Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis
Abstract
1. Introduction
2. Experiment
2.1. MoS2 Nanosheet Synthesis
2.2. gC3N4 Nanosheet Synthesis
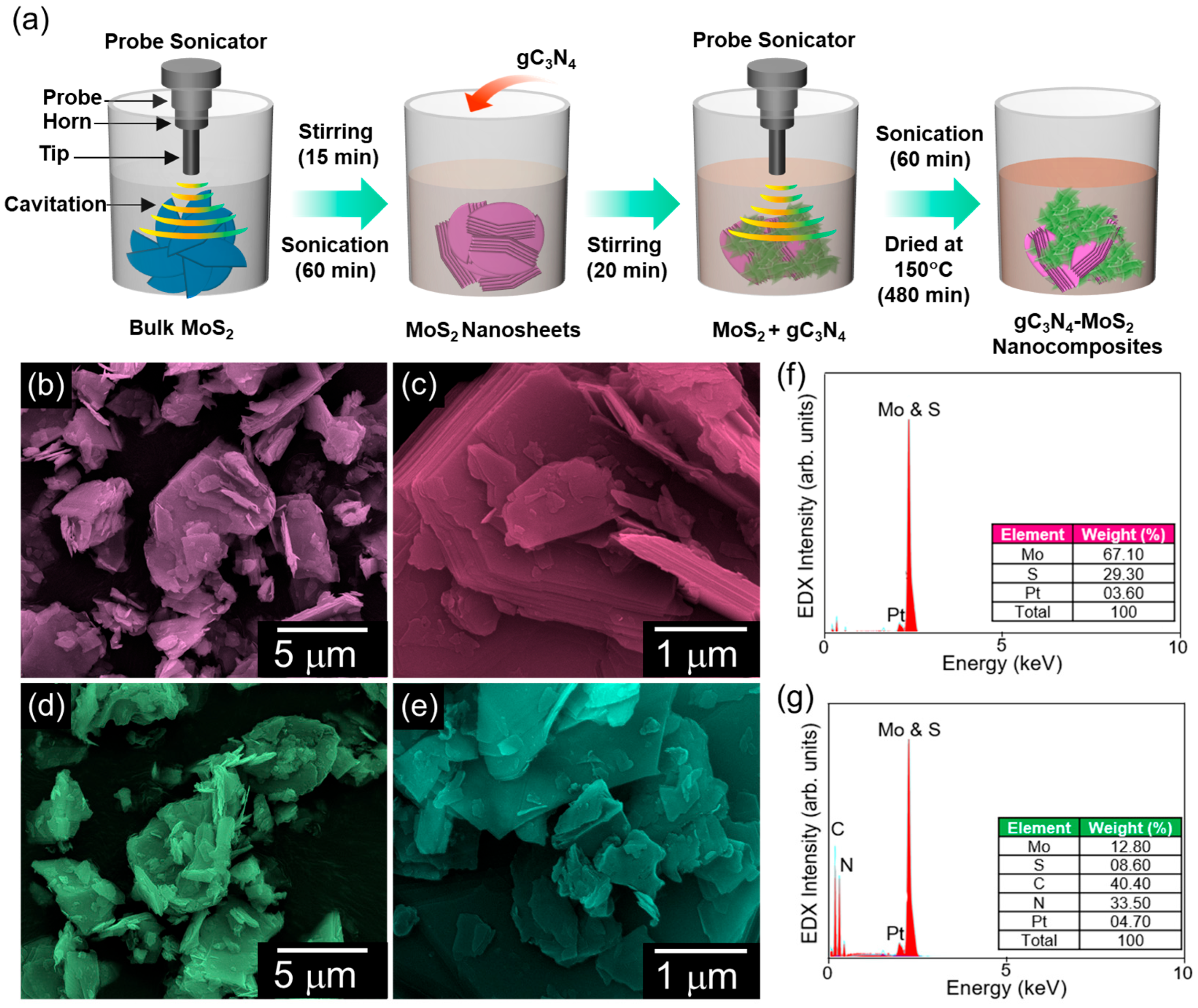
2.3. gC3N4–MoS2 Nanocomposite Fabrication
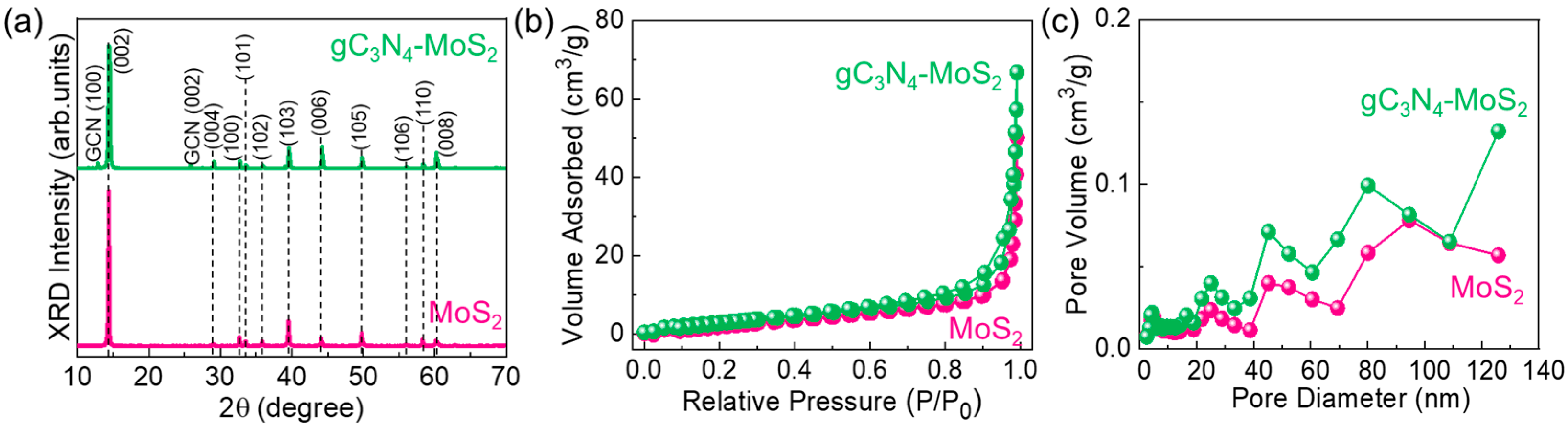
2.4. Material Characterization
2.5. Electrocatalytic Measurements
3. Results and Discussion
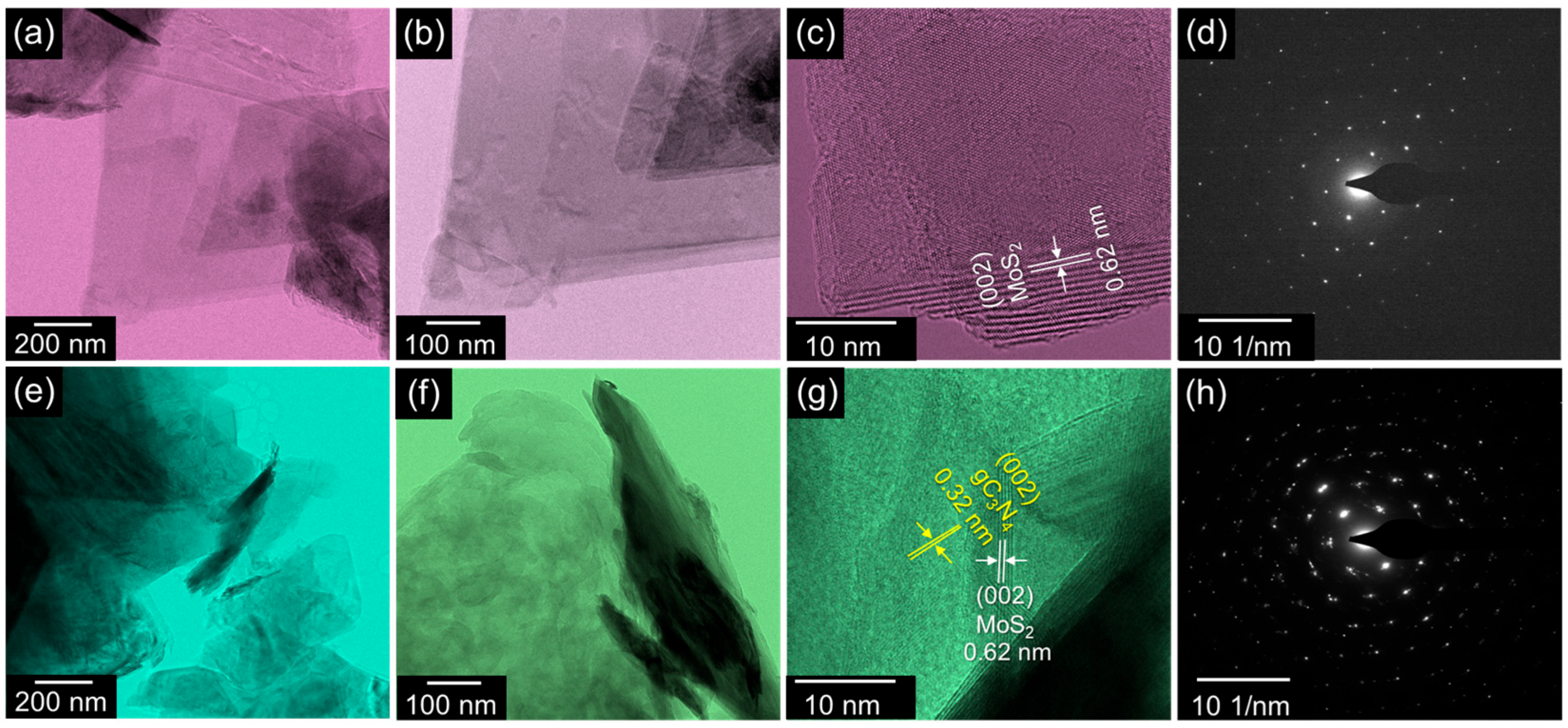

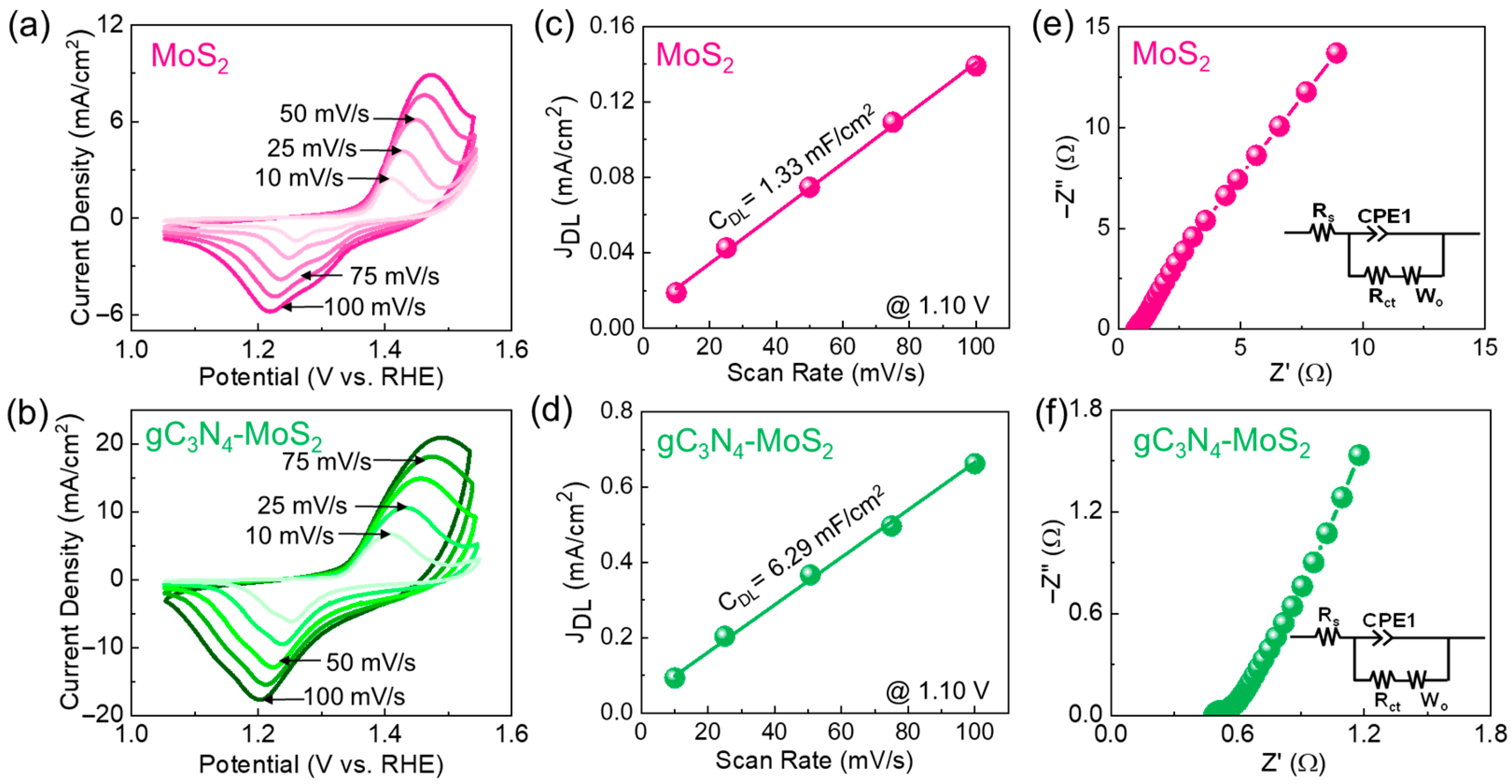
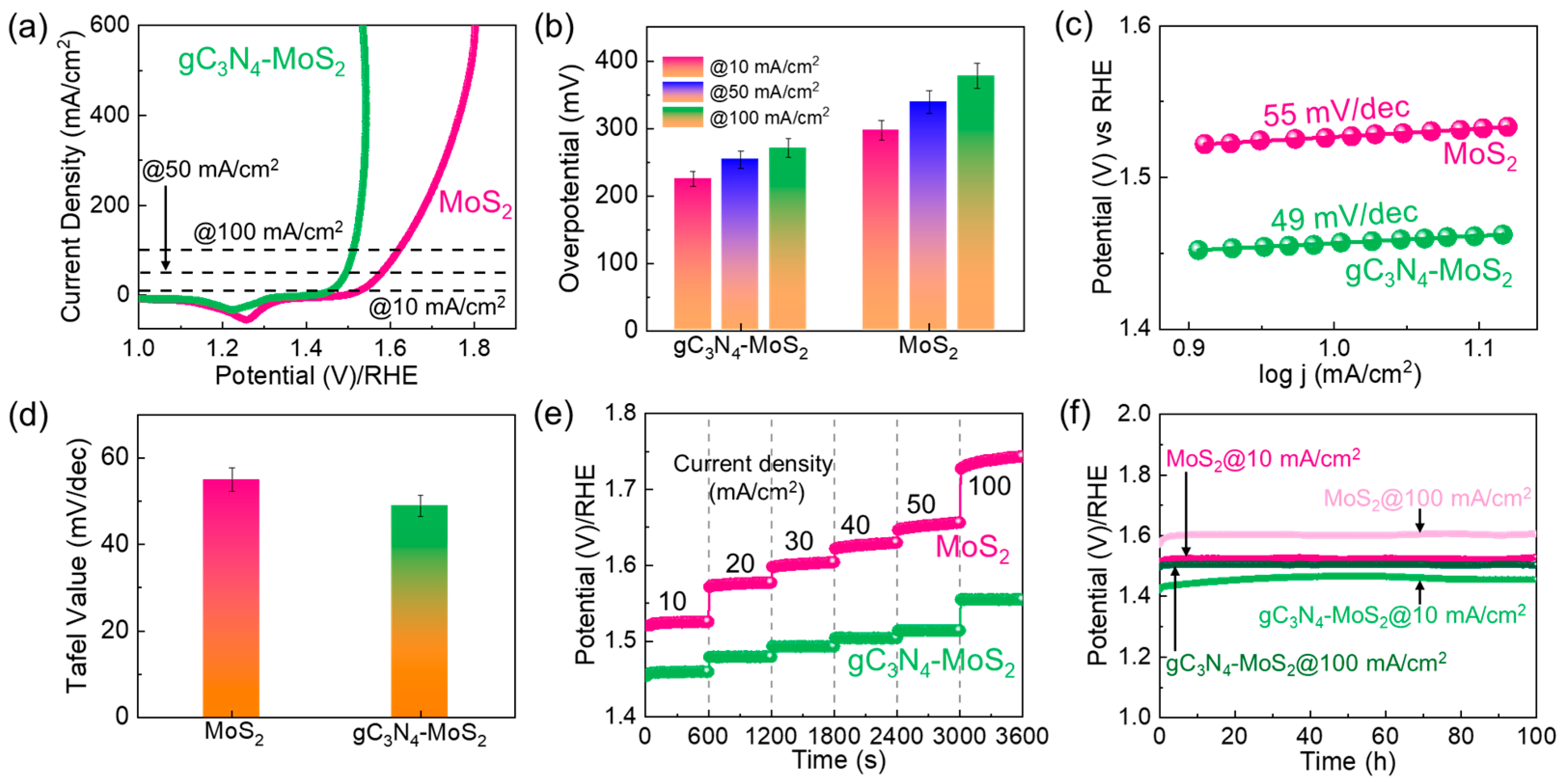

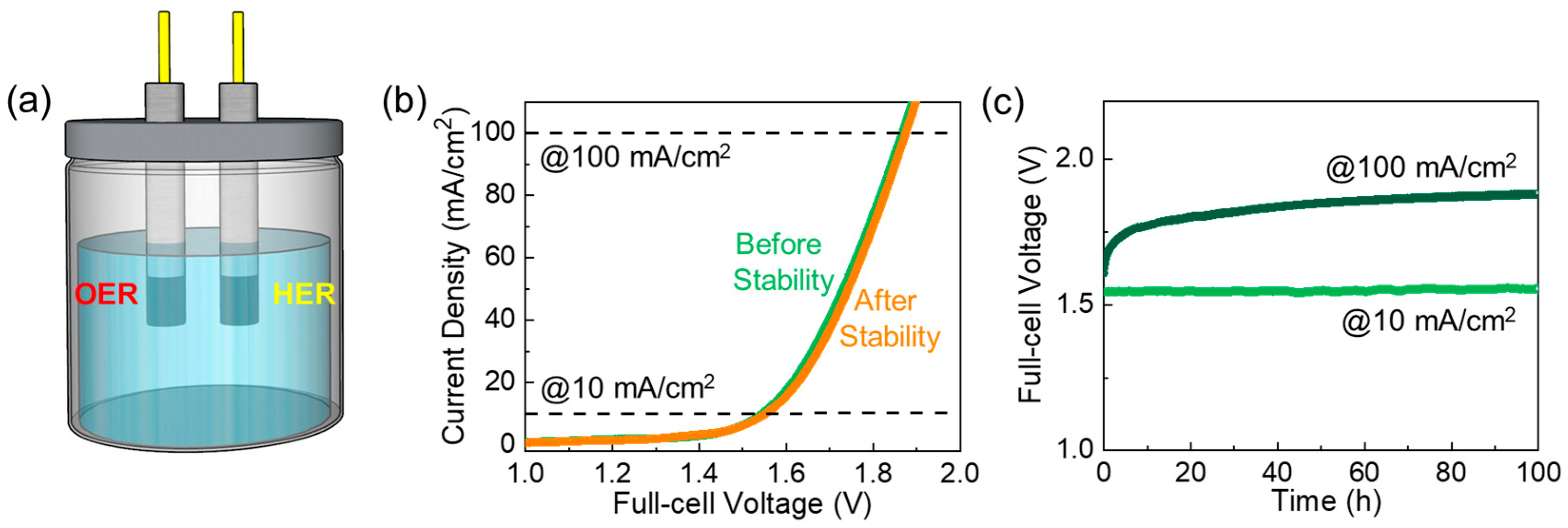
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Q.; Wang, Y.; Liu, P.-F.; Zheng, L.-R.; Wang, G.; Yang, H.-G.; Wong, P.-K.; Zhang, H.; Zhao, H. Cobalt Covalent Doping in MoS2 to Induce Bifunctionality of Overall Water Splitting. Adv. Mater. 2018, 30, 1801450. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Xu, S.; Zaidi, S.A.H. Environmental impact of energy imports: Natural resources income and natural gas production profitability in the Asia-Pacific Economic Cooperation Countries. Geosci. Front. 2024, 15, 101756. [Google Scholar] [CrossRef]
- Manikandan, R.; Sadhasivam, S.; Lee, S.; Seung-Cheol, C.; Ashok Kumar, K.; Bathula, C.; Gopalan Sree, V.; Young Kim, D.; Sekar, S. Deep eutectic solvents assisted synthesis of AC-decorated NiO nanocomposites for hydrogen evolution reaction. J. Mol. Liq. 2023, 375, 121338. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Yu, Q.; Qu, X.; Wan, J.; Xiao, Z.; Chi, J.; Wang, L. Recent advances in design of hydrogen evolution reaction electrocatalysts at high current density: A review. Chin. J. Catal. 2024, 63, 33–60. [Google Scholar] [CrossRef]
- Han, N.; Yang, K.R.; Lu, Z.; Li, Y.; Xu, W.; Gao, T.; Cai, Z.; Zhang, Y.; Batista, V.S.; Liu, W.; et al. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 924. [Google Scholar] [CrossRef]
- Li, Y.; Peng, C.-K.; Hu, H.; Chen, S.-Y.; Choi, J.-H.; Lin, Y.-G.; Lee, J.-M. Interstitial boron-triggered electron-deficient Os aerogels for enhanced pH-universal hydrogen evolution. Nat. Commun. 2022, 13, 1143. [Google Scholar] [CrossRef]
- Ma, N.; Zhao, W.; Wang, W.; Li, X.; Zhou, H. Large scale of green hydrogen storage: Opportunities and challenges. Int. J. Hydrogen Energy 2024, 50, 379–396. [Google Scholar] [CrossRef]
- Ceballos-Alvarez, C.; Maziar, J.; Moradizadeh, L.; Siaj, M.; Shahgaldi, S.; Izquierdo, R. Enhanced Graphene Oxide-Nafion® membranes for proton exchange membrane water electrolysis. J. Membr. Sci 2025, 734, 124267. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cheng, J.; Ding, L.; Lv, H.; Zhang, K.; Hu, A.; Yang, X.; Sun, W.; Mao, Y. Interfacial engineering for promoting charge transfer in MoS2/CoFeLDH heterostructure electrodes for overall water splitting. Int. J. Hydrogen Energy 2024, 49, 897–906. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, E.; Yun, J.; Arumugasamy, S.K.; Choi, M.-J.; Lee, Y.; Lee, S. High-performance electrocatalyst of activated carbon-decorated molybdenum trioxide nanocomposites for effective production of H2 and H2O2. Sep. Purif. Technol. 2025, 361, 131614. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Lee, Y.; Lee, S. Enhanced bifunctional water electrolysis activities of activated carbon-decorated trimetallic Ni-Al-La nanocomposites. Appl. Surf. Sci. 2025, 698, 163098. [Google Scholar] [CrossRef]
- Slobodkin, I.; Davydova, E.; Sananis, M.; Breytus, A.; Rothschild, A. Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte. Nat. Mater. 2024, 23, 398–405. [Google Scholar] [CrossRef]
- Sekar, S.; Park, S.; Jung, J.; Lee, S. Superb Bifunctional Water Electrolysis Activities of Carbon Nanotube-Decorated Lanthanum Hydroxide Nanocomposites. Int. J. Energy Res. 2023, 2023, 6685726. [Google Scholar] [CrossRef]
- Rong, C.; Dastafkan, K.; Wang, Y.; Zhao, C. Breaking the Activity and Stability Bottlenecks of Electrocatalysts for Oxygen Evolution Reactions in Acids. Adv. Mater. 2023, 35, 2211884. [Google Scholar] [CrossRef]
- Xi, C.; Xu, W.; Zhou, S.; Wang, Y.; Han, S.; Jiang, J. Heterogeneous interface construction of P-doped MoS2 based on the N-doped graphene oxide aerogels for efficient hydrogen evolution. Int. J. Hydrogen Energy 2024, 58, 1596–1607. [Google Scholar] [CrossRef]
- Sankar, B.D.; Sekar, S.; Vignesh, V.; Ruan, J.; Nirmala, R.; Lee, Y.; Lee, S.; Tsai, P.-C.; Chen, S.-C.; Lin, Y.-C.; et al. A dual-purpose binder-free FeNiS2-Decorated Ti3C2Tx nanocomposite for supercapacitor and catalytic hydrogen evolution reaction. J. Power Sources 2025, 649, 237412. [Google Scholar] [CrossRef]
- Sekar, S.; Shanmugam, A.; Senthilkumar, G.; Thangasami, K.; Jung, H.; Lee, Y.; Lee, S. Enhanced Hydrogen Evolution Reaction Using Biomass-Activated Carbon Nanosheets Derived from Eucalyptus Leaves. Materials 2025, 18, 670. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Lee, S.C.; Sankar, K.V.; Jin, B.; Lee, J.; Park, J.H.; Jun, S.C. Amorphous Phosphorus-Incorporated Cobalt Molybdenum Sulfide on Carbon Cloth: An Efficient and Stable Electrocatalyst for Enhanced Overall Water Splitting over Entire pH Values. ACS Appl. Mater. Interfaces 2017, 9, 37739–37749. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Jose, V.; Lee, J.-M. Design Strategies for Development of TMD-Based Heterostructures in Electrochemical Energy Systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Manikandan, R.; Arumugasamy, S.K.; Sekar, S.; Lee, Y.; Chang, S.-C.; Lee, S. Rapid Microwave-Assisted Synthesis of CuSe Nanoparticles for High-Sensitivity Serotonin Biosensing in Serum. Chemosensors 2025, 13, 264. [Google Scholar] [CrossRef]
- Huang, X.; Xu, H.; Cao, D.; Cheng, D. Interface construction of P-Substituted MoS2 as efficient and robust electrocatalyst for alkaline hydrogen evolution reaction. Nano Energy 2020, 78, 105253. [Google Scholar] [CrossRef]
- Chen, W.; Gu, J.; Du, Y.; Song, F.; Bu, F.; Li, J.; Yuan, Y.; Luo, R.; Liu, Q.; Zhang, D. Achieving Rich and Active Alkaline Hydrogen Evolution Heterostructures via Interface Engineering on 2D 1T-MoS2 Quantum Sheets. Adv. Funct. Mater. 2020, 30, 2000551. [Google Scholar] [CrossRef]
- Ren, X.; Yang, F.; Chen, R.; Ren, P.; Wang, Y. Improvement of HER activity for MoS2: Insight into the effect and mechanism of phosphorus post-doping. New J. Chem. 2020, 44, 1493–1499. [Google Scholar] [CrossRef]
- Nayana, K.; Sunitha, A.P. MoS2−x/GCD-MoS2−x nanostructures for tuning the overpotential of Volmer-Heyrovsky reaction of electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 55, 422–431. [Google Scholar] [CrossRef]
- Sheelam, A.; Bell, J.G. Uniform electrodeposition of Pd nanoparticles on MoS2-x nanosheets using magnetic fields for improved mass utilization in hydrogen evolution. Int. J. Hydrogen Energy 2024, 56, 348–357. [Google Scholar] [CrossRef]
- Thomas, S.A.; Roy, N.; Sharaf Saeed, W.; Sreedhar, A.; Cherusseri, J. Hydrothermally synthesized MoS2/ZnS heterostructure with efficient catalytic performance for hydrogen evolution. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Sathiyan, K.; Mondal, T.; Mukherjee, P.; Patra, S.G.; Pitussi, I.; Kornweitz, H.; Bar-Ziv, R.; Zidki, T. Enhancing the catalytic OER performance of MoS2 via Fe and Co doping. Nanoscale 2022, 14, 16148–16155. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Li, W.; Zhou, M.; Gao, W.; Liu, B.; Wei, J.; Zhang, X.; Liu, H.; Liu, Y.; Zeng, X. MoS2 nanosheets grown on nickel chalcogenides: Controllable synthesis and electrocatalytic origins for the hydrogen evolution reaction in alkaline solution. J. Mater. Chem. A 2019, 7, 21514–21522. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Zhang, Y.; Yang, B.; Qi, Q.; Sun, M.; Zi, F.; Leung, M.K.H.; Huang, B. Interface Modulation of MoS2/Metal Oxide Heterostructures for Efficient Hydrogen Evolution Electrocatalysis. Small 2020, 16, 2002212. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Zhang, L. In situ electrochemical metal (Co, Ni) oxide deposition on MoS2 nanosheets for highly efficient electrocatalytic water splitting. New J. Chem. 2023, 47, 4430–4438. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.-M.; Hossain, M.S.A.; Sugahara, Y.; et al. Mesoporous Iron-doped MoS2/CoMo2S4 Heterostructures through Organic–Metal Cooperative Interactions on Spherical Micelles for Electrochemical Water Splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar] [CrossRef]
- Fageria, P.; Sudharshan, K.Y.; Nazir, R.; Basu, M.; Pande, S. Decoration of MoS2 on g-C3N4 surface for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 258, 1273–1283. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, P.; Sun, Y.; Wang, Y.; Li, J.; Guo, J. MoS2 nanosheets on C3N4 realizing improved electrochemical hydrogen evolution. Mater. Lett. 2017, 197, 41–44. [Google Scholar] [CrossRef]
- Khan, M.S.; Noor, T.; Pervaiz, E.; Iqbal, N.; Zaman, N. Fabrication of MoS2/rGO hybrids as electrocatalyst for water splitting applications. RSC Adv. 2024, 14, 12742–12753. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhou, C.; Bai, S.; Yang, S.; Yang, F.; Yang, B.; Zeng, X. Multi-interface and rich-defects 1T phase MoS2 combine with FeS anchored on reduced graphene oxide for efficient alkaline hydrogen evolution. Int. J. Hydrogen Energy 2024, 90, 191–198. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Pan, F.; Khan, M.; Lei, T.; Kamli, M.R.; M Sabir, J.S.; Khan, I.; Ansari, M.Z. Visible light driven hydrogen generation and pollutant degradation with Au loaded 2D/2D heterojunctional nanocomposite of MoS2 and g-C3N4. Int. J. Hydrogen Energy 2024, 51, 1141–1153. [Google Scholar] [CrossRef]
- Azhar, A.; Basit, M.A.; Mehmood, W.; Ali, M.A.; Zahid, S.; Ahmad, M.; Zaidi, S.J.A.; Park, T.J. Synchronized wet-chemical development of 2-dimensional MoS2 and g-C3N4/MoS2 QDs nanocomposite as efficient photocatalysts for detoxification of aqueous dye solutions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130581. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.O.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B Environ. 2018, 239, 334–344. [Google Scholar] [CrossRef]
- He, L.; Cui, B.; Liu, J.; Wang, M.; Zhang, Z.; Zhang, H. Fabrication of Porous CoOx/mC@MoS2 Composite Loaded on g-C3N4 Nanosheets as a Highly Efficient Dual Electrocatalyst for Oxygen Reduction and Hydrogen Evolution Reactions. ACS Sustain. Chem. Eng. 2018, 6, 9257–9268. [Google Scholar] [CrossRef]
- Mehtab, A.; Ali, S.A.; Ingole, P.P.; Mao, Y.; Alshehri, S.M.; Ahmad, T. MoS2 Nanoflower-Deposited g-C3N4 Nanosheet 2D/2D Heterojunction for Efficient Photo/Electrocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2023, 6, 12003–12012. [Google Scholar] [CrossRef]
- Devi, S.B.; Sekar, S.; Kowsuki, K.; Maiyalagan, T.; Preethi, V.; Nirmala, R.; Lee, S.; Navamathavan, R. Graphitic carbon nitride encapsulated sonochemically synthesized β-nickel hydroxide nanocomposites for electrocatalytic hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 40349–40358. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Xiao, S.; Bi, F.; Zhao, L.; Li, Y.; Zhang, X.; Gai, G.; Dong, X. One-step, high-yield synthesis of g-C3N4 nanosheets for enhanced visible light photocatalytic activity. RSC Adv. 2019, 9, 39304–39314. [Google Scholar] [CrossRef]
- Ul Hassan, H.M.; Tawab, S.A.; Khan, M.I.; Hussain, A.; Elsaeedy, H.I.; Almutairi, B.S.; Hassan, A.; Raza, A. Reduce the recombination rate by facile synthesis of MoS2/g-C3N4 heterostructures as a solar light responsive catalyst for organic dye degradation. Diam. Relat. Mater 2023, 140, 110420. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, H.; Bai, K.; Chen, X.; Li, E.; Shen, Y.; Wang, M. Fabrication of a g-C3N4/MoS2 photocatalyst for enhanced RhB degradation. Phys. E Low-Dimens. Syst. Nanostructures 2022, 144, 115361. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wei, T.; Wang, C.; Wan, J.; Tang, Y.; Guo, M.; Ma, Y.; Yang, Y. Boosting the Photocatalytic Performance of g-C3N4 through MoS2 Nanotubes with the Cavity Enhancement Effect. Langmuir 2024, 40, 11160–11172. [Google Scholar] [CrossRef]
- Ryaboshapka, D.; Bargiela, P.; Piccolo, L.; Afanasiev, P. On the electronic properties and catalytic activity of MoS2–C3N4 materials prepared by one-pot reaction. Int. J. Hydrogen Energy 2022, 47, 34012–34024. [Google Scholar] [CrossRef]
- Singh, J.; Akhtar, S.; Tran, T.T.; Kim, J. MoS2 nanoflowers functionalized with C3N4 nanosheets for enhanced photodecomposition. J. Alloys Compd. 2023, 954, 170206. [Google Scholar] [CrossRef]
- Lan, Z.; Yu, Y.; Yao, J.; Cao, Y. The band structure and photocatalytic mechanism of MoS2-modified C3N4 photocatalysts with improved visible photocatalytic activity. Mater. Res. Bull. 2018, 102, 433–439. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Shanmugam, A.; Saravanan, S.; Pugazhendi, I.; Lee, Y.; Kim, D.Y.; Manikandan, R.; Chang, S.-C.; Lee, S. Enhanced bifunctional water electrolysis performance of spherical ZnMn2O4 nanoparticles. Int. J. Hydrogen Energy 2025, 141, 721–728. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Ansari, A.S.; Sree, V.G.; Jana, A.; Meena, A.; Sekar, S.; Cho, S.; Kim, H.; Im, H. Nitrogen-Doped CuO@CuS Core–Shell Structure for Highly Efficient Catalytic OER Application. Nanomaterials 2023, 13, 3160. [Google Scholar] [CrossRef]
- Manikandan, R.; Sekar, S.; Mani, S.P.; Lee, S.; Kim, D.Y.; Saravanan, S. Bismuth tungstate-anchored PEDOT: PSS materials for high performance HER electrocatalyst. Int. J. Hydrogen Energy 2023, 48, 11746–11753. [Google Scholar] [CrossRef]
- Karmakar, A.; Durairaj, M.; Madhu, R.; De, A.; Dhandapani, H.N.; Spencer, M.J.S.; Kundu, S. From Proximity to Energetics: Unveiling the Hidden Compass of Hydrogen Evolution Reaction. ACS Mater. Lett. 2024, 6, 3050–3062. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Natraj, V.; Mohan, V.; Chennakrishnan, J.; Kim, S.J. Unveiling the bi-functional electrocatalytic properties of rhenium di-sulfide nanostructures towards the development of high-rate alkaline water electrolyzer. Chem. Eng. J. 2024, 500, 156356. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Swaminathan, R.; Mohan, V.; Kim, S.-J. Unravelling the Bi-Functional Electrocatalytic Properties of {Mo72Fe30} Polyoxometalate Nanostructures for Overall Water Splitting Using Scanning Electrochemical Microscope and Electrochemical Gating Methods. Adv. Sci. 2024, 11, 2401073. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Ilanchezhiyan, P.; Ahmed, A.T.A.; Lee, Y.; Lee, S. Substantial Electrocatalytic Oxygen Evolution Performances of Activated Carbon-Decorated Vanadium Pentoxide Nanocomposites. Int. J. Energy Res. 2024, 2024, 9953038. [Google Scholar] [CrossRef]
- Dong, G.; Zhou, T.; Zhang, C.; Wang, Y.; Wang, Y.; Shi, W.; Su, X.; Zeng, T.; Chen, Y. Role of Charge Accumulation in MoS2/Graphitic Carbon Nitride Nanostructures for Photocatalytic N2 Fixation. ACS Appl. Nano Mater. 2024, 7, 15406–15415. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Oshima, R.; Maeda, Y.; Nagata, Y. Preparation of Platinum Nanoparticles by Sonochemical Reduction of the Pt (II) Ion. Langmuir 1999, 15, 2733–2737. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Zhang, J.; Du, J.; Han, B.; Liu, Z.; Jiang, T.; Zhang, Z. Sonochemical Formation of Single-Crystalline Gold Nanobelts. Angew. Chem. Int. Ed. 2006, 45, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Nemamcha, A.; Rehspringer, J.-L.; Khatmi, D. Synthesis of Palladium Nanoparticles by Sonochemical Reduction of Palladium (II) Nitrate in Aqueous Solution. J. Phys. Chem. B 2006, 110, 383–387. [Google Scholar] [CrossRef]
- Su, C.-H.; Wu, P.-L.; Yeh, C.-S. Sonochemical Synthesis of Well-Dispersed Gold Nanoparticles at the Ice Temperature. J. Phys. Chem. B 2003, 107, 14240–14243. [Google Scholar] [CrossRef]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent Oxygen Evolution Reaction of Activated Carbon-Anchored NiO Nanotablets Prepared by Green Routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef]
- Zuo, L.-X.; Jiang, L.-P.; Zhu, J.-J. A facile sonochemical route for the synthesis of MoS2/Pd composites for highly efficient oxygen reduction reaction. Ultrason. Sonochem. 2017, 35, 681–688. [Google Scholar] [CrossRef]
- Wang, W.; Kapitanova, O.O.; Ilanchezhiyan, P.; Xi, S.; Panin, G.N.; Fu, D.; Kang, T.W. Self-assembled MoS2/rGO nanocomposites with tunable UV-IR absorption. RSC Adv. 2018, 8, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nakamura, R. Structural change of molybdenum sulfide facilitates the electrocatalytic hydrogen evolution reaction at neutral pH as revealed by in situ Raman spectroscopy. Chin. J. Catal. 2018, 39, 401–406. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.-F.; Alothman, A.A.; Alshgari, R.A.; Ganesh, P.S. Synergetic effect of the ultrasonic-assisted hydrothermal process on the photocatalytic performance of MoS2 and WS2 nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 8858–8867. [Google Scholar] [CrossRef]
- Chen, K.; Feng, H.; Li, L.; Luo, M.; Xue, S.; Sang, J. Synthesis of dual Z-scheme flower-like spherical Ag3PO4/MoS2/g-C3N4 photocatalyst with high photocatalytic performance. J. Mater. Sci. Mater. Electron. 2022, 33, 16077–16098. [Google Scholar] [CrossRef]
- Elavarasan, N.; Palanisamy, G.; Kumar, P.S.; Venkatesh, G.; Vignesh, S.; Bhuvaneswari, K.; Rangasamy, G. Construction of a ternary g-C3N4/MoS2/MWCNTs nanocomposite for the enhanced photocatalytic performance against organic dye. Appl. Nanosci. 2023, 13, 5851–5863. [Google Scholar] [CrossRef]
- Baig, M.M.; Pervaiz, E.; Yang, M.; Gul, I.H. High-Performance Supercapacitor Electrode Obtained by Directly Bonding 2D Materials: Hierarchal MoS2 on Reduced Graphene Oxide. Front. Mater. 2020, 7, 580424. [Google Scholar] [CrossRef]
- Lu, X.; Lin, Y.; Dong, H.; Dai, W.; Chen, X.; Qu, X.; Zhang, X. One-Step Hydrothermal Fabrication of Three-dimensional MoS2 Nanoflower using Polypyrrole as Template for Efficient Hydrogen Evolution Reaction. Sci. Rep. 2017, 7, 42309. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Li, Y.; Yu, H. Hydrothermal synthesis of Co2P-modified MoS2: A highly efficient non-precious metal catalyst of light. Ionics 2019, 25, 5003–5011. [Google Scholar] [CrossRef]
- Wei, Z.; Shen, X.; Ji, Y.; Yang, Z.; Wang, T.; Li, S.; Zhu, M.; Tian, Y. Synthesis of novel MoS2/g-C3N4 nanocomposites for enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 15885–15895. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Q.; Zhou, Y.; Wan, J.; Hu, Z. Preparation and high-efficient hydrogen evolution reaction of hydrangea-like MoS2 hollow microspheres modified by needle-like g-C3N4. J. Mater. Sci. Mater. Electron. 2019, 30, 16446–16451. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, J.; Gao, Q.; Lei, H. A 2H-MoS2/carbon cloth composite for high-performance all-solid-state supercapacitors derived from a molybdenum dithiocarbamate complex. Dalton Trans. 2021, 50, 11954–11964. [Google Scholar] [CrossRef]
- Li, N.; Zhou, J.; Sheng, Z.; Xiao, W. Molten salt-mediated formation of g-C3N4-MoS2 for visible-light-driven photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 430, 218–224. [Google Scholar] [CrossRef]
- Archana, C.; Abinaya, R.; Mani, N.; Santhana Krishnan, H. Strain Effect in the Layered MoS2-rGO Heterostructure with Enhanced Performance for Flexible Thermoelectric Applications. ACS Appl. Energy Mater. 2025, 8, 1589–1597. [Google Scholar]
- Cao, Y.; Gao, Q.; Li, Q.; Jing, X.; Wang, S.; Wang, W. Synthesis of 3D porous MoS2/g-C3N4 heterojunction as a high efficiency photocatalyst for boosting H2 evolution activity. RSC Adv. 2017, 7, 40727–40733. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.I.; Keegan, M.; van den Bosch, B.; Yan, N.; Rothenberg, G. The Influence of Metal Impurities on NiOOH Electrocatalytic Activity in the Oxygen Evolution Reaction. ChemElectroChem 2024, 11, e202400223. [Google Scholar] [CrossRef]
- Dhanda, M.; Arora, R.; Yadav, M.; Pahuja, P.; Ahlawat, S.; Nehra, S.P.; Lata, S. Calibration of varying MoS2 amount with dual fabrication GCN/CQDs for supercapacitor’s electrode and strengthening of the energy parameters. Mater. Sci. Eng. B 2024, 300, 117055. [Google Scholar] [CrossRef]
- Wang, Y.; Williams, T.; Gengenbach, T.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Unique hybrid Ni2P/MoO2@MoS2 nanomaterials as bifunctional non-noble-metal electro-catalysts for water splitting. Nanoscale 2017, 9, 17349–17356. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, S.; Liu, Y.; Gao, N.; Wang, G.; Lian, J.; Jiang, Q. Charge Storage by Electrochemical Reaction of Water Bilayers Absorbed on MoS2 Monolayers. Sci. Rep. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Bai, J.; Lv, W.; Ni, Z.; Wang, Z.; Chen, G.; Xu, H.; Qin, H.; Zheng, Z.; Li, X. Integrating MoS2 on sulfur-doped porous g-C3N4 iostype heterojunction hybrids enhances visible-light photocatalytic performance. J. Alloys Compd. 2018, 768, 766–774. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Gao, L.-J.; Ren, J.-T.; Yuan, Z.-Y. Engineering g-C3N4 based materials for advanced photocatalysis: Recent advances. Green Energy Environ. 2024, 9, 166–197. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Wen, Z.; Cui, S.; Yuan, C.; Chen, J. N-doped graphene/porous g-C3N4 nanosheets supported layered-MoS2 hybrid as robust anode materials for lithium-ion batteries. Nano Energy 2014, 8, 157–164. [Google Scholar] [CrossRef]
- Ling, G.Z.S.; Oh, V.B.-Y.; Haw, C.Y.; Tan, L.-L.; Ong, W.-J. g-C3N4 Photocatalysts: Utilizing Electron–Hole Pairs for Boosted Redox Capability in Water Splitting. Energy Mater. Adv. 2023, 4, 0038. [Google Scholar] [CrossRef]
- Ghanashyam, G.; Jeong, H.K. Size Effects of MoS2 on Hydrogen and Oxygen Evolution Reaction. J. Electrochem. Sci. Technol. 2022, 13, 120–127. [Google Scholar] [CrossRef]
- Sekar, S.; Sim, D.H.; Lee, S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials 2022, 12, 531. [Google Scholar] [CrossRef]
- Chen, B.; Hu, P.; Yang, F.; Hua, X.; Yang, F.F.; Zhu, F.; Sun, R.; Hao, K.; Wang, K.; Yin, Z. In Situ Porousized MoS2 Nano Islands Enhance HER/OER Bifunctional Electrocatalysis. Small 2023, 19, 2207177. [Google Scholar] [CrossRef]
- Sadighi, Z.; Liu, J.; Zhao, L.; Ciucci, F.; Kim, J.-K. Metallic MoS2 nanosheets: Multifunctional electrocatalyst for the ORR, OER and Li–O2 batteries. Nanoscale 2018, 10, 22549–22559. [Google Scholar] [CrossRef]
- Sekar, S.; Yun, J.-S.; Park, S.; Kim, D.Y.; Lee, Y.; Lee, S. Excellent Bifunctional Water Electrolysis Activities of α-MoO3/AC Nanocomposites. Int. J. Energy Res. 2024, 2024, 3167699. [Google Scholar] [CrossRef]
- Ahmed, I.; Biswas, R.; Patil, R.A.; Halder, K.K.; Singh, H.; Banerjee, B.; Kumar, B.; Ma, Y.-R.; Haldar, K.K. Graphitic Carbon Nitride Composites with MoO3-Decorated Co3O4 Nanorods as Catalysts for Oxygen and Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 12672–12681. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R. Constructing MoS2/g-C3N4 heterojunction with enhanced oxygen evolution reaction activity: A theoretical insight. Appl. Surf. Sci. 2020, 510, 145489. [Google Scholar] [CrossRef]
- Cheng, P.; Yuan, C.; Zhou, Q.; Hu, X.; Li, J.; Lin, X.; Wang, X.; Jin, M.; Shui, L.; Gao, X.; et al. Core–Shell MoS2@CoO Electrocatalyst for Water Splitting in Neural and Alkaline Solutions. J. Phys. Chem. C 2019, 123, 5833–5839. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Huang, H.; Wang, C.; Gao, L.; Ma, T. One-dimensional MoO2–Co2Mo3O8@C nanorods: A novel and highly efficient oxygen evolution reaction catalyst derived from metal–organic framework composites. Chem. Commun. 2018, 54, 2739–2742. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yu, Z.G.; Seng, H.L.; Zhang, N.; Liu, X.; Zhang, Y.-W.; Yang, W.; Gong, H. Simultaneous edge and electronic control of MoS2 nanosheets through Fe doping for an efficient oxygen evolution reaction. Nanoscale 2018, 10, 20113–20119. [Google Scholar] [CrossRef]
- Kwon, I.S.; Debela, T.T.; Kwak, I.H.; Park, Y.C.; Seo, J.; Shim, J.Y.; Yoo, S.J.; Kim, J.-G.; Park, J.; Kang, H.S. Ruthenium Nanoparticles on Cobalt-Doped 1T′ Phase MoS2 Nanosheets for Overall Water Splitting. Small 2020, 16, 2000081. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Wang, J.; Da, Y.; Zhang, J.; Li, L.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Sequential Electrodeposition of Bifunctional Catalytically Active Structures in MoO3/Ni–NiO Composite Electrocatalysts for Selective Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 2003414. [Google Scholar] [CrossRef]
- Ji, X.; Lin, Y.; Zeng, J.; Ren, Z.; Lin, Z.; Mu, Y.; Qiu, Y.; Yu, J. Graphene/MoS2/FeCoNi(OH)x and Graphene/MoS2/FeCoNiPx multilayer-stacked vertical nanosheets on carbon fibers for highly efficient overall water splitting. Nat. Commun. 2021, 12, 1380. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Tran, D.T.; Doan, T.L.L.; Kim, D.H.; Kim, N.H.; Lee, J.H. Rational Design of Core@shell Structured CoS@Cu2MoS4 Hybridized MoS2/N,S-Codoped Graphene as Advanced Electrocatalyst for Water Splitting and Zn-Air Battery. Adv. Energy Mater. 2020, 10, 1903289. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Doan, T.L.L.; Nguyen, D.C.; Prabhakaran, S.; Kim, D.H.; Tran, D.T.; Kim, N.H.; Lee, J.H. Single-Atom Co-Decorated MoS2 Nanosheets Assembled on Metal Nitride Nanorod Arrays as an Efficient Bifunctional Electrocatalyst for pH-Universal Water Splitting. Adv. Funct. Mater. 2021, 31, 2100233. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Ding, H.; He, L.; Wang, E.; Wang, B.; Fan, S.; Liu, K. A lightly Fe-doped (NiS2/MoS2)/carbon nanotube hybrid electrocatalyst film with laser-drilled micropores for stabilized overall water splitting and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 17527–17536. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, Q.; Li, W.; Li, J.; Fu, X.; Cerqueira, M.F.; Alpuim, P.; Liu, L. Atomic-layer-deposited ultrafine MoS2 nanocrystals on cobalt foam for efficient and stable electrochemical oxygen evolution. Nanoscale 2017, 9, 2711–2717. [Google Scholar] [CrossRef]
- Denisdon, S.; Senthil Kumar, P.; Rangasamy, G. High-Performance NiO/GCN Nanohybrids Synthesized under Different Thermal Conditions for Efficient Electrocatalytic Oxygen Evolution Reaction. Ind. Eng. Chem. Res. 2024, 63, 6951–6959. [Google Scholar] [CrossRef]
- Zabielaite, A.; Balciunaite, A.; Upskuviene, D.; Simkunaite, D.; Levinas, R.; Niaura, G.; Vaiciuniene, J.; Jasulaitiene, V.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Investigation of Hydrogen and Oxygen Evolution on Cobalt-Nanoparticles-Supported Graphitic Carbon Nitride. Materials 2023, 16, 5923. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Cao, T.; Xia, T.; Wu, C.; Feng, M.; Li, X.; Mei, Z.; Gao, H.; Huo, D.; Ren, X.; et al. MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting. Nanomaterials 2023, 13, 752. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-Rich Ultrathin Cobalt–Iron Layered Double Hydroxide for Electrochemical Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Nangai, E.K.; Saravanan, S.; Kim, D.Y.; Lee, S. Enhanced Hydrogen Evolution Reaction Performances of Ultrathin CuBi2O4 Nanoflakes. Int. J. Energy Res. 2023, 2023, 5038466. [Google Scholar] [CrossRef]
- Kashif, M.; Thangarasu, S.; Oh, T.-H. Enriching the active sites of nanosheets assembled as flower-like MoS2 microstructure by controllable morphology for electrochemical hydrogen evolution reaction. Int. J. Hydrog. Energy 2024, 82, 47–52. [Google Scholar] [CrossRef]
- Srividhya, G.; Viswanathan, C.; Ponpandian, N. Interfacing NiV layered double hydroxide with sulphur-doped g-C3N4 as a novel electrocatalyst for enhanced hydrogen evolution reaction through Volmer–Heyrovský mechanism. Energy Adv. 2023, 2, 1464–1475. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tekalgne, M.; Tran, C.V.; Nguyen, T.P.; Dao, V.; Le, Q.V.; Ahn, S.H.; Kim, S.Y. Synthesis of MoS2/WS2 Nanoflower Heterostructures for Hydrogen Evolution Reaction. Int. J. Energy Res. 2024, 2024, 3192642. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, M.; Sheng, W.; Yan, Y. Hollow Chevrel-Phase NiMo3S4 for Hydrogen Evolution in Alkaline Electrolytes. Angew. Chem. Int. Ed. 2016, 55, 15240–15245. [Google Scholar] [CrossRef]
- Ji, S.; Guo, J.; Yang, N.; Song, J.; Wang, Y.; Xing, G. Improvement of hydrogen evolution catalytic performance of MoS2 nanoflowers by constructing MoS2/MXene Ti3C2 heterostructure petals. Funct. Mater. Lett. 2024, 17, 2451011. [Google Scholar] [CrossRef]
- Feng, Y.-Y.; Deng, G.; Wang, X.-Y.; Zhu, M.; Bian, Q.-N.; Guo, B.-S. MoS2/NiFeS2 heterostructure as a highly efficient electrocatalyst for overall water splitting at high current densities. Int. J. Hydrogen Energy 2023, 48, 12354–12363. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ali, S.; Zada, A.; Ilyas, N.; Shaha, R.; Wang, Y.; Qiao, L. Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion. Mater. Des. 2021, 204, 109674. [Google Scholar]
- Zhou, F.; Zhang, X.; Sa, R.; Zhang, S.; Wen, Z.; Wang, R. The electrochemical overall water splitting promoted by MoS2 in coupled nickel–iron (oxy)hydride/molybdenum sulfide/graphene composite. J. Chem. Eng. 2020, 397, 125454. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface Engineering of MoS2/Ni3S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Ed. Angew. Chem. Int. Ed. 2016, 55, 6702–6707. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Li, S.; Zhou, L.; Li, Z.; Li, J.; Shao, M. Interface engineering of (Ni, Fe)S2@MoS2 heterostructures for synergetic electrochemical water splitting. Appl. Catal. B 2019, 247, 107–114. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, K.; Fu, X.; Guan, S.; Li, X.; Peng, Z. Novel three-dimensional Ni2P-MoS2 heteronanosheet arrays for highly efficient electrochemical overall water splitting. J. Alloys Compd. 2021, 856, 158094. [Google Scholar] [CrossRef]
- Mekete Meshesha, M.; Gautam, J.; Chanda, D.; Gwon Jang, S.; Lyong Yang, B. Enhancing the electrochemical activity of zinc cobalt sulfide via heterojunction with MoS2 metal phase for overall water splitting. J. Alloys Compd. 2023, 652, 272–284. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Wan, H.; Ji, X.; Xu, K.; Zha, D.; Miao, L.; Jiang, J. Mutually beneficial Co3O4@MoS2 heterostructures as a highly efficient bifunctional catalyst for electrochemical overall water splitting. J. Mater. Chem. A 2018, 6, 2067–2072. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.-C.; Cheng, F.; Xi, P. Epitaxial Heterogeneous Interfaces on N-NiMoO4/NiS2 Nanowires/Nanosheets to Boost Hydrogen and Oxygen Production for Overall Water Splitting. Adv. Funct. Mater. 2019, 29, 1805298. [Google Scholar] [CrossRef]
- Banti, B.F.; Goddati, M.; Nwaji, N.; Gwak, J.; Gicha, B.B.; Kang, H.; Asgaran, S.; Chun, H.-J.; Lee, J. Defect Engineered Ru-CoMOF@MoS2 Heterointerface Facilitate Water Oxidation Process. ChemSusChem 2024, e202402533. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Shanmugam, A.; Lee, Y.; Lee, S. Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis. Materials 2025, 18, 3775. https://doi.org/10.3390/ma18163775
Sekar S, Shanmugam A, Lee Y, Lee S. Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis. Materials. 2025; 18(16):3775. https://doi.org/10.3390/ma18163775
Chicago/Turabian StyleSekar, Sankar, Atsaya Shanmugam, Youngmin Lee, and Sejoon Lee. 2025. "Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis" Materials 18, no. 16: 3775. https://doi.org/10.3390/ma18163775
APA StyleSekar, S., Shanmugam, A., Lee, Y., & Lee, S. (2025). Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis. Materials, 18(16), 3775. https://doi.org/10.3390/ma18163775







