Cellulolytic Potential of Newly Isolated Alcohol-Tolerant Bacillus methylotrophicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Molecular Identification of the Bacterial Strain
2.2. Evaluation of Alcohol Tolerance
2.3. Bacterial Culture in CMC Medium
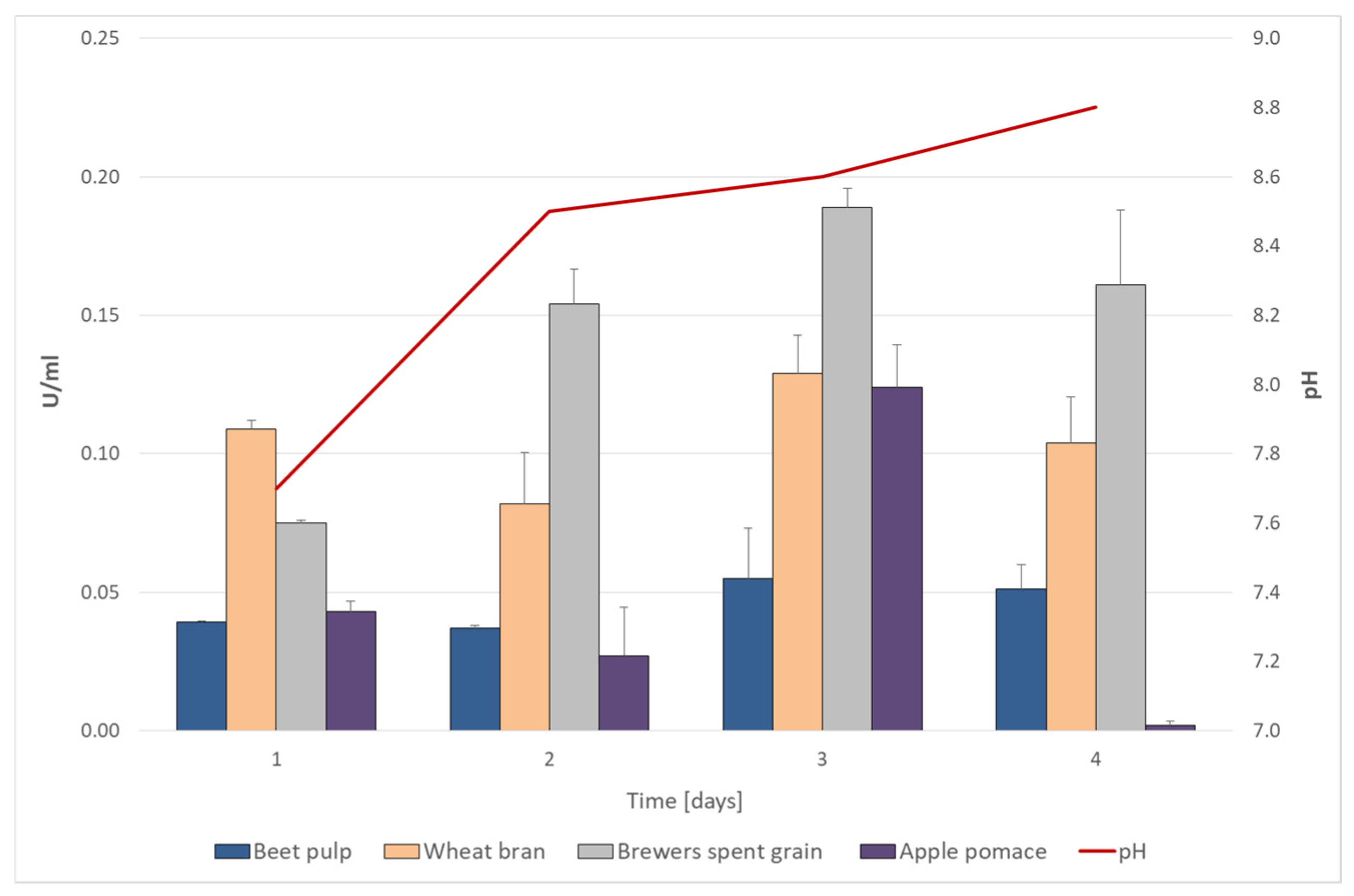
2.4. Selection of Lignocellulosic Substrate
2.5. Determination of Cellulolytic Activity
2.6. Screening of Process Parameters
2.7. Optimization of Culture Medium for Cellulase Production
2.8. Enzymatic Hydrolysis of Lignocellulose
2.9. Purification of Cellulase
2.10. Characterization of Cellulase
2.11. Determination of the Chemical Composition of the Waste Material
2.12. SEM Analysis
3. Results and Discussion
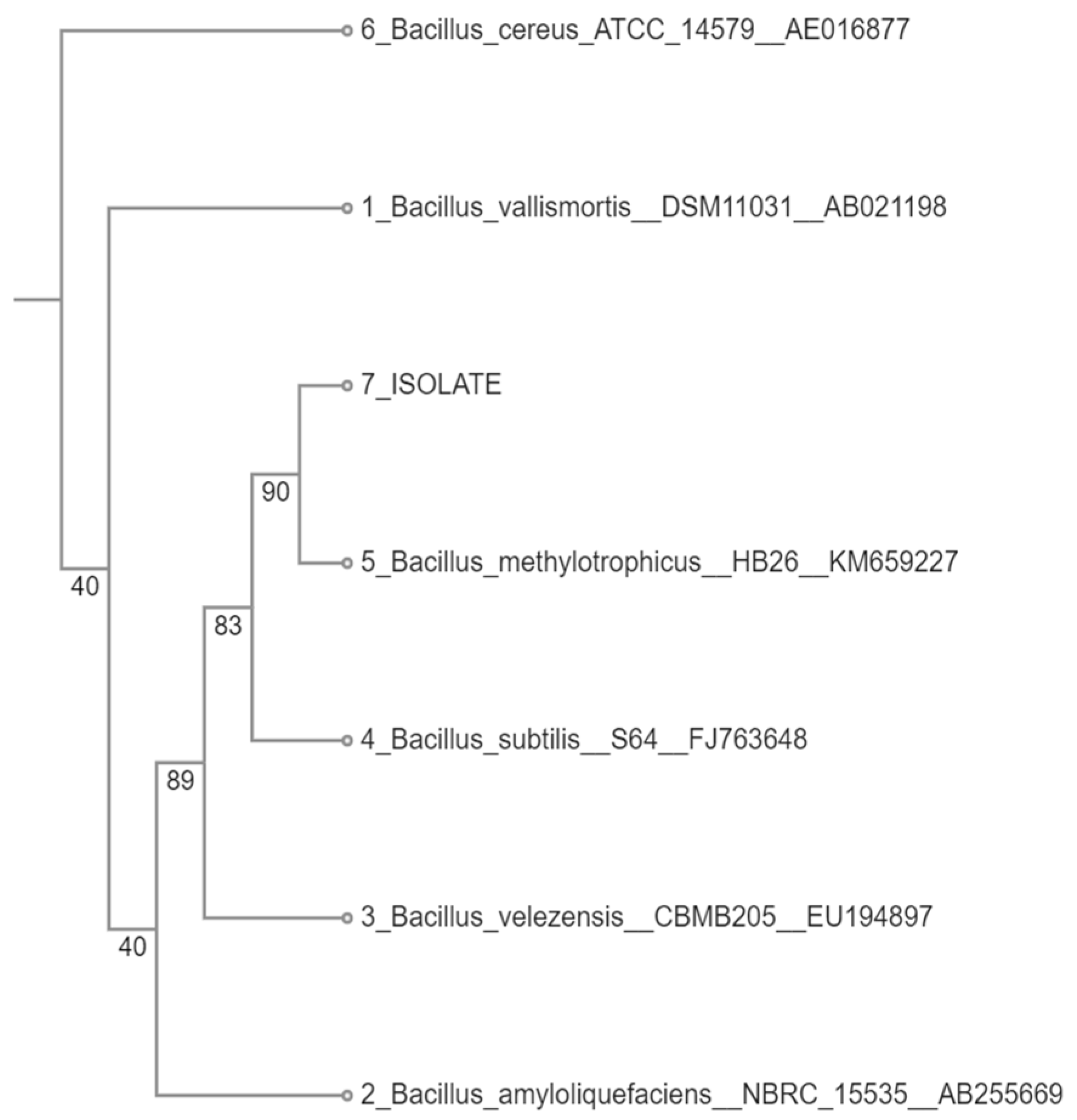
3.1. Identification of the Bacterial Isolate
3.2. Alcohol Tolerance of the Tested Isolate
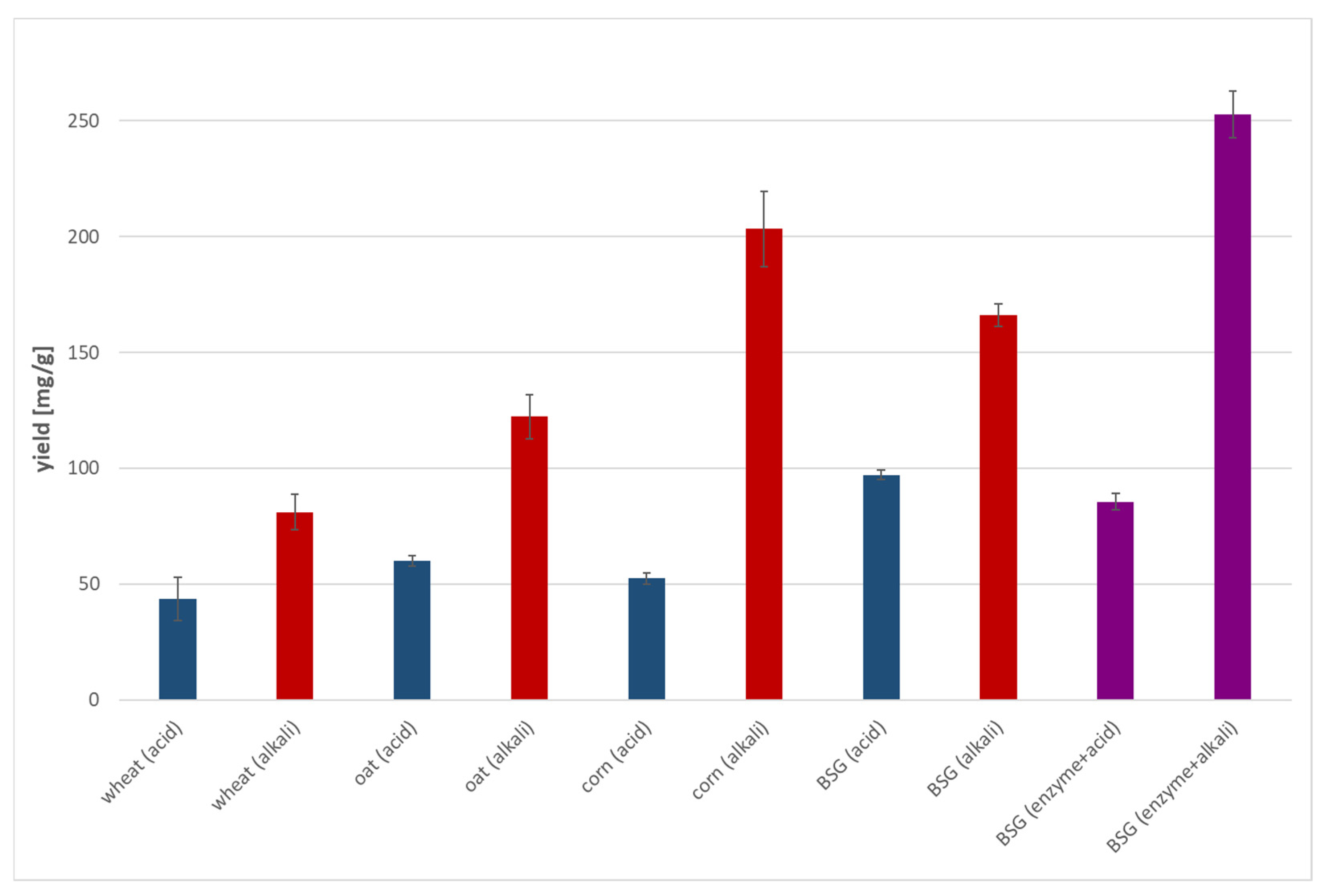
3.3. Cellulolytic Capability of the Isolate
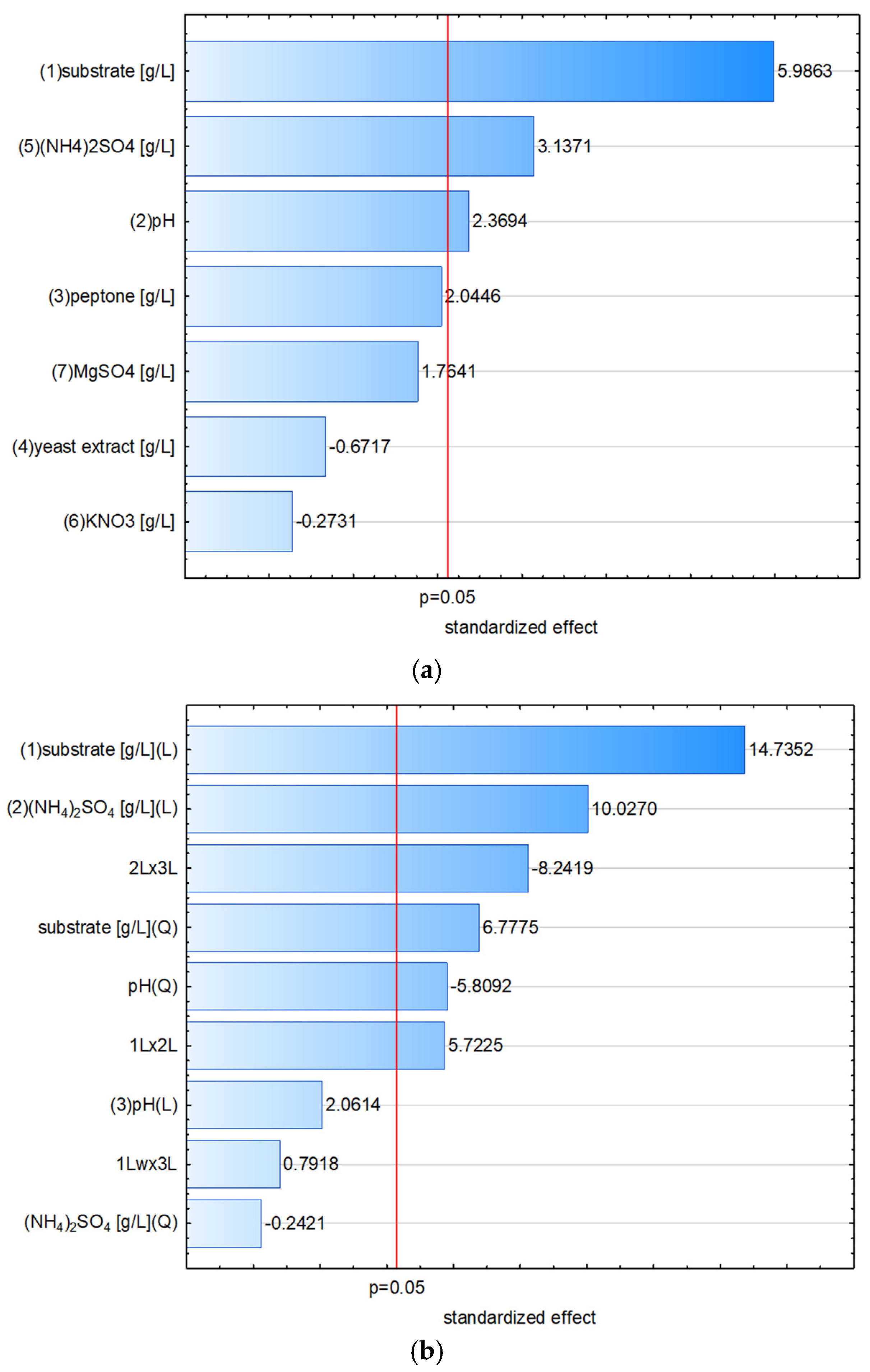
3.4. Screening of Process Parameters with the Plackett–Burman Design
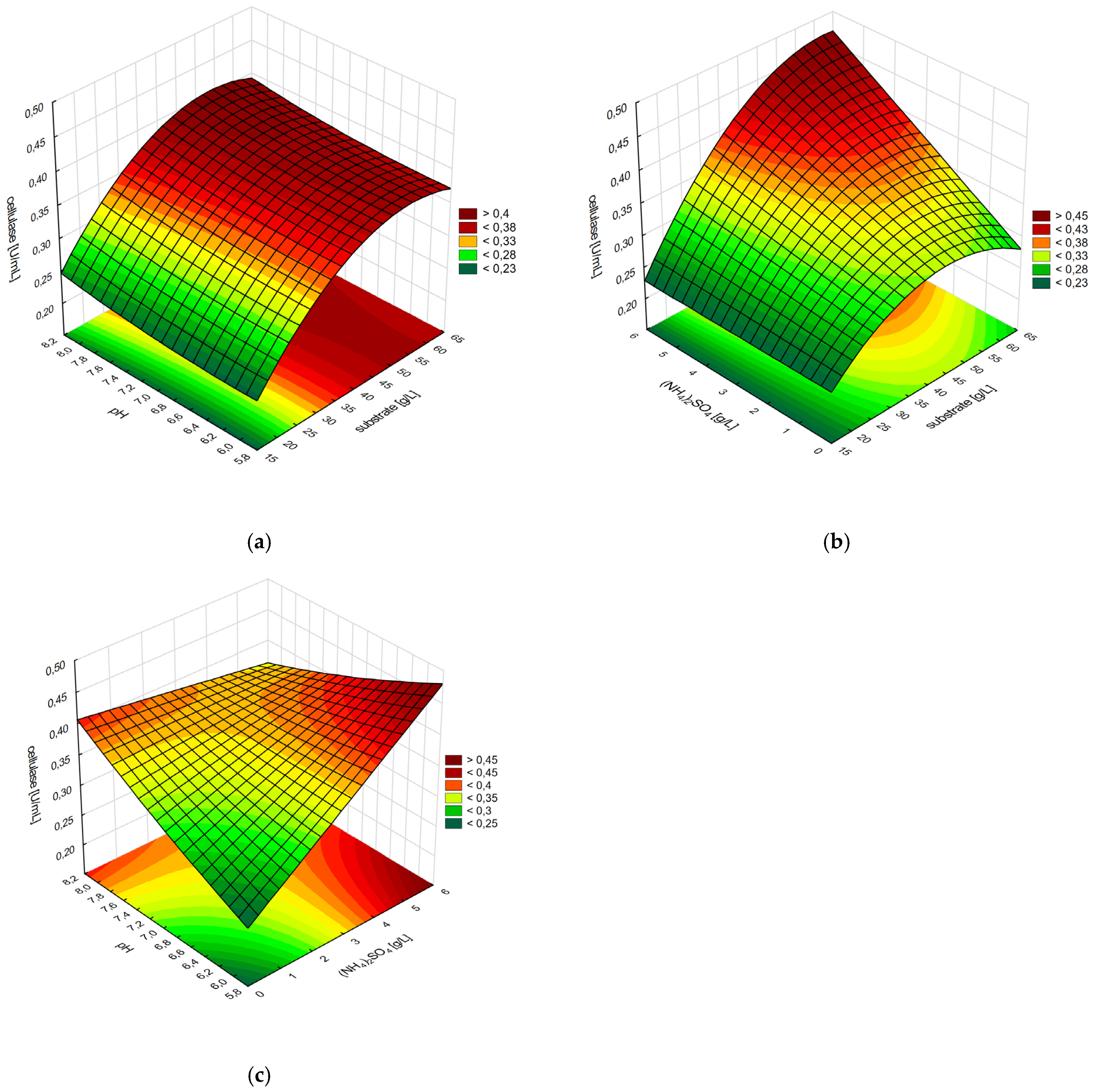
3.5. Optimization of Cellulase Production with Box–Behnken Design
3.6. Raw BSG Hydrolysis with Concentrated Enzyme
3.7. Pretreated BSG Hydrolysis with Concentrated Enzyme
3.8. Characterization of the Partially Purified Cellulase
3.9. Chemical Composition Analysis of Spent Grain
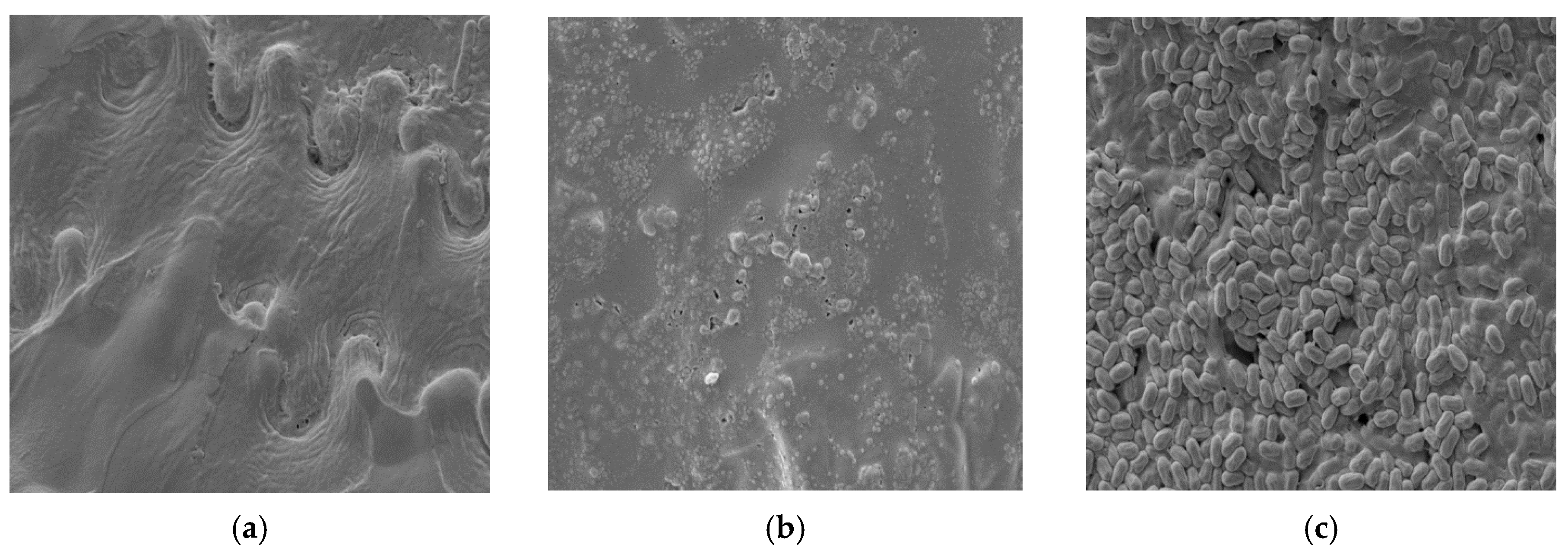
3.10. SEM Analysis of Raw and Enzymatically Treated BSG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSG | Brewer’s spent grain |
| CMC | Carboxymethyl cellulose |
| DNS | 3,5-Dinitrosalicylic acid |
| YE | Yeast extract |
| PCR | Polymerase chain reaction |
| SEM | Scanning electron microscopy |
| TFF | Tangential flow filtration |
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food industry by-products used as a functional food ingredients. Int. J. Waste Resour. 2016, 6, 248–253. [Google Scholar] [CrossRef]
- Farcas˛, A.; Socaci, S.; Mudura, E.; Dulf, F.; Vodnar, D.; Tofana, M.; Salanta, L.C. Brewing technology. In Exploitation of Brewing Industry Wastes to Produce Functional Ingredients; IntechOpen: Budapest, Hungary, 2017. [Google Scholar]
- Ezebuiro, V.; Ogugbue, C.J.; Oruwari, B.; Ire, F.S. Bioethanol production by an ethanol-tolerant Bacillus cereus strain GBPS9 using sugarcane bagasse and cassava peels as feedstocks. J. Biotechnol. Biomater. 2015, 5, 213. [Google Scholar] [CrossRef]
- Fabbrizi, G.; Giannoni, T.; Lorenzi, L.; Nicolini, A.; Iodice, P.; Coccia, V.; Cavalaglio, G.; Gelosia, M. High solid and low cellulase enzymatic hydrolysis of cardoon stems pretreated by acidified γ-valerolactone/water solution. Energies 2022, 15, 2600. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and chemical pretreatment of lignocellulosic biomass. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 6; pp. 143–196. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 2009, 25, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Talukdar, S.A.; Mohsina, K.; Sarker, P.K.; Sayem, S.M.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus 2013, 2, 154. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R.; Cranenburgh, R. Bacillus protein secretion: An unfolding story. Trends Microbiol. 2008, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 30, 304–314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pietraszek, P.; Walczak, P. Characteristic and applications of Bacillus strains isolated from soil. Pol. J. Agron. 2014, 16, 37–44. [Google Scholar]
- Azadian, F.; Badoei-Dalfard, A.; Namaki-Shoushtari, A.; Hassanshahian, M. Purification and biochemical properties of a thermostable, haloalkaline cellulase from Bacillus licheniformis AMF-07 and its application for hydrolysis of different cellulosic substrates to bioethanol production. Mol. Biol. Res. Commun. 2016, 5, 143–155. [Google Scholar] [PubMed] [PubMed Central]
- Gozan, M.; Harahap, A.F.; Bakti, C.P.; Setyahadi, S. Optimization of cellulase production by Bacillus sp. BPPT CC RK2 with pH and temperature variation using response surface methodology. Web Conf. 2018, 67, 02051. [Google Scholar] [CrossRef]
- Hao, C.; Yu, X.B.; Yan, Z.L. Optimization of the medium for the production of cellulase by the mutant Trichoderma reesei WX-112 using response surface methodology. Food Technol. Biotech. 2006, 44, 89–94. [Google Scholar]
- Otajevwo, F.D.; Aluyi, H.S.A. Cultural conditions necessary for optimal cellulase yield by cellulolytic bacterial organisms as they relate to residual sugars released in broth medium. Mod. Appl. Sci. 2011, 5, 141–151. [Google Scholar] [CrossRef]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2014, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng. 2010, 105, 871–879. [Google Scholar] [CrossRef]
- Tu, M.; Chandra, R.P.; Saddler, J.N. Evaluating the distribution of cellulases and the recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnol. Prog. 2007, 23, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Maeda, T.; Furusawa, C. Understanding and engineering alcohol-tolerant bacteria using OMICS technology. World J. Microbiol. Biotechnol. 2018, 34, 157. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Storms, R.; Tsang, A. Microplate-based carboxymethylcellulose assay for endoglucanase activity. Anal. Biochem. 2005, 342, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Lemões, J.S.; Lemons e Silva, C.F.; Peres Farias Avila, S.; Scherrer Montero, C.R.; Delmar dos Anjos e Silva, S.; Samios, D.; Carmo Ruaro Peralba, M. Chemical pretreatment of Arundo donax L. for second-generation ethanol production. Electron. J. Biotechnol. 2018, 31, 67–74. [Google Scholar] [CrossRef]
- Prosiński, S. 1984 Chemia Drewna Part I; Państwowe Wydawnictwo Rolnicze i Leśnicze: Warsaw, Poland, 1984. [Google Scholar]
- Klason, P. Über lignin und lignin-reaktionen. Ber. Dtsch. Chem. Ges. 1920, 53, 706–711. [Google Scholar] [CrossRef][Green Version]
- TAPPIT 222 om-02. Acid-Insoluble Lignin in Wood and Pulp. 2006. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T222.aspx (accessed on 4 April 2025).
- Dunlap, C.H.; Kim, S.J.; Kwon, S.W.; Rooney, A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.-T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef]
- Narkthewan, P.; Makkapan, W. Cellulase activity of Bacillus velezensis isolated from soil in a dairy farm. IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012040. [Google Scholar] [CrossRef]
- Sarangthem, I.; Rajkumari, L.; Ngashangva, N.; Nandeibam, J.; Yendrembam, R.B.S.; Mukherjee, P.K. Isolation and characterization of bacteria from natural hot spring and insights into the thermophilic cellulase production. Curr. Microbiol. 2023, 80, 64. [Google Scholar] [CrossRef]
- Nair, A.S.; Al-Battashi, H.; Al-Akzawi, A.; Annamalai, N.; Gujarathi, A.; Al-Bahry, S.; Dhillon, G.S.; Sivakumar, N. Waste office paper: A potential feedstock for cellulase production by a novel strain Bacillus velezensis ASN1. Waste Manag. 2018, 79, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Abood, M.F.; Hamzah, H.M.; Al-Rawii, D.F. Production of cellulase and bioethanol by ethanol-tolerant co-culture of Bacillus cereus and Fusarium solani. J. Phys.Conf. Ser. 2021, 1879, 022016. [Google Scholar] [CrossRef]
- Singh, S.; Vidyarthi, A.S.; Nigam, V.K.; Dev, A. Extracellular facile biosynthesis, characterization and stability of gold nanoparticles by Bacillus licheniformis. Artif. Cells Nanomed. Biotechnol. 2014, 42, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.Y. Purification and characterization of an organic-solvent-tolerant cellulase from a halotolerant isolate, Bacillus sp. L1. J. Ind. Microbiol. Biotechnol. 2012, 39, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Anchal Akhtar, N.; Kanika Goyal, D.; Goyal, A. Response surface methodology for optimization of microbial cellulase production. Rom. Biotechnol. Lett. 2016, 21, 11832–11841. [Google Scholar]
- Pramanik, S.K.; Mahmud, S.; Paul, G.K.; Jabin, T.; Naher, K.; Uddin, M.S.; Zaman, S.; Saleh, M.A. Fermentation optimization of cellulase production from sugarcane bagasse by Bacillus pseudomycoides and molecular modeling study of cellulase. Curr. Res. Microb. Sci. 2020, 27, 100013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abada, E.A.; Elbaz, R.M.; Sonbol, H.; Korany, S.M. Optimization of cellulase production from Bacillus albus (MN755587) and its involvement in bioethanol production. Pol. J. Environ. Stud. 2021, 30, 2459–2466. [Google Scholar] [CrossRef]
- Shah, F.; Mishra, S. In vitro optimization for enhanced cellulose degrading enzyme from Bacillus licheniformis KY962963 associated with a microalgae Chlorococcum sp. using OVAT and statistical modeling. SN Appl. Sci. 2020, 2, 1923. [Google Scholar] [CrossRef]
- Tabssum, F.; Irfan, M.; Shakir, H.A.; Qazi, J.I. RSM based optimization of nutritional conditions for cellulase mediated saccharification by Bacillus cereus. J. Biol. Eng. 2018, 12, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, W.A.; Javed, S. Biochemical Characterization of Cellulase From Bacillus subtilis Strain and its Effect on Digestibility and Structural Modifications of Lignocellulose Rich Biomass. Front. Bioeng. Biotechnol. 2021, 9, 800265. [Google Scholar] [CrossRef]
- Sajith, S.; Sreedevi, S.; Priji, P.; Unni, K.N.; Benjamin, S. Production and partial purification of cellulase from a novel fungus, Aspergillus flavus BS1. Ann. Microbiol. 2014, 64, 763–771. [Google Scholar] [CrossRef]
- Moreau, A.; Montplaisir, D.; Sparling, R.; Barnabé, S. Hydrogen, ethanol and cellulase production from pulp and paper primary sludge by fermentation with Clostridium thermocellum. Biomass Bioenergy 2015, 72, 256–262. [Google Scholar] [CrossRef]
- Adsul, M.; Sandhu, S.K.; Singhania, R.R.; Gupta, R.; Puri, S.K.; Mathur, A. Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme Microb. Technol. 2020, 133, 109442. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Uppugundla, N.; Bowman, M.J.; Cavalier, D.; Sousa, L.D.C.; Dale, B.E.; Balan, V. Sugar loss and enzyme inhibition due to oligosaccharide accumulation during high solids-loading enzymatic hydrolysis. Biotechnol. Biofuels 2015, 8, 195. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, H.J.; Bang, W.G.; Choi, I.G.; Kim, K.H. Functional characterization of a bacterial expansin from Bacillus subtilis for enhanced enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2009, 102, 1342–1353. [Google Scholar] [CrossRef]
- Lugani, Y.; Singla, R.; Sooch, B.S. Optimization of cellulase production from newly isolated Bacillus Sp. Y3. J. Bioprocess Biotech. 2015, 5, 264. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Development and validation of a stochastic molecular model of cellulose hydrolysis by action of multiple cellulase enzymes. Bioresour. Bioprocess. 2017, 4, 54. [Google Scholar] [CrossRef]
- Irfan, M.; Mushtaq, Q.; Tabssum, F.; Shakir, H.A.; Quazi, J.I. Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Expr. 2017, 7, 29. [Google Scholar] [CrossRef]
- Dar, M.A.; Pawar, K.D.; Rajput, B.P.; Rahi, P.; Pandit, R.S. Purification of a cellulase from cellulolytic gut bacterium, Bacillus tequilensis G9 and its evaluation for valorization of agro-wastes into added value byproducts. Biocatal. Agric. Biotechnol. 2019, 20, 101219. [Google Scholar] [CrossRef]
- Thakkar, A.; Saraf, M. Application of statistically based experimental designs to optimize cellulase production and identification of gene. Nat. Prod. Bioprospect. 2014, 4, 341–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva, T.S.; Silva, D.A.K.; Nogueira, A.L. Metformin hydrochloride sustained release biopolymeric system composed by PLLA-CMC microparticles. J. Appl. Polym. Sci. 2021, 138, 50806. [Google Scholar] [CrossRef]
- Ghazanfar, M.; Irfan, M.; Shakir, H.A.; Khan, M.; Nadeem, M.; Irfan, A. Cellulase production optimization by Bacillus aerius through response surface methodology in submerged fermentation. Cellulose Chem. Technol. 2022, 56, 321–330. [Google Scholar] [CrossRef]
- Vyas, A.K.; Putatunda, C.; Singh, J.; Vyas, D. Cellulase production by Bacillus subtilis M1 using pretreated groundnut shell based liquid state fermentation. BIOTROPIA—Southeast Asian J. Trop. Med. Public Health. 2016, 23, 28–34. [Google Scholar] [CrossRef]
- BalaKumaran, M.D.B.; Kalaichelvan, P.T.; Santhi, R. Exploitation of Agro-industrial wastes as substrates for cellulase production by Bacillus licheniformis MTCC 429. Microbiol. J. 2015, 5, 36–42. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Coconut mesocarp-based lignocellulosic waste as a substrate for cellulase production from high promising multienzyme-producing Bacillus amyloliquefaciens FW2 without pretreatments. Microorganisms 2022, 10, 327. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, X. Alkali-stable cellulase from a halophilic isolate, Gracilibacillus sp. SK1 and its application in lignocellulosic saccharification for ethanol production. Biomass Bioenergy 2015, 18, 19–25. [Google Scholar] [CrossRef]
- Agrawal, R.; Semwal, S.; Kumar, R.; Mathur, A.; Gupta, R.P.; Tuli, D.K.; Satlewal, A. Synergistic Enzyme Cocktail to Enhance Hydrolysis of Steam Exploded Wheat Straw at Pilot Scale. Front. Energy Res. 2018, 6, 122. [Google Scholar] [CrossRef]
- Takano, M.; Hoshino, K. Bioethanol production from rice straw by simultaneous saccharification and fermentation with statistical optimized cellulase cocktail and fermenting fungus. Bioresour. Bioprocess. 2018, 5, 16. [Google Scholar] [CrossRef]
- Rastogi, G.; Bhalla, A.; Adhikari, A.; Bischoff, K.M.; Hughes, S.R.; Christopher, L.P. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol. 2010, 101, 8798–8806. [Google Scholar] [CrossRef]
- Singh, J.; Batra, N.; Sobti, R.C. Purification and characterization of alkaline cellulase produced by a novel isolate, Bacillus sphaericus JS1. J. Ind. Microbiol. Biotechnol. 2004, 31, 51–56. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hur, S.H.; Hong, J.H. Purification and characterization of an alkaline cellulase from a newly isolated alkalophilic Bacillus sp. HSH-810. Biotechnol. Lett. 2005, 27, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Harnvoravongchai, P.; Singwisut, R.; Ounjai, P.; Aroonnual, A.; Kosiyachinda, P.; Janvilisri, T.; Chankhamhaengdecha, S. Isolation and characterization of thermophilic cellulose and hemicellulose degrading bacterium, Thermoanaerobacterium sp. R63 from tropical dry deciduous forest soil. PLoS ONE 2020, 15, e0236518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, A.; Jeske, L.; Ulbrich, S.; Hofmann, J.; Koblitz, J.; Schomburg, I.; Neumann-Schaal, M.; Jahn, D.; Schomburg, D. BRENDA, the ELIXIR core data resource in 2021: New developments and updates. Nucleic Acids Res. 2021, 49, D498–D508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Guo, H.; Gu, J.; Qin, W. Optimize purification of a cellulase from Bacillus velezensis A4 by aqueous two-phase system (ATPS) using response surface methodology. Process Biochem. 2019, 87, 196–203. [Google Scholar] [CrossRef]
- Duman, Y.; Karakuş, Y.Y.; Sertel, A.; Polat, F. Production, purification, and characterization of a thermo-alkali stable and metal-tolerant carboxymethylcellulase from newly isolated Bacillus methylotrophicus Y37. Turk. J. Chem. 2016, 40, 802–815. [Google Scholar] [CrossRef]
- Niyonzima, F.N. Detergent-compatible bacterial cellulases. J. Basic Microbiol. 2019, 59, 134–147. [Google Scholar] [CrossRef]
- Klapiszewski, Ł. Advanced Silica/Lignin Hybrida. Ph.D. Thesis, Poznań University of Technology, Poznan, Poland, 2014. Available online: https://sin.put.poznan.pl/dissertations/details/d430 (accessed on 12 December 2024).
- Shimizu, F.L.; Monteiro, P.Q.; Ghiraldi, P.H.C.; Melati, R.B.; Pagnocca, F.C.; de Souza, W.; Sant’aNna, C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crops Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef]
- Potprommanee, L.; Wang, X.Q.; Han, Y.J.; Nyobe, D.; Peng, Y.P.; Huang, Q.; Liu, J.Y.; Liao, Y.L.; Chang, K.L. Characterization of a thermophilic cellulase from Geobacillus sp. HTA426, an efficient cellulase-producer on alkali pretreated of lignocellulosic biomass. PLoS ONE 2017, 12, e0175004. [Google Scholar] [CrossRef]
| Run | Substrate [g/L] | (NH4)2SO4 [g/L] | pH | Cellulase [U] Observed | Cellulase [U] Predicted |
|---|---|---|---|---|---|
| 1 | 20 | 0.5 | 7 | 0.265 | 0.259 |
| 2 | 60 | 0.5 | 7 | 0.268 | 0.312 |
| 3 | 20 | 5.5 | 7 | 0.291 | 0.275 |
| 4 | 60 | 5.5 | 7 | 0.422 | 0.455 |
| 5 | 20 | 3.0 | 6 | 0.246 | 0.263 |
| 6 | 60 | 3.0 | 6 | 0.403 | 0.379 |
| 7 | 20 | 3.0 | 8 | 0.276 | 0.281 |
| 8 | 60 | 3.0 | 8 | 0.451 | 0.397 |
| 9 | 40 | 0.5 | 6 | 0.308 | 0.274 |
| 10 | 40 | 5.5 | 6 | 0.469 | 0.451 |
| 11 | 40 | 0.5 | 8 | 0.393 | 0.389 |
| 12 | 40 | 5.5 | 8 | 0.370 | 0.372 |
| 13 | 40 | 3.0 | 7 | 0.342 | 0.367 |
| 14 | 40 | 3.0 | 7 | 0.344 | 0.367 |
| 15 | 40 | 3.0 | 7 | 0.362 | 0.367 |
| Model Component | SS | df | MS | F | p |
|---|---|---|---|---|---|
| substrate [g/L] (L) | 0.0272 | 1 | 0.0272 | 217.13 | 0.0046 |
| substrate [g/L] (Q) | 0.0058 | 1 | 0.0058 | 46.46 | 0.0209 |
| (NH4)2SO4 [g/L] (L) | 0.0126 | 1 | 0.0126 | 100.54 | 0.0098 |
| pH (Q) | 0.0042 | 1 | 0.0042 | 33.73 | 0.0284 |
| 1 L × 2 L | 0.0041 | 1 | 0.0041 | 32.75 | 0.0292 |
| 2 L × 3 L | 0.0085 | 1 | 0.0085 | 67.93 | 0.0144 |
| lack of fit | 0.0068 | 6 | 0.0011 | 9.02 | 0.1031 |
| pure error | 0.0003 | 2 | 0.0001 | ||
| total SS | 0.0702 | 14 |
| Parameter | Raw BSG * % a.d.w | Hydrolyzed BSG % a.d.w |
|---|---|---|
| Moisture | 4.66 ± 0.14 | 5.94 ± 0.08 |
| Moisture after ethanol extraction | 7.14 ± 0.16 | 6.48 ± 0.13 |
| Extractives | 14.24 ± 0.21 | 21.09 ± 0.13 |
| Cellulose | 12.74 ± 0.06 | 18.47 ± 0.16 |
| Klason lignin | 10.25 ± 0.68 | 15.12 ± 0.04 |
| Hemicellulose | 74.35 ± 0.13 | 74.24 ± 0.24 |
| Pentosans | 24.79 ± 0.08 | 25.26 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choińska-Pulit, A.; Sobolczyk-Bednarek, J.; Łaba, W. Cellulolytic Potential of Newly Isolated Alcohol-Tolerant Bacillus methylotrophicus. Materials 2025, 18, 3256. https://doi.org/10.3390/ma18143256
Choińska-Pulit A, Sobolczyk-Bednarek J, Łaba W. Cellulolytic Potential of Newly Isolated Alcohol-Tolerant Bacillus methylotrophicus. Materials. 2025; 18(14):3256. https://doi.org/10.3390/ma18143256
Chicago/Turabian StyleChoińska-Pulit, Anna, Justyna Sobolczyk-Bednarek, and Wojciech Łaba. 2025. "Cellulolytic Potential of Newly Isolated Alcohol-Tolerant Bacillus methylotrophicus" Materials 18, no. 14: 3256. https://doi.org/10.3390/ma18143256
APA StyleChoińska-Pulit, A., Sobolczyk-Bednarek, J., & Łaba, W. (2025). Cellulolytic Potential of Newly Isolated Alcohol-Tolerant Bacillus methylotrophicus. Materials, 18(14), 3256. https://doi.org/10.3390/ma18143256





