Synergistic Effect of Calcination Temperature and Silver Doping on Photocatalytic Performance of ZnO Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Methods
2.2. Material Characterization
2.3. Photocatalytic Test
3. Results and Discussion
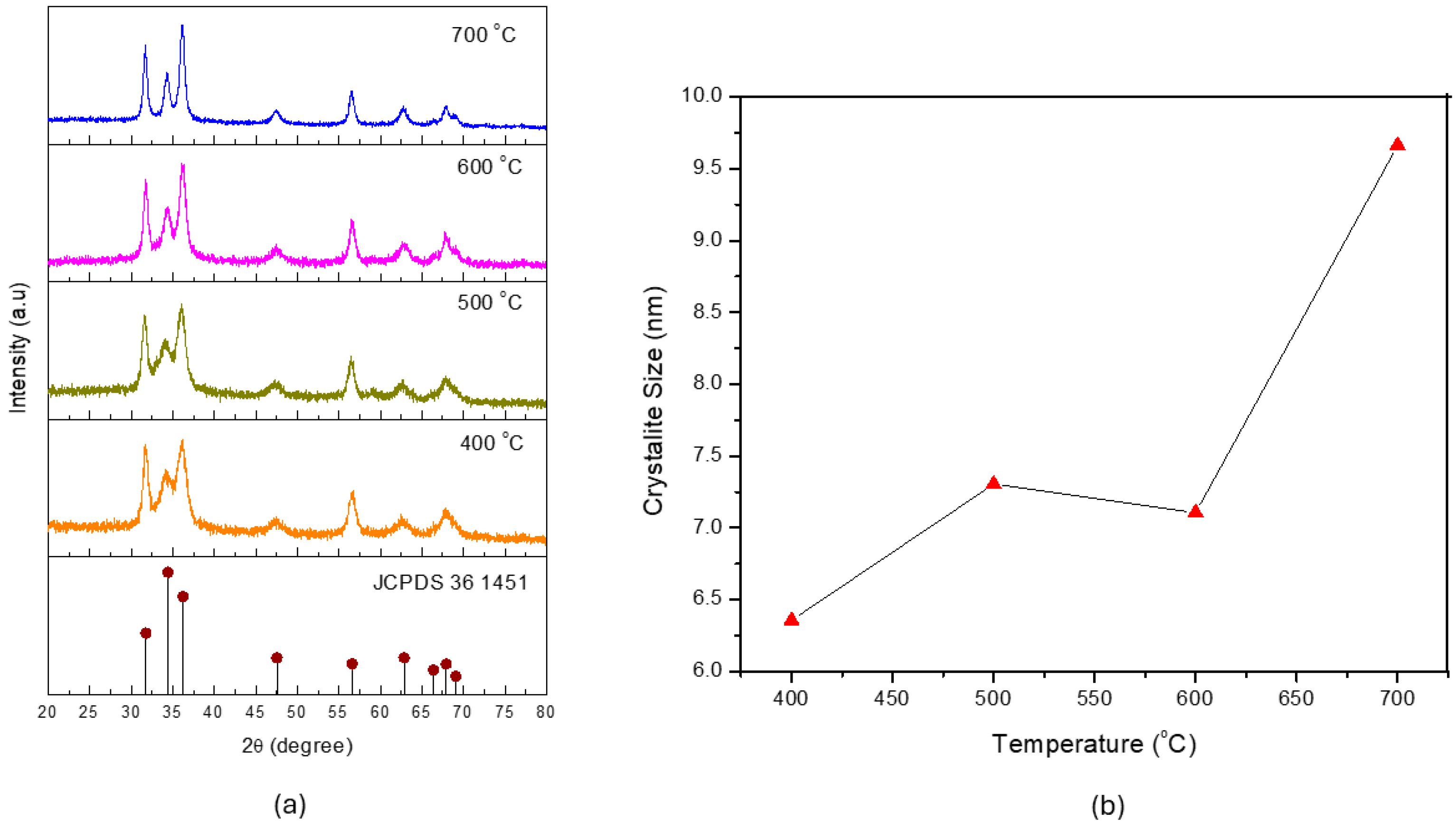
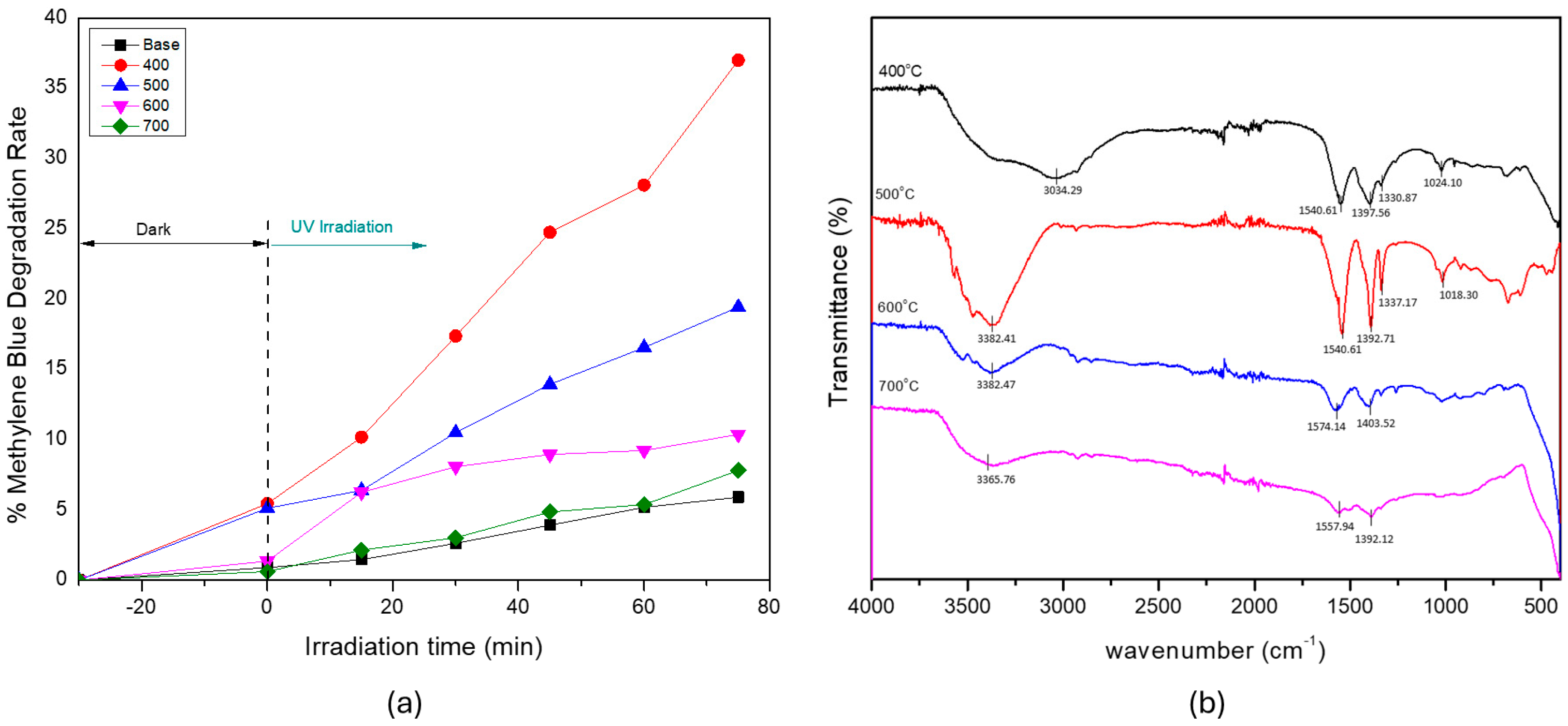
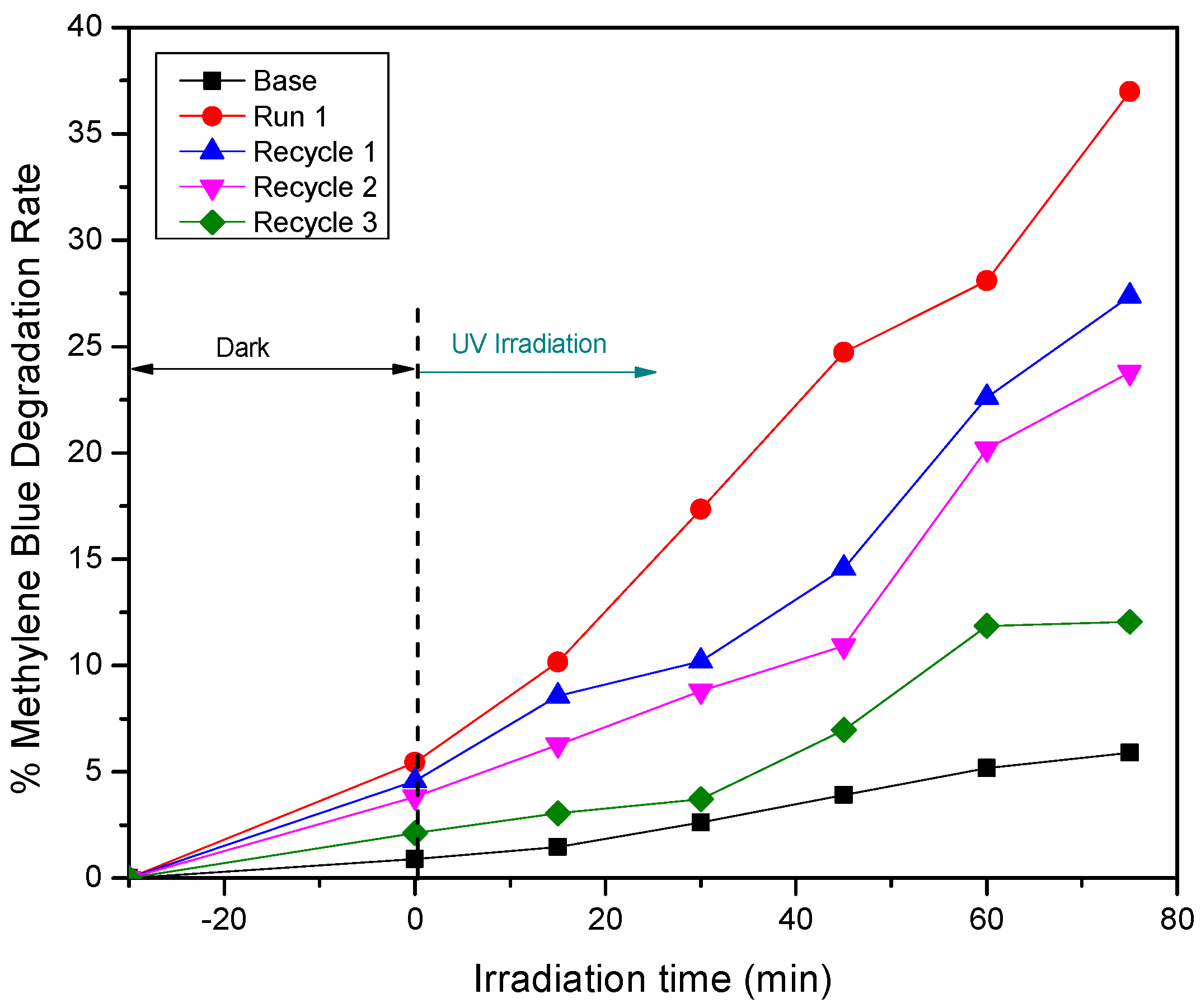
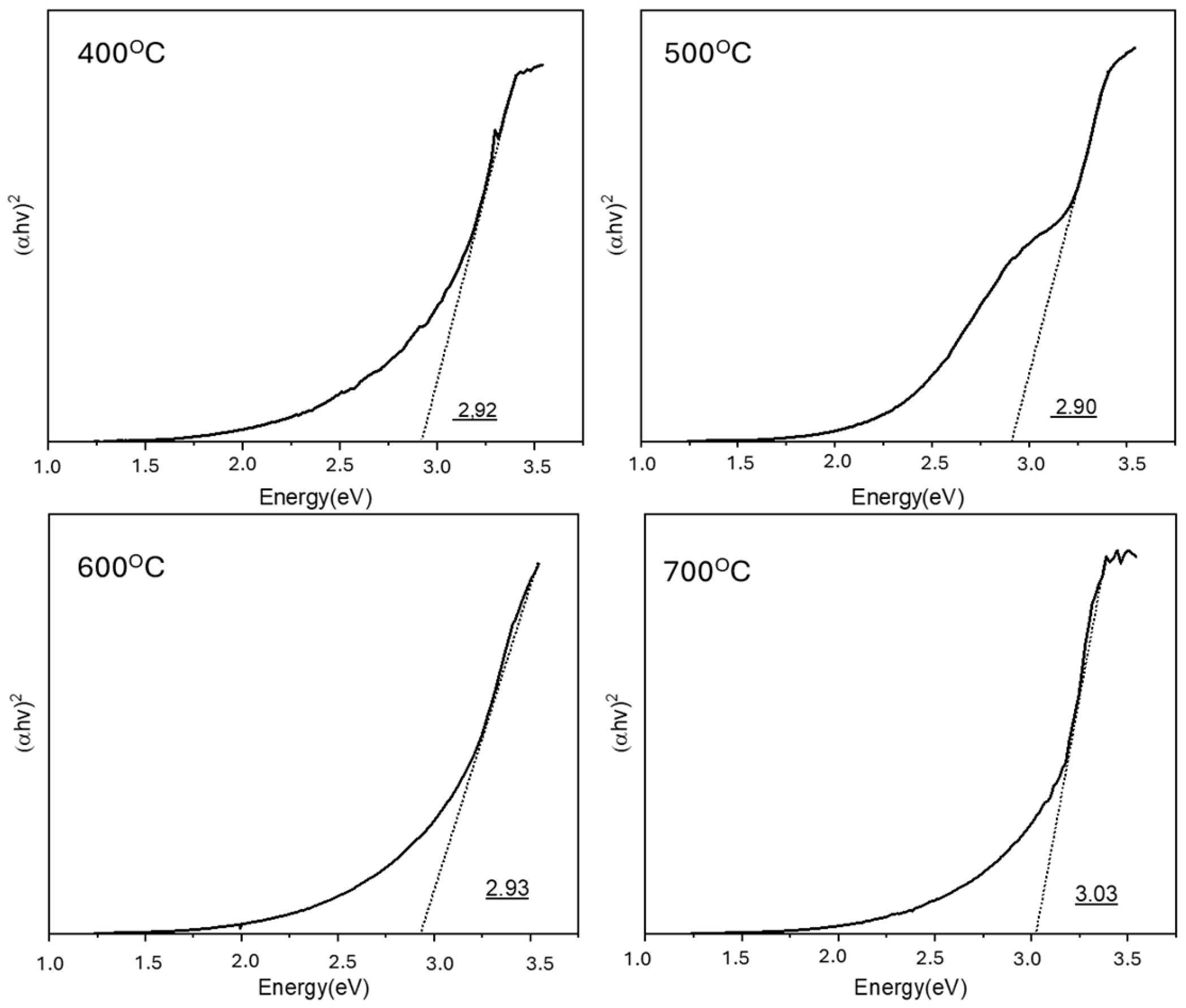
3.1. Effect of Calcination Temperature on ZnO’s Properties and Photocatalytic Activity
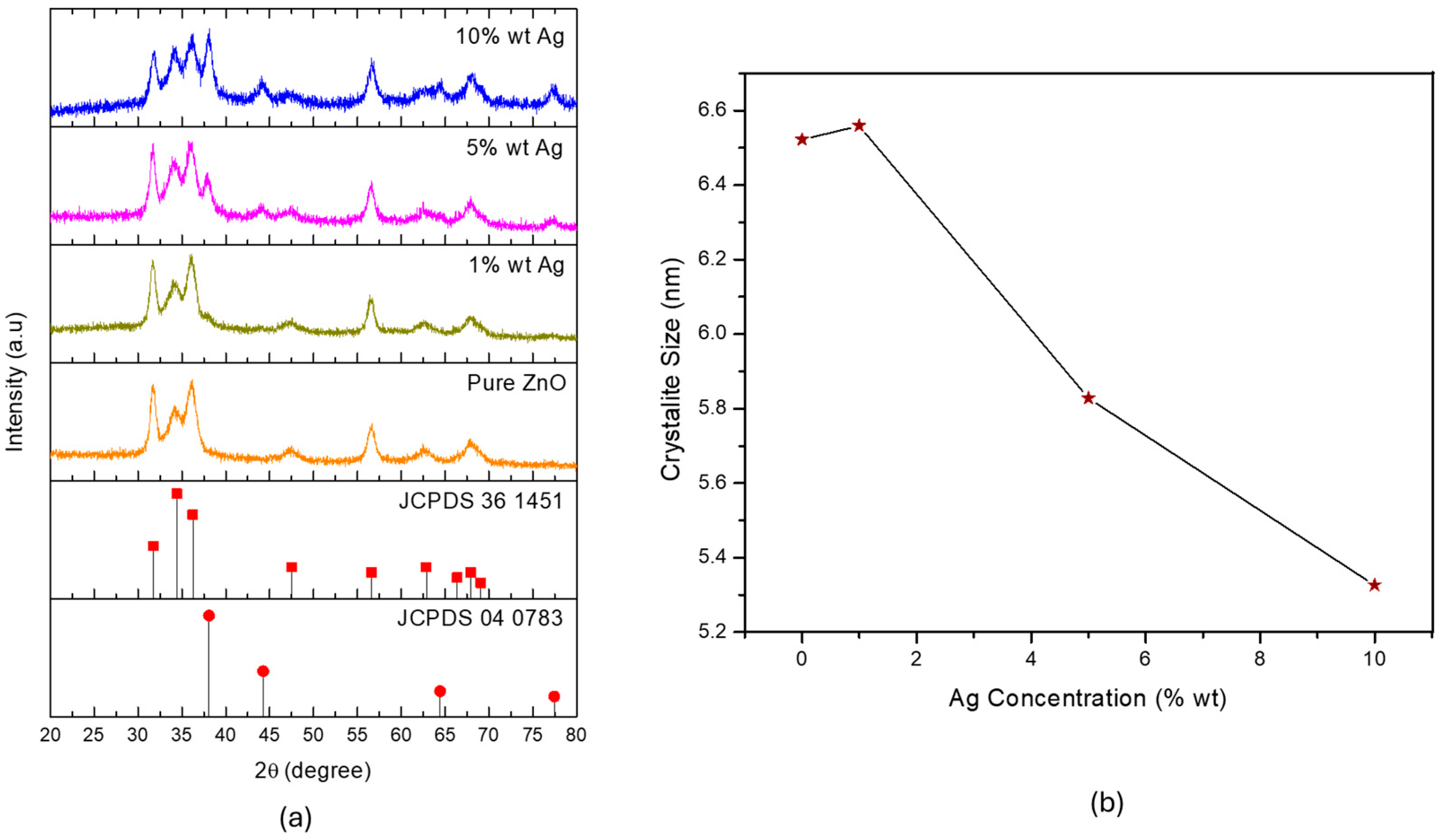
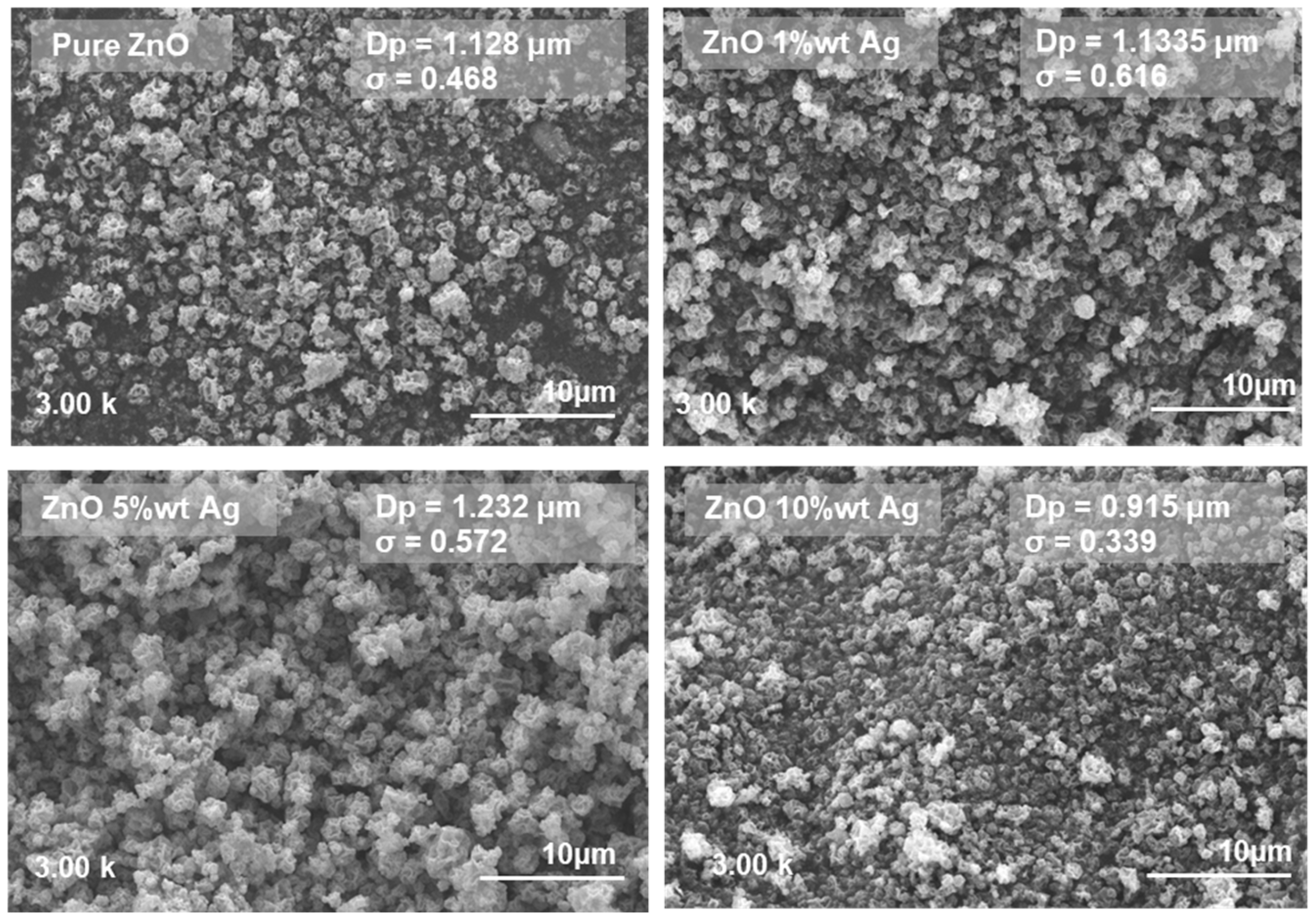
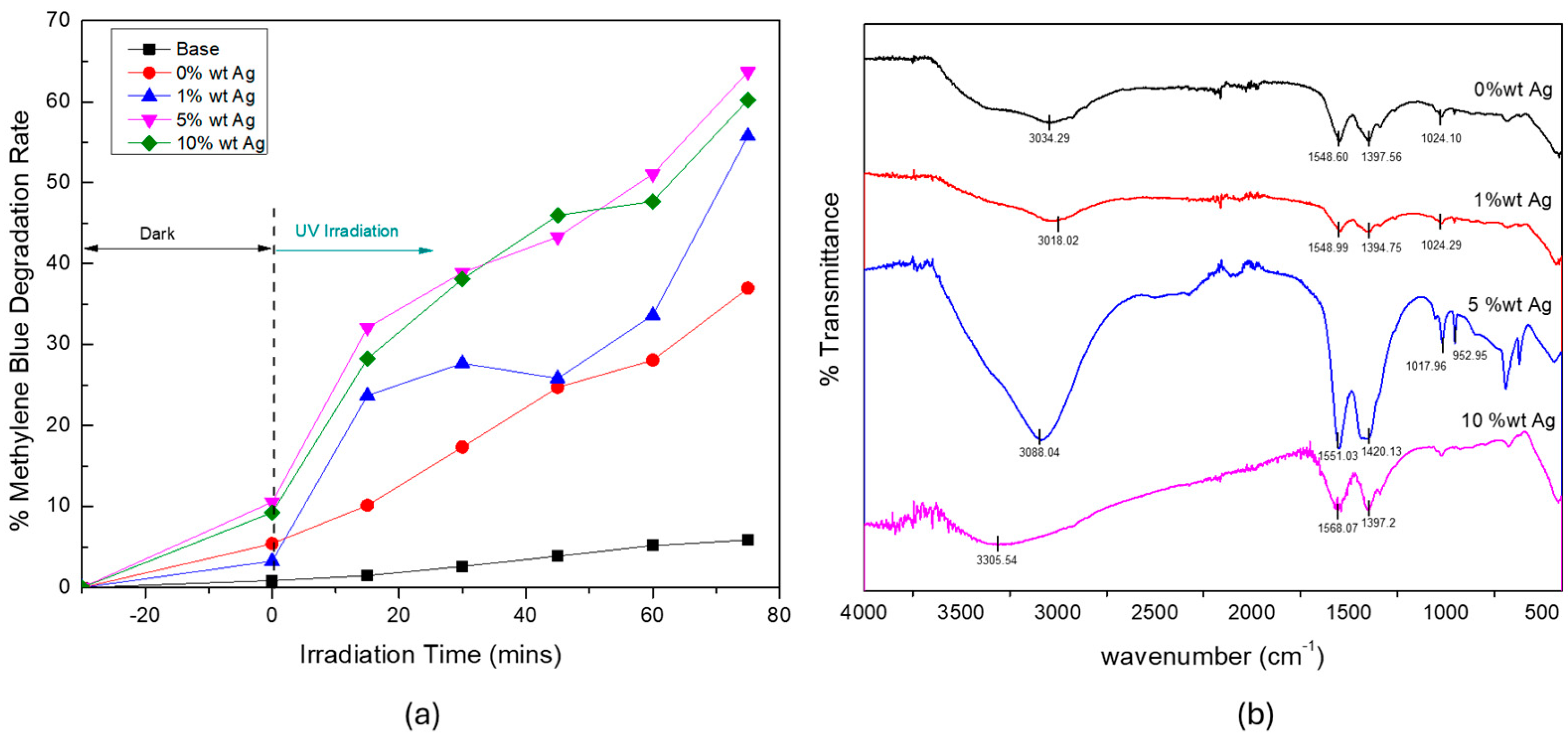
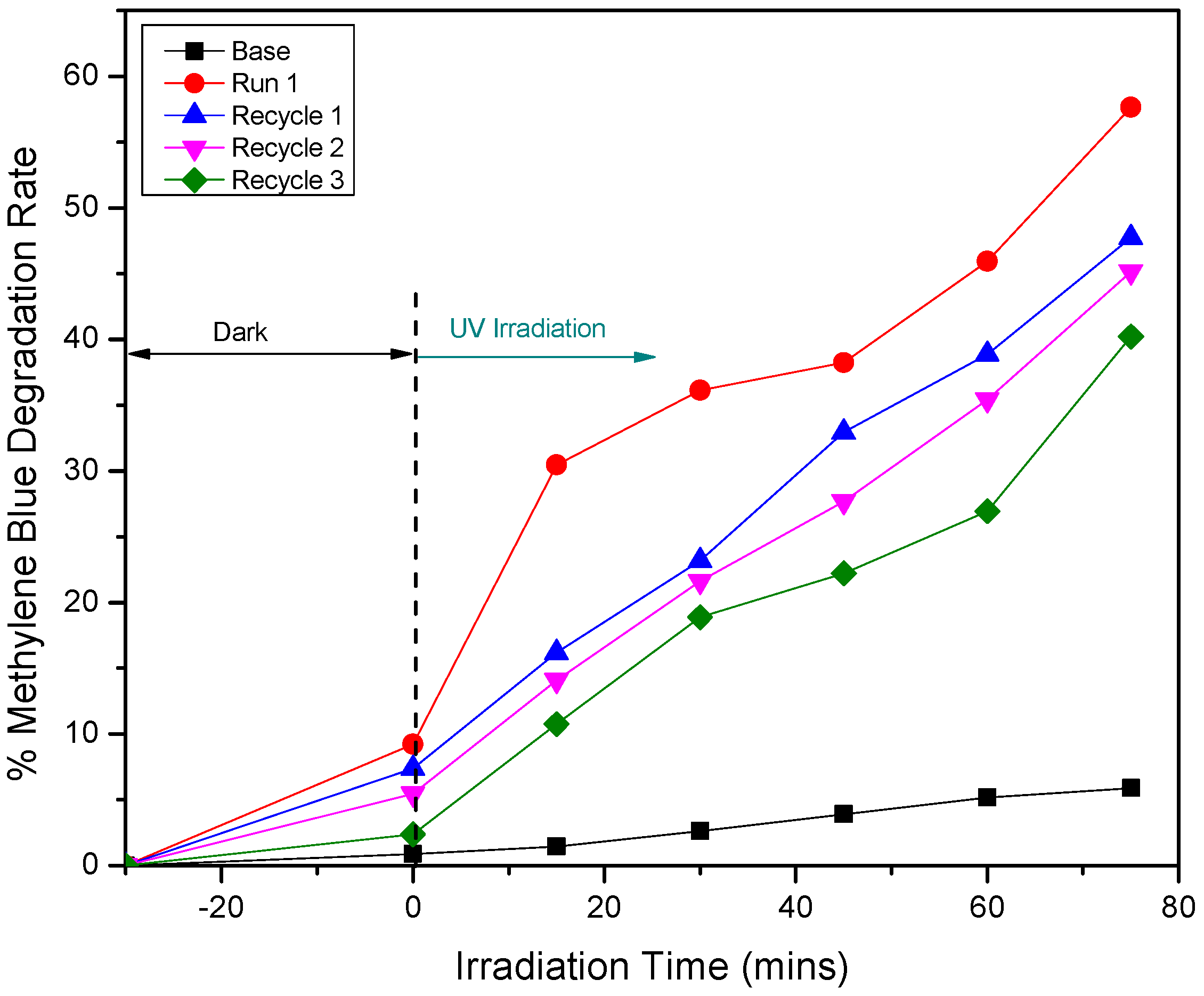
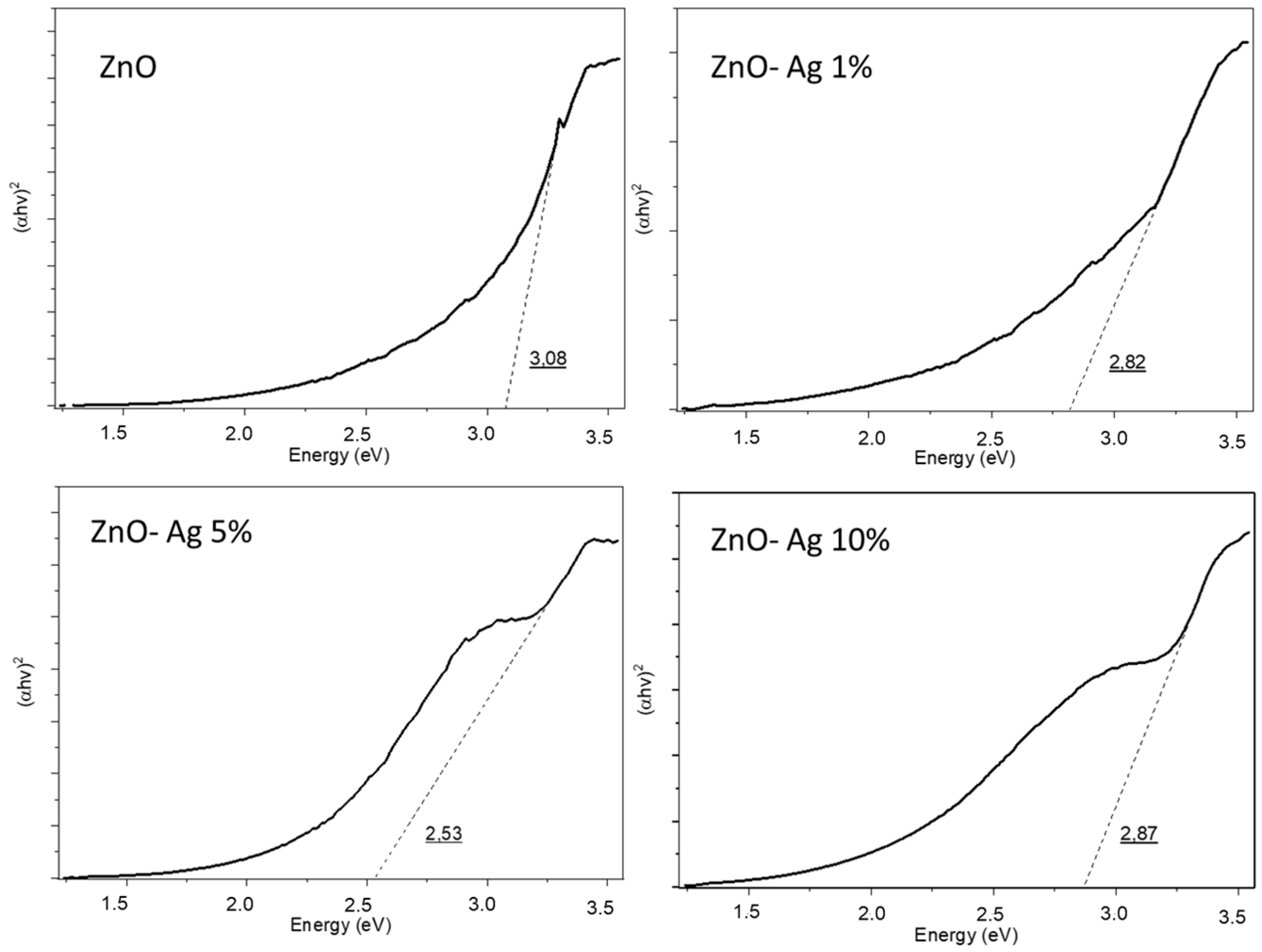
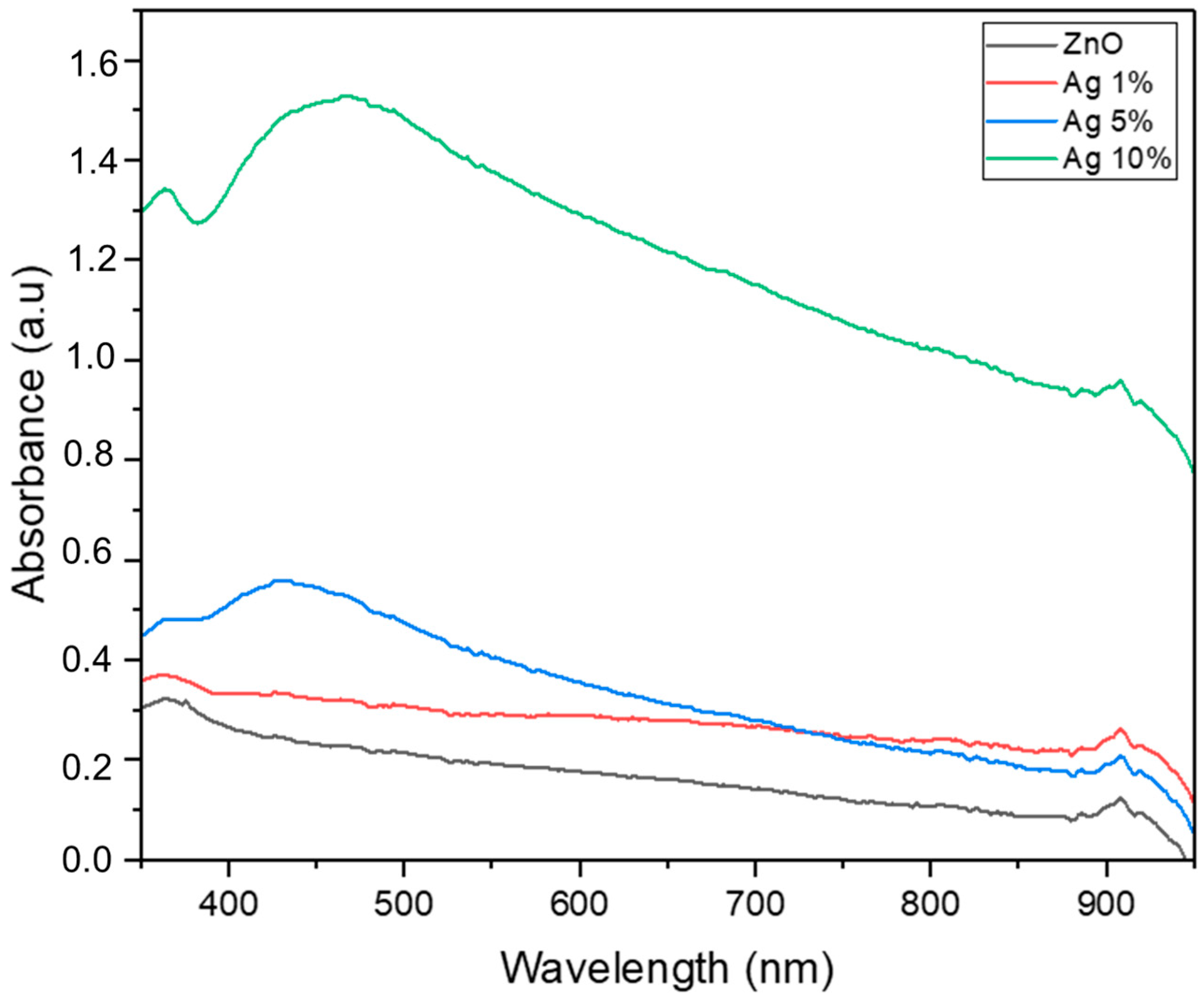
3.2. Effect of Silver Dopant Concentration on ZnO’s Properties and Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FTIR | Fourier transform infrared |
| FWHM | Full width at half maximum |
| LSPR | Localized surface plasmon resonance |
| MB | Methylene blue |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| USP | Ultrasonic spray pyrolysis |
| UV | Ultraviolet |
| XRD | X-ray diffraction |
References
- Liu, C.; Xiao, C.; Li, W. Zinc oxide nanoparticles as electron transporting interlayer in organic solar cells. J. Mater. Chem. C 2021, 9, 14093–14114. [Google Scholar] [CrossRef]
- Bhandari, K.P.; Sapkota, D.R.; Jamarkattel, M.K.; Stillion, Q.; Collins, R.W. Zinc Oxide Nanoparticles-Solution-Based Synthesis and Characterizations. Nanomaterials 2023, 13, 1795. [Google Scholar] [CrossRef]
- Riwayati, I.; Winardi, S.; Madhania, S.; Shimada, M. Green synthesis of ZnO nanoparticles using Cosmos caudatus: Effects of calcination temperature and precursor type on photocatalytic and antimicrobial activities. Results Eng. 2024, 24, 103594. [Google Scholar] [CrossRef]
- Riwayati, I.; Winardi, S.; Winardi, S.; Shimada, M. Cosmos caudatus extract-intervened zinc oxide nanoparticles: Reaction mechanism. molecular docking, and optical properties. Int. J. Appl. Sci. Eng. 2024, 21, 2024177. [Google Scholar] [CrossRef]
- Otalora, C.; Botero, M.A.; Ordoñez, G. ZnO compact layers used in third-generation photovoltaic devices: A review. J. Mater. Sci. 2021, 56, 15538–15571. [Google Scholar] [CrossRef]
- Waqas Alam, M.; Sharma, A.; Sharma, A.; Kumar, S.; Mohammad Junaid, P.; Awad, M. VOC Detection with Zinc Oxide Gas Sensors: A Review of Fabrication, Performance, and Emerging Applications. Electroanalysis 2024, 37, 202400246. [Google Scholar] [CrossRef]
- Winardi, S.; Qomariyah, L.; Widiyastuti, W.; Kusdianto, K.; Nurtono, T.; Madhania, S. The role of electro-sprayed silica-coated zinc oxide nanoparticles to hollow silica nanoparticles for optical devices material and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125327. [Google Scholar] [CrossRef]
- Kusdianto, K.; Kusuma, T.C.; Hudandini, M.; Widiyastuti, W.; Madhania, S.; Machmudah, S.; Nurtono, T.; Winardi, S. Characterizations of Ag doped ZnO particles via flame pyrolysis method for degradation of methylene blue. Conf. Ser. Mater. Sci. Eng. 2019, 673, 12012. [Google Scholar] [CrossRef]
- Nurtono, T.; Kusuma, T.C.; Hudandini, M.; Widiyastuti, W.; Shimada, M.; Madhania, S.; Machmudah, S.; Puspitasari, D.; Winardi, S.; Kusdianto, K. Effect of Ag content in ZnO-Ag nanocomposites prepared by spray pyrolysis method for degradation of textile dye waste. AIP Conf. Proc. 2020, 2219, 30005. [Google Scholar] [CrossRef]
- Hussain, A.; Fiaz, S.; Almohammedi, A.; Waqar, A. Optimizing photocatalytic performance with Ag-doped ZnO nanoparticles: Synthesis and characterization. Heliyon 2024, 10, e35725. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, M.D.; Van Hoang, T.; Trinh, D.T.; Pham, D.T.; Nguyen, D.L. Experimental and numerical study on photocatalytic activity of the ZnO nanorods/CuO composite film. Sci. Rep. 2020, 10, 7792. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, Q.; Liu, H.; Wang, X. Preparations and applications of zinc oxide based photocatalytic materials. Adv. Sens. Energy Mater. 2023, 2, 100069. [Google Scholar] [CrossRef]
- Kumar, S.; Curda, P.; Maskova, H.; Doudová, L.; Pleskunov, P.; Kuzminova, A.; Hejduk, L.; Sterba, J.; Kylián, O.; Rego, R.O.; et al. Ag-Cu Nanoarchitecture for Enhanced LSPR Absorption: The Role of Surface Roughness and near-field Interactions. Adv. Mater. Interfaces 2025, 2400785. [Google Scholar] [CrossRef]
- Workie, A.B.; Ningsih, H.S.; Shih, S.-J. An comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Za’aBar, F.I.; Bahrudin, M.S.; Arsad, A.Z.; Azman, N.I.; Rahman, M.N.; Abdullah, W.S.; Chau, C.F.; Zuhdi, A.W. Systematic optimization of sol–gel processed Al-doped ZnO for cost-effective transparent conductive oxide. J. Sol-Gel Sci. Technol. 2024, 110, 52–61. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Abu Nayem, S.M.; Alam, S.; Islam, I.; Seong, G.; Chowdhury, A.-N. ZnO Nanomaterials: Tailored Properties and Infinite Possibilities. Nanomaterials 2025, 15, 609. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Kontoliou, K.; Volla, E.; Karachalios, K.; Raptopoulos, G.; Paraskevopoulou, P.; Lagopati, N.; Pavlatou, E.A. Optimization of ZnO Nanoparticles’ Synthesis via Precipitation Method Applying Taguchi Robust Design. Catalysts 2023, 13, 1367. [Google Scholar] [CrossRef]
- Miao, H.; Wang, H.; Feng, X.; Jiao, H. One-step spray pyrolysis synthesis of ZnO/Ag hollow spheres for enhanced visible-light-driven antibacterial applications and wound healing. Dalton Trans. 2025, 54, 2574–2583. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Radha, S.; Muthumeenakshi, K. Environmentally friendly synthesis of Ag–ZnO nanocomposite-modified graphite electrode for copper ion quantification. J. Mater. Sci. Mater. Electron. 2025, 36, 697. [Google Scholar] [CrossRef]
- Suresh, P.; Doss, A.; Pole, R.P.; Devika, M. Green synthesis, characterization and antioxidant activity of bimetallic (Ag-ZnO) nanoparticles using Capparis zeylanica leaf extract. Biomass Convers. Biorefinery 2024, 14, 16451–16459. [Google Scholar] [CrossRef]
- Hudandini, M.; Puri, N.R.; Winardi, S.; Widiyastuti, W.; Shimada, M.; Kusdianto, K. Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents. Catalysts 2022, 12, 1374. [Google Scholar] [CrossRef]
- Fatimah, S.; Ragadhita, R.; Al Husaeni, D.F.; Nandiyanto, A.B.D. How to Calculate Crystallite Size from X-Ray Diffraction (XRD) using Scherrer Method. ASEAN J. Sci. Eng. 2022, 2, 65–76. [Google Scholar] [CrossRef]
- Cardenas-Flechas, L.J.; Raba, A.M.; Barba-Ortega, J.; Moreno, L.C.; Joya, M.R. Effect of Calcination Temperature on the Behavior of the Agglomerated Co 3O 4 Nanoparticles Obtained Through the Sol–Gel Method. J. Inorg. Organomet. Polym. Mater. 2021, 31, 121–128. [Google Scholar] [CrossRef]
- Ashraf, R.; Riaz, S.; Kayani, Z.N.; Naseem, S. Effect of Calcination on Properties of ZnO Nanoparticles. Mater. Today Proc. 2015, 2 Pt B, 5468–5472. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Zhang, J.; Luo, J.; Zhang, Z.; Shen, Z. A general mechanism of grain growth—I. Theory. J. Mater. 2021, 7, 1007–1013. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Recrystallization mechanisms and microstructure development in emerging metallic materials: A review. J. Sci. Adv. Mater. Devices 2019, 4, 19–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Guo, M.; Li, Z.; Xu, Z.; Ye, J.; Li, W.; Wei, S. Effects of calcination temperature on grain growth and phase transformation of nano-zirconia with different crystal forms prepared by hydrothermal method. J. Mater. Res. Technol. 2022, 19, 4003–4017. [Google Scholar] [CrossRef]
- Bhardwaj, J.S.; Singh, A.; Agarwal, P. Work function tunning of directly grown multi-layer graphene on silicon by PECVD and fabrication of Ag/ITO/Gr/n-Si/Ag solar cell. Diam. Relat. Mater. 2025, 155, 112271. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Yudasari, N.; Tahir, D. Optical and structural investigation of synthesis ZnO/Ag Nanoparticles prepared by laser ablation in liquid. Mater. Sci. Semicond. Process. 2020, 105, 104712. [Google Scholar] [CrossRef]
- Kusdianto, K.; Sari, T.D.; A Laksono, M.; Madhania, S.; Winardi, S. Fabrication and application of ZnO-Ag nanocomposite materials prepared by gas-phase methods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 12023. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Manzoor, F.; Zafar, A.; Mahmood, M.; Rasheed, M.; Anwar, M. Impact of Ag doping on structural, optical, morphological, optical and photoluminescent properties of ZnO nanoparticles. Opt. Quantum Electron. 2020, 52, 344. [Google Scholar] [CrossRef]
- Jiang, D.; Hudandini, M.; Masaki, Y.; Kusdianto, K.; Kubo, M.; Shimada, M. Visible-Light-Driven Photocatalytic Activity of Ag-Loaded TiO2 Nanoparticulate Thin Film Fabricated via PECVD-PVD Method. J. Chem. Eng. Jpn. 2024, 57, 2331105. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag-ZnO heterojunctions under UV-visible light. RSC. Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- Seong, S.; Park, I.-S.; Jung, Y.C.; Lee, T.; Kim, S.Y.; Park, J.S.; Ko, J.-H.; Ahn, J. Synthesis of Ag-ZnO core-shell nanoparticles with enhanced photocatalytic activity through atomic layer deposition. Mater. Des. 2019, 177, 107831. [Google Scholar] [CrossRef]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Darvishi, S.M.R. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Uribe-López, M.; Hidalgo-López, M.; López-González, R.; Frías-Márquez, D.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Kusdianto, K.; Widiyastuti, W.; Shimada, M.; Nurtono, T.; Machmudah, S.; Winardi, S. Photocatalytic activity of ZnO-Ag nanocomposites prepared by a one-step process using flame pyrolysis. Int. J. Technol. 2019, 10, 571–581. [Google Scholar] [CrossRef]
- Jarvin, M.; Kumar, S.A.; Rosaline, D.R.; Foletto, E.L.; Dotto, G.L.; Inbanathan, S.S.R. Remarkable sunlight-driven photocatalytic performance of Ag-doped ZnO nanoparticles prepared by green synthesis for degradation of emerging pollutants in water. Environ. Sci. Pollut. Res. 2022, 29, 57330–57344. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusdianto, K.; Puri, N.R.; Shimada, M.; Madhania, S.; Winardi, S. Synergistic Effect of Calcination Temperature and Silver Doping on Photocatalytic Performance of ZnO Material. Materials 2025, 18, 3362. https://doi.org/10.3390/ma18143362
Kusdianto K, Puri NR, Shimada M, Madhania S, Winardi S. Synergistic Effect of Calcination Temperature and Silver Doping on Photocatalytic Performance of ZnO Material. Materials. 2025; 18(14):3362. https://doi.org/10.3390/ma18143362
Chicago/Turabian StyleKusdianto, K., Nurdiana Ratna Puri, Manabu Shimada, Suci Madhania, and Sugeng Winardi. 2025. "Synergistic Effect of Calcination Temperature and Silver Doping on Photocatalytic Performance of ZnO Material" Materials 18, no. 14: 3362. https://doi.org/10.3390/ma18143362
APA StyleKusdianto, K., Puri, N. R., Shimada, M., Madhania, S., & Winardi, S. (2025). Synergistic Effect of Calcination Temperature and Silver Doping on Photocatalytic Performance of ZnO Material. Materials, 18(14), 3362. https://doi.org/10.3390/ma18143362






