Efficiency of Spirulina sp. in the Treatment of Model Wastewater Containing Ni(II) and Pb(II)
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Biomass
2.2. Characteristics of Algae Biomass
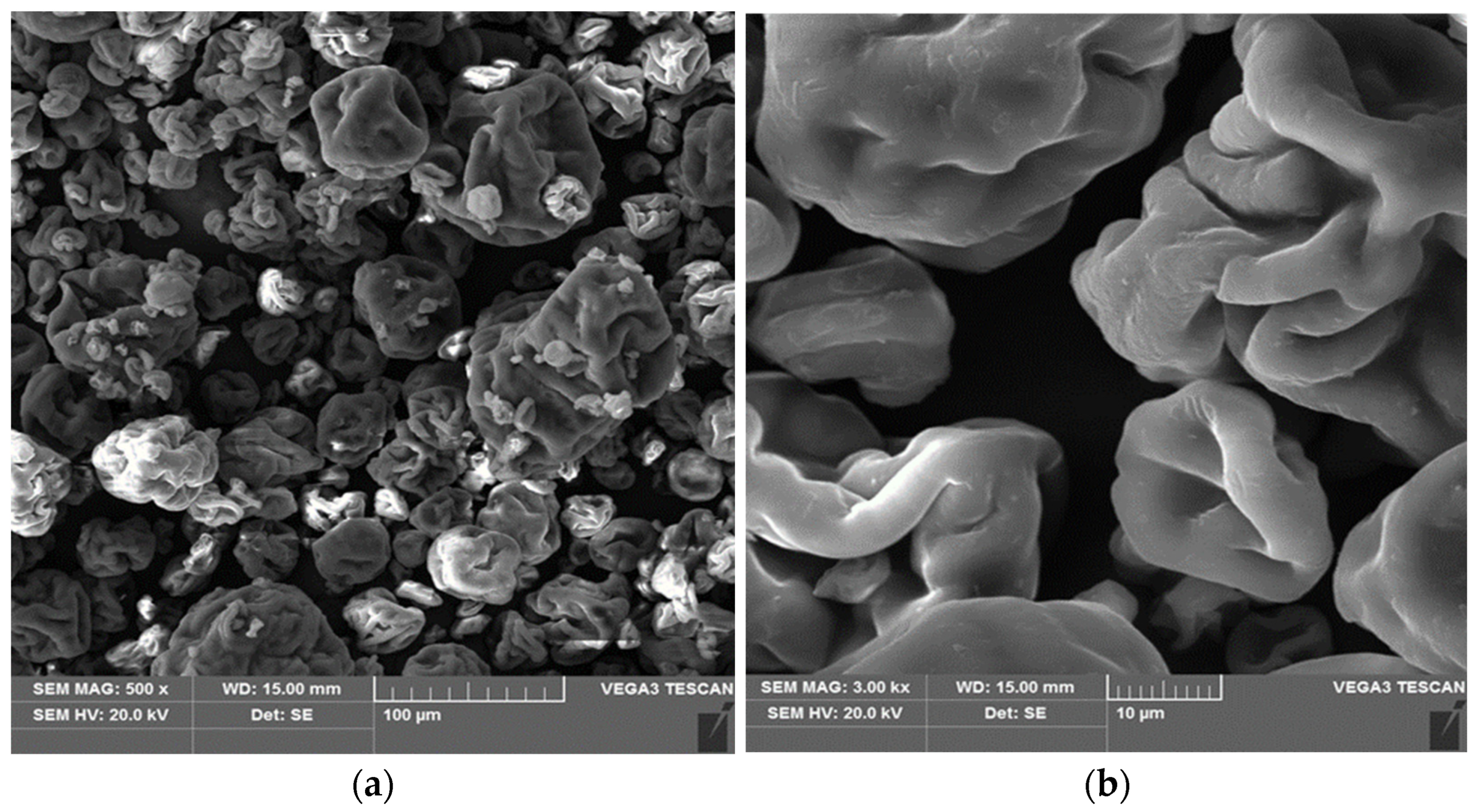
2.2.1. Analysis of Surface Morphology and Chemical Composition of the Biosorbent
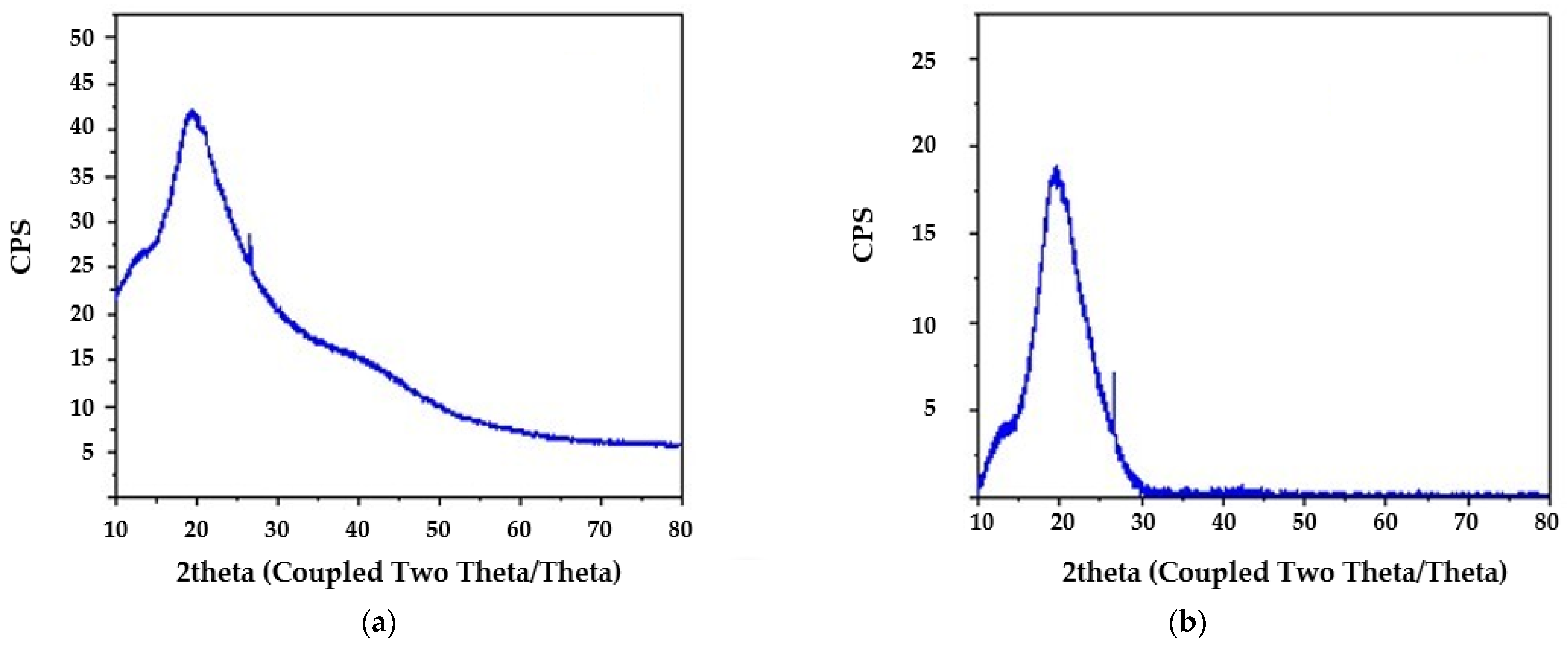
2.2.2. X-Ray Diffraction Analysis (XRD)
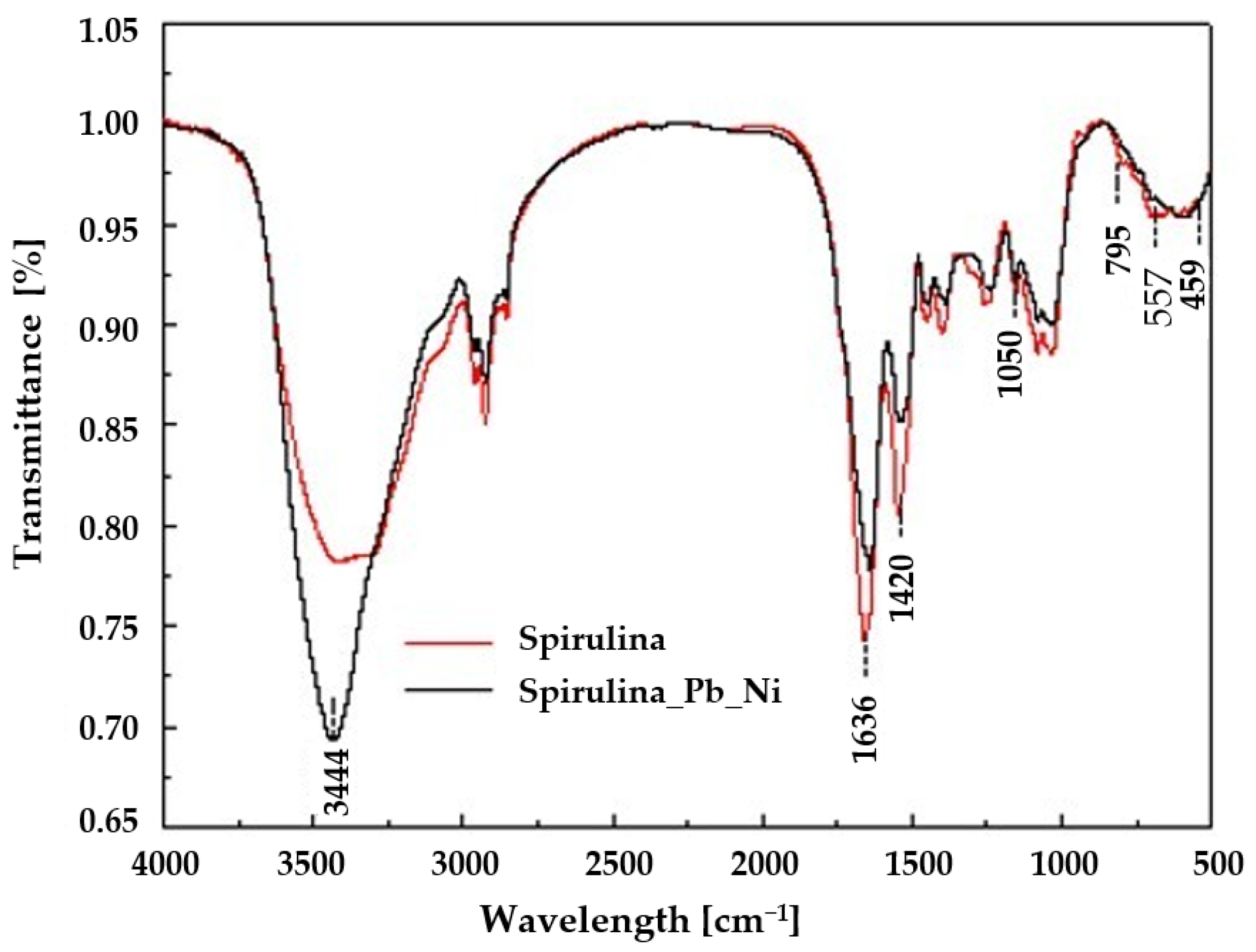
2.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
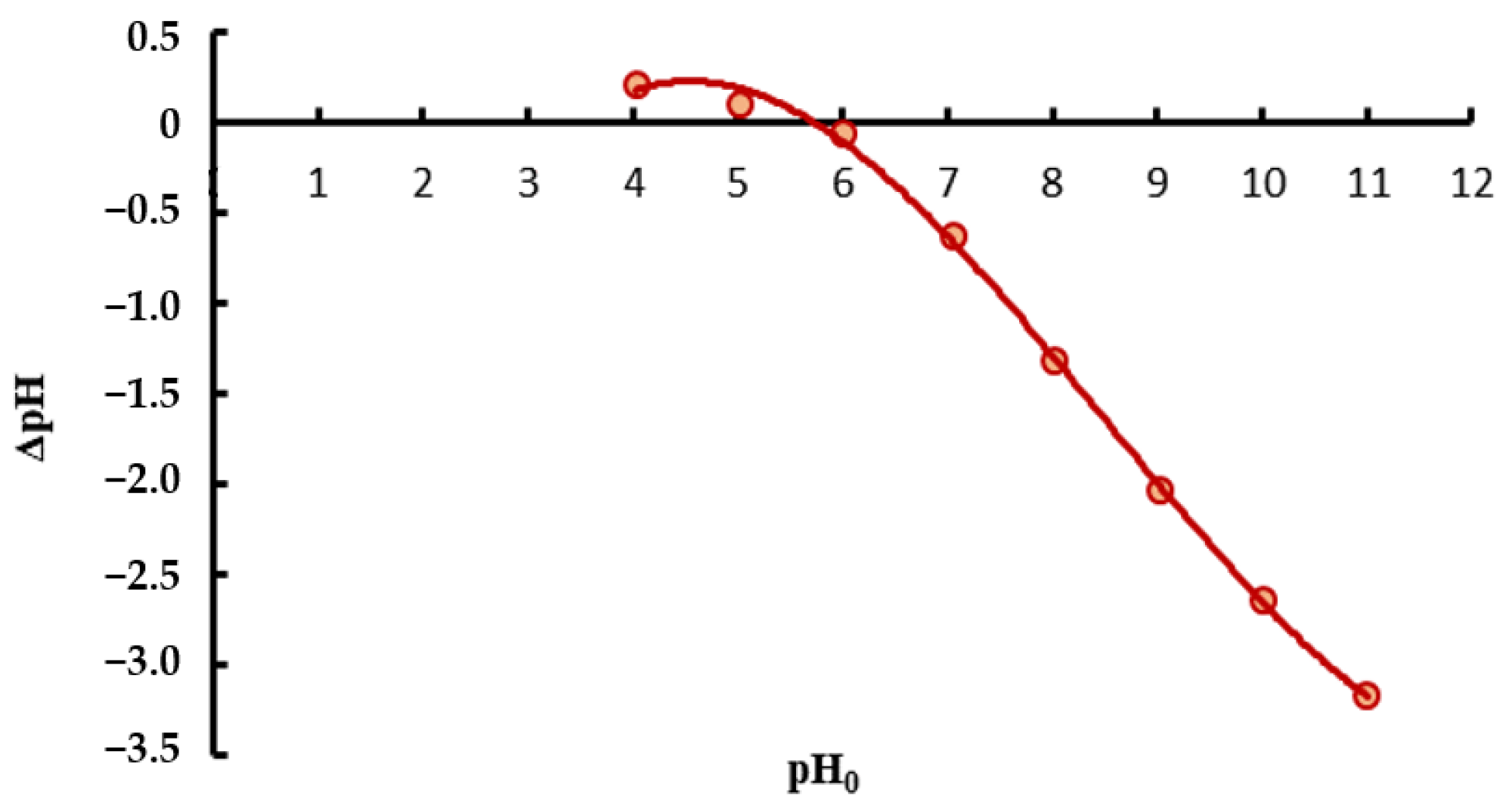
2.2.4. Point of Zero Charge Analysis
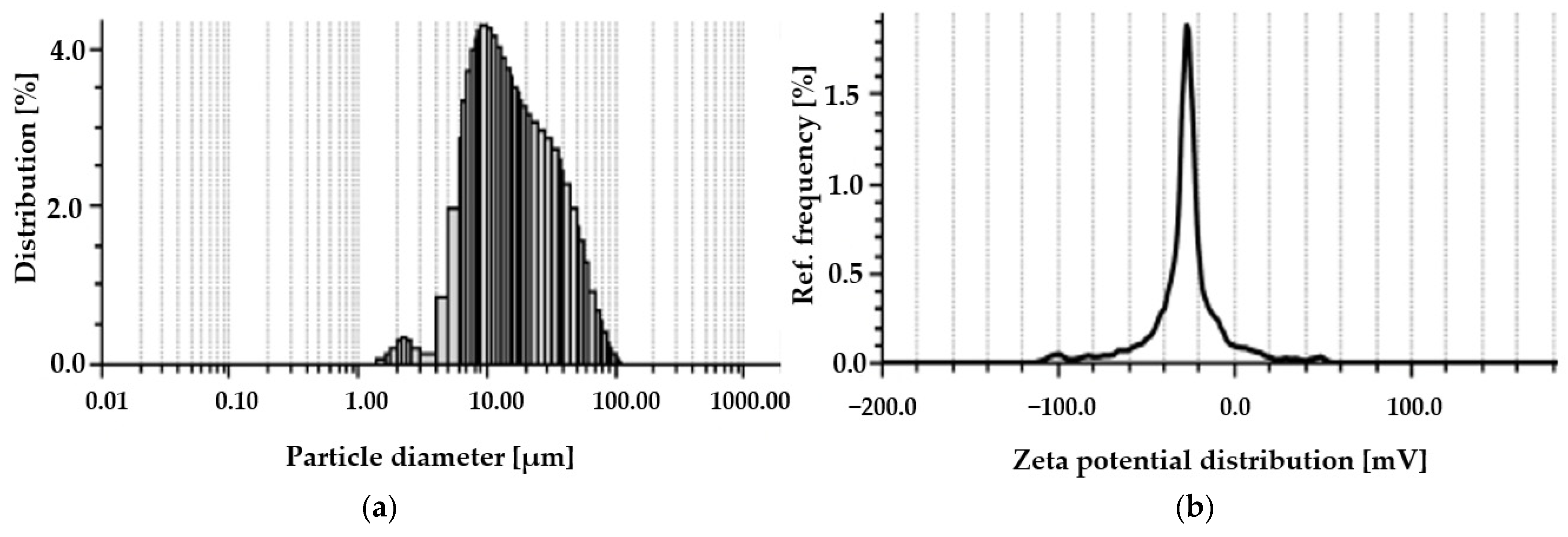
2.2.5. Particle Size and Zeta Potential Analysis
2.3. Metal Ions Speciation Analysis
2.4. Batch Adsorption Studies
2.5. Equilibrium Isotherm Studies for Adsorption
2.6. Adsorption Kinetics Analysis
3. Results and Discussion
3.1. Characteristics of Spirulina sp.
3.2. Batch Adsorption Experiments
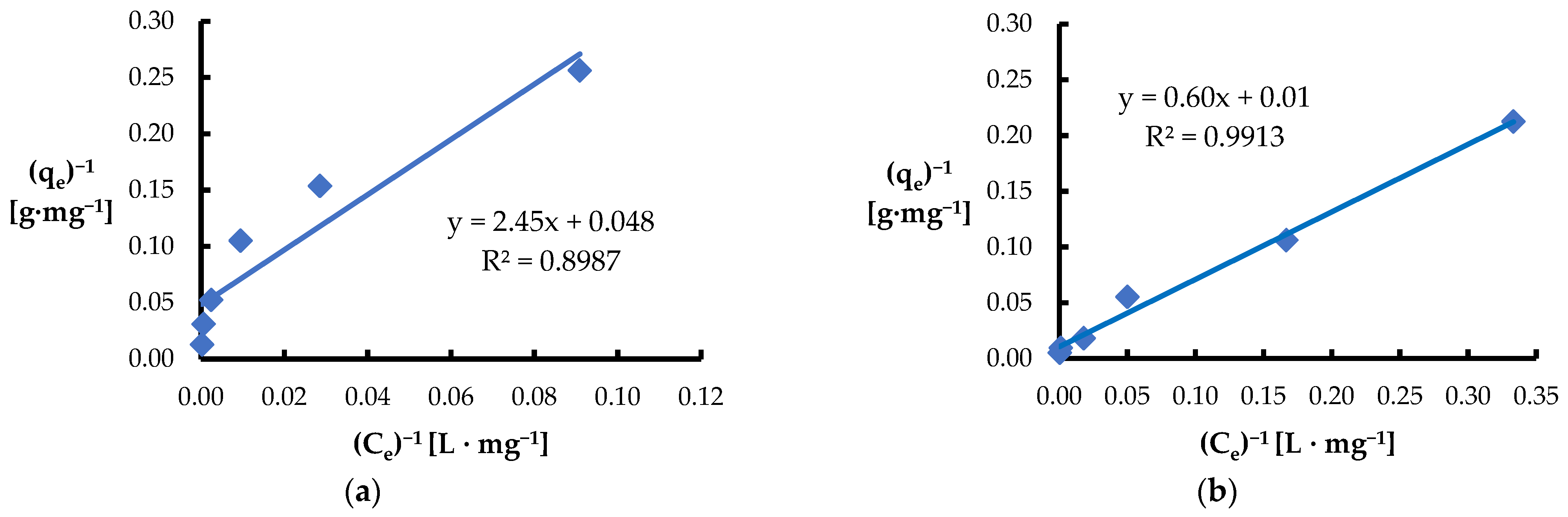
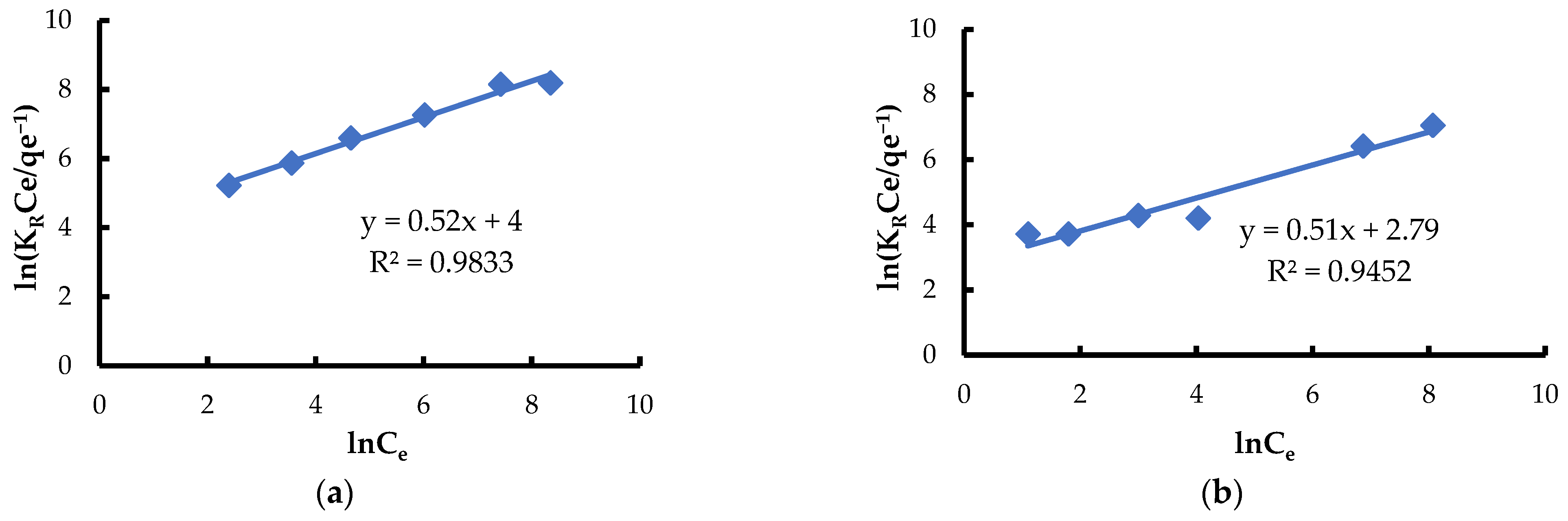
3.3. Equilibrium Adsorption
3.4. Kinetic Adsorption
| Heavy Metals | Adsorption Capacity | Experimental Conditions | |
|---|---|---|---|
| Ni(II) Pb(II) | 20.8 mg·g−1 93.5 mg·g−1, | Spirulina sp. dry biomass; pH: 6 and 20 °C; Contact time: 120 min; Biomass dosage: 10 g·L−1; Initial concentration: 50–5000 mg·L−1 | [this study] |
| Cd | 98 mg·g−1 | Spirulina (Arthrospira) platensis TISTR 8217 dry biomass; pH: 7 and 26 °C; Contact time: 30 min (equilibrium reached); Biomass dosage: 0.5 g·L−1 | [35] |
| Cr(III) | 185 mg·g−1 | Spirulina sp. (Lyophilized form–L and Photoautotrophic form–A); pH: 7 and 35 °C; Contact time: 30 min (kinetic equilibrium reached within ca. 10 min); Biomass dosage: 1 g·L−1 | [36] |
| Cd(II) | 159 mg·g−1 | ||
| Cu(II) | 196 mg·g−1 | ||
| Cd(II) | 625 mg·g−1 | Live Spirulina sp. and Dead Spirulina sp. (sun dried); pH: 6.0 and 35–38 °C; Contact time: About 960 min (for final reading, kinetics measured up to 240 min); Biomass dosage: 12 g·L−1 | [61] |
| Cd(II) | 355 mg·g−1 | ||
| Cr(VI) | 189 mg·g−1 | Fresh Spirulina platensis (dried at 60 °C) and Spent Spirulina platensis biomass (after b-carotene extraction with acetone, dried at 60 °C); pH: 1.5 and 25 °C; Contact time: kinetics studied up to 600 min; Biomass dosage: 1.0 g·L−1 | [37] |
| Cr(VI) | 213 mg·g−1 | ||
| Cr(VI) | 99% removal | Spirulina platensis nanoparticles (prepared by mechanical agitation at 10,000 rpm, 20 min; mean diameter 215.6 nm, PDI 0.151); pH: 4 and 25 °C; Contact time: Agitated until equilibrium; Initial concentration: 250 mg· L−1; Biomass dosage: 1.0 g·L−1 | [38] |
| Pb(II) | 253 mg·g−1 | Vacuum freeze-dried Spirulina platensis (Vsp), prepared at <10 Pa and ~60 °C for 12 h after pre-freezing at ~80 °C; pH: 5.0 and 25 °C; Contact time: 720 min; Initial concentration: from 0 to 100 mg·L−1; Biomass dosage: 0.5 g·L−1 | [39] |
| As(V) | 25 mg·g−1 | Spirulina platensis modified with 0.5 mol/L ZnCl2 for 24 h at 25 °C, dried at 80 °C, sieved (<500 µm); Research conducted in the binary system: As (V) and Cd(II); pH: 6.0 and 25 °C; Contact time: 480 min; Initial concentration: from 10 to 300 mg·L−1; Biomass dosage: 4 g·L−1 | [40] |
| Cd(II) | 29 mg·g−1 | ||
| Zn(II) | 51 mg·g−1 | Arthrospira (Spirulina) platensis biomass dried at 60 °C and ground into powder; pH: 6 and 25 °C; Contact time: 60 min; Initial concentration: from 20 to 100 mg·L−1; Biomass dosage: 3 g·L−1 | [41] |
| Cr(VI) | 4 mg·g−1 | Spirulina platensis modified with 5% Na2CO3, 5% KCl, and 5% Na2CO3, dried at 80 °C, grounded, sieved (<10 mm); Research conducted in the binary system: Cr(VI) and Cu(II), Fe(II) and Cr(VI), Cu (II) and Cr (VI), Ni (II) and Cr (VI); Initial concentration: from 25 to 150 mg·L−1; pH: 4 and 25 °C; Biomass dosage: 6 g·L−1; Contact time: 120 min | [42] |
| Fe(II) | 18 mg·g−1 | ||
| Cu(II) | 6 mg·g−1 | ||
| Ni(II) | 12 mg·g−1 | ||
| Pb(II) | 88 mg·g−1 | Spirulina platensis immobilized in alginate beads (S.P@Ca-SA), prepared with 2% S. platensis, 2% SA, 4% CaCl2; pH: 5 and 25 °C; Contact time: Equilibrium time, within 6 h; Biomass dosage: 1, 5, and 10 g·L−1 | [101] |
| Pb(II) | 115 mg·g−1 | Spirulina (Free biomass); pH 5.2 and 25 °C; Contact time: 72 h; Initial concentration: 0.6 to 5.6 mg· L−1; Biomass dosage: 50 mg·L−1 | [102] |
| Pb(II) | 282 mg·g−1 | Spirulina (Immobilized on alginate); pH: 5.2 and 25 °C; Contact time: Within 360 min (in fixed-bed column); Initial concentration: Varied for isotherm (20 to 150 mg·L−1), varied for column (4 to 20 mg·L−1) | |
| Pb | 0.6 mg/105 alga cells | Spirulina (Live biomass); Contact time: Equilibrium within 1440 min (rapid adsorption in first 0–12 min); Temperature: 25 °C; Initial concentration: from 10 to 50 mg·L−1 | [103] |
| Zr(IV) | 110 mg·g−1 | Spirulina platensis (Dry biomass); pH 2 and 25 °C; Contact time: 35 min; Initial concentration: 150 mg·L−1; Biomass dosage: 2.5 g·L−1 | [104] |
| Cr(III) Mn(II) Mg(II) | 45 mg·g−1 44 mg·g−1 42 mg·g−1 | Raw Arthrospira (Spirulina) platensis biomass; pH: 5; Biomass dosage: 1 g· L−1; Equilibrium time: ~50 min; Equilibrium conditions tested for concentrations 10–300 mg·L−1 | [105] |
| Material | Adsorption Capacity | Experimental Conditions | |
|---|---|---|---|
| SYNTHETIC ADSORBENTS | |||
| Diethylene triamine penta (methylene phosphonic acid) functionalized magnetic nanoadsorbent Fe3O4@SiO2-DTPMP | 13.28 mg·g−1 (Pb) 8.56 mg·g−1 (Ni) | For Pb at pH 6, for Ni at pH 5; Temperature 25 °C; Biomass dosage: 1 g·L−1; Initial concentration: 10–50 mg·L−1 | [97] |
| Semicarbazide modified poly(methylmethacrylate) | 32.36 mg·g−1 (Pb) 11.53 mg·g−1 (Ni) | pH 5.0; Temperature 25 °C; Biomass dosage: 1,5 g·L−1 (Pb), 1.0 g·L−1 (Ni); Initial concentration: 10–60 mg·L−1 | [98] |
| BIOADSORBENTS | |||
| Spirulina platensis dry biomass | 20.8 mg·g−1 (Ni) 93.5 mg·g−1 (Pb) | pH: 6; Temperature 20 °C; Contact time: 120 min Biomass dosage: 10 g·L−1; Initial concentration: 50–5000 mg·L−1 | [This study] |
| Bioadsorbents composed of Cannabis sativa L. leaves extract and fibers | 15.384 mg·g−1 (Pb) 17.137 mg·g−1 (Ni) | Temperature 25 °C; Biomass dosage: 0.33–0.25 g·L−1; Initial concentration: 4–8 mg·L−1 | [94] |
| Sugarcane bagasse | 44.053 mg·g−1 (Pb) 56.818 mg·g−1 (Ni) | pH 5.0; Temperature 25 °C; Biomass dosage: 0.1 g·L−1; Initial concentration: 2–60 mg·L−1; Contact time: 60 min | [106] |
| Sargassum filipendula-brown alga | 367.9 mg·g−1 (Pb) 34.3 mg·g−1 (Ni) | pH 5.0; Temperature 25 °C; Biomass dosage: 2 g·L−1; Initial concentration: 50–150 mg·L−1; Contact time: 85 min | [107] |
| Milkweed fibers | 18.79 mg·g−1 (Pb) 3.67 mg·g−1 (Ni) | pH 6.0; Temperature 25 °C; Biomass dosage: 2 g·L−1; Initial concentration: 5–80 mg·L−1 | [95] |
| Biochar produced from Eucalyptus camdulensis sawdust | 193.95 mg·g−1 (Pb) 55.21 mg·g−1 (Ni) | pH 6.0; Temperature 25 °C; Biomass dosage: 0.4 g·L−1 (Pb) and 0.2 g·L−1 (Ni); Initial concentration: 20 mg·L−1 (Pb) and 40 mg·L−1 (Ni) | [108] |
| Cocoa (Theobroma cacao L.) pod husks | 25.2 mg·g−1 (Pb) 14.31 mg·g−1 (Ni) | pH 6.0; Temperature 25 °C; Initial concentration: 100 mg·L−1 (Pb) and 40 mg·L−1 (Ni); Fixed-Bed Column Study (Bed depth: 4 and 7.5 cm; diameter of 6.6 cm; flow rate of 1 mL/s) | [96] |
| MINERAL ADSORBENTS | |||
| Alkali-activated Egyptian calcium bentonite | 13.0 mg·g−1 (Pb) 12.2 mg·g−1 (Ni) | pH 7.0; Temperature 20 °C; Biomass dosage: 1 g·L−1; Initial concentration: 10–50 mg·L−1; Contact time: 120 min | [109] |
| Manganese oxides (α-MnO2, β-MnO2, γ-MnO2, δ-MnO2, λ-MnO2) | 40.3 ÷299.2 mg·g−1 (Pb) | pH 4.0; room temperature; Biomass dosage: 0.5 g·L−1; Initial concentration: 45 mg·L−1 for α-, β-, γ-, and λ-MnO2, and 150 mg·L−1 for δ-MnO2 | [110] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musah, B.I.; Wan, P.; Xu, Y.; Liang, C.; Peng, L. Biosorption of Chromium (VI) and Iron (II) by Acid-Based Modified Chlorella Vulgaris and Spirulina Platensis: Isotherms and Thermodynamics. Int. J. Environ. Sci. Technol. 2022, 19, 11087–11102. [Google Scholar] [CrossRef]
- Kunoh, T.; Nakanishi, M.; Kusano, Y.; Itadani, A.; Ando, K.; Matsumoto, S.; Tamura, K.; Kunoh, H.; Takada, J. Biosorption of Metal Elements by Exopolymer Nanofibrils Excreted from Leptothrix Cells. Water Res. 2017, 122, 139–147. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Wang, W.; Li, Y.; Chang, W.; Zhou, A.; Mu, R. Heavy Metal Distribution Characteristics, Water Quality Evaluation, and Health Risk Evaluation of Surface Water in Abandoned Multi-Year Pyrite Mine Area. Water 2023, 15, 3138. [Google Scholar] [CrossRef]
- Bhuyan, M.d.S.; Haider, S.M.B.; Meraj, G.; Bakar, M.A.; Islam, M.d.T.; Kunda, M.; Siddique, M.d.A.B.; Ali, M.M.; Mustary, S.; Mojumder, I.A.; et al. Assessment of Heavy Metal Contamination in Beach Sediments of Eastern St. Martin’s Island, Bangladesh: Implications for Environmental and Human Health Risks. Water 2023, 15, 2494. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Li, J.; Gao, X.; Zhang, J.; Liu, Z. Ecological and Health Risk Assessments and Water Quality Criteria of Heavy Metals in the Haihe River. Environ. Pollut. 2021, 290, 117971. [Google Scholar] [CrossRef]
- Rezaei, H. Biosorption of Chromium by Using Spirulina Sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Ali, I. The Quest for Active Carbon Adsorbent Substitutes: Inexpensive Adsorbents for Toxic Metal Ions Removal from Wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Pagnanelli, F.; Cibati, A.; Uccelletti, D.; Palleschi, C.; Toro, L. Biotreatment and Bioassessment of Heavy Metal Removal by Sulphate Reducing Bacteria in Fixed Bed Reactors. Water Res. 2010, 44, 151–158. [Google Scholar] [CrossRef]
- Wang, W.; Lu, N.; Pan, H.; Wang, Z.; Han, X.; Zhu, Z.; Guan, J. Heavy Metal Pollution and Its Prior Pollution Source Identification in Agricultural Soil: A Case Study in the Qianguo Irrigation District, Northeast China. Sustainability 2022, 14, 4494. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłońska, J. Pollution Assessment and Spatial Distribution of Heavy Metals in Surface Waters and Bottom Sediments of the Krzna River (Poland). Water 2024, 16, 1008. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy Metal Pollution in the Aquatic Environment: Efficient and Low-Cost Removal Approaches to Eliminate Their Toxicity: A Review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, K.; Kamala, K.; Ramasamy, P.; Musthafa, M.S.; Almujri, S.S.; Asdaq, S.M.B.; Sivaperumal, P. Unveiling the Silent Threat: Heavy Metal Toxicity Devastating Impact on Aquatic Organisms and DNA Damage. Mar. Pollut. Bull. 2024, 200, 116139. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Butkovskyi, A.; Leal, L.H.; Zeeman, G.; Rijnaarts, H.H.M. Micropollutants in Source Separated Wastewater Streams and Recovered Resources of Source Separated Sanitation. Environ. Res. 2017, 156, 434–442. [Google Scholar] [CrossRef]
- Daud, M.K.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W.; et al. Drinking Water Quality Status and Contamination in Pakistan. BioMed Res. Int. 2017, 2017, 7908183. [Google Scholar] [CrossRef]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I. Conventional methods of wastewater treatment. In Cyanobacteria for Bioremediation of Wastewaters; Zinicovscaia, I., Cepoi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–25. ISBN 978-3-319-26749-4. [Google Scholar]
- Elkady, M.; Shokry, H.; Hamad, H. New Activated Carbon from Mine Coal for Adsorption of Dye in Simulated Water or Multiple Heavy Metals in Real Wastewater. Materials 2020, 13, 2498. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Costas, A.; Cristóbal, A. Agro-Industrial Waste as Potential Heavy Metal Adsorbents and Subsequent Safe Disposal of Spent Adsorbents. Water 2022, 14, 3298. [Google Scholar] [CrossRef]
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability 2023, 15, 5999. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Sun, C.-H.; Fu, Q.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X.; Reungsang, A.; Chang, H.-X. Life-Cycle Assessment of Biofuel Production from Microalgae via Various Bioenergy Conversion Systems. Energy 2019, 171, 1033–1045. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Xu, Y.; Wang, J.; Chu, R.; Yin, Z.; Mo, F.; Zhu, L. A Review on Flocculation as an Efficient Method to Harvest Energy Microalgae: Mechanisms, Performances, Influencing Factors and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110005. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R.; Lopez-Elias, J.A.; Porchas-Cornejo, M.A. Bioremediation of aquaculture effluents. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 539–553. ISBN 978-0-12-800021-2. [Google Scholar]
- Al-Homaidan, A.A.; Al-Abbad, A.F.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabdullatif, J.A. Lead Removal by Spirulina Platensis Biomass. Int. J. Phytoremediation 2016, 18, 184–189. [Google Scholar] [CrossRef]
- Urréjola-Madriñán, S.; Paz-Armada, I.; Cameselle, C.; Gouveia, S. Application of Central Composite Design for Optimization of Adsorption of Chromium(VI) by Spirulina Platensis Algae Biomass. Water 2022, 14, 2539. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Bio-Sorption of Toxic Metals from Industrial Wastewater by Algae Strains Spirulina Platensis and Chlorella Vulgaris: Application of Isotherm, Kinetic Models and Process Optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Kumar Ghodke, P.; Manna, S.; Chen, W.-H. Emerging Technologies for Sustainable Production of Biohydrogen Production from Microalgae: A State-of-the-Art Review of Upstream and Downstream Processes. Bioresour. Technol. 2021, 342, 126057. [Google Scholar] [CrossRef] [PubMed]
- Rangsayatorn, N.; Upatham, E.S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.R. Phytoremediation Potential of Spirulina (Arthrospira) Platensis: Biosorption and Toxicity Studies of Cadmium. Environ. Pollut. 2002, 119, 45–53. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ Ions by Blue–Green Algae Spirulina Sp.: Kinetics, Equilibrium and the Mechanism of the Process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, S.V.; Jyoti, K.K.; Lele, S.S. Kinetic and Equilibrium Modeling of Chromium (VI) Biosorption on Fresh and Spent Spirulina Platensis/Chlorella Vulgaris Biomass. Bioresour. Technol. 2008, 99, 3600–3608. [Google Scholar] [CrossRef]
- Dotto, G.L.; Lima, E.C.; Pinto, L.A.A. Biosorption of Food Dyes onto Spirulina Platensis Nanoparticles: Equilibrium Isotherm and Thermodynamic Analysis. Bioresour. Technol. 2012, 103, 123–130. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhao, D.; Lin, J.; Gao, P.; Yao, L. Adsorption of Pb2+ onto Freeze-Dried Microalgae and Environmental Risk Assessment. J. Environ. Manag. 2020, 265, 110472. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, Y.; Liang, C.; Musah, B.I.; Peng, L. Simultaneous Biosorption of Arsenic and Cadmium onto Chemically Modified Chlorella Vulgaris and Spirulina platensis. Water 2021, 13, 2498. [Google Scholar] [CrossRef]
- Alharbi, R.M.; Sholkamy, E.N.; Alsamhary, K.I.; Abdel-Raouf, N.; Ibraheem, I.B.M. Optimization Study of the Capacity of Chlorella Vulgaris as a Potential Bio-Remediator for the Bio-Adsorption of Arsenic (III) from Aquatic Environments. Toxics 2023, 11, 439. [Google Scholar] [CrossRef]
- Musah, B.I.; Xu, Y.; Liang, C.; Peng, L. Biosorption of Chromium (VI), Iron (II), Copper (II), and Nickel (II) Ions onto Alkaline Modified Chlorella Vulgaris and Spirulina Platensis in Binary Systems. Environ. Sci. Pollut. Res. 2022, 29, 62514–62536. [Google Scholar] [CrossRef]
- Robledo-Padilla, F.; Aquines, O.; Silva-Núñez, A.; Alemán-Nava, G.S.; Castillo-Zacarías, C.; Ramirez-Mendoza, R.A.; Zavala-Yoe, R.; Iqbal, H.M.N.; Parra-Saldívar, R. Evaluation and Predictive Modeling of Removal Condition for Bioadsorption of Indigo Blue Dye by Spirulina Platensis. Microorganisms 2020, 8, 82. [Google Scholar] [CrossRef]
- Deniz, F.; Kepekci, R.A. Bioremediation of Contaminated Water with Unnatural Dye Using Blue-green Alga Spirulina Platensis. Environ. Prog. Sustain. Energy 2015, 34, 1414–1419. [Google Scholar] [CrossRef]
- Garza-González, M.T.; Alcalá-Rodríguez, M.M.; Pérez-Elizondo, R.; Cerino-Córdova, F.J.; Garcia-Reyes, R.B.; Loredo-Medrano, J.A.; Soto-Regalado, E. Artificial Neural Network for Predicting Biosorption of Methylene Blue by Spirulina Sp. Water Sci. Technol. 2011, 63, 977–983. [Google Scholar]
- Ayachi, F.; Lima, E.C.; Sakly, A.; Mejri, H.; Ben Lamine, A. Modeling of Adsorption Isotherms of Reactive Red RR-120 on Spirulina Platensis by Statistical Physics Formalism Involving Interaction Effect between Adsorbate Molecules. Prog. Biophys. Mol. Biol. 2019, 141, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ismail, G.A.; Allam, N.G.; El-Gemizy, W.M.; Salem, M.A. The Role of Silver Nanoparticles Biosynthesized by Anabaena Variabilis and Spirulina Platensis Cyanobacteria for Malachite Green Removal from Wastewater. Environ. Technol. 2021, 42, 4475–4489. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.A.; Calvete, T. Comparison of Spirulina Platensis Microalgae and Commercial Activated Carbon as Adsorbents for the Removal of Reactive Red 120 Dye from Aqueous Effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kausar, R.A.; Buhani; Suharso. Methylene Blue Adsorption Isotherm on Spirulina Sp. Microalgae Biomass Coated by Silica-Magnetite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 857, 012019. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes From Wastewater: A Review. Front. Environ. Sci. 2021, 9, 764958. [Google Scholar] [CrossRef]
- Dotto, G.L.; Esquerdo, V.M.; Vieira, M.L.G.; Pinto, L.A.A. Optimization and Kinetic Analysis of Food Dyes Biosorption by Spirulina Platensis. Colloids Surf. B Biointerfaces 2012, 91, 234–241. [Google Scholar] [CrossRef]
- Shanmugam, D.; Alagappan, M.; Rajan, R.K. Bench-Scale Packed Bed Sorption of Cibacron Blue F3GA Using Lucrative Algal Biomass. Alex. Eng. J. 2016, 55, 2995–3003. [Google Scholar] [CrossRef]
- Guler, U.A.; Ersan, M.; Tuncel, E.; Dügenci, F. Mono and Simultaneous Removal of Crystal Violet and Safranin Dyes from Aqueous Solutions by HDTMA-Modified Spirulina Sp. Process Saf. Environ. Prot. 2016, 99, 194–206. [Google Scholar]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive Removal of Dye Using Biochar Derived from Residual Algae after In-Situ Transesterification: Alternate Use of Waste of Biodiesel Industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef]
- Pedrosa, M.; Ribeiro, R.S.; Guerra-Rodríguez, S.; Rodríguez-Chueca, J.; Rodríguez, E.; Silva, A.M.T.; Ðolic, M.; Rita Lado Ribeiro, A. Spirulina-Based Carbon Bio-Sorbent for the Efficient Removal of Metoprolol, Diclofenac and Other Micropollutants from Wastewater. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100720. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.-J.; Park, S.-J.; Lee, C.-G. Enhanced Removal of Micropollutants from Water Using ZnCl2-Modified Spirulina Sp.-Based Biochar. J. Appl. Phycol. 2024, 36, 167–179. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.-J.; Choi, M.-Y.; Lee, C.-G.; Park, S.-J. Adsorption of Heavy Metals and Bisphenol A from Wastewater Using Spirulina Sp.-Based Biochar as an Effective Adsorbent: A Preliminary Study. J. Appl. Phycol. 2023, 35, 2257–2269. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, D.; Wu, W.; Li, F. The Different Toxicological Effects and Removal Efficiencies of Norfloxacin and Sulfadiazine in Culturing Arthrospira (Spirulina) Platensis. Ecotoxicol. Environ. Saf. 2023, 250, 114468. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Choi, T.-R.; Gurav, R.; Bhatia, S.K.; Park, Y.-L.; Kim, H.J.; Kan, E.; Yang, Y.-H. Adsorption Behavior of Tetracycline onto Spirulina Sp. (Microalgae)-Derived Biochars Produced at Different Temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef]
- Ismail, G.A.; Allam, N.G.; El-Gemizy, W.M.; Salem, M.A.-S. Potential Role of Spirulina (Arthrospira) Platensis Biomass for Removal of TiO2NPs -MG Hybrid Nanocomposite Produced after Wastewater Treatment by TiO2 Nanoparticles. An. Acad. Bras. Ciênc. 2021, 93, e20201669. [Google Scholar] [CrossRef]
- Doshi, H.; Ray, A.; Kothari, I.L. Biosorption of Cadmium by Live and Dead Spirulina: IR Spectroscopic, Kinetics, and SEM Studies. Curr. Microbiol. 2007, 54, 213–218. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Application of the Biochar Derived from Orange Peel for Effective Biosorption of Cpper and Cadmium in Batch Studies: Isotherm Models and Kinetic Studies. Arab. J. Geosci. 2019, 12, 46. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water—A Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, A.; Soltan Mohammadzadeh, J.S.; Khudiev, A. What Is the Correct Form of BET Isotherm for Modeling Liquid Phase Adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys.Chem. 1906, 57, 385–471. [Google Scholar]
- Nitzsche, R.; Gröngröft, A.; Kraume, M. Separation of Lignin from Beech Wood Hydrolysate Using Polymeric Resins and Zeolites—Determination and Application of Adsorption Isotherms. Sep. Purif. Technol. 2019, 209, 491–502. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Removal of Cadmium(II) and Zinc(II) Metal Ions from Binary Aqueous Solution by Rice Husk Ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 172–184. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Jovanovich, D.S. Physical Adsorption of Gases I: Isotherms for Monolayer and Multilayer Adsorption. Colloid Polym. Sci. 1969, 235, 1203–1214. [Google Scholar]
- Halsey, G.D. The role of surface heterogeneity in adsorption. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1952; Volume 4, pp. 259–269. ISBN 978-0-12-007804-2. [Google Scholar]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gel’ster Stoffe, Kungliga Svenska Vetenskapsakademines. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-Order Kinetic Model for the Sorption of Cadmium onto Tree Fern: A Comparison of Linear and Non-Linear Methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Weber, W.J.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. ASCE 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Cabal, B.; Ania, C.O.; Parra, J.B.; Pis, J.J. Kinetics of Naphthalene Adsorption on an Activated Carbon: Comparison between Aqueous and Organic Media. Chemosphere 2009, 76, 433–438. [Google Scholar] [CrossRef]
- Figaro, S.; Avril, J.P.; Brouers, F.; Ouensanga, A.; Gaspard, S. Adsorption Studies of Molasse’s Wastewaters on Activated Carbon: Modelling with a New Fractal Kinetic Equation and Evaluation of Kinetic Models. J. Hazard. Mater. 2009, 161, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Malletzidou, L.; Kyratzopoulou, E.; Kyzaki, N.; Nerantzis, E.; Kazakis, N.A. Towards the Sustainable Removal of Heavy Metals from Wastewater Using Arthrospira Platensis: A Laboratory-Scale Approach in the Context of a Green Circular Economy. Appl. Sci. 2025, 15, 791. [Google Scholar] [CrossRef]
- Phiri, J.T.; Oh, S. Biosorption of Cd(II), Co(II), and Cu(II) onto Microalgae under Acidic and Neutral Conditions. Sustainability 2024, 16, 6342. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Portela, R.; Baeza, P.; Alcolea-Rodriguez, V.; Villarroel, M.; Ávila, P. Zeta Potential as a Tool for Functional Materials Development. Catal. Today 2023, 423, 113862. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of Phosphate Anions Using the Modified Chitosan Beads: Adsorption Kinetic, Isotherm and Mechanism Studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Qu, B.; Zhou, J.; Xiang, X.; Zheng, C.; Zhao, H.; Zhou, X. Adsorption Behavior of Azo Dye C. I. Acid Red 14 in Aqueous Solution on Surface Soils. J. Environ. Sci. 2008, 20, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive Removal of Basic Dyes from Aqueous Solutions by Surfactant Modified Bentonite Clay (Organoclay): Kinetic and Competitive Adsorption Isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-Activated Carbon Prepared from Sucrose Spherical Carbon: Adsorption Equilibrium, Kinetic and Thermodynamic Studies for Methylene Blue Removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü. Anionic Dye Removal from Aqueous Solutions Using Modified Zeolite: Adsorption Kinetics Isotherm Studies. Chem. Eng. J. 2012, 200, 59–67. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead Removal from Aqueous Solution by Natural Pretreated Clinoptilolite: Adsorption Equilibrium Kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Özdemir, Y.; Doğan, M.; Alkan, M. Adsorption of Cationic Dyes from Aqueous Solutions by Sepiolite. Microporous Mesoporous Mater. 2006, 96, 419–427. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Physicochim. USSR 1940, 12, 327. [Google Scholar]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic Removal Using Mesoporous Alumina Prepared via a Templating Method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Aslam, Z.; Shawabkeh, R.A.; Hussein, I.A.; Al-Baghli, N.; Eic, M. Synthesis of Activated Carbon from Oil Fly Ash for Removal of H2S from Gas Stream. Appl. Surf. Sci. 2015, 327, 107–115. [Google Scholar] [CrossRef]
- Maldonado_Rojas, W.; Velilla-Plaza, C.; Suarez-Rodriguez, A.; Carrasquilla-Tejedor, Y.; Caro Álvarez, J.; Arroyo, B.; Tejeda-Benitez, L. Synthesis of a Biosorbent from Cannabis sativa L. Extracts and Fibers for the Removal of Mercury, Nickel, Cadmium, and Lead from Water; SSRN: Rochester, NY, USA, 2024. [Google Scholar]
- Eftekhari, E.; Hasani, H.; Fashandi, H. Removal of Heavy Metal Ions (Pb2+ and Ni2+) from Aqueous Solution Using Nonwovens Produced from Lignocellulosic Milkweed Fibers. J. Ind. Text. 2021, 51, 695–713. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, Á. Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials 2022, 15, 6937. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Kuvarega, A.T.; Mbule, P.; Mishra, A.K. Diethylene Triamine Penta (Methylene Phosphonic Acid) Functionalized Magnetic Nanosilica for Adsorptive Removal of Heavy Metals (Pb2+ and Ni2+) from Aqueous Solution. J. Mol. Liq. 2025, 426, 127468. [Google Scholar] [CrossRef]
- Shukur, S.A.; Zageer, D.; Ahmed, A. Adsorption Study of Heavy Metal Ions from Aqueous Solution by Modified Poly (Methyl Methacrylate) with Semicarbazide as Environmental Sustainability. IOP Conf. Ser. Earth Environ. Sci. 2025, 1507, 012023. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Liu, H.; Chen, Q.; Liu, Z. Lead Biosorption and Desorption by Intact and Pretreated Spirulina Maxima Biomass. Chemosphere 2005, 58, 125–130. [Google Scholar] [CrossRef]
- Rezaei, H.; Kulkarni, S.D.; Saptarshi, P.G. Study of Physical Chemistry on Biosorption of Copper by Using Sprulina Sp. Int. J. ChemTech Res. 2011, 3, 1429–1439. [Google Scholar]
- Purev, O.; Park, C.; Kim, H.; Myung, E.; Choi, N.; Cho, K. Spirulina Platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions. Int. J. Environ. Res. Public Health 2023, 20, 1106. [Google Scholar] [CrossRef] [PubMed]
- Villen-Guzman, M.; Jiménez, C.; Rodriguez-Maroto, J.M. Batch and Fixed-Bed Biosorption of Pb (II) Using Free and Alginate-Immobilized Spirulina. Processes 2021, 9, 466. [Google Scholar] [CrossRef]
- Chen, H.; Pan, S. Bioremediation Potential of Spirulina: Toxicity and Biosorption Studies of Lead. J. Zhejiang Univ. Sci. B 2005, 6, 171–174. [Google Scholar] [CrossRef]
- Abd El-Reheem Mohammed, A.; Muhammad, S.S.; Mohamed, H.A.; El-Agamy, H.H. Potentiality of Spirulina Platensis in Zirconium Ions Biosorption and Removal from Aqueous Solution as an Eco-Friendly Process. Sep. Sci. Technol. 2025, 60, 1101–1112. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Godlewska, K.; Trynda, J.; Marycz, K. Arthrospira (Spirulina) Platensis: An Effective Biosorbent for Nutrients. Process Biochem. 2020, 88, 129–137. [Google Scholar] [CrossRef]
- Lang’at, E.K.; Omolo, J.O.; Ongoma, P.O. Sugarcane Bagasse Based Adsorbents and Their Adsorption Efficacy on Removal of Heavy Metals from Nakuru Industrial Wastewater: Optimization, Kinetic and Thermodynamic Aspects. Asian J. Appl. Chem. Res. 2024, 15, 276–293. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Balomajumder, C.; Kumar, S. Efficacy of Sargassum Filipendula for the Removal of Pb2+, Cd2+ and Ni2+ Ions from Aqueous Solution: A Comparative Study. Desalin. Water Treat. 2018, 129, 216–226. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus Camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Eleraky, M.I.; Razek, T.M.A.; Hasani, I.W.; Fahim, Y.A. Adsorptive Removal of Lead, Copper, and Nickel Using Natural and Activated Egyptian Calcium Bentonite Clay. Sci. Rep. 2025, 15, 13050. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, A.; Fu, H.; Zhang, L.; Liu, H.; Zheng, S.; Wan, H.; Xu, Z. Efficient Removal of Pb(II) Ions Using Manganese Oxides: The Role of Crystal Structure. RSC Adv. 2017, 7, 41228–41240. [Google Scholar] [CrossRef]


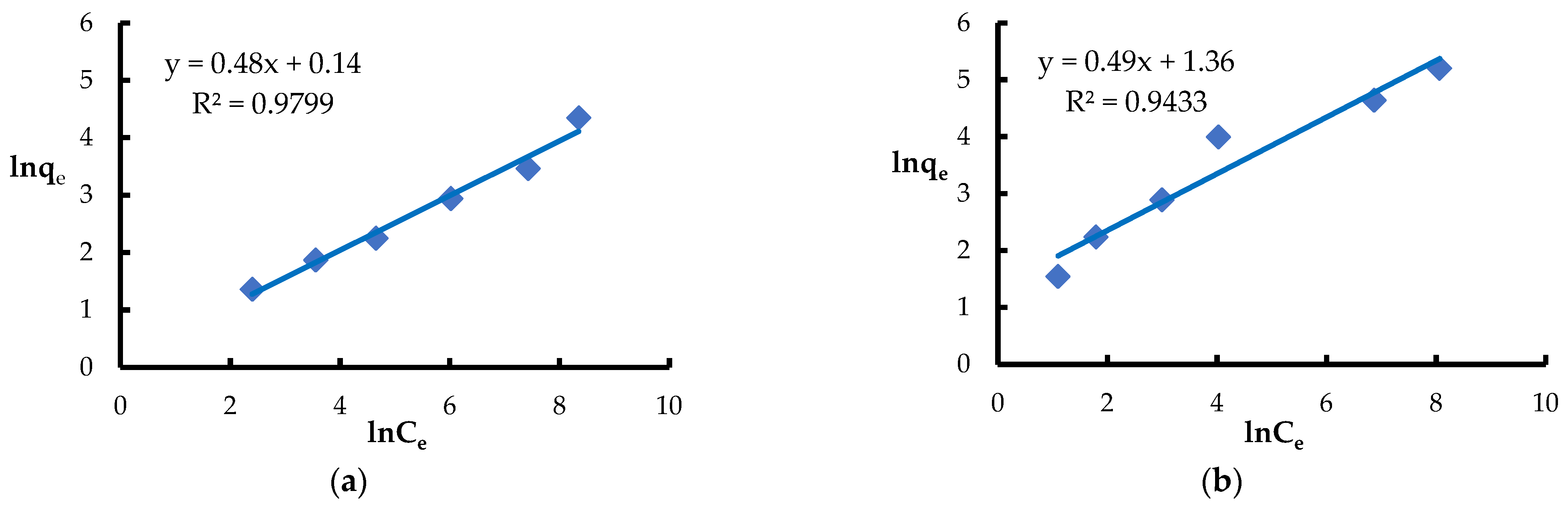
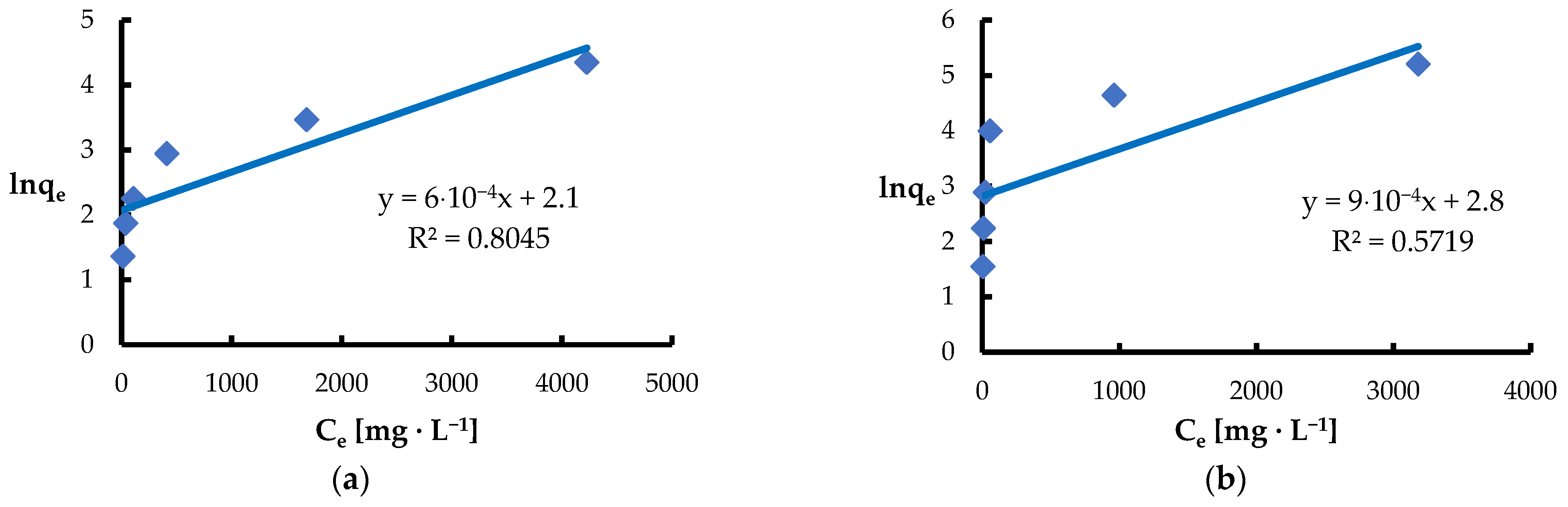
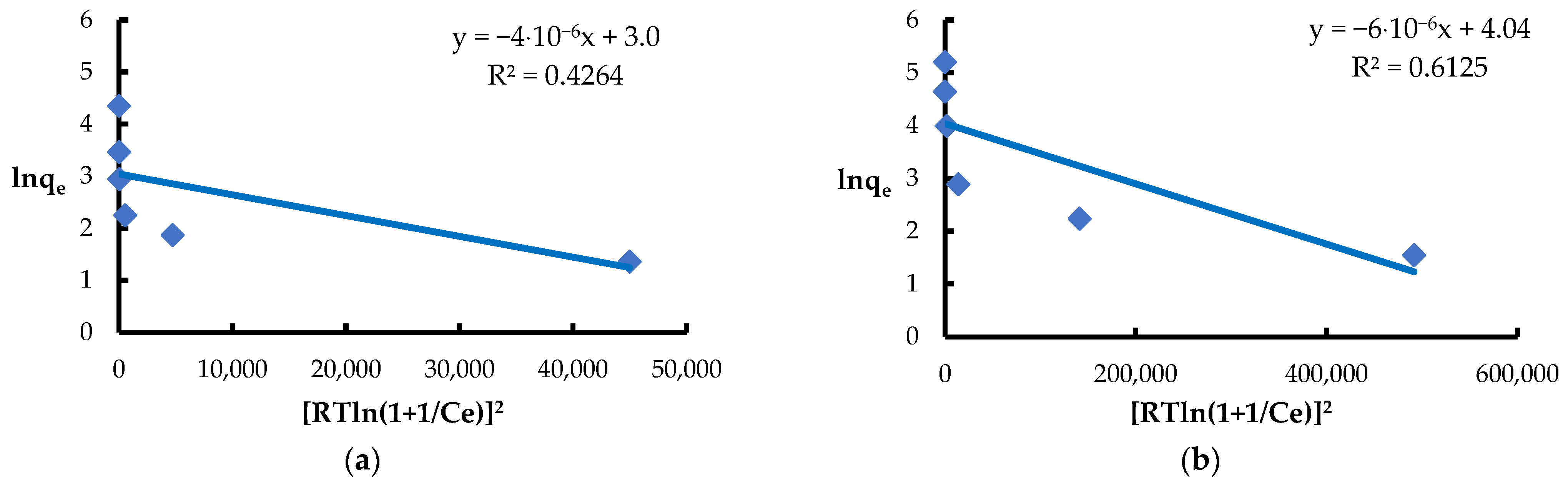
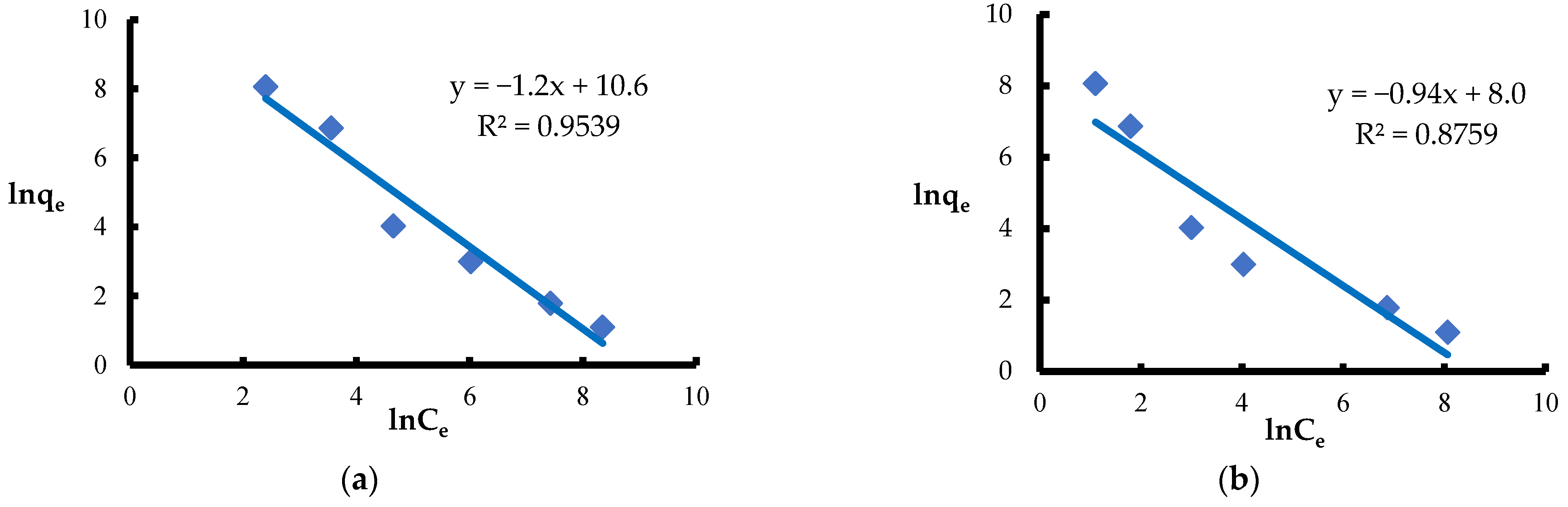

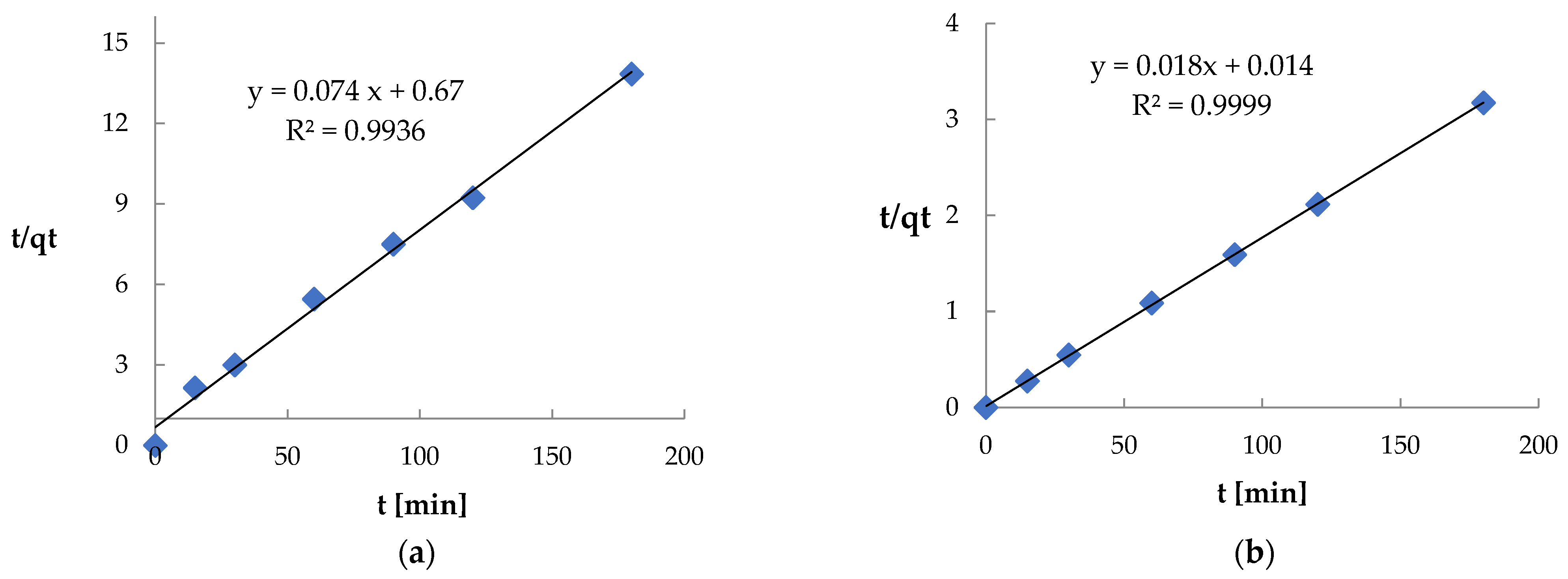
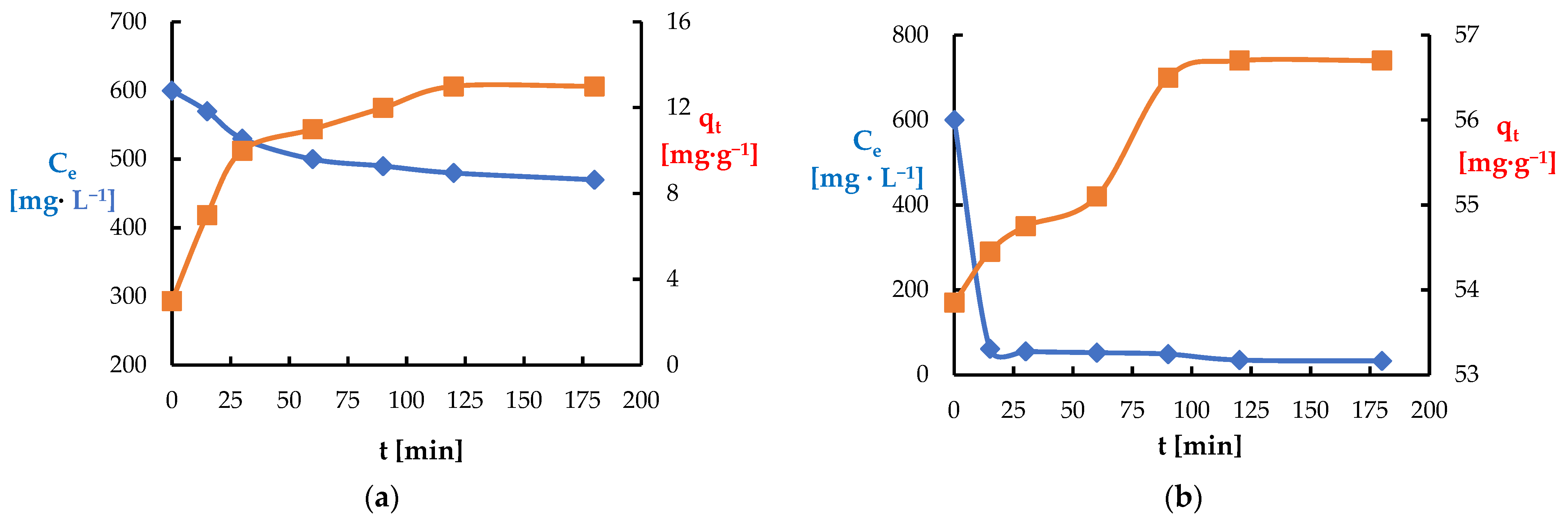
| Isotherm | Equation |
|---|---|
| Freundlich | |
| Langmuir | |
| Redlich-Peterson | |
| Jovanović | |
| Halsey | |
| Temkin | |
| Dubinin–Radushkevich | |
| Brunauer, Emmett, and Teller |
| Isotherm | Parameter | Ni(II) | Pb(II) |
|---|---|---|---|
| Freundlich | KF [mg1−1/n·L1/n·g−1] | 1.15 | 3.90 |
| n | 2.1 | 2.0 | |
| R2 | 0.9799 | 0.9433 | |
| Langmuir | KL [L·mg−1] | 0.12 | 6.4·10−3 |
| qmax [mg·g−1] | 20.8 | 93.5 | |
| R2 | 0.8987 | 0.9913 | |
| Redlich and Peterson | KR [L·g−1] | 7.3 | 4.3 |
| aR [(L·mg−1)β] | 54.6 | 16.3 | |
| β | 0.52 | 0.51 | |
| R2 | 0.9833 | 0.9452 | |
| Jovanović | KJ [L·mg−1] | 6.0·10−4 | 9.0·10−4 |
| qmax [mg·g−1] | 8.2 | 16.4 | |
| R2 | 0.8045 | 0.5719 | |
| Halsey | KH [mgn−1·g−n·L] | 1.5·10−4 | 2.0·10−4 |
| nH | 0.83 | 1.06 | |
| R2 | 0.9539 | 0.8759 | |
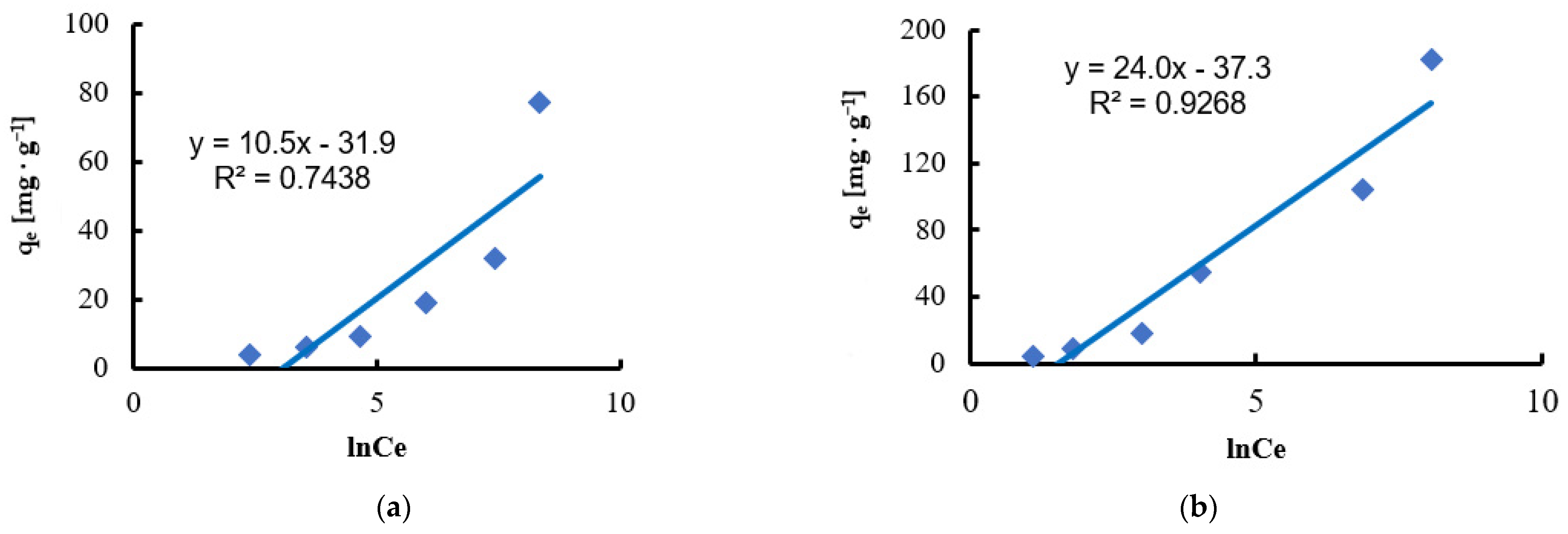
| Temkin | KT [L·mg−1] | 0.05 | 0.21 |
| BT [J·mol−1] | 10.5 | 24 | |
| b | 232 | 101 | |
| R2 | 0.7438 | 0.9268 | |
| Dubinin and Radushkevich | KDR [mol2·J−2] | 4.9·10−6 | 6·10−6 |
| qmax [mg·g−1] | 20.1 | 56.8 | |
| E [J·mol−1] | 353.5 | 288.7 | |
| R2 | 0.4264 | 0.6125 | |
| Brunauer, Emmett, and Teller | KBET [L·mg−1] | 1.00 | 1.00 |
| qmax [mg·g−1] | 6·10−2 | 7·10−3 | |
| R2 | 0.2520 | 0.0595 |
| Kinetic Model | Parameter | Ni(II) | Pb(II) |
|---|---|---|---|
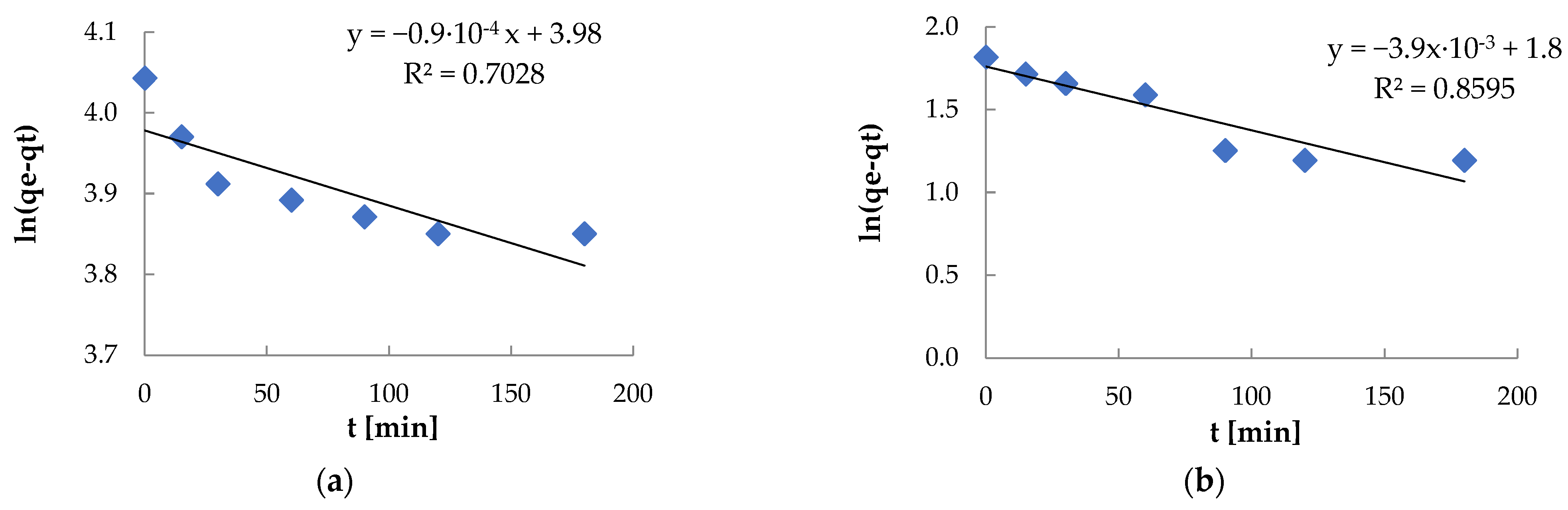
| Pseudo-first-order | k1 [min−1] R2 | 9.0·10−4 0.7028 | 3.9·10−3 0.8595 |
| Pseudo-second-order | k2 [g·mg−1·min−1] R2 | 4.1·10−4 0.9936 | 1.8·10−2 0.9999 |
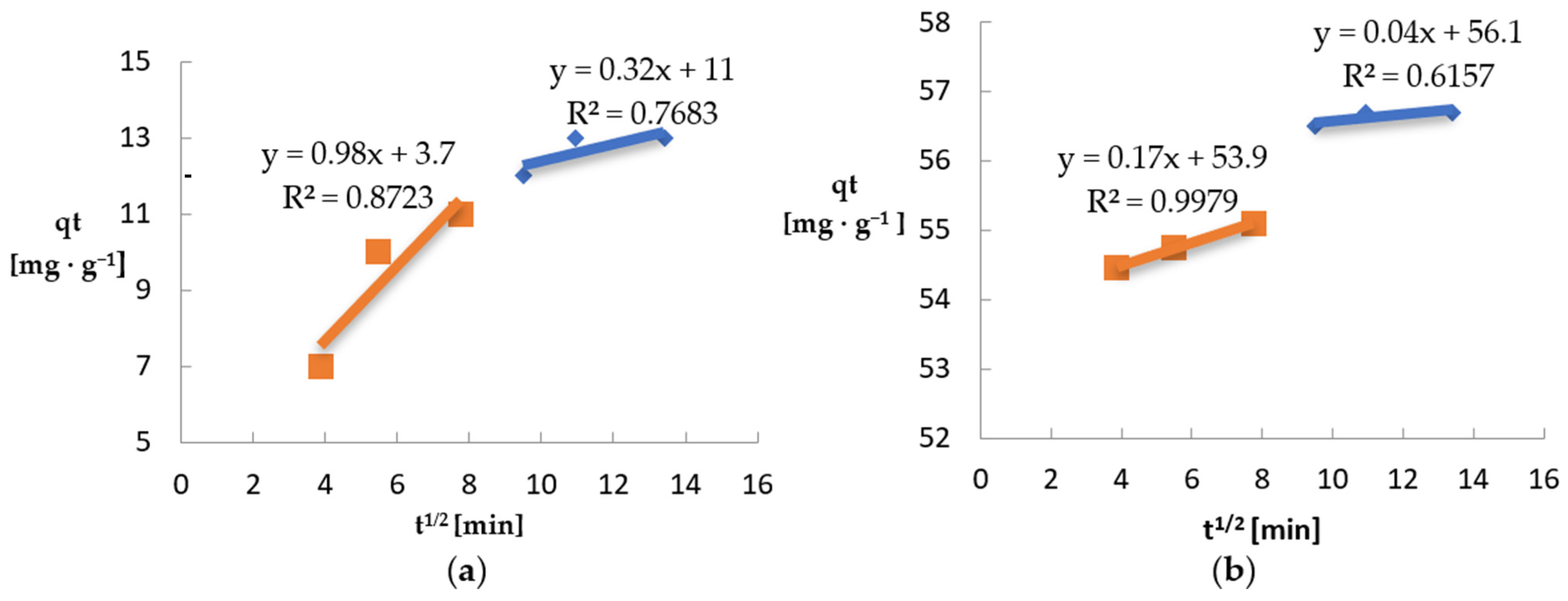
| Intra-particle diffusion | k’1 [mg·g−1·min−1/2] b1 [mg·g−1] R2 | 0.98 3.7 0.8723 | 0.17 53.9 0.9979 |
| k’2 [mg·g−1·min−1/2] b2 [mg·g−1] R2 | 0.32 10.0 0.7683 | 0.04 56.1 0.6157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sočo, E.; Domoń, A.; Azizi, M.; Pająk, D.; Cieniek, B.; Michel, M.M.; Papciak, D. Efficiency of Spirulina sp. in the Treatment of Model Wastewater Containing Ni(II) and Pb(II). Materials 2025, 18, 3639. https://doi.org/10.3390/ma18153639
Sočo E, Domoń A, Azizi M, Pająk D, Cieniek B, Michel MM, Papciak D. Efficiency of Spirulina sp. in the Treatment of Model Wastewater Containing Ni(II) and Pb(II). Materials. 2025; 18(15):3639. https://doi.org/10.3390/ma18153639
Chicago/Turabian StyleSočo, Eleonora, Andżelika Domoń, Mostafa Azizi, Dariusz Pająk, Bogumił Cieniek, Magdalena M. Michel, and Dorota Papciak. 2025. "Efficiency of Spirulina sp. in the Treatment of Model Wastewater Containing Ni(II) and Pb(II)" Materials 18, no. 15: 3639. https://doi.org/10.3390/ma18153639
APA StyleSočo, E., Domoń, A., Azizi, M., Pająk, D., Cieniek, B., Michel, M. M., & Papciak, D. (2025). Efficiency of Spirulina sp. in the Treatment of Model Wastewater Containing Ni(II) and Pb(II). Materials, 18(15), 3639. https://doi.org/10.3390/ma18153639













