Evaluation of Polymethyl Methacrylate as a Provisional Material in a Fully Digital Workflow for Immediate-Load Complete-Arch Implant-Supported Prostheses over Three Months
Highlights
- PMMA provisionals ensure stability and function in immediate-loading protocols.
- Digital workflows enhance precision in full-arch prosthetic rehabilitation.
- Immediate loading reduces treatment time without compromising outcomes.
- Provisional PMMA supports soft tissue contouring and esthetics.
- PMMA materials provide predictable results in full-arch prosthesis cases.
Abstract
1. Introduction
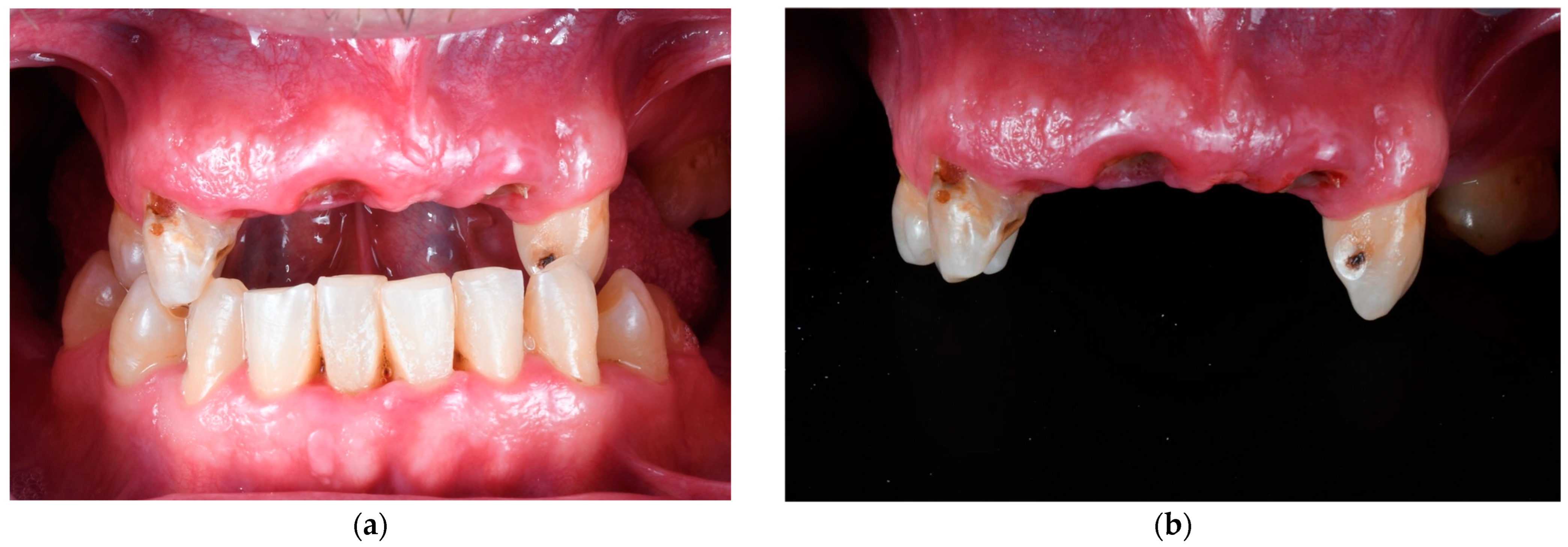
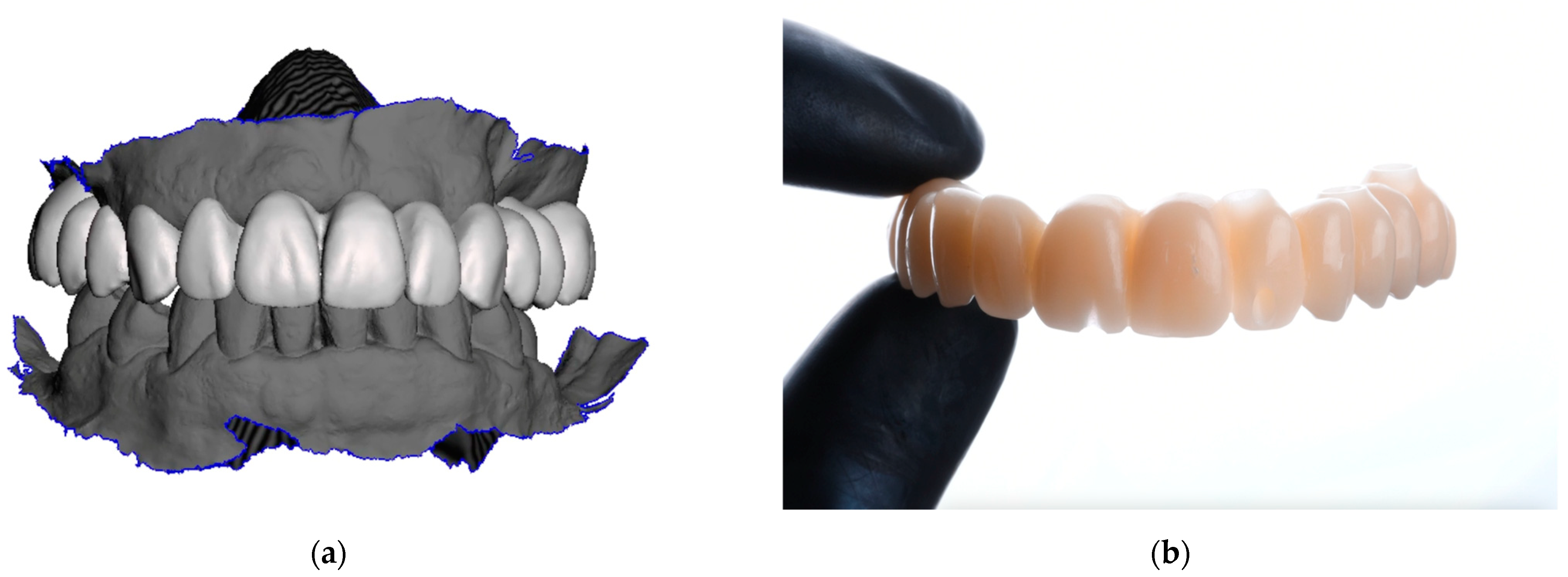
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelara, K.; Bratos, M.; Sorensen, J.A. Comparison of strength of milled and conventionally processed PMMA complete-arch implant-supported immediate interim fixed dental prostheses. J. Prosthet. Dent. 2023, 129, 221–227. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Bedrossian, A.; De Souza, A.; Bokhary, A.; Gonzaga, L.; Chochlidakis, K. Digital Workflow in Implant Treatment Planning for Terminal Dentition Patients. J. Prosthodont. 2022, 31, 543–548. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. PMMA-Based Nanocomposites for Odontology Applications: A State-of-the-Art. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- García, I.; Cortés, J.; Jiménez, J.; Peláez, J.; Suárez, M.J. Precision and practical usefulness of intraoral scanners in implant dentistry: A systematic literature review. J. Clin. Exp. Dent. 2020, 12, e784–e793. [Google Scholar] [CrossRef] [PubMed]
- Venezia, P.; Torsello, F.; Cavalcanti, R.; D’Amato, S. Retrospective analysis of 26 complete-arch implant-supported monolithic zirconia prostheses with feldspathic porcelain veneering limited to the facial surface. J. Prosthet. Dent. 2015, 114, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- De Angelis, N.; Pesce, P.; De Lorenzi, M.; Menini, M. Evaluation of Prosthetic Marginal Fit and Implant Survival Rates for Conventional and Digital Workflows in Full-Arch Immediate Loading Rehabilitations: A Retrospective Clinical Study. J. Clin. Med. 2023, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Francetti, L.; Romeo, D.; Corbella, S.; Taschieri, S.; Del Fabbro, M. Bone level changes around axial and tilted implants in full-arch fixed immediate restorations. Interim results of a prospective study. Clin. Implant Dent. Relat. Res. 2012, 14, 646–654. [Google Scholar] [CrossRef]
- Agliardi, E.; Clericò, M.; Ciancio, P.; Massironi, D. Immediate loading of full-arch fixed prostheses supported by axial and tilted implants for the treatment of edentulous atrophic mandibles. Quintessence Int. 2010, 41, 285–293. [Google Scholar]
- Viswambaran, M.; Kapri, A.; D’Souza, D.; Kumar, M. An evaluation of fracture resistance of interim fixed partial denture fabricated using polymethylmethacrylate and reinforced by different fibres for its optimal placement: An in vitro study. Med. J. Armed Forces India 2011, 67, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Kotina, E.; Hamilton, A.; Lee, J.D.; Lee, S.J.; Grieco, P.C.; Pedrinaci, I. Milled PMMA: A Material for Long-Term Implant-Supported Fixed Complete Dental Prostheses. Int. J. Prosthodont 2024, 37, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.S.Y.; de Magalhães, K.M.F.; Rocha, E.P.; Dos Santos, P.H.; Assunção, W.G. Oral health-related quality of life and satisfaction in edentulous patients rehabilitated with implant-supported full dentures all-on-four concept: A systematic review. Clin. Oral. Investig. 2022, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Espona, J.; Vidal-Ponsoda, C.; Quintana, P.; Henarejos-Domingo, V.; Roig, M. A fully digital protocol to provide a fixed interim complete denture for immediate loading for a completely edentulous patient: A dental technique. J. Prosthet. Dent. 2023, 130, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Roig, E.; Roig, M.; Garza, L.C.; Costa, S.; Maia, P.; Espona, J. Fit of complete-arch implant-supported prostheses produced from an intraoral scan by using an auxiliary device and from an elastomeric impression: A pilot clinical trial. J. Prosthet. Dent. 2022, 128, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, H.; Jansen, V.K.; Richter, E.J. A 10-year follow-up study of IMZ and TPS implants in the edentulous mandible using bar-retained overdentures. Int. J. Oral. Maxillofac. Implant. 1995, 10, 231–243. [Google Scholar]
- Di, P.; Lin, Y.; Li, J.H.; Luo, J.; Qiu, L.X.; Chen, B. The All-on-Four implant therapy protocol in the management of edentulous Chinese patients. Int. J. Prosthodont. 2013, 26, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Balshi, S.F.; Wolfinger, G.J.; Balshi, T.J. A prospective study of immediate functional loading, following the Teeth in a Day protocol: A case series of 55 consecutive edentulous maxillas. Clin. Implant. Dent. Relat. Res. 2005, 7, 24–31. [Google Scholar] [CrossRef]
- Tischler, M.; Patch, C.; Bidra, A.S. Rehabilitation of edentulous jaws with zirconia complete-arch fixed implant-supported prostheses: An up to 4-year retrospective clinical study. J. Prosthet. Dent. 2018, 120, 204–209. [Google Scholar] [CrossRef]
- Uesugi, T.; Shimoo, Y.; Munakata, M.; Sato, D.; Yamaguchi, K.; Fujimaki, M. The All-on-four concept for fixed full-arch rehabilitation of the edentulous maxilla and mandible: A longitudinal study in Japanese patients with 3-17-year follow-up and analysis of risk factors for survival rate. Int. J. Implant. Dent. 2023, 9, 43. [Google Scholar] [CrossRef]
- Velasco, E.; Cracel, J.L.; Matos, N.; Jiménez, A.; Ortiz, I.; Moreno, J. Immediate Functional Loading with Full-Arch Fixed Implant-Retained Rehabilitation in Periodontal Patients: Clinical Study. Int. J. Environ. Res. Public Health 2022, 19, 13162. [Google Scholar] [CrossRef] [PubMed]
- Kioleoglou, I.; Pissiotis, A.; Michalakis, K. Accuracy of fit of implant-supported bars fabricated on definitive casts made by different dental stones. J. Clin. Exp. Dent. 2018, 10, e252–e263, Erratum in J. Clin. Exp. Dent. 2019, 11, e104. [Google Scholar] [CrossRef] [PubMed]
- Figueras, O.; Cantó, O.; Real, F.; Roig, M. Protocol for the clinical assessment of passive fit for multiple implant-supported prostheses: A dental technique. J Prosthet Dent 2021, 126, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ghoul, W.E.; Chidiac, J.J. Prosthetic requirements for immediate implant loading: A review. J. Prosthodont. 2012, 21, 141–154. [Google Scholar] [CrossRef]
- Durkan, R.; Oyar, P.; Deste, G. Effects of Cantilever Length and Implant Inclination on the Stress Distribution of Mandibular Prosthetic Restorations Constructed from Monolithic Zirconia Ceramic. Int. J. Oral. Maxillofac Implant. 2020, 35, 121–129. [Google Scholar] [CrossRef]
- Kumari, A.; Malhotra, P.; Phogat, S.; Yadav, B.; Yadav, J.; Phukela, S.S. A finite element analysis to study the stress distribution on distal implants in an all-on-four situation in atrophic maxilla as affected by the tilt of the implants and varying cantilever lengths. J. Indian Prosthodont. Soc. 2020, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Apicella, A.; Nicolais, L.; Aversa, R.; Sorrentino, R. Mandibular flexure and stress build-up in mandibular full-arch fixed prostheses supported by osseointegrated implants. Clin. Oral. Implant. Res. 2003, 14, 103–114. [Google Scholar] [CrossRef]
- Lerner, H.; Hauschild, U.; Sader, R.; Ghanaati, S. Complete-arch fixed reconstruction by means of guided surgery and immediate loading: A retrospective clinical study on 12 patients with 1 year of follow-up. BMC Oral. Health 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Liu, J.Z.; Feng, H.L.; Heng, M.D.; Wang, B.; Pan, S.X. Time Efficiency of Immediate Loading of Full-arch Implant Reconstructions Using Prefabricated Prostheses Located by an Anchor Pin: A Pilot Study. Chin. J. Dent. Res. 2021, 24, 257–265. [Google Scholar]
- Pomares, C. A retrospective study of edentulous patients rehabilitated according to the ‘all-on-four’ or the ‘all-on-six’ immediate function concept using flapless computer-guided implant surgery. Eur. J. Oral. Implantol. 2010, 3, 155–163. [Google Scholar] [PubMed]
- Romanos, G.E.; Gaertner, K.; Aydin, E.; Nentwig, G.H. Long-term results after immediate loading of platform-switched implants in smokers versus nonsmokers with full-arch restorations. Int. J. Oral. Maxillofac Implant. 2013, 28, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Kowar, J.; Eriksson, A.; Jemt, T. Fixed implant-supported prostheses in elderly patients: A 5-year retrospective comparison between partially and completely edentulous patients aged 80 years or older at implant surgery. Clin. Implant Dent. Relat. Res. 2013, 15, 37–46. [Google Scholar] [CrossRef]
- Kwon, T.; Bain, P.A.; Levin, L. Systematic review of short- (5-10 years) and long-term (10 years or more) survival and success of full-arch fixed dental hybrid prostheses and supporting implants. J. Dent. 2014, 42, 1228–1241. [Google Scholar] [CrossRef]
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018, 37, 526–533. [Google Scholar] [CrossRef]
- Giannetti, L.; Apponi, R.; Mordini, L.; Presti, S.; Breschi, L.; Mintrone, F. The occlusal precision of milled versus printed provisional crowns. J. Dent. 2022, 117, 103924. [Google Scholar] [CrossRef]
- Singh, P.; Shenoy, A.; Nallaswamy, D.; Maiti, S. Comparative Evaluation of Microbial Adhesion on Provisional Crowns Fabricated with Milled Polymethyl Methacrylate (PMMA) and Conventional Acrylic Resin: A Prospective Clinical Trial. Cureus 2024, 16, e64469. [Google Scholar] [CrossRef]
- Mendonça, C.; de Macedo, D.; Nicolai, C.; Madeira, H.; Van Dooren, E.; Norré, D. Digital Full-Arch Implant-Supported Polymethyl Methacrylate Interim Prosthesis: A Practice-Based Cohort Study on Survival and Quality of Life. Int. J. Prosthodont. 2024, 37, 394–403. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into their Modern Manufacturing Techniques. Materials 2020, 13. [Google Scholar] [CrossRef]
- Bangera, M.K.; Kotian, R.; Madhyastha, P. Effects of silver nanoparticle-based antimicrobial formulations on the properties of denture polymer: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2023, 129, 310–321. [Google Scholar] [CrossRef]
- Siebel, T.; Kelm, J.; Porsch, M.; Regitz, T.; Neumann, W.H. Two-stage exchange of infected knee arthroplasty with an prosthesis-like interim cement spacer. Acta Orthop. Belg. 2002, 68, 150–156. [Google Scholar] [PubMed]
- Bacevic, M.; Dethier, F.; Lecloux, G.; Seidel, L.; Rompen, E.; Lambert, F. The Effects of Direct Polymethyl Methacrylate and Zirconia-on-Ti-Base Abutments on Peri-implant Soft Tissue Integration: A Study in Minipigs. Int. J. Prosthodont. 2023, 36, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Braian, M.; Wennerberg, A. Trueness and precision of 5 intraoral scanners for scanning edentulous and dentate complete-arch mandibular casts: A comparative in vitro study. J. Prosthet. Dent. 2019, 122, 129–136. [Google Scholar] [CrossRef]
- García, I.; Cortés, J.; Martínez, M.; Sánchez, A.; Peláez, J.; Suárez, M.J. Implant-supported full-arch rehabilitation with immediate loading using two different digital impression techniques: A case report with 2-year follow-up. Quintessence Int. 2023, 54, 844–851. [Google Scholar]
- Pellegrino, G.; Ferri, A.; Cercenelli, L.; Marcelli, E.; Marchetti, C.; Tarsitano, A. 3D planning of ear prosthesis and navigated flapless surgery for craniofacial implants: A pilot study. J. Stomatol. Oral. Maxillofac. Surg. 2021, 122, 391–396. [Google Scholar] [CrossRef]
- Loubele, M.; Jacobs, R.; Maes, F.; Denis, K.; White, S.; Coudyzer, W. Image quality vs radiation dose of four cone beam computed tomography scanners. Dentomaxillofac Radiol. 2008, 37, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Polo, M.; Barmak, A.B.; Ortega, R.; Rutkunas, V.; Kois, J.C.; Revilla-León, M. Accuracy, scanning time, and patient satisfaction of stereophotogrammetry systems for acquiring 3D dental implant positions: A systematic review. J. Prosthodont. 2023, 32, 208–224. [Google Scholar] [CrossRef]
- Antonacci, D.; Caponio, V.C.A.; Troiano, G.; Pompeo, M.G.; Gianfreda, F.; Canullo, L. Facial scanning technologies in the era of digital workflow: A systematic review and network meta-analysis. J. Prosthodont. Res. 2023, 67, 321–336. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, L.; Cao, D.; Izadikhah, I.; Gao, P.; Zhao, Y. Accuracy of three-dimensional photogrammetry and cone beam computed tomography based on linear measurements in patients with facial deformities. Dentomaxillofac. Radiol. 2021, 50, 20200001. [Google Scholar] [CrossRef]
- Coskunses, F.M.; Tak, Ö. Clinical performance of narrow-diameter titanium-zirconium implants in immediately loaded fixed full-arch prostheses: A 2-year clinical study. Int. J. Implant. Dent. 2021, 7, 30. [Google Scholar] [CrossRef]
- Canullo, L.; Tallarico, M.; Pradies, G.; Marinotti, F.; Loi, I.; Cocchetto, R. Soft and hard tissue response to an implant with a convergent collar in the esthetic area: Preliminary report at 18 months. Int. J. Esthet. Dent. 2017, 12, 306–323. [Google Scholar] [PubMed]
- Finelle, G.; Papadimitriou, D.E.V.; Souza, A.B.; Katebi, N.; Gallucci, G.O.; Araújo, M.G. Peri-implant soft tissue and marginal bone adaptation on implant with non-matching healing abutments: Micro-CT analysis. Clin. Oral. Implants Res. 2015, 26, e42–e46. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, R.J.; Porter, S.S. Platform switching: A new concept in implant dentistry for controlling postrestorative crestal bone levels. Int. J. Periodontics Restor. Dent. 2006, 26, 9–17. [Google Scholar]
- Dhima, M.; Salinas, T.J.; Rieck, K.L. Virtual surgical planning for treatment of severe mandibular retrognathia with collapsed occlusion using contemporary surgical and prosthodontic protocols. J. Oral. Maxillofac. Surg. 2013, 71, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Albiero, A.M.; Benato, R.; Benato, A.; Degidi, M. Guided-welded approach planning using digital scanning technology for combined screw- and conometric-retained implant-supported maxillary prosthesis. Int. J. Comput. Dent. 2020, 23, 325–333. [Google Scholar]
- Joda, T.; Ferrari, M.; Gallucci, G.O.; Wittneben, J.G.; Brägger, U. Digital technology in fixed implant prosthodontics. Periodontol. 2000 2017, 73, 178–192. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Chuang, S.K.; Weber, H.P. Implant loading protocols for edentulous patients with fixed prostheses: A systematic review and meta-analysis. Int. J. Oral. Maxillofac. Implants 2014, 29, 256–270. [Google Scholar] [CrossRef]
- Bouhy, A.; Lamy, M.; Altaep, Y.; Lambert, F. Maxillary implant overdenture retained by four unsplinted attachments and opposed by a natural or fixed dentition: Five-year clinical outcomes. A prospective case series. Clin. Oral. Implants Res. 2023, 34, 285–296. [Google Scholar] [CrossRef]
- Zembic, A.; Tahmaseb, A.; Wismeijer, D. Within-Subject Comparison of Maxillary Implant-Supported Overdentures with and without Palatal Coverage. Clin. Implant Dent. Relat. Res. 2015, 17, 570–579. [Google Scholar] [CrossRef]
- Ni Riordain, R.; Moloney, E.; O’Sullivan, K.; McCreary, C. Validity and reliability of patient-centered outcome measures in oral dysesthesia. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 112, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; López, R.; Fernández, M.; Ramírez, L.; Sanz, M.; López, J. Usefulness of implementing the OHIP-14 questionnaire to assess the impact of xerostomia and hyposalivation on quality of life in patients with primary Sjögren’s syndrome. J. Oral. Pathol. Med. 2022, 51, 810–817. [Google Scholar] [CrossRef]
- Adamo, D.; Pecoraro, G.; Fortuna, G.; Amato, M.; Marenzi, G.; Aria, M. Assessment of oral health-related quality of life, measured by OHIP-14 and GOHAI, and psychological profiling in burning mouth syndrome: A case-control clinical study. J. Oral. Rehabil. 2020, 47, 42–52. [Google Scholar] [CrossRef]



| Variable | Level | Number | Percentage |
|---|---|---|---|
| Sex | Male | 36 | 50 |
| Female | 36 | 50 | |
| Range of age | <45 years old | 54 | 12.5 |
| Between 45 and 60 | 168 | 38.88 | |
| >60 years old | 210 | 48.61 | |
| Smoking habit | Smokers | 24 | 33.3 |
| Non-smokers | 48 | 66.7 | |
| Location | Maxilla | 37 | 51.4 |
| Mandible | 35 | 48.6 | |
| Implant diameter * | 3.75 | 39 | 9.02 |
| 4.3 | 275 | 63.65 | |
| 5 | 118 | 27.31 | |
| Abutment * | 1.5 | 29 | 6.71 |
| 2.5 | 286 | 66.2 | |
| 3.5 | 104 | 24.07 | |
| Angulated | 13 | 3 |
| Variable | Level | Mean MBL * |
|---|---|---|
| Sex | Male | −0.432 ± 0.036 |
| Female | −0.272 ± 0.038 | |
| Range of age | <45 years old | −0.257 ± 0.074 |
| Between 45 and 60 | −0.363 ± 0.038 | |
| >60 years old | −0.377 ± 0.043 | |
| Smoking habit | Smokers | −0.392 ± 0.045 |
| Non-smokers | −0.335 ± 0.033 | |
| Resin | −0.798 ± 0.099 | |
| Opposing | Teeth | −0.366 ± 0.050 |
| dentition | Ceramic | −0.335 ± 0.099 |
| Zirconia | −0.300 ± 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza, L.C.; Crooke, E.; Vallés, M.; Soliva, J.; Rodríguez, X.; Rodeja, M.; Roig, M. Evaluation of Polymethyl Methacrylate as a Provisional Material in a Fully Digital Workflow for Immediate-Load Complete-Arch Implant-Supported Prostheses over Three Months. Materials 2025, 18, 562. https://doi.org/10.3390/ma18030562
Garza LC, Crooke E, Vallés M, Soliva J, Rodríguez X, Rodeja M, Roig M. Evaluation of Polymethyl Methacrylate as a Provisional Material in a Fully Digital Workflow for Immediate-Load Complete-Arch Implant-Supported Prostheses over Three Months. Materials. 2025; 18(3):562. https://doi.org/10.3390/ma18030562
Chicago/Turabian StyleGarza, Luis Carlos, Eduardo Crooke, Marta Vallés, Joan Soliva, Xavier Rodríguez, Mariona Rodeja, and Miguel Roig. 2025. "Evaluation of Polymethyl Methacrylate as a Provisional Material in a Fully Digital Workflow for Immediate-Load Complete-Arch Implant-Supported Prostheses over Three Months" Materials 18, no. 3: 562. https://doi.org/10.3390/ma18030562
APA StyleGarza, L. C., Crooke, E., Vallés, M., Soliva, J., Rodríguez, X., Rodeja, M., & Roig, M. (2025). Evaluation of Polymethyl Methacrylate as a Provisional Material in a Fully Digital Workflow for Immediate-Load Complete-Arch Implant-Supported Prostheses over Three Months. Materials, 18(3), 562. https://doi.org/10.3390/ma18030562






