Effects of Red Mud on Cement Mortar Based on Sodium Salt Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Testing Methods
3. Results and Discussion
3.1. Properties of LRM+N and LRM+S
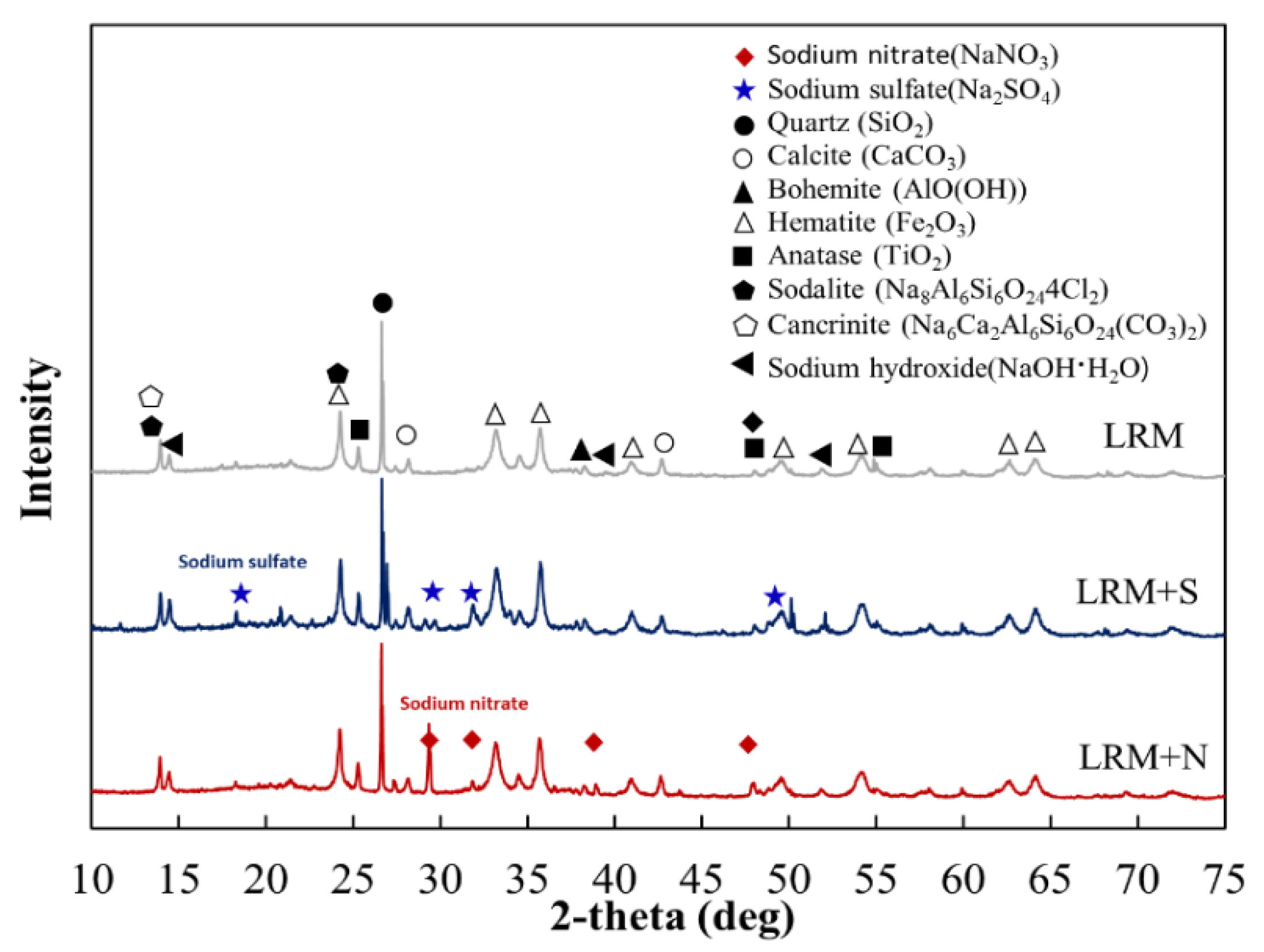
3.1.1. XRD
3.1.2. Physical Properties
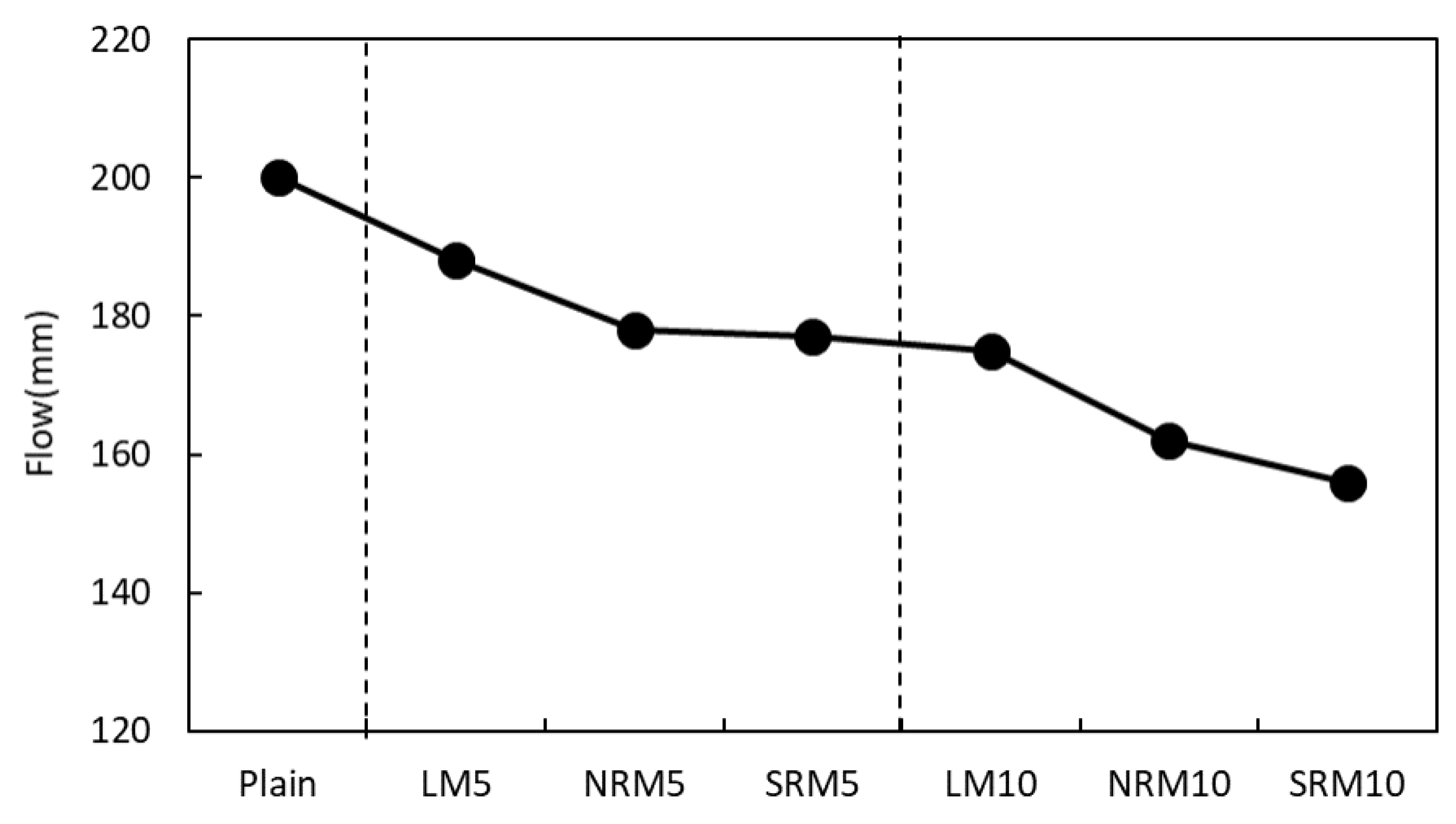
3.2. Flow
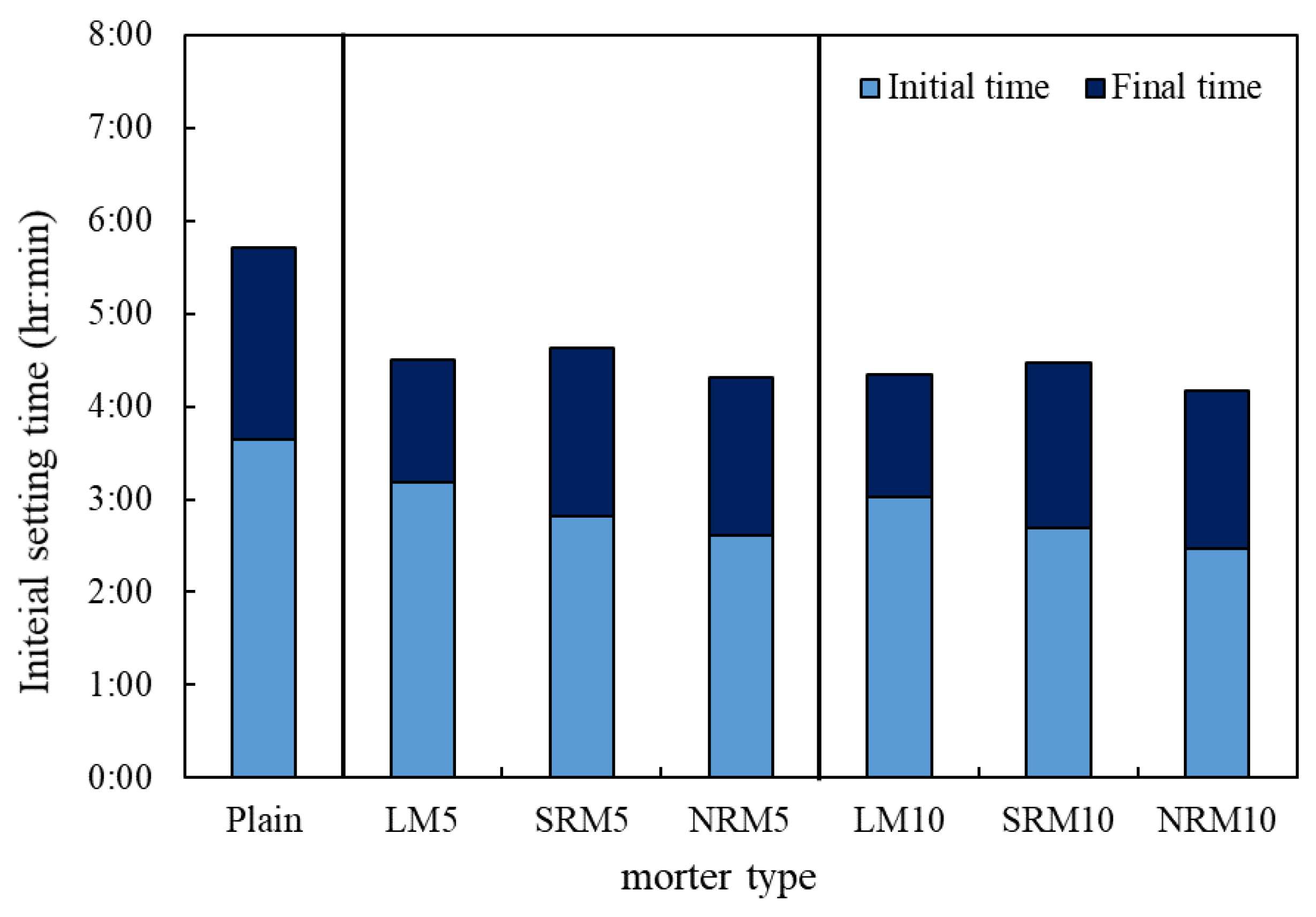
3.3. Setting Time
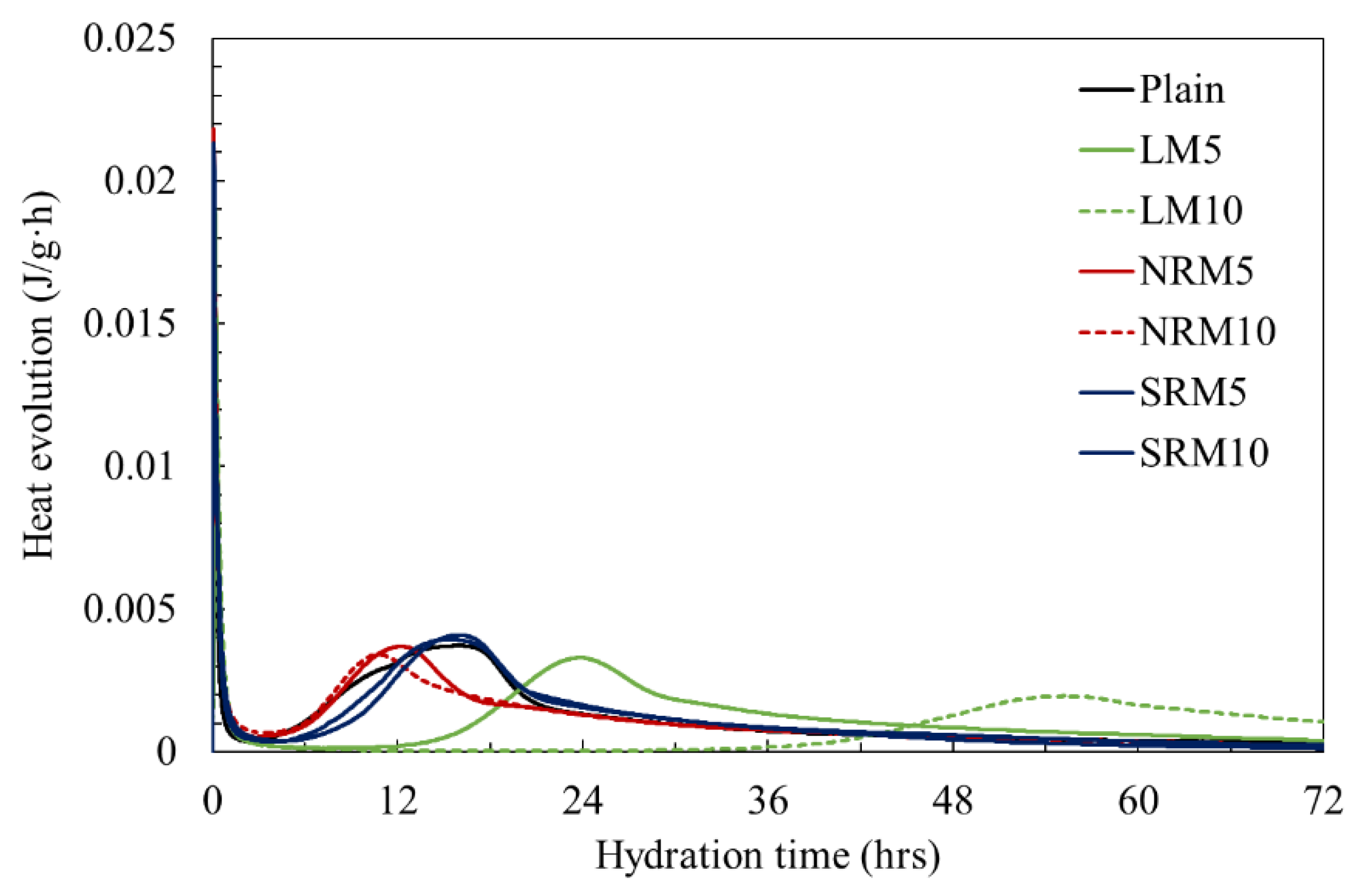
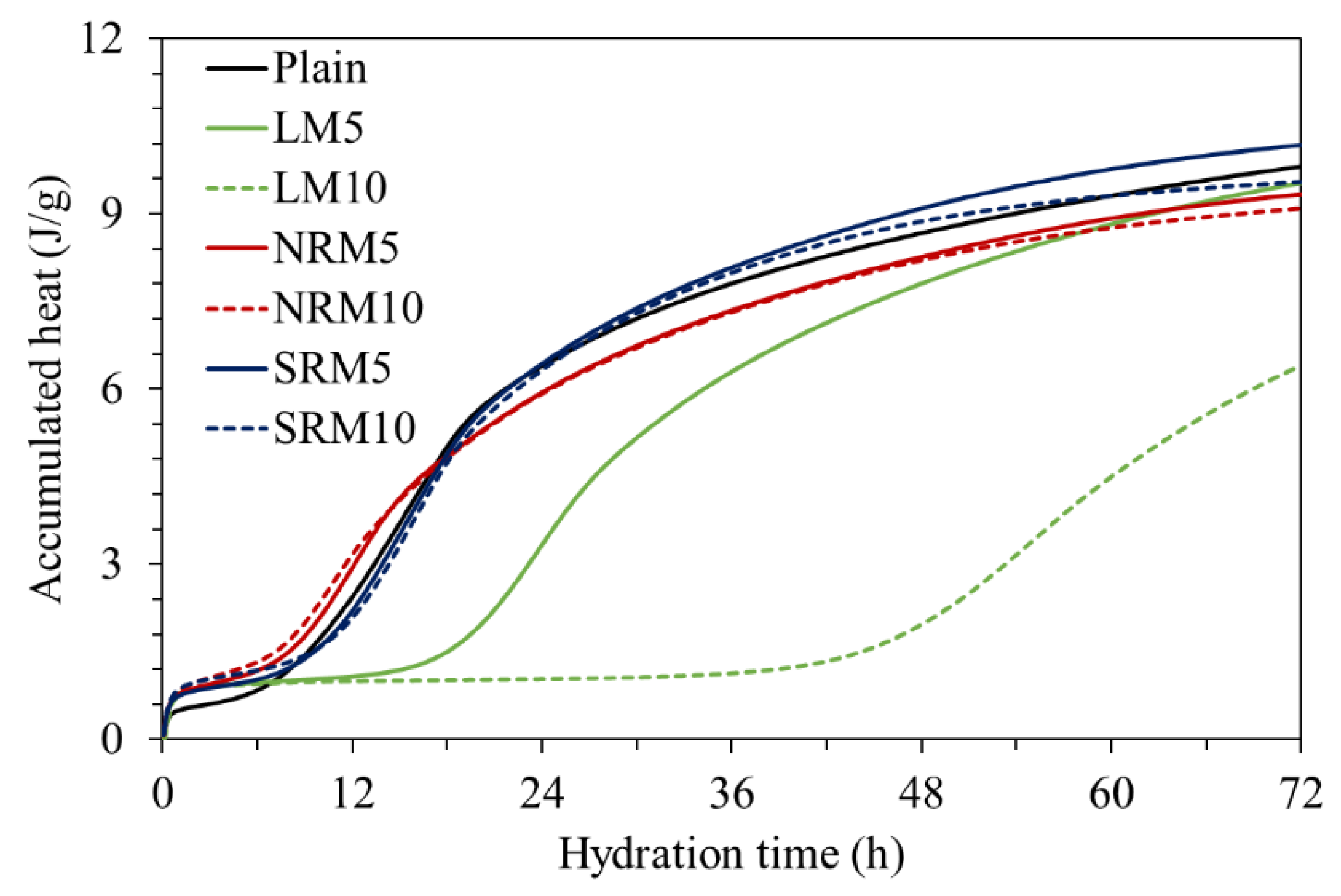
3.4. Hydration Heat
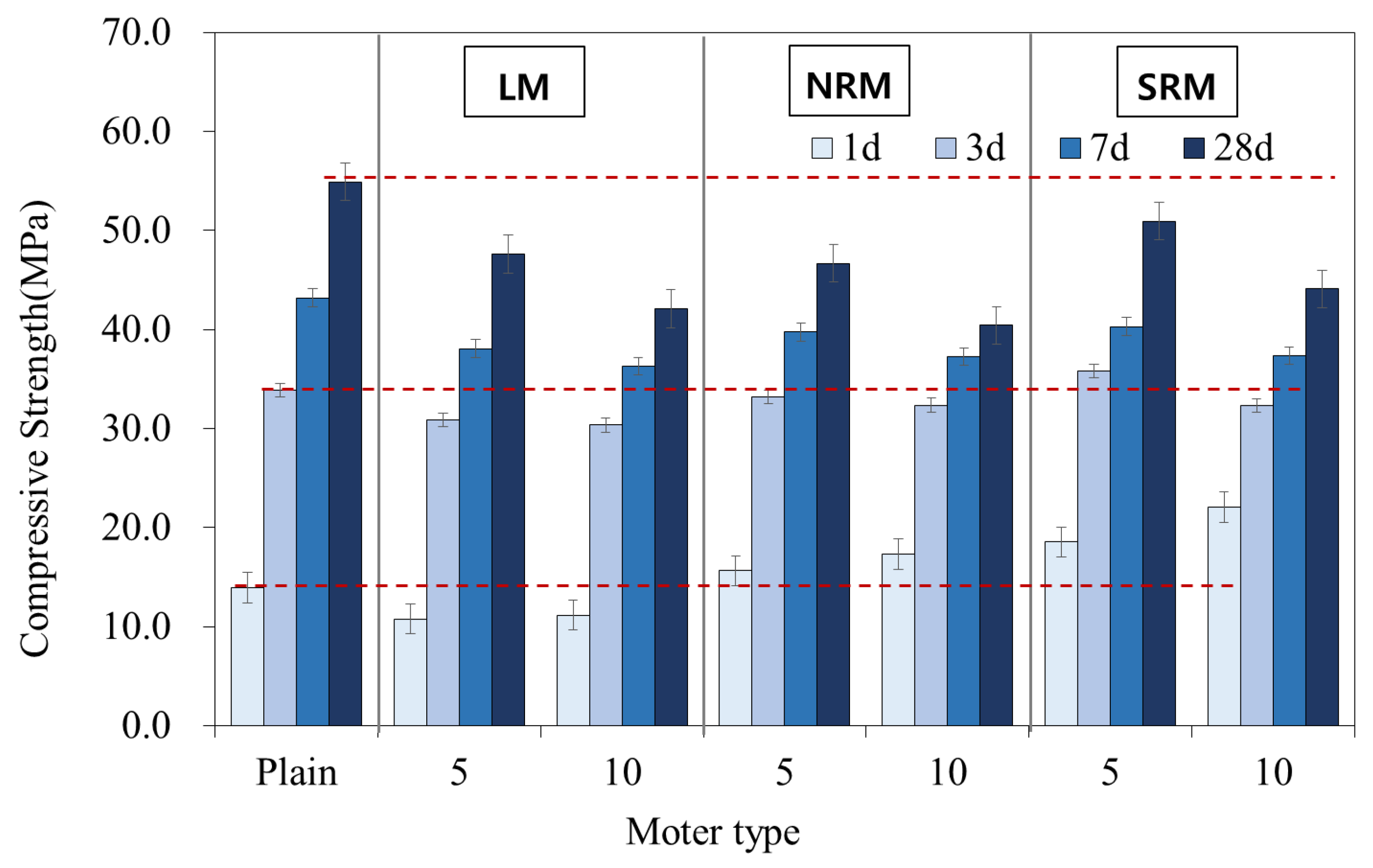
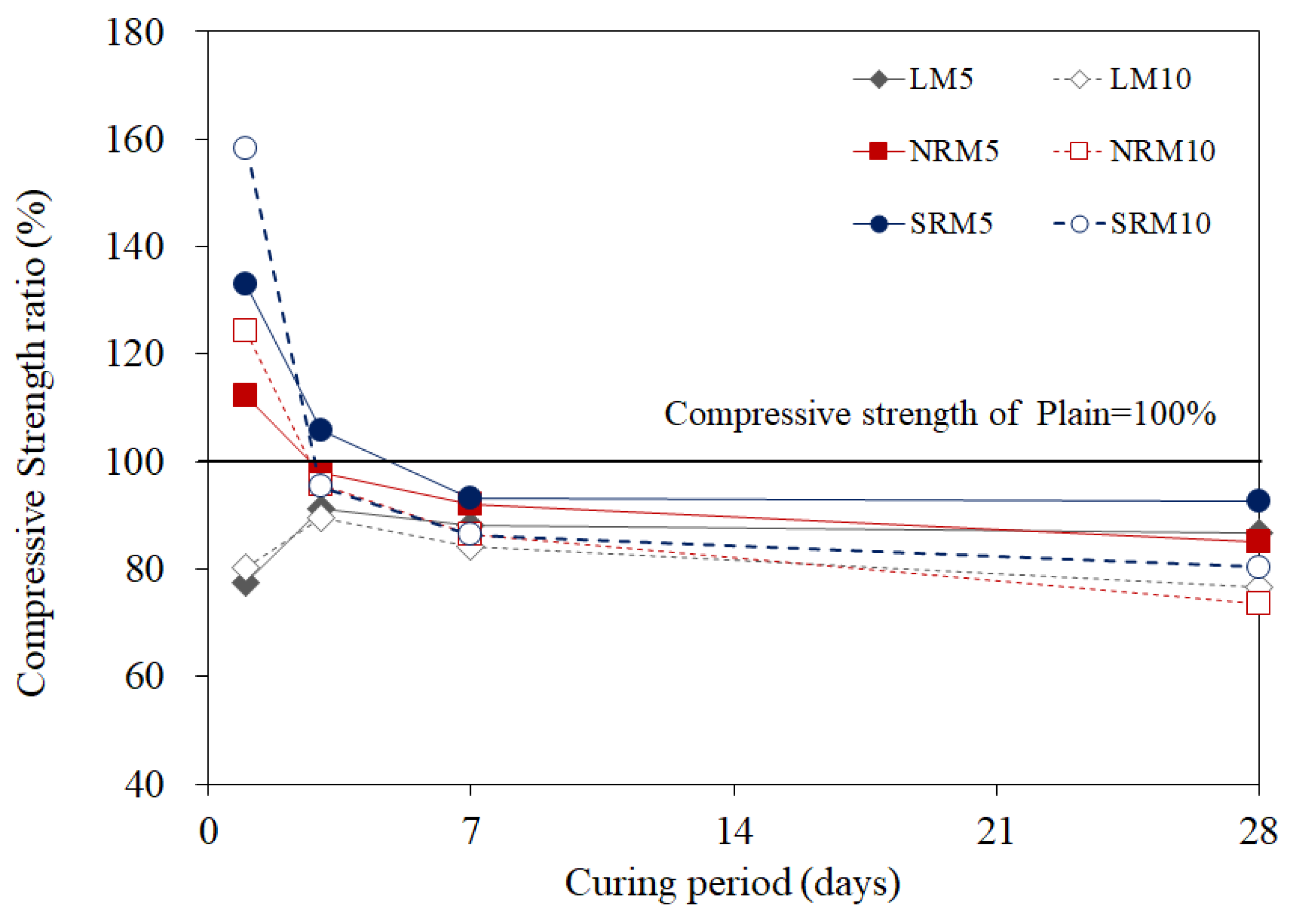
3.5. Compressive Strength
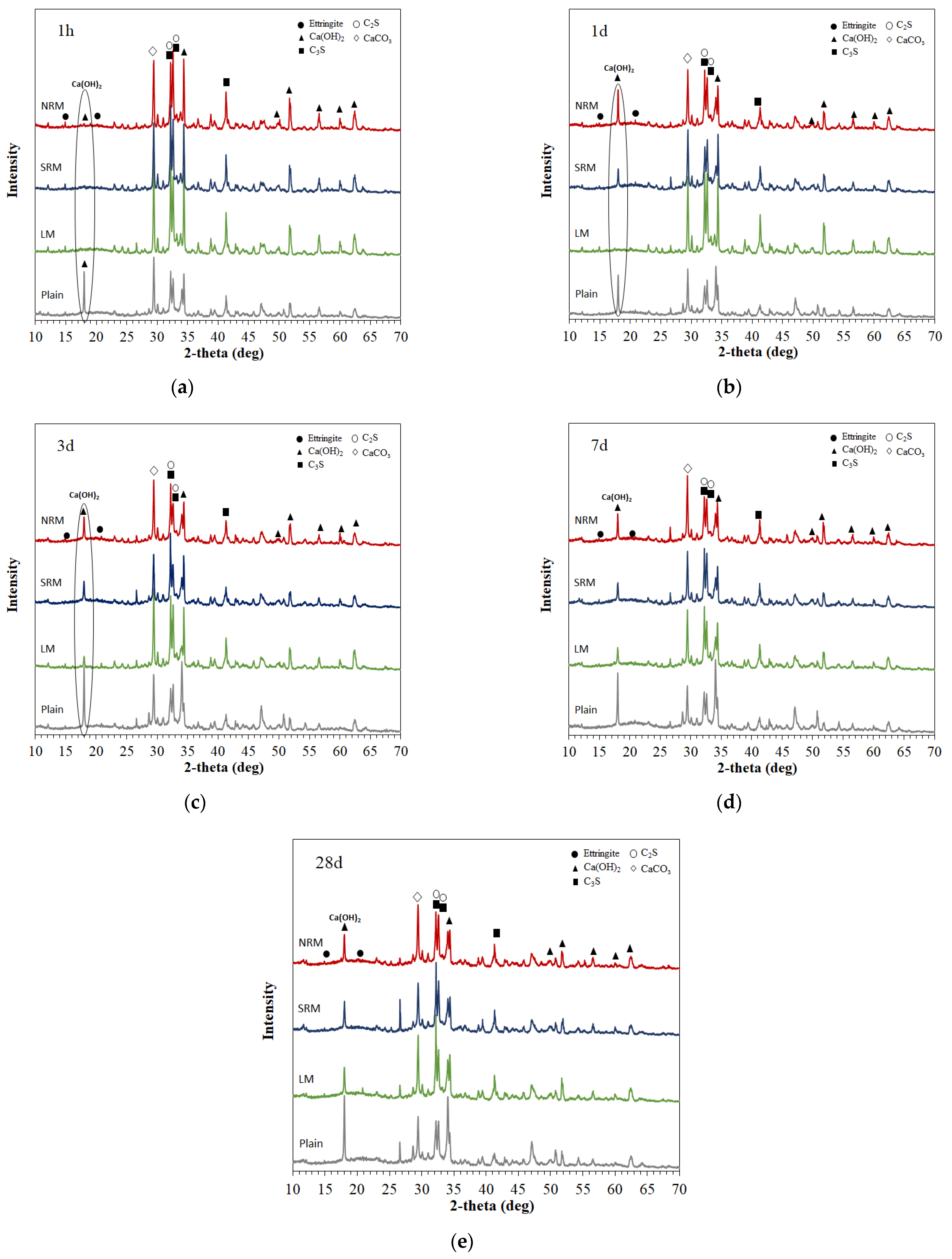
3.6. XRD Observations
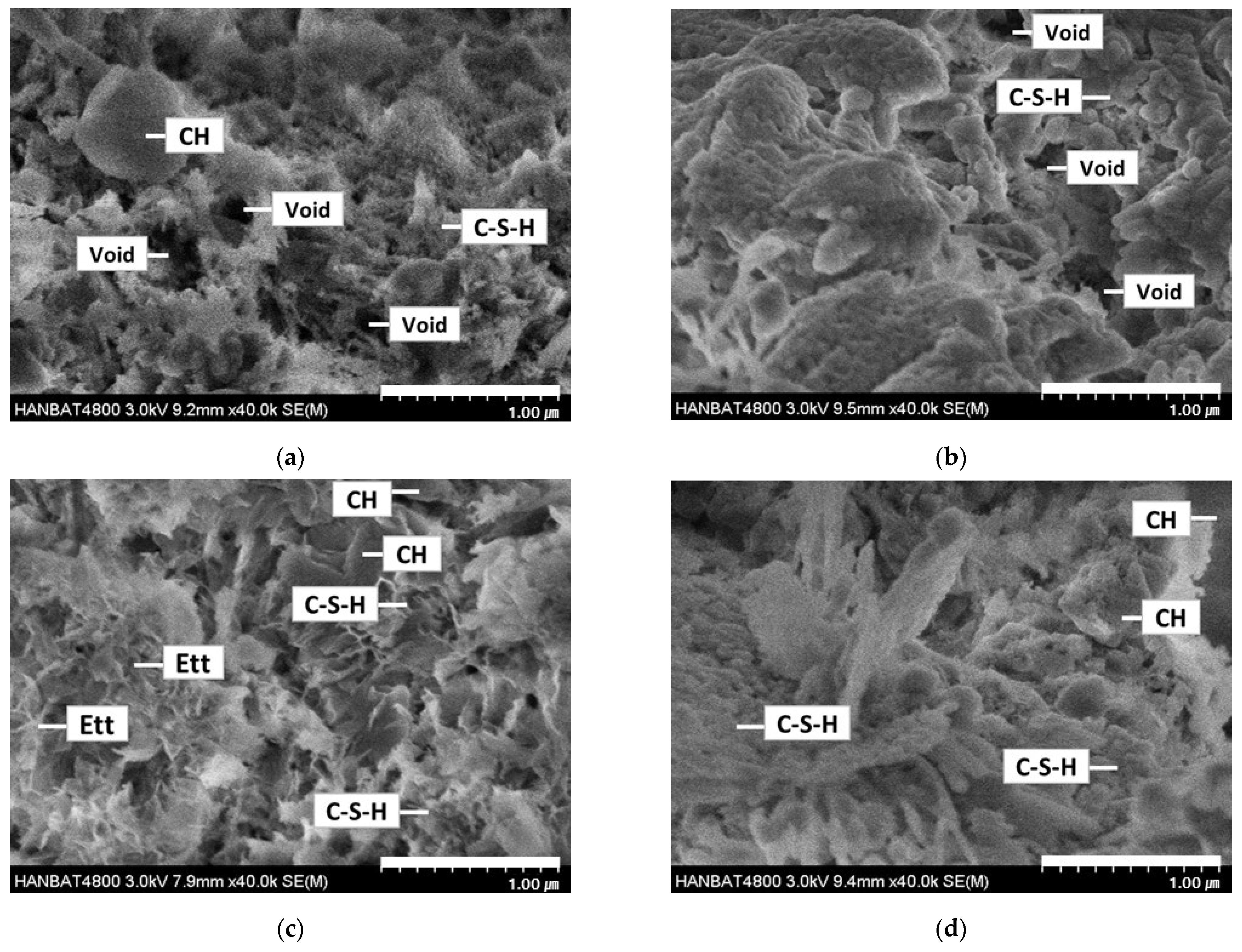
3.7. SEM Observations
4. Conclusions
- (1)
- LRM was neutralized with nitric acid and sulfuric acid, confirming the production of NaNO3 and Na2SO4 and stabilizing the pH between 7 and 8.
- (2)
- NRM and SRM, with the addition of LRM+N and LRM+S, had a shorter initial setting time and a similar final time compared to LM. The hydration heat peak of NRM and SRM appeared earlier than that of LM and was comparable to Plain.
- (3)
- XRD analysis at 1 day revealed that the Ca(OH)2 peak in NRM and SRM was similar to Plain but absent in LM. SEM observations result at 1 day showed reduced pore structures and the presence of Ca(OH)2 in NRM and SRM compared to LM.
- (4)
- The 1-day compressive strength of NRM and SRM exceeded that of LM, with SRM demonstrating a 35–60% strength increase over Plain, depending on dosage. These findings suggest that LRM treated with nitric or sulfuric acid has potential as a setting accelerator for cement mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| LRM | Liquefied red mud |
| NRM | Neutralized red mud |
| OPC | Ordinary Portland cement |
| SRM | Sulfuric-treated red mud |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
References
- Jiang, T.; Cui, K.; Chang, J. Development of low-carbon cement: Carbonation of compounded C2S by β-C2S and γ-C2S. Cem. Concr. Compos. 2023, 139, 105071. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Provis, J.L.; Tsang, D.C.W.; Poon, C.S. Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 Gas. Cem. Concr. Compos. 2020, 106, 103489. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Jiang, T.; Zhang, J.; Lau, D.; Chang, J. Understanding the role of carbon nanotubes in low-carbon concrete: From experiment to molecular dynamics. Cem. Concr. Compos. 2023, 142, 105189. [Google Scholar] [CrossRef]
- Cui, K.; Lu, D.; Jiang, T.; Zhang, J.; Jiang, Z.; Zhang, G.; Chang, J.; Lau, D. Understanding the role of carbon nanotubes in low carbon sulfoaluminate cement-based composite. J. Clean. Prod. 2023, 416, 137843. [Google Scholar] [CrossRef]
- Lima, M.S.S.; Thives, L.P. Evaluation of red mud as filler in Brazilian dense graded asphalt mixtures. Constr. Build. Mater. 2020, 260, 119894. [Google Scholar] [CrossRef]
- Menzie, W.D.; Barry, J.J.; Bleiwas, D.I.; Bray, E.L.; Goonan, T.G.; Matos, G. The Global Flow of Aluminum from 2006 Through 2025; United States Department of the Interior, United States Geological Survey: Reston, VA, USA, 2010.
- Liu, X.; Zhang, N.; Yao, Y.; Sun, H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef]
- Hildebrando, E.A.; Souza, J.A.d.S.; Angélica, R.S.; Neves, R.F. Application of bauxite waste from Amazon region in the heavy clay industry. Mater. Res. 2013, 16, 1418–1422. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.-a.; Lyu, G.; Guo, F.; Zhang, W.; Zhang, Y. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Phan, T.M.; Kang, S.P.; Vo, H.V.; Park, D.W. Evaluation on performances of cold asphalt mixture containing recycled waste glass and red mud. Case Stud. Constr. Mater. 2024, 20, e03194. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization processes of bauxite residue: A com prehensive review. Materials 2021, 403, 123671. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ounoughene, G.; Borra, C.R.; Binnemans, K.; Van Gerven, T. Neutralisation of bauxite residue by carbon dioxide prior to acidic leaching for metal recovery. Miner. Eng. 2017, 112, 92–102. [Google Scholar] [CrossRef]
- Xue, S.-g.; Wu, Y.-j.; Li, Y.-w.; Kong, X.-f.; Zhu, F.; William, H.; Li, X.-f.; Ye, Y.-z. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review. J. Cent. South Univ. 2019, 26, 268–288. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 on the kinetics and microstructural development of white cement hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Wu, P.; Wei, C.; Liu, X.; Zhang, Z.; Xue, Y.; Liu, X. Impact of hematite in red mud on hydration characteristics and environmental performance of cementitious materials. Cem. Concr. Compos. 2025, 160, 106035. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Ge, Z.; Man, T.; Huppert, H.E. Infiltration characteristics of slurries in porous media based on the coupled Lattice-Boltzmann discrete element method. Comput. Geotech. 2025, 177, 106865. [Google Scholar] [CrossRef]
- ASTM C1437-20; Standard Test Method for the Flow of Hydraulic Cement Mortar. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM C191-21; Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Li, Z.; Afshinnia, K.; Rangaraju, P.R. Effect of alkali content of cement on properties of high performance cementitious mortar. Constr. Build. Mater. 2016, 102, 631–639. [Google Scholar] [CrossRef]
- ASTM C349-18; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Kang, S.; Kang, H.; Lee, B. Hydration properties of cement with liquefied red mud neutralized by nitric acid. Materials 2021, 14, 2641. [Google Scholar] [CrossRef]
- Kang, S.; Kang, H.; Lee, B. Effects of adding neutralized red mud on the hydration properties of cement paste. Materials 2020, 13, 4107. [Google Scholar] [CrossRef]
- Kang, S.P.; Kwon, S.J. Effects of red mud and alkali-activated slag cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Sun, H.; Zhang, J.; Li, L. Structural investigation relating to the cementitious activity of bauxite residue—Red mud. Cem. Concr. Res. 2011, 41, 847–853. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Mechanical properties of concrete containing liquefied red mud subjected to uniaxial compression loads. Materials 2020, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, Y.; Cui, M.; Yang, J.; Wang, H.; Ma, X.; Chen, Y. Insights into the effect of NaOH on the hydration products of solidified cement-NaNO3 matrices and leaching behavior of Sr2+. Sci. Total Environ. 2021, 755, 142581. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Characterization of slag cement mortar containing nonthermally treated dried Red Mud. Appl. Sci. 2019, 9, 2510. [Google Scholar] [CrossRef]

| Type | Chemical Composition (wt.%) | Moisture Content Ratio (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | MnO | Cr2O3 | ||
| Red mud sludge | 38.8 | 16.1 | 22.8 | 3.4 | 0.21 | 0.29 | 10.0 | 0.4 | 6.2 | 0.1 | 0.1 | 36 |
| Type | Blaine (cm2/g) | Setting Time | Density (g/cm3) | Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial (min) | Final (h) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Lg. Loss | |||
| OPC * | 3300 | 200 | 5.5 | 3.15 | 21.7 | 5.7 | 3.2 | 63.1 | 2.8 | 2.2 | 1.3 |
| Type | Color | Specific Gravity | Residue Content (%) | Viscosity (cP) | NaCl (wt.%) | Moisture (wt.%) |
|---|---|---|---|---|---|---|
| Polycarboxylic acid series | Light brown | 1.136 | 40.7 | 180 | - | - |
| Methyl cellulose | White | - | - | 32,900 | 1.36 | 1.40 |
| Mix ID | W/C (%) | Mix Design (g) | |||||
|---|---|---|---|---|---|---|---|
| Cement | Sand | Water | LRM | LRM+N | LRM+S | ||
| Plain | 50 | 100 | 300 | 50 | - | - | - |
| LM5 * | 95 | 45.27 | 9.72 | - | - | ||
| LM10 | 90 | 40.54 | 19.46 | - | - | ||
| NRM5 ** | 95 | 45.51 | - | 9.49 | - | ||
| NRM10 | 90 | 41.02 | - | 18.98 | - | ||
| SRM5 | 95 | 46.06 | - | 8.94 | |||
| SRM10 | 90 | 42.12 | - | 17.88 | |||
| Type of Red Mud | Moisture Content (%) | pH | Density (g/cm3) | Viscosity (cP) |
|---|---|---|---|---|
| LRM | 48.6 | 11.5 | 1.50 | 36,670 |
| LRM+N | 47.3 | 7.5 | 1.50 | 43,650 |
| LRM+S | 44.1 | 7.6 | 1.54 | 60,670 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-P.; Kim, S.-J.; Lee, B.-K.; Kang, H.-J. Effects of Red Mud on Cement Mortar Based on Sodium Salt Type. Materials 2025, 18, 3563. https://doi.org/10.3390/ma18153563
Kang S-P, Kim S-J, Lee B-K, Kang H-J. Effects of Red Mud on Cement Mortar Based on Sodium Salt Type. Materials. 2025; 18(15):3563. https://doi.org/10.3390/ma18153563
Chicago/Turabian StyleKang, Suk-Pyo, Sang-Jin Kim, Byoung-Ky Lee, and Hye-Ju Kang. 2025. "Effects of Red Mud on Cement Mortar Based on Sodium Salt Type" Materials 18, no. 15: 3563. https://doi.org/10.3390/ma18153563
APA StyleKang, S.-P., Kim, S.-J., Lee, B.-K., & Kang, H.-J. (2025). Effects of Red Mud on Cement Mortar Based on Sodium Salt Type. Materials, 18(15), 3563. https://doi.org/10.3390/ma18153563





