Linear Actuation of Dielectrophoretic Formed Multi-Walled Carbon Nanotube Fiber with Carbide-Derived Carbon in Polar Aprotic and Polar Protic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNT and CNTCDC Fiber Generation
2.3. Electromechanical Deformation
2.4. Characterizations
3. Results and Discussion
3.1. Characterizations of CNT and CNTCDC Fiber
3.2. EMD Measurements of CNT and CNTCDC Fiber
3.2.1. Cyclic Voltammetric Studies
3.2.2. Chronoamperometric EMD Measurements
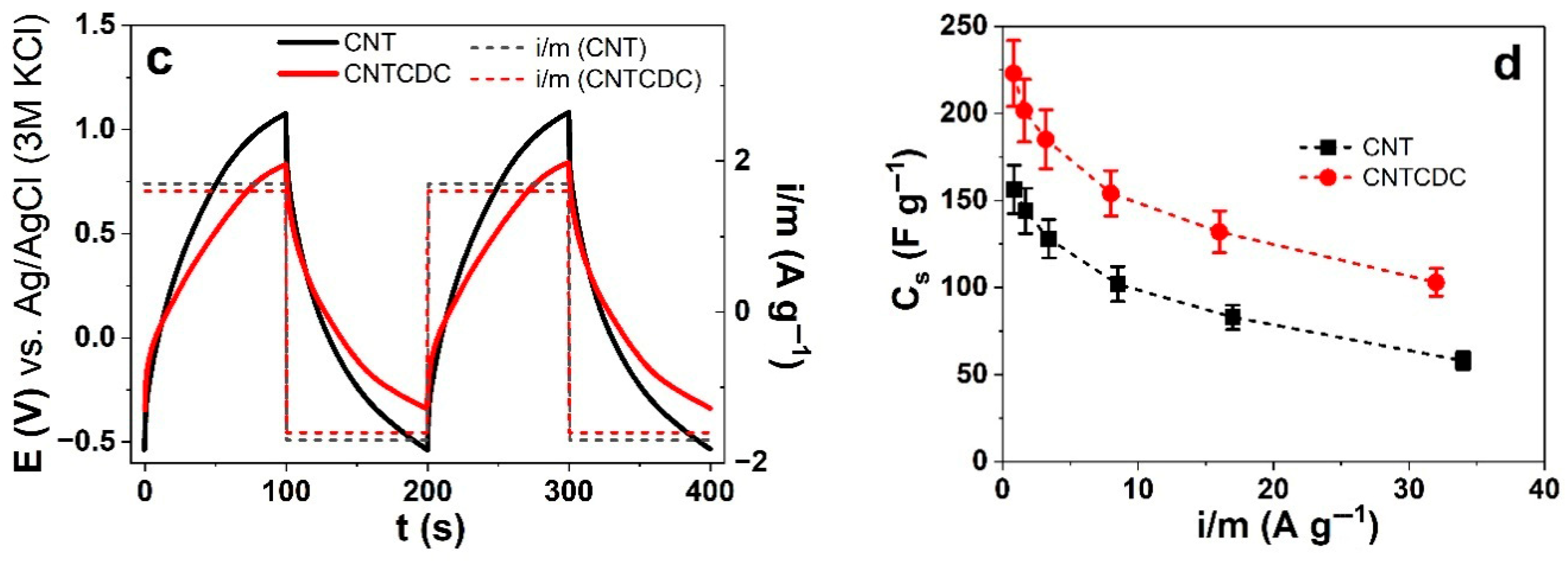
3.3. Energy Storage of CNT and CNTCDC Fiber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.; Chao, Y.; Ge, Y.; Foroughi, J.; Zhao, Y.; Wang, C.; Long, H.; Wallace, G.G. High-Performance Hybrid Carbon Nanotube Fibers for Wearable Energy Storage. Nanoscale 2017, 9, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Mirfakhrai, T.; Oh, J.; Kozlov, M.; Fok, E.C.W.; Zhang, M.; Fang, S.; Baughman, R.H.; Madden, J.D.W. Electrochemical Actuation of Carbon Nanotube Yarns. Smart Mater. Struct. 2007, 16, S243–S249. [Google Scholar] [CrossRef]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mäder, E.; Seide, G.; Gries, T. Novel Carbon Nanotube/Cellulose Composite Fibers As Multifunctional Materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Lee, Y.H. Carbon-Based Electrochemical Capacitors. ChemSusChem 2012, 5, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yue, H.; Aslam, M.J.; Bai, Y.; Zhu, Z.; Wei, F. Controllable Preparation and Strengthening Strategies towards High-Strength Carbon Nanotube Fibers. Nanomaterials 2022, 12, 3478. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Zhou, G.; Li, Q. Understanding the Mechanical and Conductive Properties of Carbon Nanotube Fibers for Smart Electronics. Adv. Mater. 2020, 32, 1902028. [Google Scholar] [CrossRef]
- Lu, W.; Zu, M.; Byun, J.H.; Kim, B.S.; Chou, T.W. State of the Art of Carbon Nanotube Fibers: Opportunities and Challenges. Adv. Mater. 2012, 24, 1805–1833. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.H. Carbon Nanotube Yarns. Korean J. Chem. Eng. 2012, 29, 277–287. [Google Scholar] [CrossRef]
- Fallah Gilvaei, A.; Hirahara, K.; Nakayama, Y. In-Situ Study of the Carbon Nanotube Yarn Drawing Process. Carbon 2011, 49, 4928–4935. [Google Scholar] [CrossRef]
- Zhang, D.; Miao, M.; Niu, H.; Wei, Z. Core-Spun Carbon Nanotube Yarn Supercapacitors for Wearable Electronic Textiles. ACS Nano 2014, 8, 4571–4579. [Google Scholar] [CrossRef]
- Im, J.; Jeong, Y.H.; Kim, M.C.; Oh, D.; Son, J.; Hyun, K.; Jeong, B.; Hong, S.; Lee, J. Wet Spinning of Multi-Walled Carbon Nanotube Fibers. Carbon 2024, 216, 118532. [Google Scholar] [CrossRef]
- Rabbani, M.T.; Sonker, M.; Ros, A. Carbon Nanotube Dielectrophoresis: Theory and Applications. Electrophoresis 2020, 41, 1893–1914. [Google Scholar] [CrossRef]
- Tang, J.; Gao, B.; Geng, H.; Velev, O.D.; Qin, L.C.; Zhou, O. Assembly of 1D Nanostructures into Sub-Micrometer Diameter Fibrils with Controlled and Variable Length by Dielectrophoresis. Adv. Mater. 2003, 15, 1352–1355. [Google Scholar] [CrossRef]
- Plaado, M.; Mononen, R.M.; Lhmus, R.; Kink, I.; Saal, K. Formation of Thick Dielectrophoretic Carbon Nanotube Fibers. Nanotechnology 2011, 22, 305711. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hennrich, F.; Flavel, B.S.; Dehm, S.; Kappes, M.; Krupke, R. Principles of Carbon Nanotube Dielectrophoresis. Nano Res. 2021, 14, 2188–2206. [Google Scholar] [CrossRef]
- Ismail, I.; Zubair, A. Alignment of Interacting Multi-Wall Carbon Nanotubes in Suspension Using Dielectrophorectic Framework. In Proceedings of the 12th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 21–23 December 2022; pp. 405–408. [Google Scholar]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2020, 32, 1902670. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G. Carbon Nanotube and Graphene Fiber Artificial Muscles. Nanoscale Adv. 2019, 1, 4592–4614. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-Derived Carbons—From Porous Networks to Nanotubes and Graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Plaado, M.; Kaasik, F.; Valner, R.; Lust, E.; Saar, R.; Saal, K.; Peikolainen, A.-L.; Aabloo, A.; Kiefer, R. Electrochemical Actuation of Multiwall Carbon Nanotube Fiber with Embedded Carbide-Derived Carbon Particles. Carbon 2015, 94, 911–918. [Google Scholar] [CrossRef]
- Kiefer, R.; Plaado, M.; Harjo, M.; Tamm, T. Tuning the Linear Actuation of Multiwall Carbon Nanotube Fibers with Carbide-Derived Carbon. Synth. Met. 2022, 288, 117099. [Google Scholar] [CrossRef]
- Kosidlo, U.; Omastova, M.; Micusik, M.; Ciric-Marjanovic, G.; Randriamahazaka, H.; Wallmersperger, T.; Aabloo, A.; Kolaric, I.; Bauernhansl, T. Nanocarbon Based Ionic Actuators-a Review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon Nanotube Actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Torop, J.; Arulepp, M.; Leis, J.; Punning, A.; Johanson, U.; Palmre, V.; Aabloo, A. Nanoporous Carbide-Derived Carbon Material-Based Linear Actuators. Materials 2010, 3, 9–25. [Google Scholar] [CrossRef]
- Torop, J.; Arulepp, M.; Sugino, T.; Asaka, K.; Ja, A.; Lust, E.; Aabloo, A. Microporous and Mesoporous Carbide-Derived Carbons for Strain Modification of Electromechanical Actuators. Langmuir 2014, 30, 2583–2587. [Google Scholar] [CrossRef]
- Kaasik, F.; Tamm, T.; Hantel, M.M.; Perre, E.; Aabloo, A.; Lust, E.; Bazant, M.Z.; Presser, V. Anisometric Charge Dependent Swelling of Porous Carbon in an Ionic Liquid. Electrochem. Commun. 2013, 34, 196–199. [Google Scholar] [CrossRef]
- Elhi, F.; Le, Q.B.; Kiefer, R. Multifunctionality of Cellulose Multiwall Carbon Nanotube Composites in Polar Aprotic and Polar Protic Solvents. J. Appl. Polym. Sci. 2025, 142, e56913. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Elhi, F.; Le, Q.B.; Kiefer, R. Sustainability of Multiwall Carbon Nanotube Fibers and Their Cellulose Composite. Sustainability 2023, 15, 9227. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K. The Relationship between Resistances Measured by Two-Probe, Wenner Probe and C1760-12 ASTM Methods in Electrically Conductive Concretes. SN Appl. Sci. 2020, 2, 10. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tang, J.; Zhang, H.; Shinya, N.; Qin, L.-C. Ultrathin Carbon Nanotube Fibrils of High Electrochemical Capacitance. ACS Nano 2009, 3, 3679–3683. [Google Scholar] [CrossRef]
- Kiefer, R.; Elhi, F.; Puust, L.; Peikolainen, A.-L.; Tamm, T. Dual Function Composite Fibers of Cellulose with Activated Carbon Aerogel and Carbide Derived Carbon. J. Appl. Polym. Sci. 2022, 139, 52297. [Google Scholar] [CrossRef]
- Kiefer, R.; Aydemir, N.; Torop, J.; Tamm, T.; Temmer, R.; Travas-Sejdic, J.; Must, I.; Kaasik, F.; Aabloo, A. Carbide-Derived Carbon as Active Interlayer of Polypyrrole Tri-Layer Linear Actuator. Sens. Actuators B Chem. 2014, 201, 100–106. [Google Scholar] [CrossRef]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and Nonspecific Molecular Interactions of Polymers with Multiwalled Carbon Nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Kikas, A.; Kisand, V.; Käärik, M.; Merisalu, M.; Treshchalov, A.; Leis, J.; Sammelselg, V.; et al. Nitrogen-Doped Carbide-Derived Carbon/carbon Nanotube Composites as Cathode Catalysts for Anion Exchange Membrane Fuel Cell Application. Appl. Catal. B Environ. 2020, 272, 119012. [Google Scholar] [CrossRef]
- Praats, R.; Käärik, M.; Kikas, A.; Kisand, V.; Aruväli, J.; Paiste, P.; Merisalu, M.; Sarapuu, A.; Leis, J.; Sammelselg, V.; et al. Electroreduction of Oxygen on Cobalt Phthalocyanine-Modified Carbide-Derived Carbon/carbon Nanotube Composite Catalysts. J. Solid State Electrochem. 2021, 25, 57–71. [Google Scholar] [CrossRef]
- Tsentalovich, D.E.; Headrick, R.J.; Mirri, F.; Hao, J.; Behabtu, N.; Young, C.C.; Pasquali, M. Influence of Carbon Nanotube Characteristics on Macroscopic Fiber Properties. ACS Appl. Mater. Interfaces 2017, 9, 36189–36198. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Giannaris, C.; Rubio, A. Single-Walled Carbon Nanotube-Polymer Composites: Strength and Weakness. Adv. Mater. 2000, 12, 750–753. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; et al. Dielectrophoretically Aligned Carbon Nanotubes to Control Electrical and Mechanical Properties of Hydrogels to Fabricate Contractile Muscle Myofibers. Adv. Mater. 2013, 25, 4028–4034. [Google Scholar] [CrossRef]
- Elhi, F.; Puust, L.; Kiefer, R.; Tamm, T. Electrolyte Contribution to the Multifunctional Response of Cellulose Carbon Nanotube Fibers. React. Funct. Polym. 2023, 182, 105480. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon at Pore Sizes Less than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G.; Asaka, K. Faradaic and Capacitive Components of the CNT Electrochemical Responses. Front. Mater. 2016, 3, 3. [Google Scholar] [CrossRef]
- Lyon, J.L.; Stevenson, K.J. Anomalous Electrochemical Dissolution and Passivation of Iron Growth Catalysts in Carbon Nanotubes. Langmuir 2007, 23, 11311–11318. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef]
- Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Madden, J.D.; Baughman, R.H. Increased Actuation Rate of Electromechanical Carbon Nanotube Actuators Using Potential Pulses with Resistance Compensation. Smart Mater. Struct. 2003, 12, 549–555. [Google Scholar] [CrossRef]
- Hughes, M.; Spinks, G.M. Multiwalled Carbon-Nanotube Actuators. Adv. Mater. 2005, 17, 443–446. [Google Scholar] [CrossRef]
- Geier, S.M.; Mahrholz, T.; Wierach, P.; Sinapius, M. Morphology- and Ion Size-Induced Actuation of Carbon Nanotube Architectures. Int. J. Smart Nano Mater. 2018, 9, 111–134. [Google Scholar] [CrossRef]
- Riemenschneider, J.; Temmen, H.; Monner, H.P. CNT Based Actuators: Experimental and Theoretical Investigation of the in-Plain Strain Generation. J. Nanosci. Nanotechnol. 2007, 7, 3359–3364. [Google Scholar] [CrossRef]
- Trunzer, T.; Fraga-García, P.; Tschuschner, M.P.A.; Voltmer, D.; Berensmeier, S. The Electrosorptive Response of a Carbon Nanotube Flow-through Electrode in Aqueous Systems. Chem. Eng. J. 2022, 428, 131009. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Luo, C.; Guo, Y.; Hou, C.; Zhang, Q.; Li, Y.; Jia, W.; Wang, H. Dielectrophoretic Assembly of Carbon Nanotube Chains in Aqueous Solution. Adv. Fiber Mater. 2021, 3, 312–320. [Google Scholar] [CrossRef]
- Wen, L.; Li, F.; Cheng, H.M. Carbon Nanotubes and Graphene for Flexible Electrochemical Energy Storage: From Materials to Devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef]
- Pandit, B.; Dhakate, S.R.; Singh, B.P.; Sankapal, B.R. Free-Standing Flexible MWCNTs Bucky Paper: Extremely Stable and Energy Efficient Supercapacitive Electrode. Electrochim. Acta 2017, 249, 395–403. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Carbon Nanotubes for Sustainable Energy Applications. ChemSusChem 2011, 4, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Hu, F.; Gao, G.; Wu, G.; Yang, X. Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes. Adv. Mater. 2018, 30, 1705713. [Google Scholar] [CrossRef]
- Su, J.; Huang, D. Coupling Transport of Water and Ions Through a Carbon Nanotube: The Role of Ionic Condition. J. Phys. Chem. C 2016, 120, 11245–11252. [Google Scholar] [CrossRef]
- Torop, J.; Palmre, V.; Arulepp, M.; Sugino, T.; Asaka, K.; Aabloo, A. Flexible Supercapacitor-like Actuator with Carbide-Derived Carbon Electrodes. Carbon 2011, 49, 3113–3119. [Google Scholar] [CrossRef]
- Pohl, M.; Kurig, H.; Tallo, I.; Jänes, A.; Lust, E. Novel Sol-Gel Synthesis Route of Carbide-Derived Carbon Composites for Very High Power Density Supercapacitors. Chem. Eng. J. 2017, 320, 576–587. [Google Scholar] [CrossRef]
- Malmberg, S.; Arulepp, M.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Krumme, A. Electrochemical Evaluation of Directly Electrospun Carbide-Derived Carbon-Based Electrodes in Different Nonaqueous Electrolytes for Energy Storage Applications. J. Carbon Res. 2020, 6, 59. [Google Scholar] [CrossRef]
- Sun, X.; Cai, M.; Chen, L.; Qiu, Z.; Liu, Z. Electrodes of Carbonized MWCNT-Cellulose Paper for Supercapacitor. J. Nanopart. Res. 2017, 19, 239. [Google Scholar] [CrossRef]
- Felhősi, I.; Keresztes, Z.; Marek, T.; Pajkossy, T. Properties of Electrochemical Double-Layer Capacitors with Carbon-Nanotubes-on-Carbon-Fiber-Felt Electrodes. Electrochim. Acta 2020, 334, 135548. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Capacitance of Two-Dimensional Titanium Carbide (MXene) and MXene/carbon Nanotube Composites in Organic Electrolytes. J. Power Sources 2016, 306, 510–515. [Google Scholar] [CrossRef]
- Borenstien, A.; Noked, M.; Okashy, S.; Aurbach, D. Composite Carbon Nano-Tubes (CNT)/Activated Carbon Electrodes for Non-Aqueous Super Capacitors Using Organic Electrolyte Solutions. J. Electrochem. Soc. 2013, 160, A1282–A1285. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance Properties of poly(3,4-Ethylenedioxythiophene)/carbon Nanotubes Composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, J.; Spinks, G.M.; Ghorbani, S.R.; Kozlov, M.E.; Safaei, F.; Peleckis, G.; Wallace, G.G.; Baughman, R.H. Preparation and Characterization of Hybrid Conducting Polymer-Carbon Nanotube Yarn. Nanoscale 2012, 4, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ofori, E.A.; Gbologah, L.; Xiong, Y.; Mensah-darkwa, K.; Tawiah, B.; Fei, B.; Zhao, X. Electrochemical Fiber Electrode Fabrication by Spinning: State-of- the-Art and Perspectives. ACS Electrochem. 2025, in press. [Google Scholar] [CrossRef]
- Hyeon, J.S.; Park, J.W.; Baughman, R.H.; Kim, S.J. Electrochemical Graphene/carbon Nanotube Yarn Artificial Muscles. Sens. Actuators B Chem. 2019, 286, 237–242. [Google Scholar] [CrossRef]
- Choi, C.; Kim, K.M.; Kim, K.J.; Lepró, X.; Spinks, G.M.; Baughman, R.H.; Kim, S.J. Improvement of System Capacitance via Weavable Superelastic Biscrolled Yarn Supercapacitors. Nat. Commun. 2016, 7, 13811. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, W.; Wei, L.; Karahan, H.E.; Yuan, Y.; Ng, A.K.; Chen, Y. All-Carbon Solid-State Yarn Supercapacitors from Activated Carbon and Carbon Fibers for Smart Textiles. Mater. Horiz. 2015, 2, 598–605. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Chen, C.; Chen, X.; Cai, Z.; Qiu, L.; Wang, Y.; Zhu, X.; Peng, H. Twisting Carbon Nanotube Fibers for Both Wire-Shaped Micro-Supercapacitor and Micro-Battery. Adv. Mater. 2013, 25, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
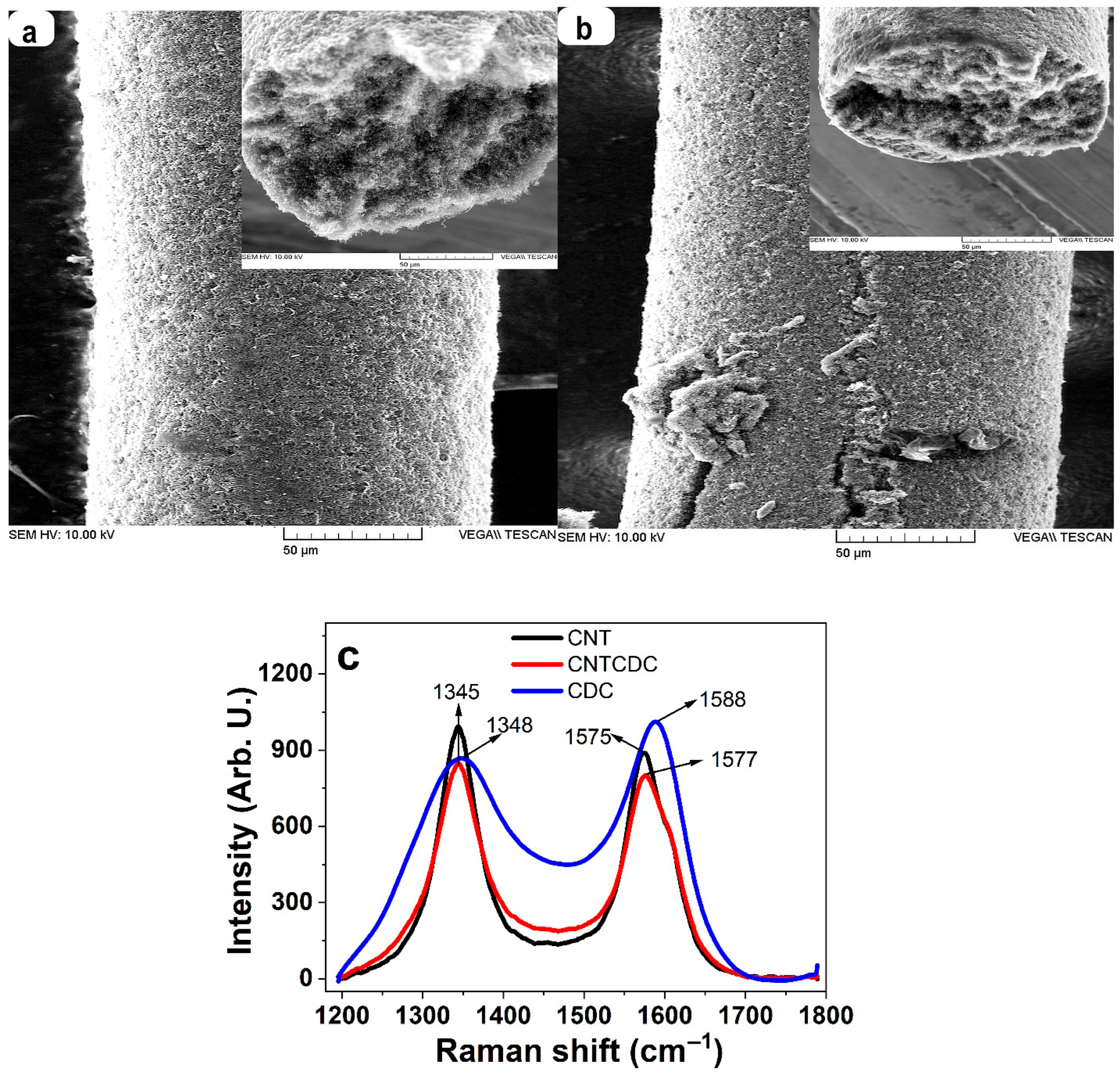
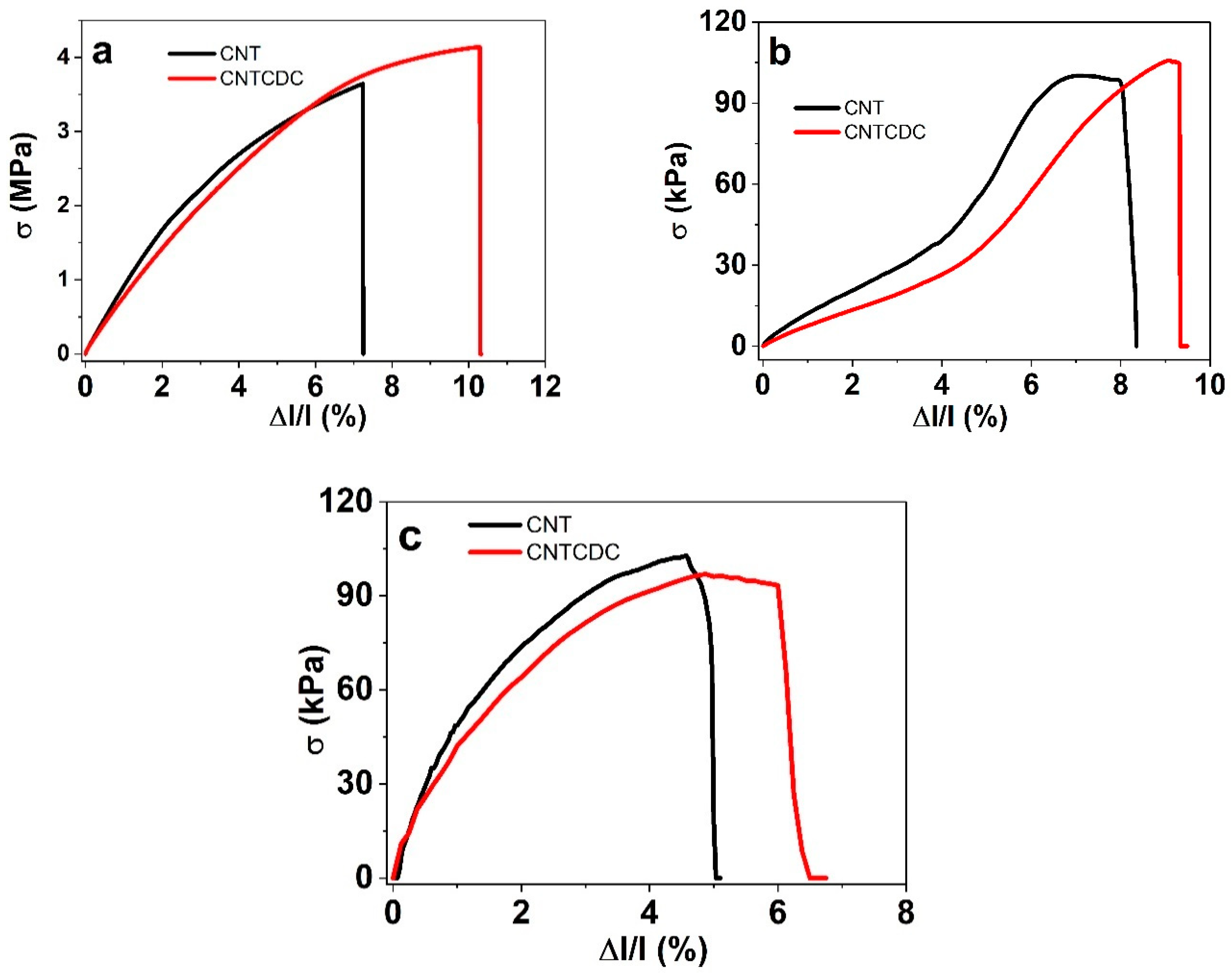


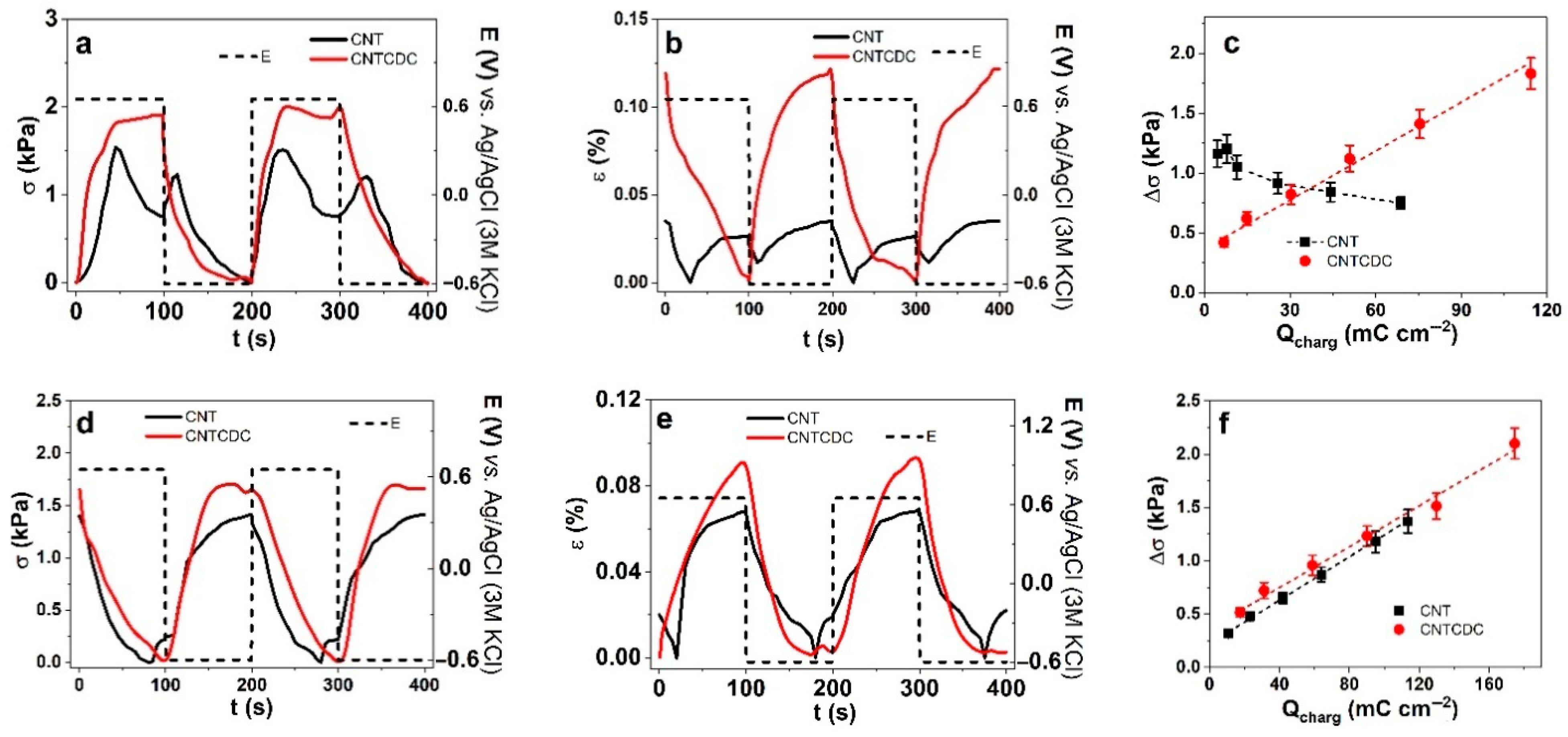
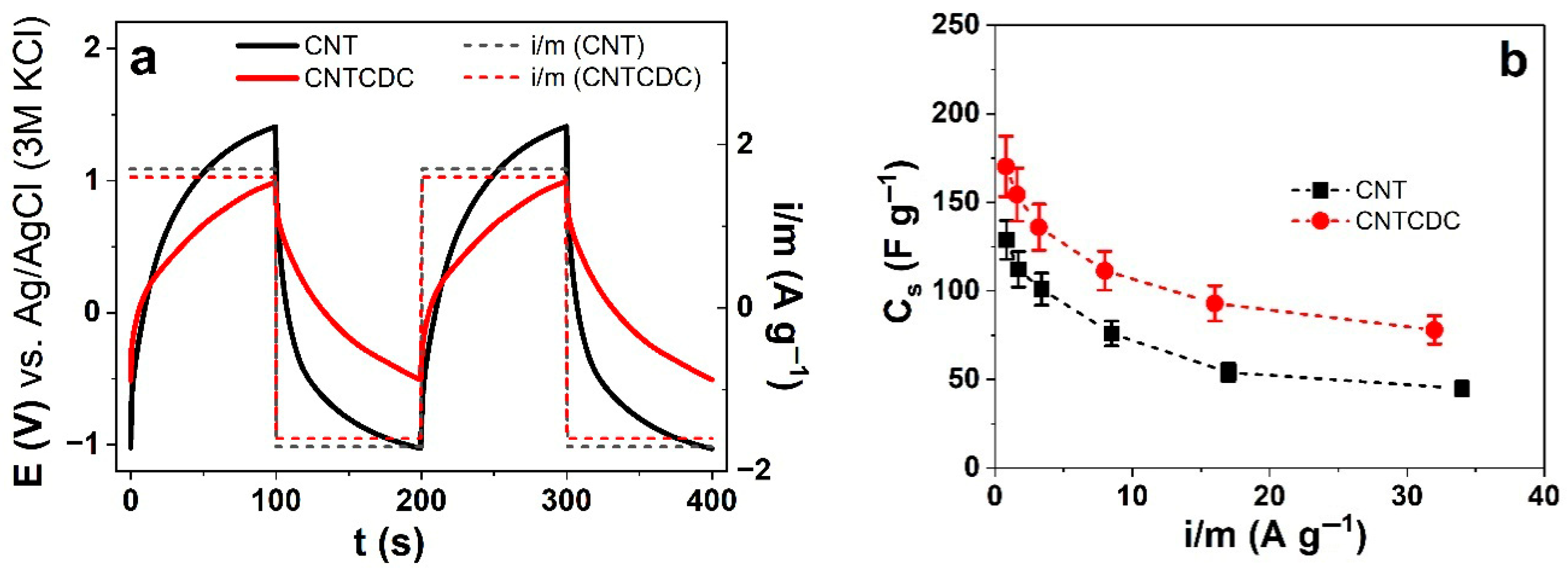
| Parameters | A (%) | σT (MPa) | Y (MPa) | |||
|---|---|---|---|---|---|---|
| CNT | CNTCDC | CNT | CNTCDC | CNT | CNTCDC | |
| dry | 7.2 ± 0.4 | 10.3 ± 0.6 | 3.6 ± 0.2 | 4.1 ± 0.2 | 44.5 ± 2.6 | 42.3 ± 0.3 |
| LiTFSI-PC | 8.0 ± 0.5 | 9.1 ± 0.5 | 0.1 ± 0.01 | 0.11 ± 0.01 | 0.88 ± 0.07 | 0.72 ± 0.04 |
| LiTFSI-aq | 4.6 ± 0.2 | 6.0 ± 0.3 | 0.1 ± 0.01 | 0.1 ± 0.01 | 1.03 ± 0.1 | 0.9 ± 0.06 |
| Fiber | Δσ (kPa) | ε (%) | Q (mC cm−2) | |
|---|---|---|---|---|
| 0.65 V | −0.6 V | |||
| CNT (PC) | 0.97 | 0.017 | 0.031 | 45.6 |
| CNTCDC (PC) | 1.64 | 0.004 | 0.1 | 77.4 |
| CNT (aq) | 0.62 | 0.04 | 0.008 | 86.7 |
| CNTCDC (aq) | 1.39 | 0.047 | 0 | 131 |
| Fiber | LiTFSI-PC | LiTFSI-aq | ||||
|---|---|---|---|---|---|---|
| Δσ (kPa) | Δε (%) | Qcharg (mC cm−2) | Δσ (kPa) | Δε (%) | Qcharg (mC cm−2) | |
| CNT | ||||||
| 2.5 mHz | 0.75 | 0.007 | 70.8 | 1.37 | 0.056 | 113.4 |
| 50 mHz | 1.21 | 0.019 | 7.8 | 0.48 | 0.021 | 23.2 |
| CNTCDC | ||||||
| 2.5 mHz | 1.83 | 0.152 | 114.4 | 2.13 | 0.095 | 174.7 |
| 50 mHz | 0.62 | 0.348 | 14.8 | 0.72 | 0.04 | 31.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, C.B.; Le, Q.B.; Kiefer, R. Linear Actuation of Dielectrophoretic Formed Multi-Walled Carbon Nanotube Fiber with Carbide-Derived Carbon in Polar Aprotic and Polar Protic Solvents. Materials 2025, 18, 3254. https://doi.org/10.3390/ma18143254
Tran CB, Le QB, Kiefer R. Linear Actuation of Dielectrophoretic Formed Multi-Walled Carbon Nanotube Fiber with Carbide-Derived Carbon in Polar Aprotic and Polar Protic Solvents. Materials. 2025; 18(14):3254. https://doi.org/10.3390/ma18143254
Chicago/Turabian StyleTran, Chau B., Quoc Bao Le, and Rudolf Kiefer. 2025. "Linear Actuation of Dielectrophoretic Formed Multi-Walled Carbon Nanotube Fiber with Carbide-Derived Carbon in Polar Aprotic and Polar Protic Solvents" Materials 18, no. 14: 3254. https://doi.org/10.3390/ma18143254
APA StyleTran, C. B., Le, Q. B., & Kiefer, R. (2025). Linear Actuation of Dielectrophoretic Formed Multi-Walled Carbon Nanotube Fiber with Carbide-Derived Carbon in Polar Aprotic and Polar Protic Solvents. Materials, 18(14), 3254. https://doi.org/10.3390/ma18143254







