Effects of Graphene-Based Nanomaterials on Anaerobic Digestion of Thermally Hydrolyzed Municipal Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene-Based Nanomaterials, Substrate, and Inoculum
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Kinetic Analysis
2.5. Calculations and Statistical Analysis
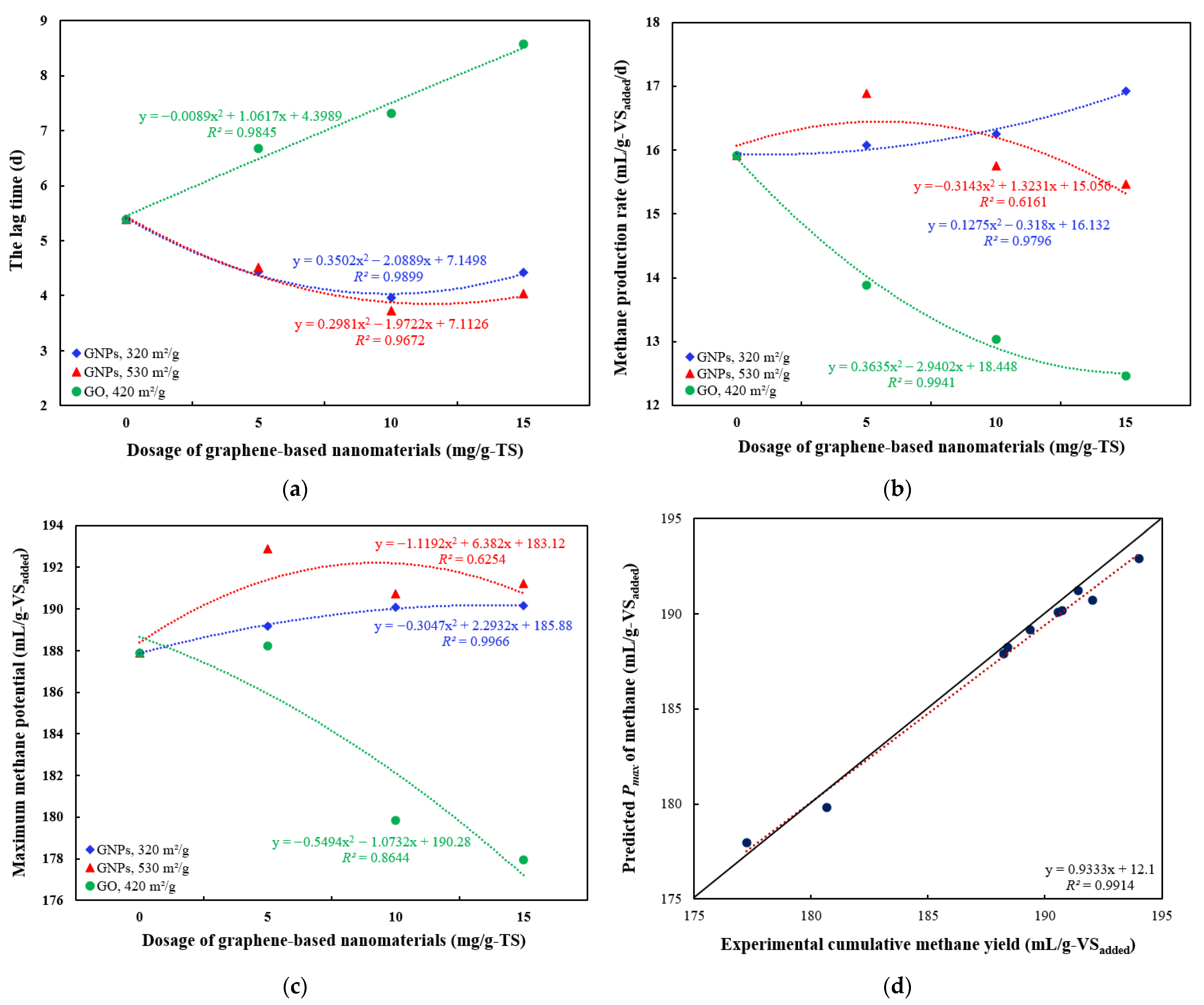
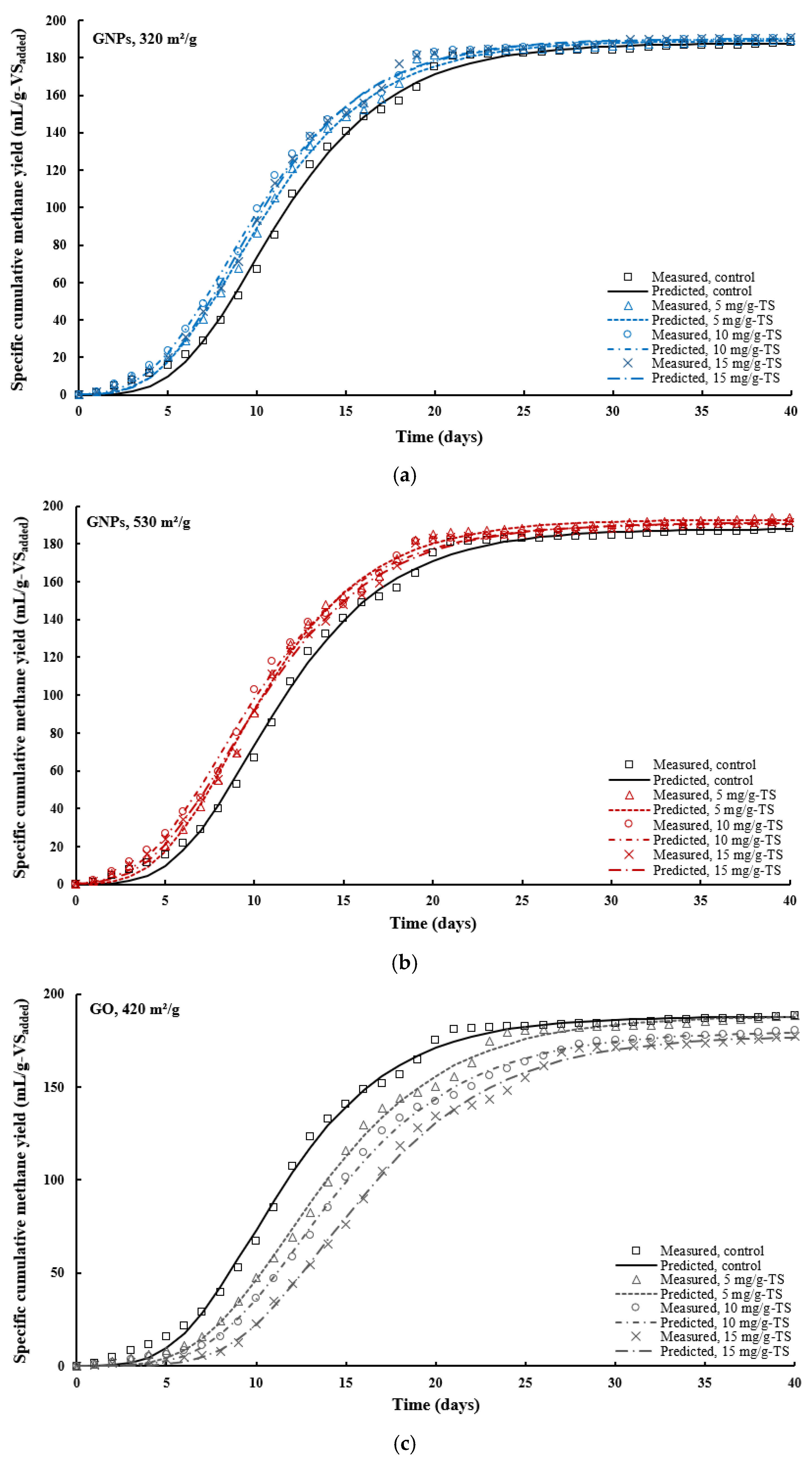
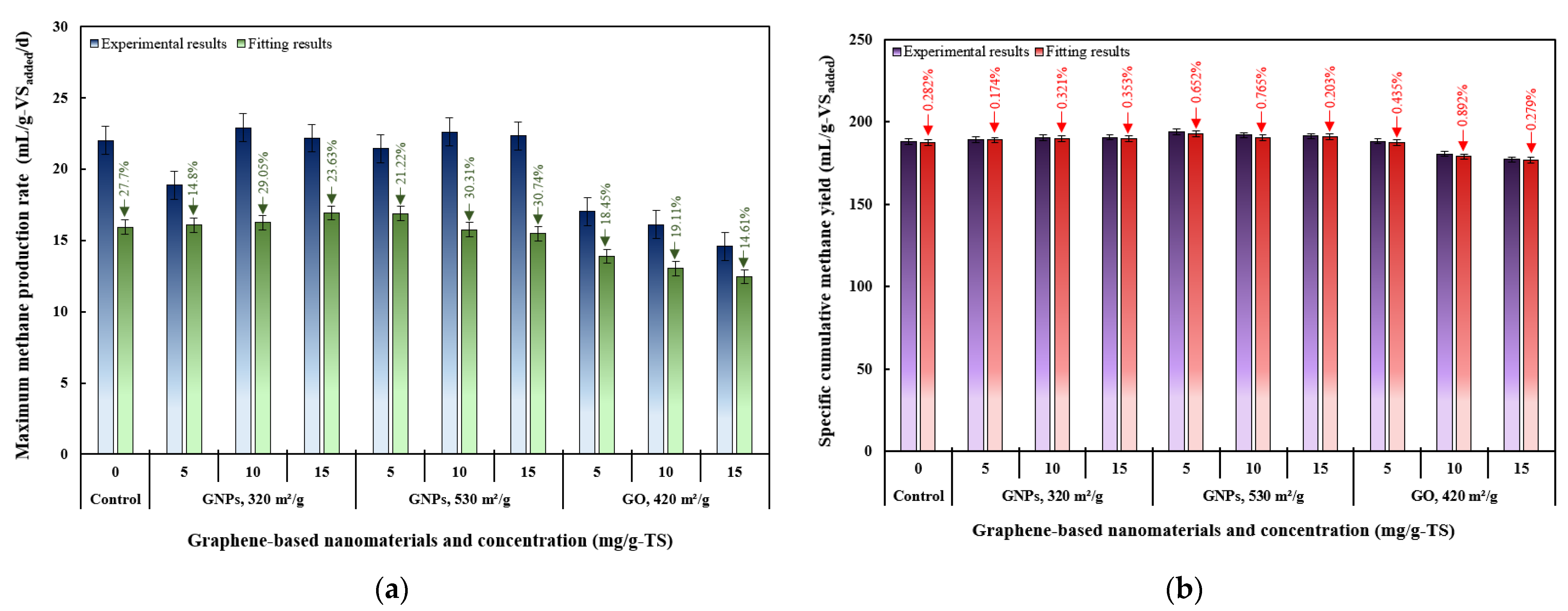
3. Results and Discussion
3.1. Characterization of Graphene and Graphene Oxide
3.2. Effects of Graphene and Graphene Oxide on AD Performance
3.3. Effects of Graphene-Based Nanomaterials on Organic Matter Degradation
3.4. Kinetic Modeling Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GNPs | Graphene nanoplatelets |
| GO | Graphene oxide |
| SS | Sewage sludge |
| THSS | Thermally hydrolyzed sewage sludge |
| AD | Anaerobic digestion |
| WWTP | Wastewater treatment plant |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive spectroscopy |
| VFAs | Volatile fatty acids |
| DIET | Direct interspecies electron transfer |
| GAC | Granular activated carbon |
| CNPs | Carbonaceous nanoparticles |
| TS | Total solids |
| VS | Volatile solids |
| COD | Chemical oxygen demand |
| SSA | Specific surface area |
| MGM | Modified Gompertz model |
| RMSE | Root mean square error |
| NRMSE | Normalized root mean square error |
| SCBY | Specific cumulative biogas yield |
| SCMY | Specific cumulative methane yield |
| FTIR | Fourier transform infrared |
References
- Ponzelli, M.; Koch, K.; Drewes, J.E.; Radjenovic, J.; Vinardell, S. The ambivalent role of graphene oxide in anaerobic digestion: A review. Bioresour. Technol. 2024, 414, 131663. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.A.; Tyagi, V.K. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; He, J.; Zhang, Y. Establishing direct interspecies electron transfer during laboratory-scale anaerobic digestion of waste activated sludge via biological ethanol-type fermentation pretreatment. ACS Sustain. Chem. Eng. 2018, 6, 13066–13077. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodward, T.L.; Nevin, K.P.; Loveley, D.P. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982. [Google Scholar] [CrossRef]
- Yadav, N.; Mohanakrishna, G.; Gandu, R.; Cahan, R.; Gandu, B. Enhancing anaerobic digestion of food waste for biogas production: Impact of graphene nanoparticles and multiwalled nanotubes on direct interspecies electron transfer mechanism. Process Saf. Environ. Prot. 2024, 191, 2335–2349. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Gan, R.; Jis, H.; Yong, X.; Yong, Y.-C.; Wu, X.; Wei, P.; Zhou, J. Enhanced biogas production from swine manure anaerobic digestion via in-situ formed graphene in electromethanogenesis system. Chem. Eng. J. 2020, 389, 124510. [Google Scholar] [CrossRef]
- Zhu, H.; Han, Y.; Ma, W.; Han, H.; Ma, W.; Xu, C. New insights into enhanced anaerobic degradation of coal gasification wastewater (CGW) with the assistance of graphene. Bioresour. Technol. 2018, 262, 302–309. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Z.; Wang, D.; Wang, Y.; Lu, Y. Enhance wastewater biological treatment through the bacteria induced graphene oxide hydrogel. Chemosphere 2018, 190, 201–210. [Google Scholar] [CrossRef]
- Bueno-Lopez, J.I.; Nguyen, C.H.; Rangel-Mendez, J.R.; Sierra-Alvarez, R.; Field, J.A.; Cervantes, F.J. Effects of graphene oxide and reduced graphene oxide on acetoclastic, hydrogenothrophic and methylotrophic methanogenesis. Biodegradation 2020, 31, 35–45. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Si, B.; Watson, J.; Zhang, Y. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Lin, P.; Li, J.; Dong, H.; Yu, H.; Qi, L.; Ren, L. Effects of graphite, graphene, and graphene oxide on the anaerobic co-digestion of sewage sludge and food waste: Attention to methane production and the fate of antibiotic resistance genes. Bioresour. Technol. 2021, 339, 125585. [Google Scholar] [CrossRef]
- Kundu, D.; Banerjee, S.; Karmakar, S.; Banerjee, R. A new insight on improved biomethanation using graphene oxide from fermented Assam lemon waste. Fuel 2022, 309, 122195. [Google Scholar] [CrossRef]
- Dong, B.; Xia, Z.; Sun, J.; Dai, X.; Chen, X.; Ni, B.-J. The inhibitory impacts of nano-graphene oxide on methane production from waste activated sludge in anaerobic digestion. Sci. Total Environ. 2019, 646, 1376–1384. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Y.; Zhong, H.; Sui, Q.; Zhang, C.; Wei, Y. Effects of graphene oxide on the performance, microbial community dynamics and antibiotic resistance genes reduction during anaerobic digestion of swine manure. Bioresour. Technol. 2017, 245, 850–859. [Google Scholar] [CrossRef]
- Lu, D.; Sun, F.; Zhou, Y. Insights into anaerobic transformation of key dissolved organic matters produced by thermal hydrolysis sludge pretreatment. Bioresour. Technol. 2018, 266, 60–67. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Zhao, L.; Lu, J.; Dong, H.; Yu, H.; Qi, L.; Ren, L. New insights of anaerobic performance, antibiotic resistance gene removal, microbial community structure: Applying graphite-based materials in wet anaerobic digestion. Environ. Technol. 2023, 44, 2781–2794. [Google Scholar] [CrossRef]
- Senol, H. Effects of NaOH, thermal, and combined NaOH-thermal pretreatments on the biomethane yields from the anaerobic digestion of walnut shells. Environ. Sci. Pollut. Res. 2021, 28, 21661–21673. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater 2540G, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Lin, Z.; Li, X.-X.; Sun, Z.-Y.; Gou, M.; Tang, Y.-Q. Straw addition improves the stability and efficiency of high-solids anaerobic digestion of dewatered sludge via creating balanced function microbial community. J. Environ. Chem. Eng. 2025, 13, 116773. [Google Scholar] [CrossRef]
- Atri, S.; Loni, E.; Zazimal, F.; Hensel, K.; Caplovicova, M.; Plesch, G.; Monfort, O. MXene-derived oxide nanoheterostructures for photocatalytic sulfamethoxazole degradation. ACS Appl. Nano Mater. 2024, 7, 16506–16515. [Google Scholar] [CrossRef]
- Dash, K.; Panda, D.K.; Yadav, K.; Meher, S.; Mishra, M. 2D material graphene as a potential antidiabetic and nontoxic compound in Drosophila melanogaster. Appl. Nanosci. 2024, 14, 423–439. [Google Scholar] [CrossRef]
- Ponzelli, M.; Zahedi, S.; Koch, K.; Drewes, J.E.; Radjenovic, J. Rapid biological reduction of graphene oxide: Impact on methane production and micropollutant transformation. J. Environ. Chem. Eng. 2022, 10, 108373. [Google Scholar] [CrossRef]
- Wrange, A.-L.; Andre, C.; Lundh, T.; Lind, U.; Blomberg, A.; Jonsson, P.J.; Havenhand, J.N. Importance of plasticity and local adaptation for coping with changing salinity in coastal areas: A test case with barnacles in the Baltic Sea. BMC Ecol. Evol. 2014, 14, 156. [Google Scholar] [CrossRef]
- Alkhrissat, T.; Matarneh, S. Impact of anaerobic co-digestion of ryegrass (RG) and cow manure (CM) on methane production and kinetic analysis. Results Eng. 2025, 25, 104028. [Google Scholar] [CrossRef]
- Leite, V.D.; Ramos, R.O.; Lopes, W.S.; Araujo, M.C.U.; Almeida, V.E.; Oliveira, N.M.; Viriato, C.L. Kinetic modeling of anaerobic co-digestion of plant solid waste with sewage sludge: Synergistic influences of total solids and substrate particle size in biogas generation. BioEnergy Res. 2024, 17, 744–755. [Google Scholar] [CrossRef]
- Gomaa, S.; Abdalla, M.; Salem, K.G.; Nasr, K.; Emara, R.; Wang, O.; El-Houshoudy, A.N. Machine learning prediction of methane, nitrogen, and natural gas mixture viscosities under normal and harsh conditions. Sci. Rep. 2024, 14, 15155. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Enhancing the production of biogas through anaerobic co-digestion of agricultural waste and chemical pre-treatments. Chemosphere 2020, 255, 126805. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Sameraie, L.; Bsoul, A.A.; Khasawneh, A.; Al-Tabbal, J. Correlation between kinetic parameters, reactor performance, and biogas and methane potential of co-digestion and mono-digestion of active sludge and olive mill wastewater. Int. J. Low Carbon Technol. 2024, 19, 1501–1515. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; Aquino, S.F. Influence of different thermal pretreatments and inoculum selection on the biomethanation of sugarcane bagasse by solid-state anaerobic digestion: A kinetic analysis. Ind. Crops Prod. 2018, 111, 684–693. [Google Scholar] [CrossRef]
- Shen, X.; Lin, X.; Yousefi, N.; Jia, J.; Kim, J.K. Wrinkling in graphene sheets and graphene oxide papers. Carbon 2014, 66, 84–92. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-friendly approach for graphene oxide synthesis by modified hummers method. Materials 2022, 15, 7228. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Cheng, J.; Xia, A.; Lens, P.N.L.; Jackson, S.A.; Dobson, A.D.W.; Murphy, J.D. Graphene facilitates biomethane production from protein-derived glycine in anaerobic digestion. iScience 2018, 10, 158–170. [Google Scholar] [CrossRef]
- Bueno-Lopez, J.I.; Rangel-Mendez, R.; Alatriste-Mondragon, F.; Perez-Rodriguez, F.; Hernandez-Montoya, V.; Cervantes, F.J. Graphene oxide triggers mass transfer limitations on the methanogenic activity of an anaerobic consortium with a particulate substrate. Chemosphere 2018, 211, 709–716. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Rodrigues, D.F. Chronic toxicity of graphene and graphene oxide in sequencing batch bioreactors: A comparative investigation. J. Hazard. Mater. 2018, 343, 200–207. [Google Scholar] [CrossRef]
- Casabella-Font, O.; Zahedi, S.; Gros, M.; Balcazar, J.L.; Radjenovic, J.; Pijuan, M. Graphene oxide addition to anaerobic digestion of waste activated sludge: Impact on methane production and removal of emerging contaminants. Environ. Pollut. 2023, 324, 121343. [Google Scholar] [CrossRef]
- Casabella-Font, O.; Riva, M.; Balcazar, J.L.; Radjenovic, J.; Pijuan, M. Distinctive effects of graphene oxide and reduced graphene oxide on methane production kinetics and pharmaceuticals removal in anaerobic reactors. Bioresour. Technol. 2024, 403, 130849. [Google Scholar] [CrossRef]
- Perrault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: Why size matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y.; Xiao, N. Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion. Bioresour. Technol. 2011, 102, 10305–10311. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Ma, C.; White, J.C.; Zhao, Z.; Duan, C.; Zhang, Y.; Adeel, M.; Rui, Y.; Li, G.; et al. Carbon nanomaterials induce residue degradation and increase methane production from livestock manure in an anaerobic digestion system. J. Clean. Prod. 2019, 240, 118257. [Google Scholar] [CrossRef]
- Muratçobanoğlu, H.; Gökçek, Ö.B.; Mert, R.A.; Zan, R.; Demirel, S. The impact of reduced graphene oxide (rGO) supplementation on cattle manure anaerobic digestion: Focusing on process performance and microbial syntrophy. Biochem. Eng. J. 2021, 173, 108080. [Google Scholar] [CrossRef]
- Muratçobanoğlu, H.; Gökçek, Ö.B.; Muratçobanoğlu, F.; Mert, R.A.; Demirel, S. Biomethane enhancement using reduced graphene oxide in anaerobic digestion of municipal solid waste. Bioresour. Technol. 2022, 354, 127163. [Google Scholar] [CrossRef]
- Fazzino, F.; Frontera, P.; Malara, A.; Pedulla, A.; Calabro, P.S. Effects of carbon-based conductive materials on semi-continuous anaerobic co-digestion of organic fraction of municipal solid waste and waste activated sludge. Chemosphere 2024, 357, 142077. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, X.; Xu, X.; Yan, X.; Bi, F.; Wu, W. Specific surface area and electron donating capacity determine biochar’s role in methane production during anaerobic digestion. Bioresour. Technol. 2020, 303, 122919. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Krause, M.C.; Moitinho, A.C.; Ferreira, L.F.R.; Souza, R.L.; Krause, L.C.; Caramao, E.B. Production and characterization of the bio-oil obtained by the fast pyrolysis of spent coffee grounds of the soluble coffee industry. J. Braz. Chem. Soc. 2019, 30, 1608–1615. [Google Scholar] [CrossRef]
- Petrovič, A.; Vohl, S.; Predikaka, T.C.; Bedoic, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of solid digestate from sewage sludge and lignocellulosic biomass: Kinetic and thermodynamic analysis, characterization of biochar. Sustainability 2021, 13, 9642. [Google Scholar] [CrossRef]
- Alghashm, S.; Qian, S.; Hua, Y.; Wu, J.; Zhang, H.; Chen, W.; Shen, G. Properties of biochar from anaerobically digested food waste and its potential use in phosphorus recovery and soil amendment. Sustainability 2018, 10, 4692. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Feng, Z.; Song, X.; Yu, Z. Seasonal and spatial distribution of matrix-bound phosphine and its relationship with the environment in the Changjiang River Estuary, China. Mar. Pollut. Bull. 2008, 56, 1630–1636. [Google Scholar] [CrossRef]
- Ran, X.; Uppuluri, N.S.T.; Deng, Y.; Zheng, Y.; Dong, R.; Muller, J.; Oechsner, H.; Li, B.; Guo, J. Comparison of phosphorus species in livestock manure and digestate by different detection techniques. Sci. Total Environ. 2023, 874, 162547. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, B.; Yao, J.; Peng, M.; Mao, L.; Zhang, W.; Shen, R. Degradation performance of estrogen during anaerobic digestion of pig manure. Waste Biomass Valorization 2024, 15, 2625–2635. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental investigation into the effect of pyrolysis on chemical forms of heavy metals in sewage sludge biochar (SSB), with brief ecological risk assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef]
- Farghali, M.; Mohamed, I.M.A.; Hassan, D.; Iwasaki, M.; Yoshida, G.; Umetsu, K.; Ihara, I. Kinetic modeling of anaerobic co-digestion with glycerol: Implications for process stability and organic overloads. Biochem. Eng. J. 2023, 199, 109061. [Google Scholar] [CrossRef]
- Alruqi, M.; Hanafi, H.A.; Sharma, P. Prognostic metamodel development for waste-derived biogas-powered dual-fuel engines using modern machine learning with K-cross fold validation. Fermentation 2023, 9, 598. [Google Scholar] [CrossRef]
- Ponzelli, M.; Radjenovic, J.; Drewes, J.E.; Koch, K. Enhanced methane production kinetics by graphene oxide in fed-batch tests. Bioresour. Technol. 2022, 360, 127642. [Google Scholar] [CrossRef] [PubMed]
- Almegbl, A.M.; Munshi, F.M.A.; Khursheed, A. Biogas enhancement in the anaerobic digestion of thermo-chemically pretreated sludge by stimulating direct interspecies electron transfer by biochar and graphene. Front. Chem. Eng. 2024, 6, 1419770. [Google Scholar] [CrossRef]
- Adelard, L.; Poulsen, T.G.; Rakotoniaina, V. Biogas and methane yield in response to co- and separate digestion of biomass wastes. Waste Manag. Res. 2015, 33, 55–62. [Google Scholar] [CrossRef] [PubMed]

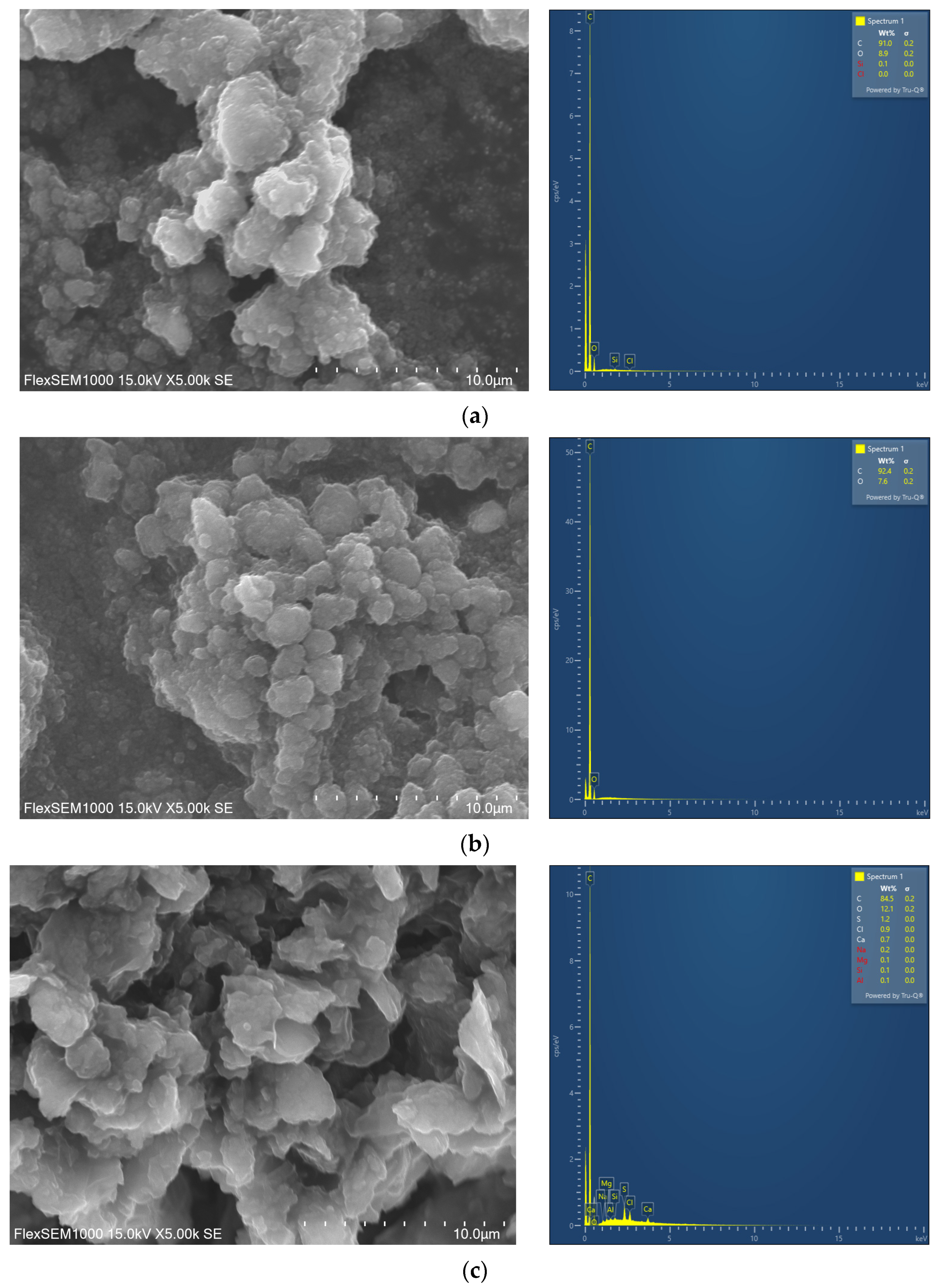
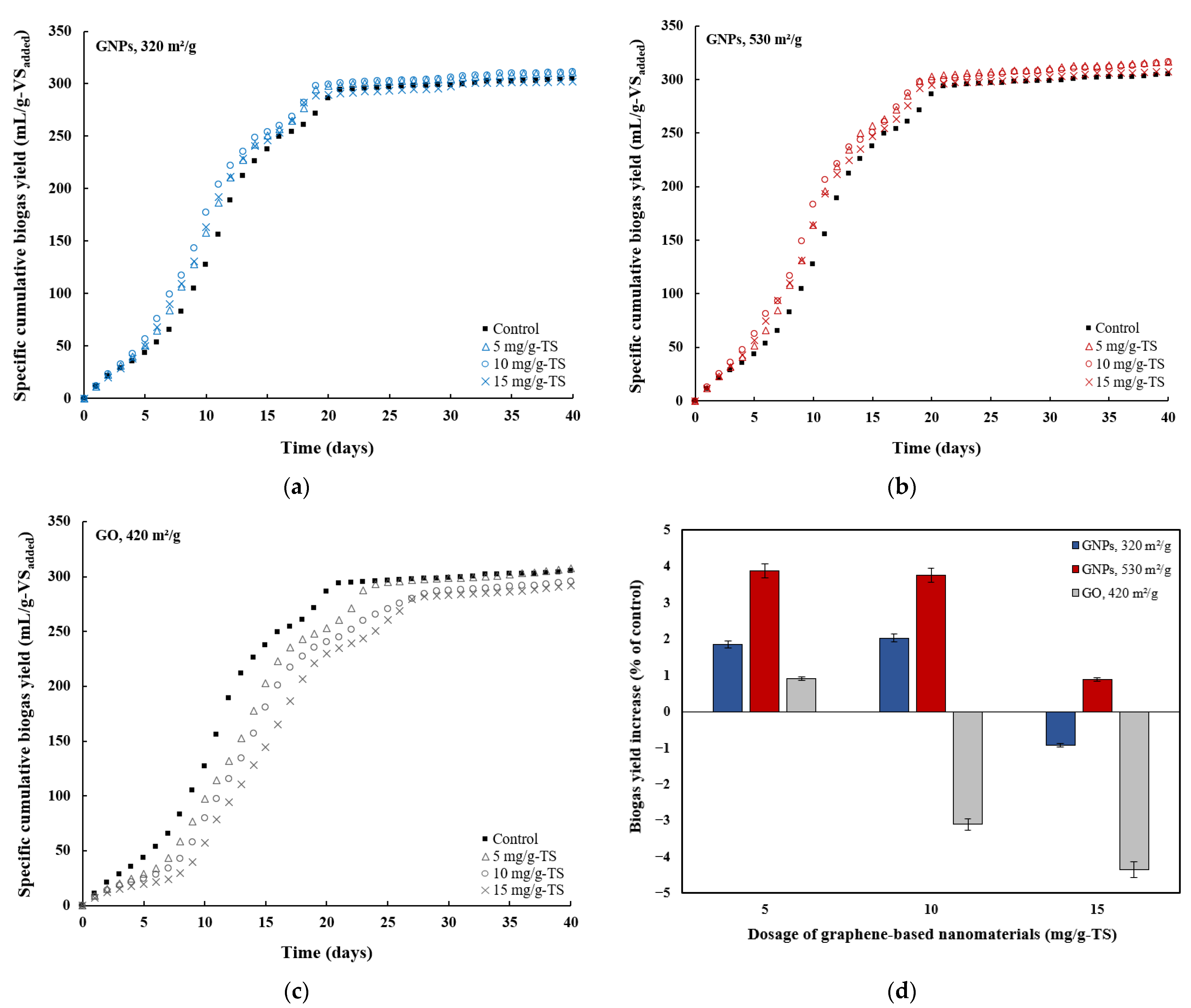
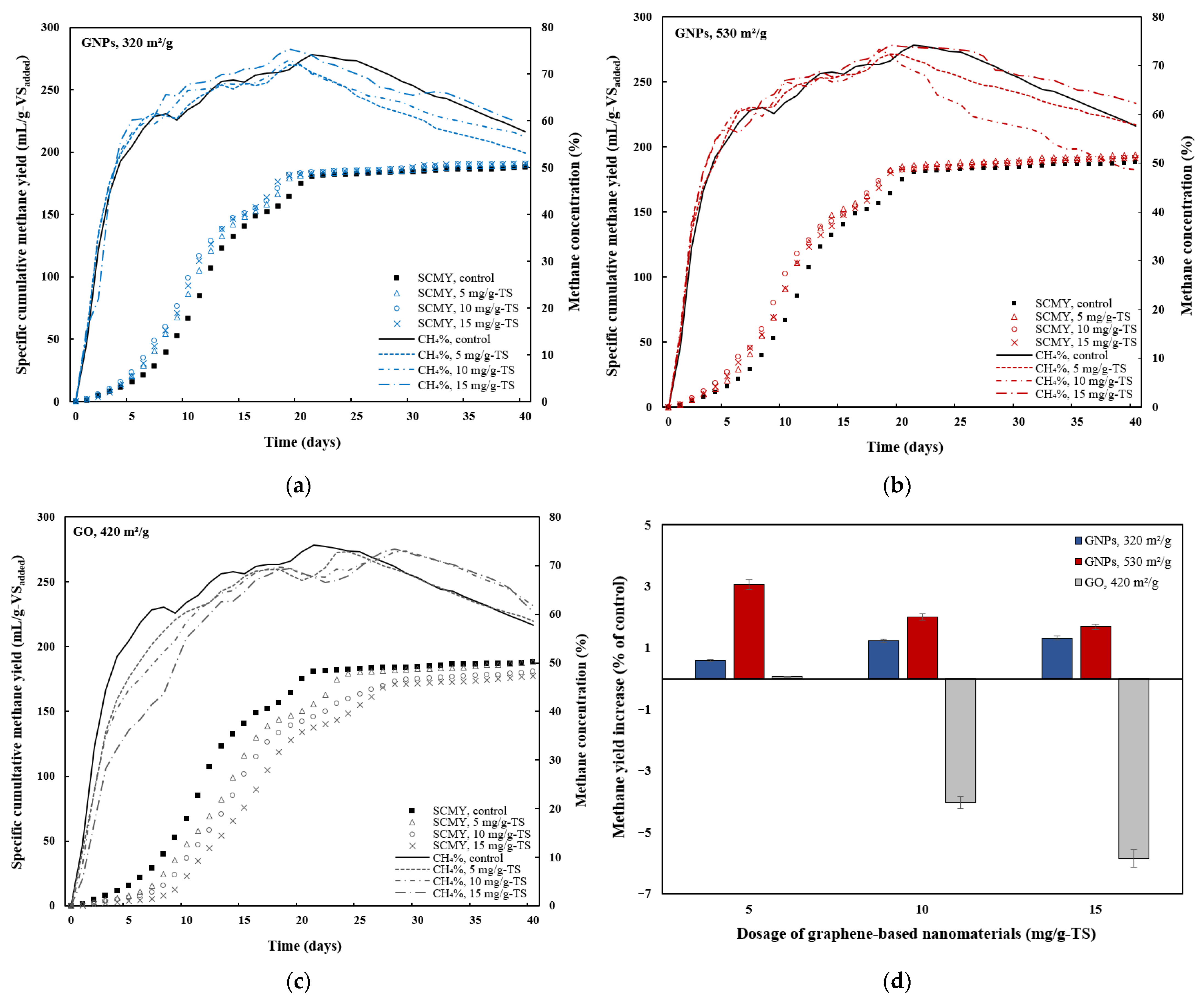

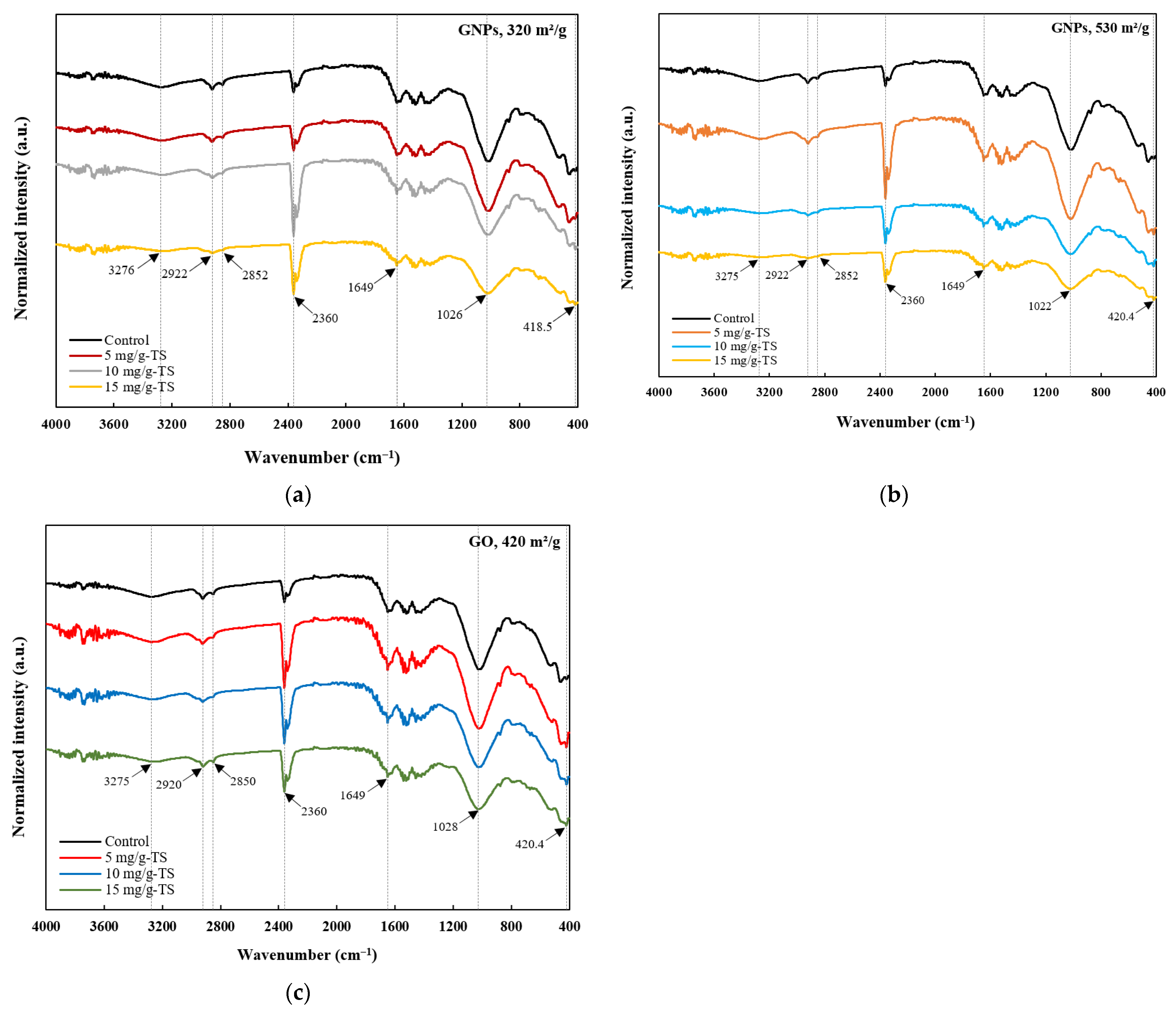
| Parameter | Substrate | Inoculum | Mixture |
|---|---|---|---|
| Total solids (TS, %) | 8.85 ± 0.14 | 4.59 ± 0.03 | 7.38 ± 0.05 |
| Volatile solids (VS, %) | 4.08 ± 0.03 | 2.64 ± 0.01 | 3.62 ± 0.01 |
| VS/TS (%) | 46.10 | 57.52 | 49.05 |
| Total chemical oxygen demand (COD, mg/L) | 70 912 ± 1281 | 45 133 ± 405 | 62 319 ± 597 |
| Electrical conductivity (mS/cm) | 2.45 ± 0.02 | 10.13 ± 0.02 | 5.36 ± 0.03 |
| pH | 5.26 ± 0.09 | 7.615 ± 0.05 | 7.009 ± 0.004 |
| C (%) | 25.33 ± 0.12 | 23.8 ± 0.10 | 24.82 ± 0.11 |
| N (%) | 3.72 ± 0.07 | 3.61 ± 0.06 | 3.69 ± 0.07 |
| C/N | 6.81 | 6.59 | 6.73 |
| Reactor Code | Added Substrate Amount (kg) | Added Inoculum Amount (kg) | Type of Graphene-Based Nanomaterial | Specific Surface Area, m2/g | Added Amount of Graphene-Based Nanomaterial (g) | Dosage of Graphene-Based Nanomaterial (mg/g-TS) |
|---|---|---|---|---|---|---|
| B0 | 1.334 | 0.667 | - | - | 0 | 0 |
| B1 | Graphene nanoplatelets (GNPs) | 320 | 0.738 | 5 | ||
| B2 | 1.476 | 10 | ||||
| B3 | 2.214 | 15 | ||||
| B4 | Graphene nanoplatelets (GNPs) | 530 | 0.738 | 5 | ||
| B5 | 1.476 | 10 | ||||
| B6 | 2.214 | 15 | ||||
| B7 | Graphene oxide (GO) nanosheets | 420 | 0.738 | 5 | ||
| B8 | 1.476 | 10 | ||||
| B9 | 2.214 | 15 |
| Statistical Parameter | Reactor Code | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B0 | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | |
| R2 | 0.994 | 0.989 | 0.986 | 0.988 | 0.989 | 0.986 | 0.987 | 0.998 | 0.999 | 0.999 |
| RMSE, mL/g-VSadded | 3.302 | 2.829 | 2.839 | 2.608 | 2.915 | 3.132 | 3.021 | 3.092 | 2.441 | 2.656 |
| NRMSE, % | 1.754 | 1.494 | 1.489 | 1.367 | 1.502 | 1.631 | 1.578 | 1.641 | 1.351 | 1.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usevičiūtė, L.; Januševičius, T.; Danila, V.; Pranskevičius, M. Effects of Graphene-Based Nanomaterials on Anaerobic Digestion of Thermally Hydrolyzed Municipal Sewage Sludge. Materials 2025, 18, 3561. https://doi.org/10.3390/ma18153561
Usevičiūtė L, Januševičius T, Danila V, Pranskevičius M. Effects of Graphene-Based Nanomaterials on Anaerobic Digestion of Thermally Hydrolyzed Municipal Sewage Sludge. Materials. 2025; 18(15):3561. https://doi.org/10.3390/ma18153561
Chicago/Turabian StyleUsevičiūtė, Luiza, Tomas Januševičius, Vaidotas Danila, and Mantas Pranskevičius. 2025. "Effects of Graphene-Based Nanomaterials on Anaerobic Digestion of Thermally Hydrolyzed Municipal Sewage Sludge" Materials 18, no. 15: 3561. https://doi.org/10.3390/ma18153561
APA StyleUsevičiūtė, L., Januševičius, T., Danila, V., & Pranskevičius, M. (2025). Effects of Graphene-Based Nanomaterials on Anaerobic Digestion of Thermally Hydrolyzed Municipal Sewage Sludge. Materials, 18(15), 3561. https://doi.org/10.3390/ma18153561







