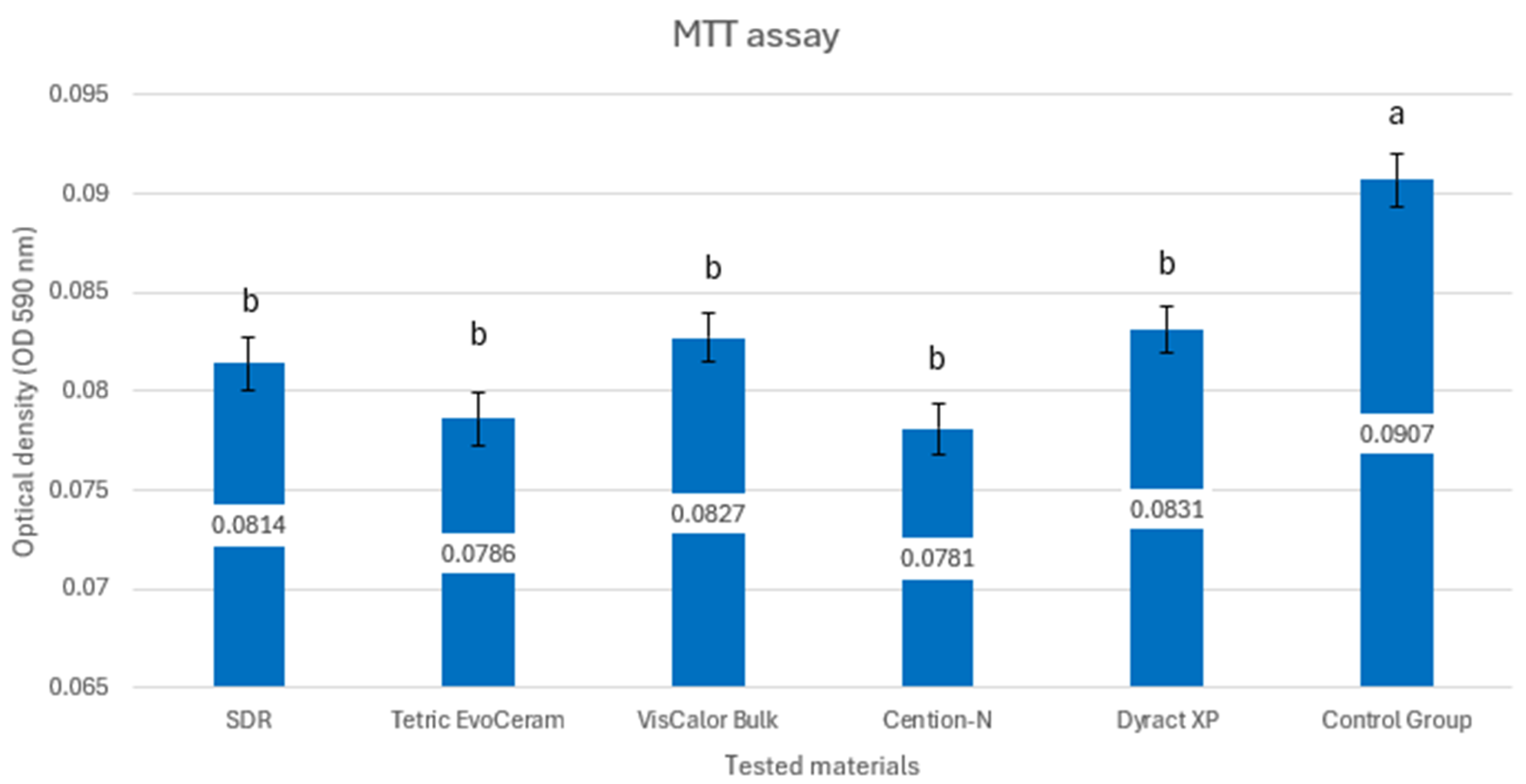
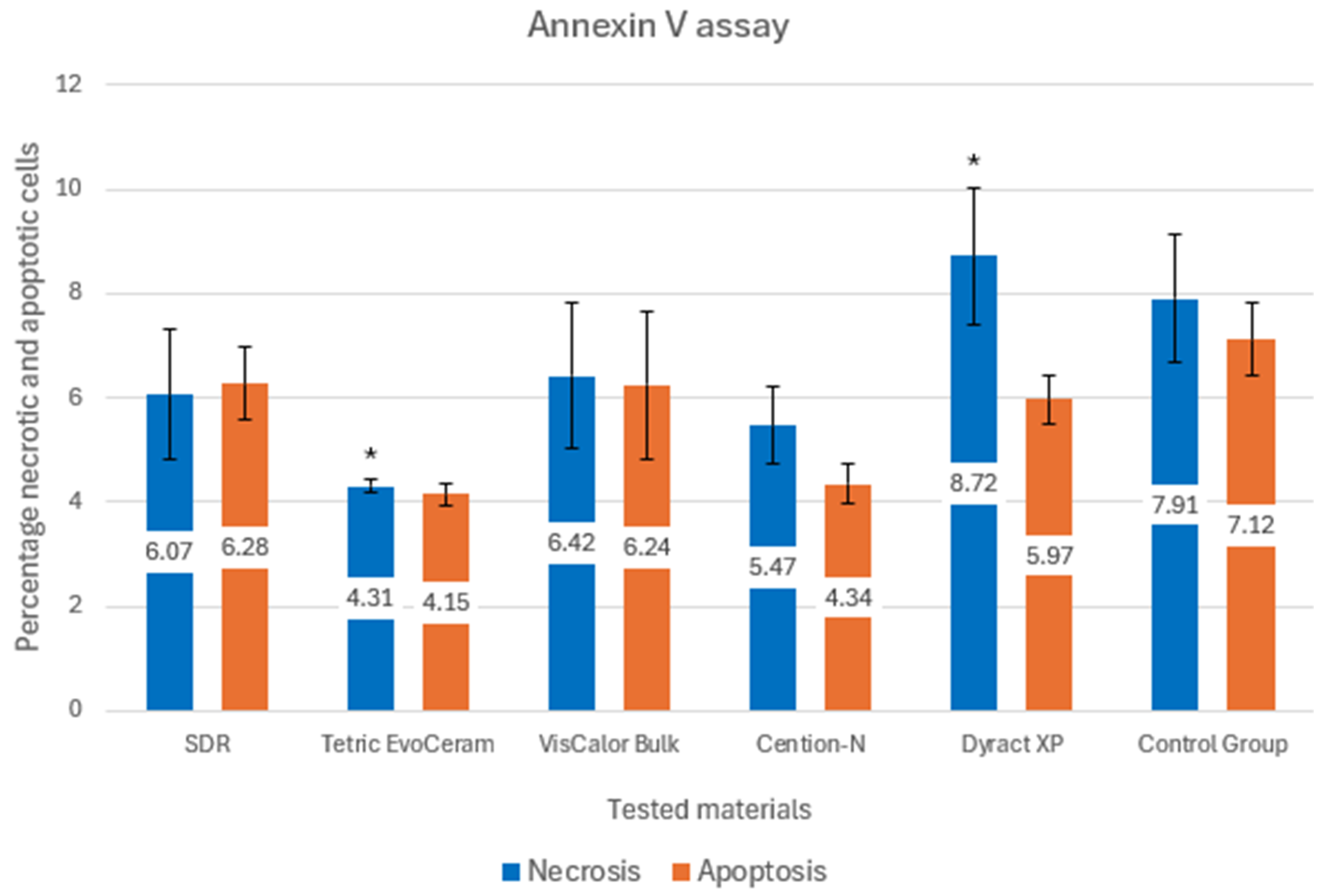
| Samples | Manufacturer | Material | Composition |
|---|---|---|---|
| 18 | Dentsply Sirona, Konstanz, Germany | SDR, Low-viscosity bulk-fill resin composite | Modified UDMA, TEGDMA, dimethacrylate and trimethacrylate resin, Silanated bariumaluminofluoroborosilicate glass, silanated strontium aluminofluoro-silicate glass, surface treated fume silicas, ytterbium fluoride, synthetic inorganic iron oxide pigments, and titanium dioxide |
| 18 | Ivoclar Vivadent, Schaan, Liechtenstein | Tetric EvoCeram Bulk-Fill, High-viscosity bulk-fill resin composite | Bisphenol-Aglycidylmethacrylat (Bis-GMA), Bis-EMA, and barium glass filler |
| 18 | VOCO, Cuxhaven, Germany | VisCalor Bulk, Thermoviscous bulk-fill resin composite | Bis-GMA, aliphatic dimethacrylate, and inorganic filler |
| 18 | Ivoclar Vivadent, Schaan, Liechtenstein | Cention-N, Moderateviscosity alkasite material | Calcium-fluoro-silicate glass, barium-aluminosilicate glass, ytterbium trifluoride, copper salt and thiocarbamide-self cure initiator (Ivocerin), acyl phosphine oxidephotoinitiator, pigment, urethane dimethacrylate (UDMA), tetramethyl Xylylendiurethane dimethacrylate, Tricyclodecandimethanol dimethacrylate (DCP), polyethylene glycol 400 dimethacrylate (PEG400 DMA), initiator (hydroperoxide—selfcuring), and stabilizer |
| 18 | Dentsply Sirona, Konstanz, Germany | Dyract XP, High-viscosity compomer material | UDMA, carboxylic acid modified dimethacrylate, TEGDMA, trimethacrylate resin (TMPTMA), dimethacrylate resins, camphorquinone, ethyl-4 (dimethylamino) benzoate, butylated hydroxy toluene (BHT), strontium-aluminosodium-fluoro phosphorsilicate glass, highly dispersed silicon dioxide, strontium fluoride, iron oxide pigments, and titanium oxide pigments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogovska-Gigova, R.; Ishkitiev, N.; Miteva, M.; Hristov, K. Cytotoxicity of Bulk-Fill Composites on Stem Cells from Human Exfoliated Deciduous Teeth—An In Vitro Study. Materials 2025, 18, 3863. https://doi.org/10.3390/ma18163863
Bogovska-Gigova R, Ishkitiev N, Miteva M, Hristov K. Cytotoxicity of Bulk-Fill Composites on Stem Cells from Human Exfoliated Deciduous Teeth—An In Vitro Study. Materials. 2025; 18(16):3863. https://doi.org/10.3390/ma18163863
Chicago/Turabian StyleBogovska-Gigova, Ralitsa, Nikolay Ishkitiev, Marina Miteva, and Krasimir Hristov. 2025. "Cytotoxicity of Bulk-Fill Composites on Stem Cells from Human Exfoliated Deciduous Teeth—An In Vitro Study" Materials 18, no. 16: 3863. https://doi.org/10.3390/ma18163863
APA StyleBogovska-Gigova, R., Ishkitiev, N., Miteva, M., & Hristov, K. (2025). Cytotoxicity of Bulk-Fill Composites on Stem Cells from Human Exfoliated Deciduous Teeth—An In Vitro Study. Materials, 18(16), 3863. https://doi.org/10.3390/ma18163863







