Corrosion Behavior of Additively Manufactured GRX-810 Alloy in 3.5 wt.% NaCl
Abstract
1. Introduction
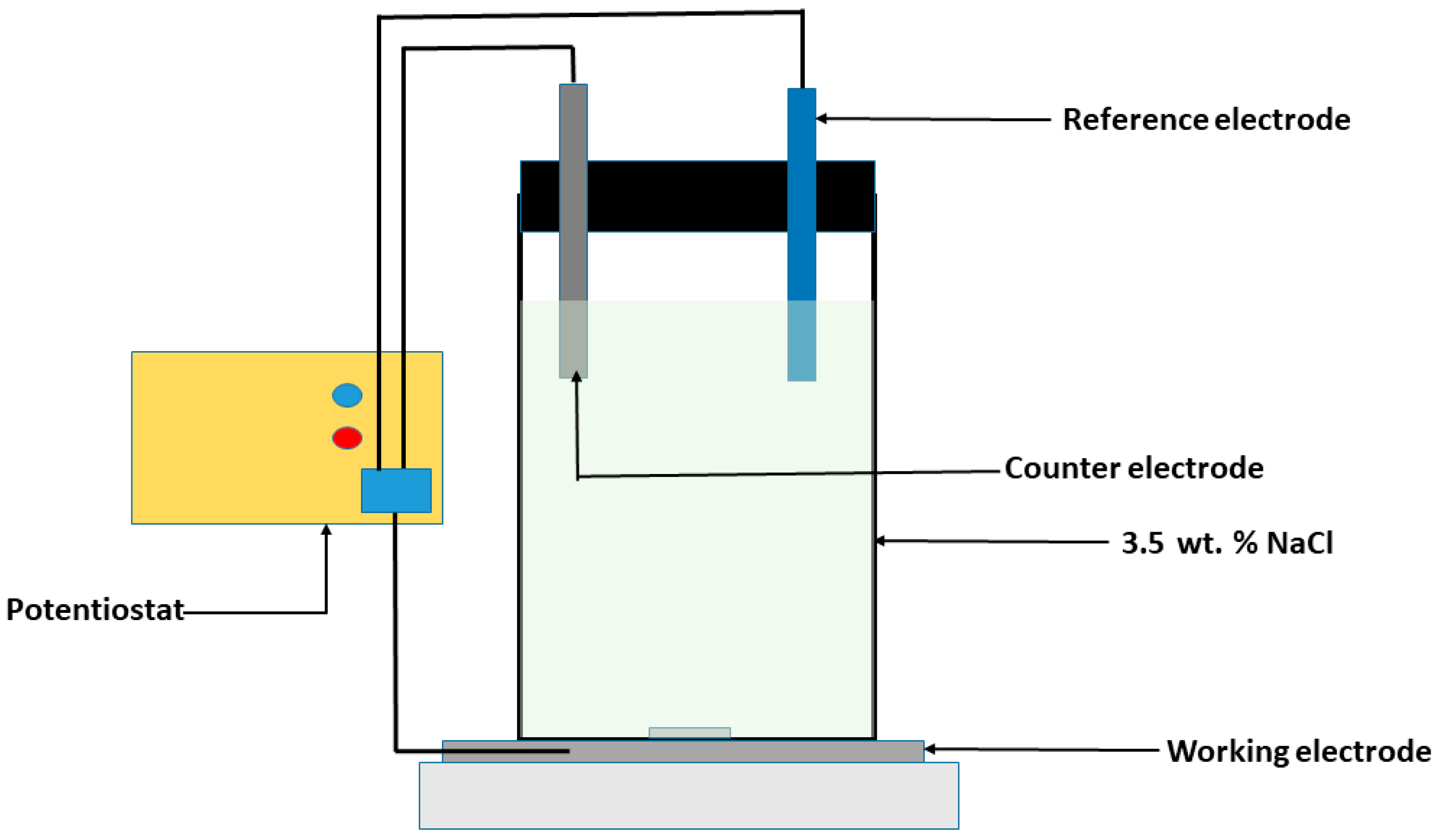
2. Materials and Methods
3. Results
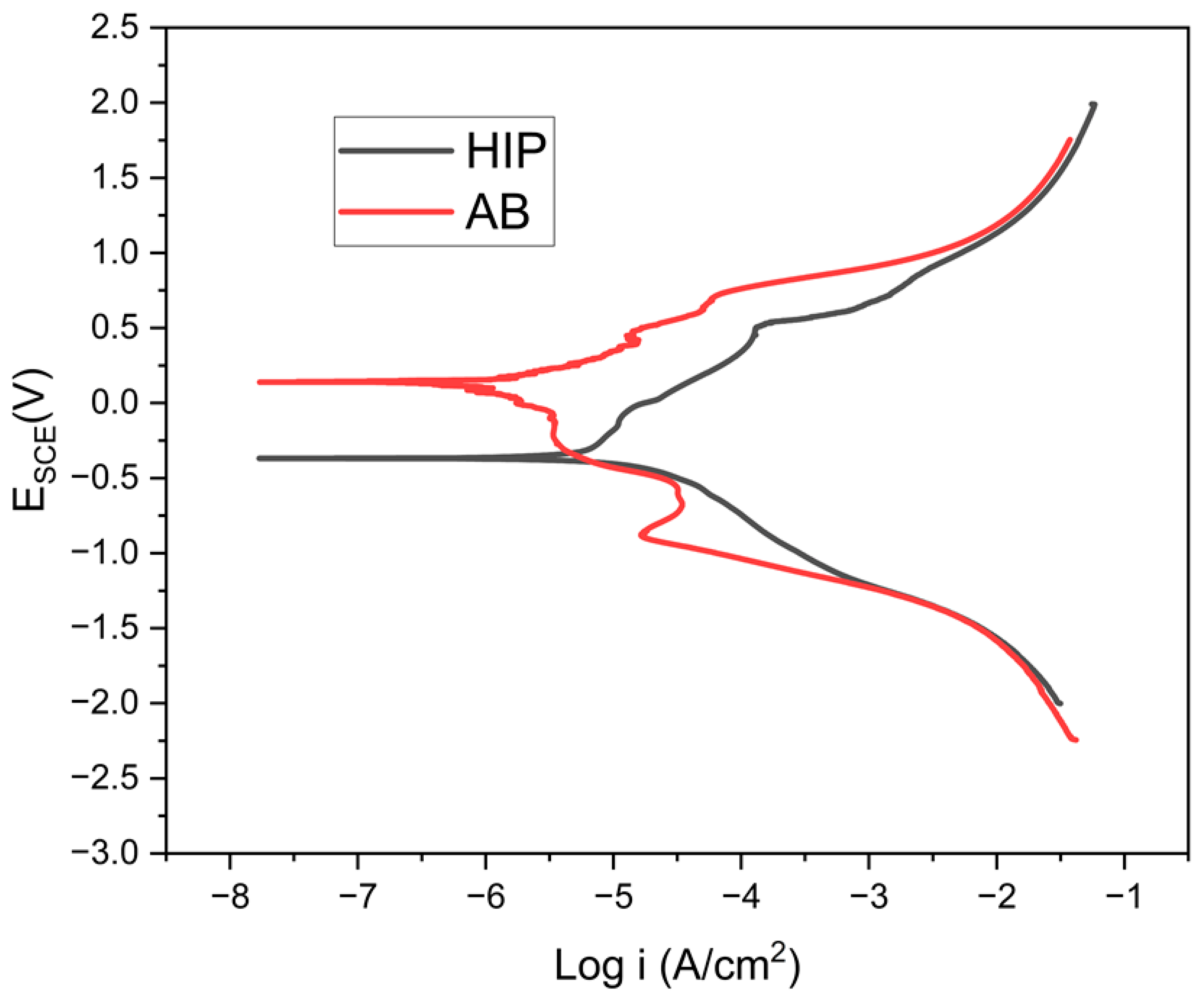
3.1. Potentiodynamic Polarization
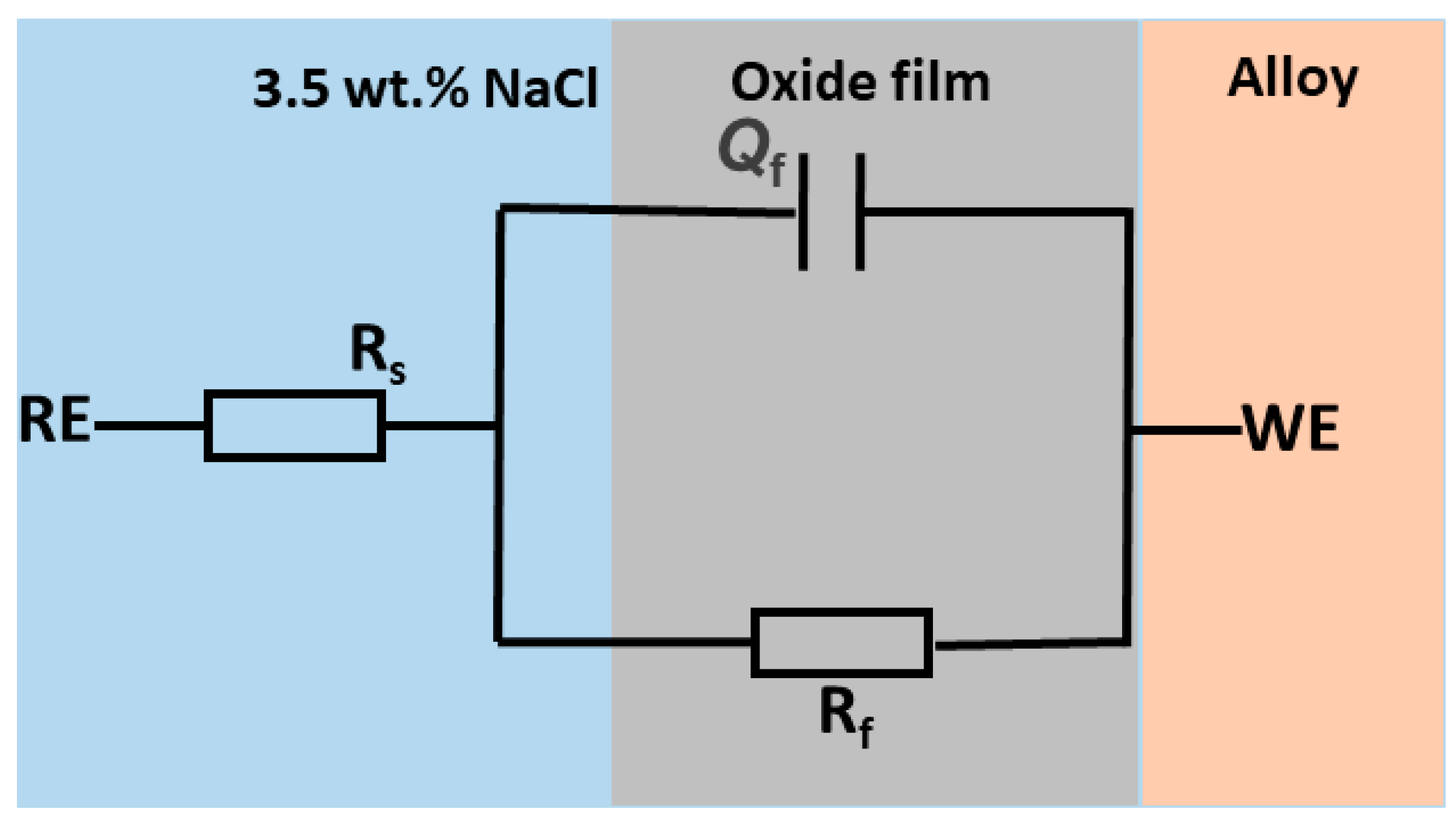
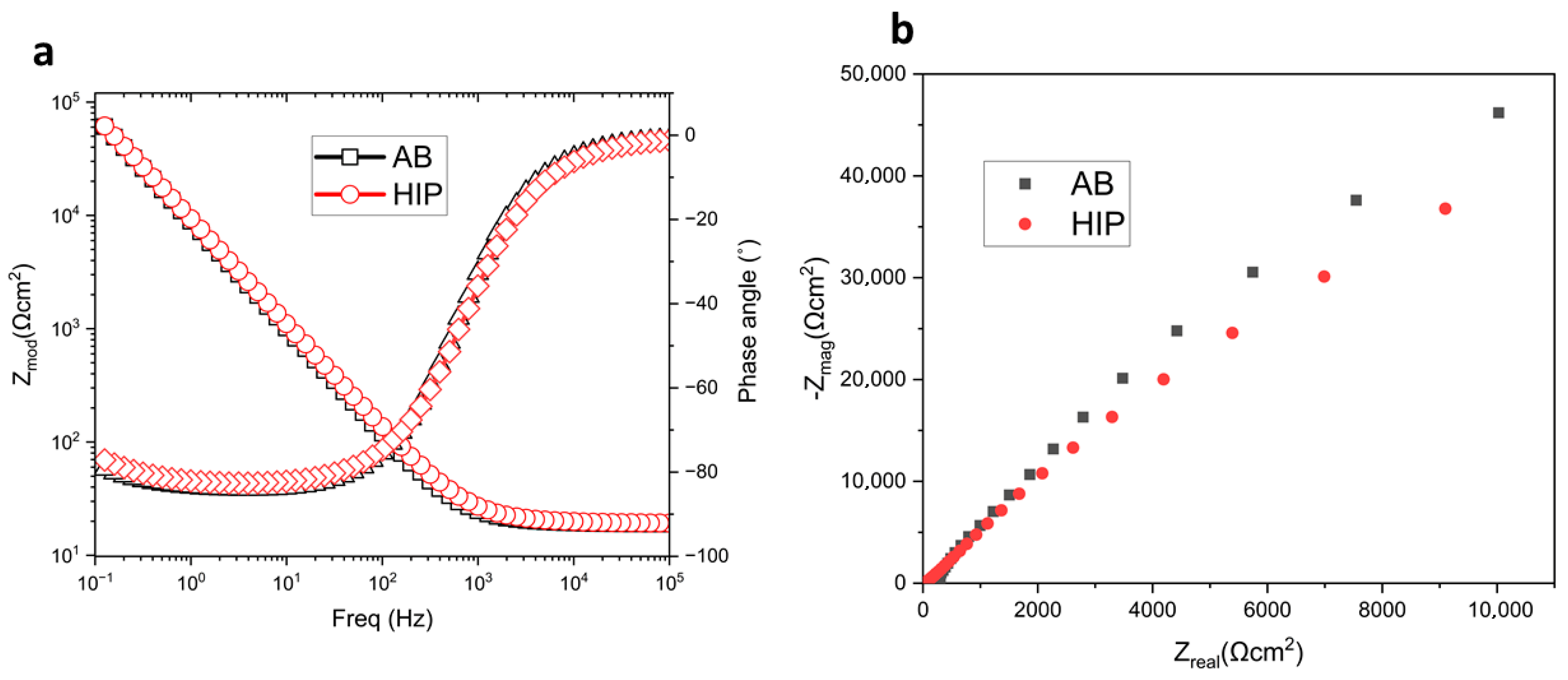
3.2. Electrochemical Impedance Spectroscopy (EIS)
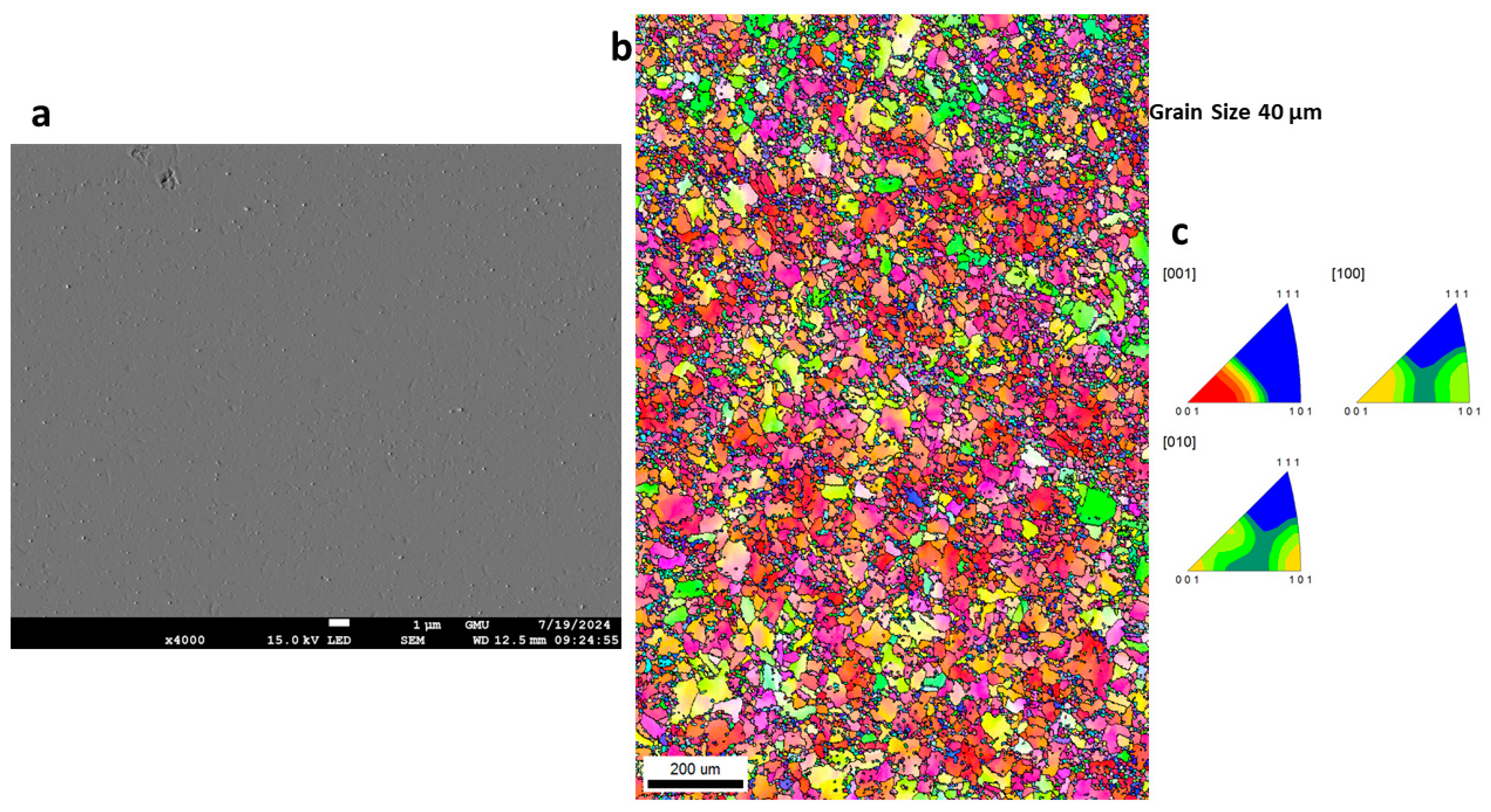
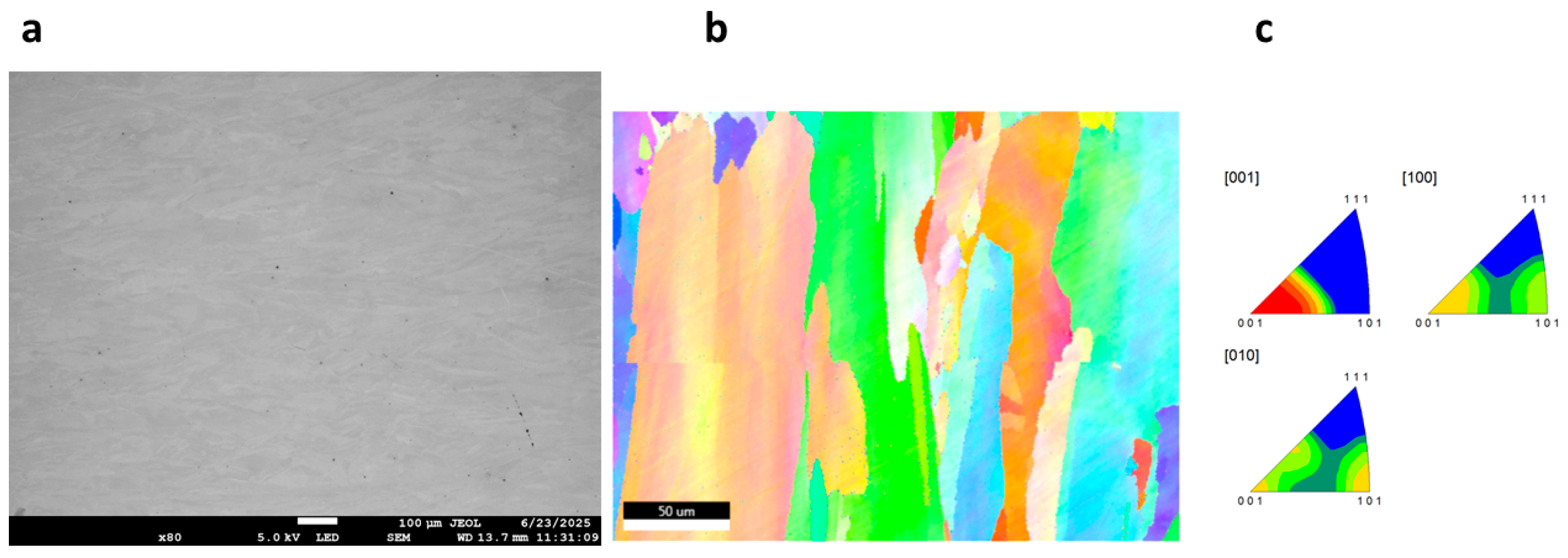
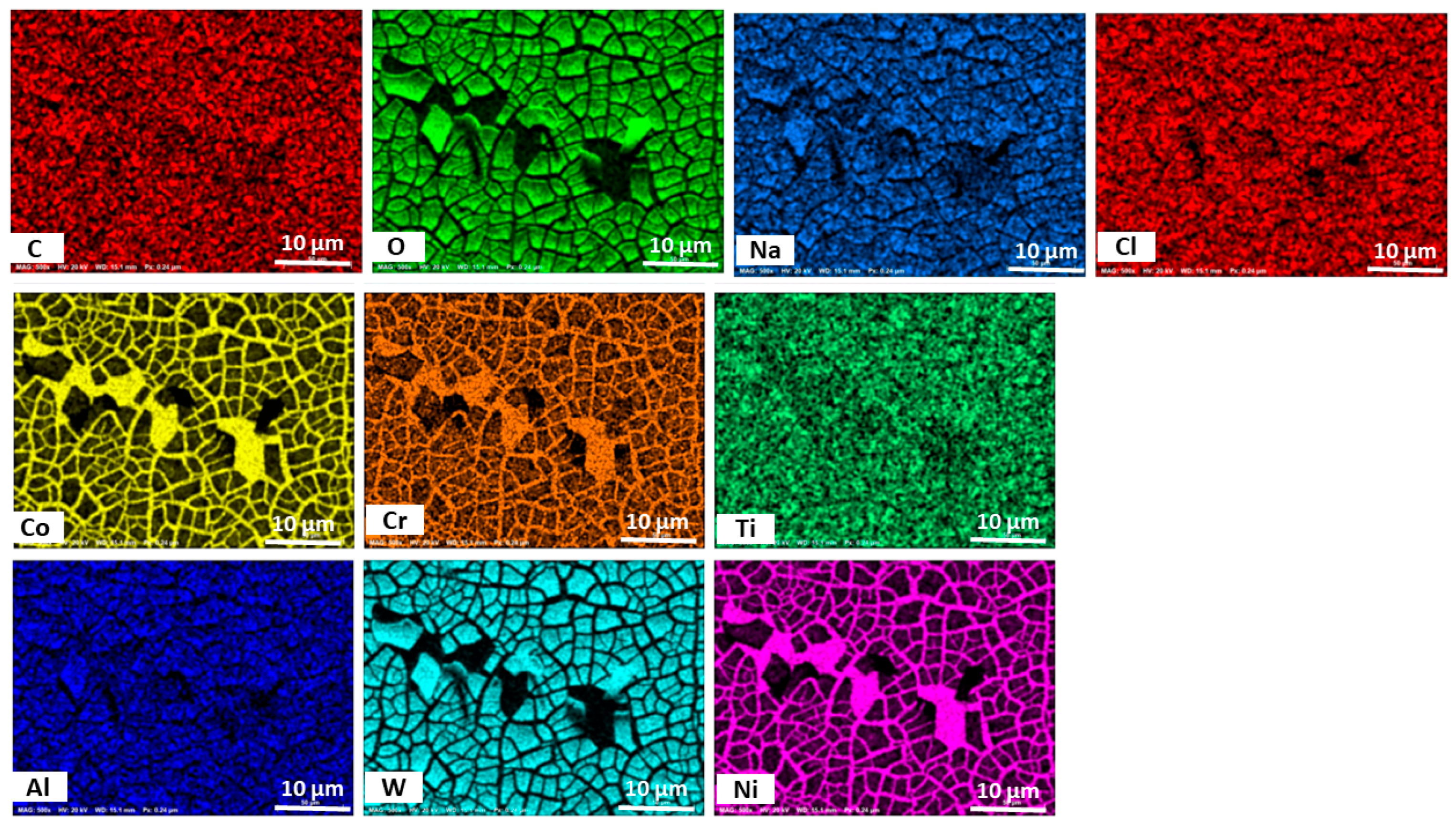
3.3. Morphology of GRX-810
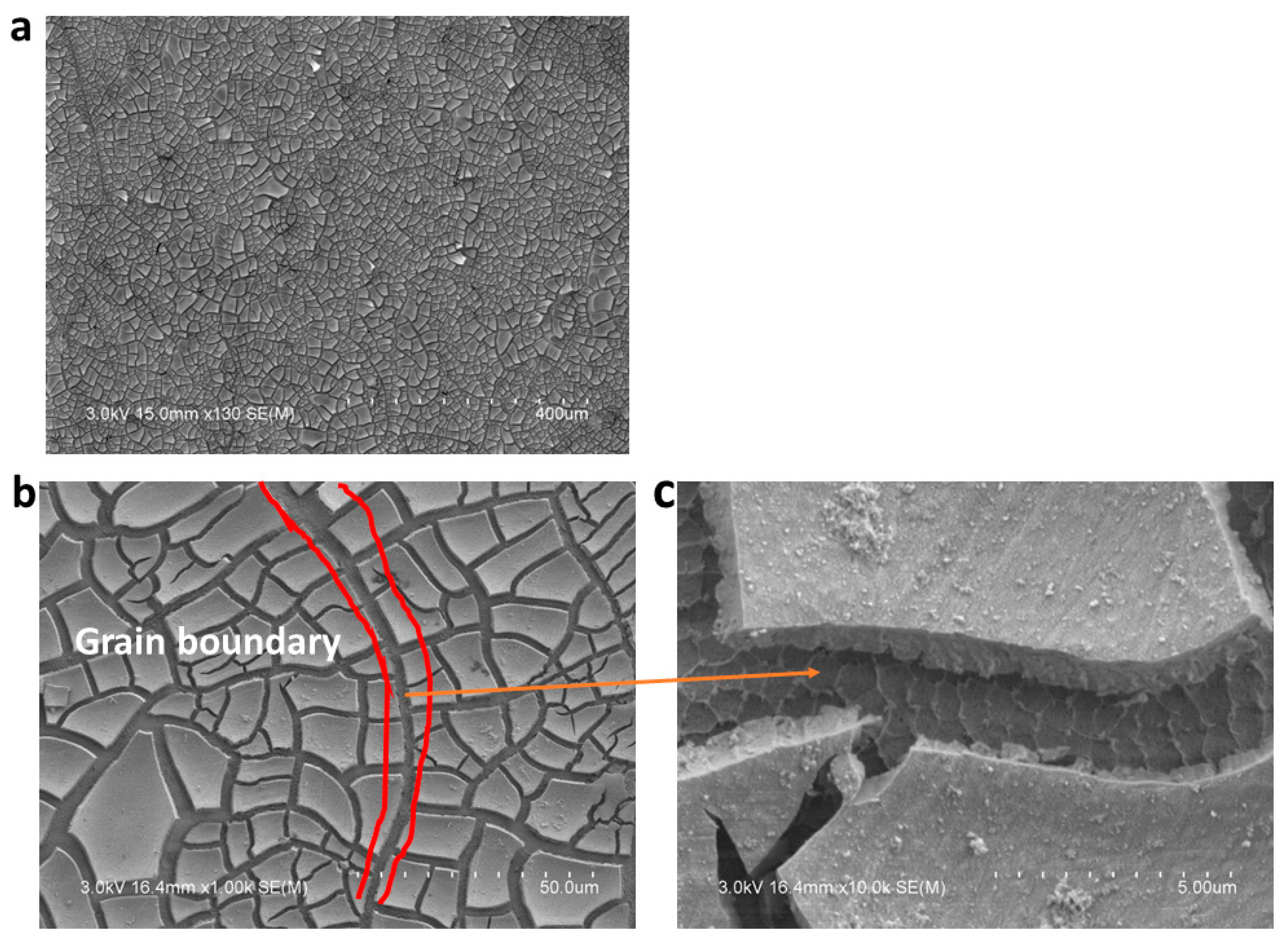
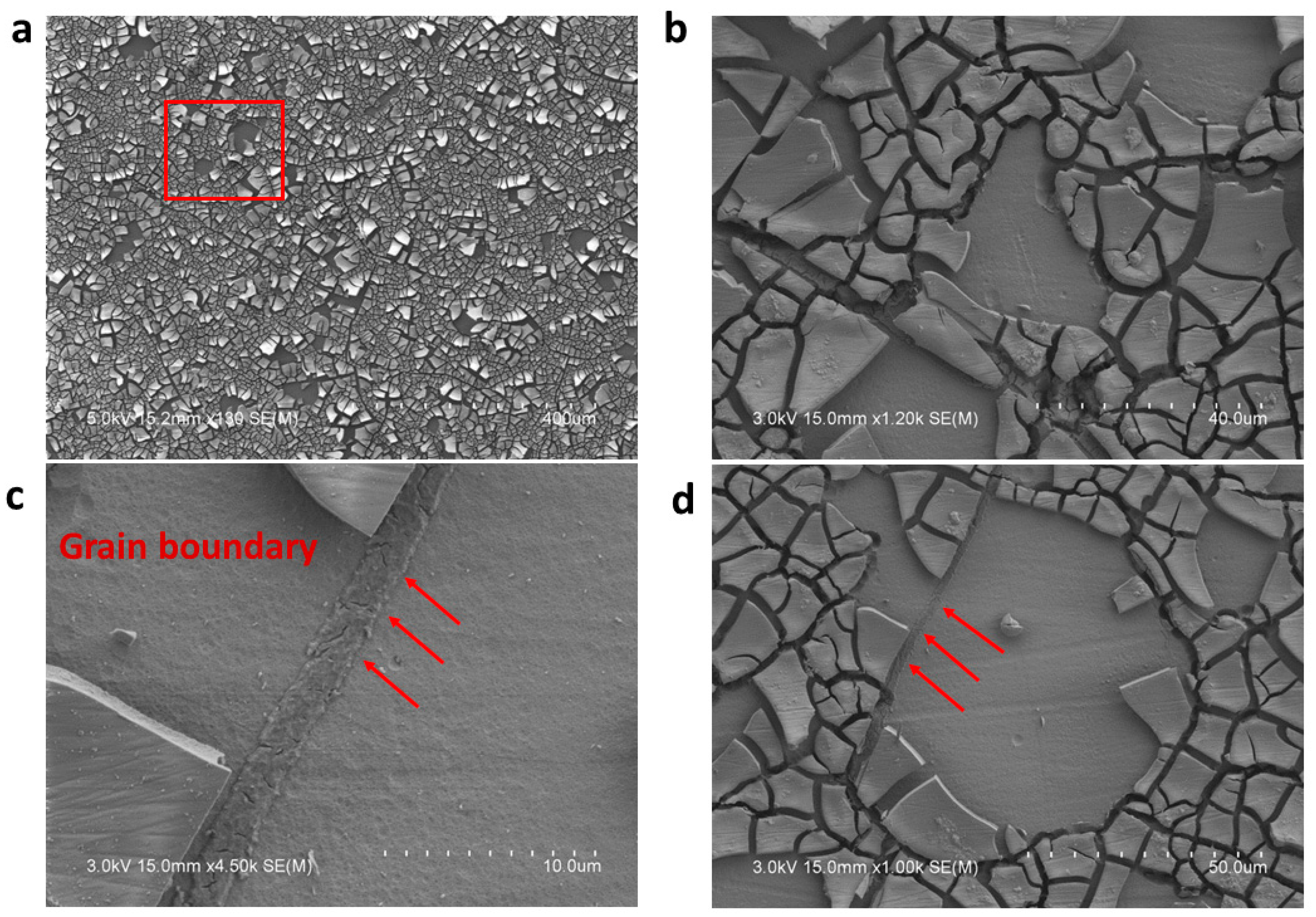
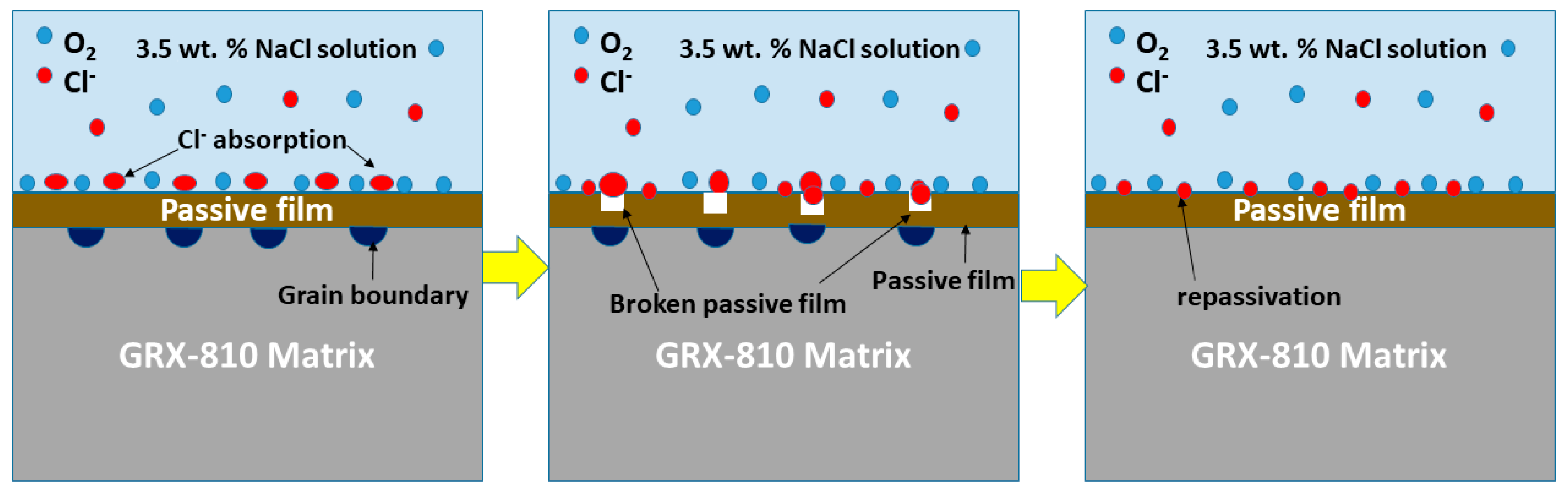
3.4. Oxide Film Formation
XRD Analysis
4. Discussion
5. Conclusions
- Based on the potentiodynamic results, the corrosion resistance rate is as follows: AB GRX-810 > HIP GRX-810. This shows decreasing Ecorr value and relative stability in marine environments. The passive film formed after repassivation was observed to be more compact in the AB GRX-810 sample compared to the HIP sample.
- Analysis of the EIS data suggested that the passive film formed on the GRX-810 alloy can be modeled as a single-layer structure, indicative of a homogenous and compact barrier layer. The high impedance values and phase angle behavior further imply that the alloy surface is densely covered with corrosion products, which may enhance the protective characteristics of the oxide film by reducing ion transport and slowing down corrosion kinetics.
- The SEM/EDS results suggest the possibility of the formation of oxide films of Cr, Ni, Co, and Ti for the GRX-810 alloy, with the XRD results confirming the formation of Cr2O3 on the surface of the alloy after polarization. However, future studies employing surface-sensitive techniques like XPS would be valuable to gain deeper insight into the atomic-level structure and composition of the oxide layer formed on the surface of the alloy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omoniyi, P.; Acharya, U.; Akinlabi, S.; Jen, T.C.; Akinlabi, E. Taguchi-Grey relational analysis driven multi-objective optimization of weld bead geometry and hardness in laser welded Ti6Al4V Alloy. Int. J. Interact. Des. Manuf. 2024, 19, 423–434. [Google Scholar] [CrossRef]
- Darabseh, T.; Yilmaz, N.; Bataineh, M. Transient thermoelasticity analysis of functionally graded thick hollow cylinder based on Green-Lindsay model. Int. J. Mech. Mater. Des. 2012, 8, 247–255. [Google Scholar] [CrossRef]
- Mahesh, R.A.; Jayaganthan, R.; Prakash, S. A study on hot corrosion behaviour of Ni-5Al coatings on Ni- and Fe-based superalloys in an aggressive environment at 900 °C. J. Alloys Compd. 2008, 460, 220–231. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Influence of micro-structure on corrosion behavior of a Ni-based superalloy in 3.5% NaCl. Electrochim. Acta 2007, 52, 7193–7202. [Google Scholar] [CrossRef]
- Chellaganesh, D.; Khan, M.A.; Jappes, J.T.W. Hot corrosion behaviour of nickel–iron-based superalloy in gas turbine application. Int. J. Ambient. Energy 2020, 41, 901–905. [Google Scholar] [CrossRef]
- Omoniyi, P.; Mahamood, R.; Arthur, N.; Pityana, S.; Skhosane, S.; Jen, T.C.; Akinlabi, E.T. Corrosion Behavior of Laser-Welded Ti6Al4V in 3.5%Wt NaCl; Springer: Singapore, 2023; pp. 395–400. Available online: https://books.google.com/books?hl=en&lr=&id=Q5-TEAAAQBAJ&oi=fnd&pg=PA395&ots=YIl1NHBmje&sig=HWOHc0BS6fyDHqgmoh67iPeecg8 (accessed on 3 November 2022).
- Osoba, L.O.; Oladoye, A.M.; Ogbonna, V.E. Corrosion evaluation of superalloys Haynes 282 and Inconel 718 in Hydrochloric acid. J. Alloys Compd. 2019, 804, 376–384. [Google Scholar] [CrossRef]
- Yanqiu, Y.; Zhixun, W.; Yanchao, Z.; Jiapo, W.; Zhenwei, L.; Zhufeng, Y. Effect of crystallographic orientation on the corrosion resistance of Ni-based single crystal superalloys. Corros. Sci. 2020, 170, 108643. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Li, Z.; Xu, H.; Li, G. Corrosion behaviors and mechanism of CrFeNi2 based high-entropy alloys. Corros. Sci. 2022, 207, 110562. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Shang, X.L.; Wang, Z.J.; He, F.; Wang, J.C.; Li, J.J.; Yu, J.K. The intrinsic mechanism of corrosion resistance for FCC high entropy alloys. Sci. China Technol. Sci. 2018, 61, 189–196. [Google Scholar] [CrossRef]
- Qiu, Y.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 2015, 31, 1235–1243. [Google Scholar] [CrossRef]
- Smith, T.M.; Kantzos, C.A.; Zarkevich, N.A.; Harder, B.J.; Heczko, M.; Gradl, P.R.; Thompson, A.C.; Mills, M.J.; Gabb, T.P.; Lawson, J.W. A 3D printable alloy designed for extreme environments. Nature 2023, 617, 513–518. [Google Scholar] [CrossRef]
- Gradl, P.; Mireles, O.R.; Katsarelis, C.; Smith, T.M.; Sowards, J.; Park, A.; Chen, P.; Tinker, D.C.; Protz, C.; Teasley, T.; et al. Advancement of extreme environment additively manufactured alloys for next generation space propulsion applications. Acta Astronaut. 2023, 211, 483–497. [Google Scholar] [CrossRef]
- Sui, F.; An, T.; Zheng, S.; Chen, L.; Li, S. Influence of effective strain on the corrosion behavior of nickel-based GH4710 superalloy in chloride solutions. Corros. Sci. 2022, 204, 110386. [Google Scholar] [CrossRef]
- Weng, F.; Chew, Y.; Ong, W.K.; Man, Y.; Sui, S.; Tan, C.; Ng, F.L.; Goh, M.H.; Bi, G. Enhanced corrosion resistance of laser aided additive manufactured CoCrNi medium entropy alloys with oxide inclusion. Corros. Sci. 2022, 195, 109965. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, B.; Guo, W.; Fu, A.; Duan, H.; Li, W. Corrosion behavior and mechanism of FeCrNi medium entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2021, 867, 159094. [Google Scholar] [CrossRef]
- Omoniyi, P.O.; Akinlabi, E.T.; Mahamood, R.M.; Jen, T.C. Corrosion Resistance of Heat Treated Ti6Al4V in NaCl. Chem. Data Collect. 2021, 36, 100780. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Davies, S. Fundamental aspects of hot isostatic pressing: An overview. Metall. Mater. Trans. A 2000, 31, 2981–3000. [Google Scholar] [CrossRef]
- Saberi, L.; Liou, F.W.; Amiri, M. Effects of Process-Induced Defects on the Corrosion of Additively Manufactured Stainless Steel 304L. J. Electrochem. Soc. 2024, 171, 051505. [Google Scholar] [CrossRef]
- Zhu, M.; He, F.; Yuan, Y.; Guo, S.; Wei, G. A comparative study on the corrosion behavior of CoCrNi medium-entropy alloy and 316L stainless steel in simulated marine environment. Intermetallics 2021, 139, 107370. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, S.; Min, Q.; Li, C.; Li, D.; Cao, B.; Ma, W.; Zhao, K.; Wang, Y.; Zhang, Z. Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions. Materials 2025, 18, 977. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.X.; Zhu, M.; Yuan, Y.F.; Guo, S.Y.; Chen, Y.B. Study on the Corrosion Behavior and Mechanism of CoCrNi Medium-Entropy Alloy in NaCl Solution with Various NaHSO3 Concentrations. J. Mater. Eng. Perform. 2023, 33, 11151–11164. [Google Scholar] [CrossRef]
- Ni, Q.; Zhu, M.; Yuan, Y.; Guo, S. Oxidation behavior and corrosion resistance of CoCrFeNi high entropy alloy compared with FeCoNi medium entropy alloy. Mater. Chem. Phys. 2024, 325, 129758. [Google Scholar] [CrossRef]
- Mohamed, O.; Hassan, M.; Egilmez, M.; Abuzaid, W.; Ibrahim, T.; Khamis, M. Corrosion behavior of CoCrNi/mild steel medium entropy alloy thin films. Mater. Today Commun. 2022, 30, 103015. [Google Scholar] [CrossRef]
- Xu, X.; Ganguly, S.; Ding, J.; Seow, C.E.; Williams, S. Enhancing mechanical properties of wire + arc additively manufactured INCONEL 718 superalloy through in-process thermomechanical processing. Mater. Des. 2018, 160, 1042–1051. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; De Vecchis, R.R.; Xiong, W. Evolution of carbide precipitates in Haynes® 282 superalloy processed by wire arc additive manufacturing. J. Mater. Process. Technol. 2022, 305, 117597. [Google Scholar] [CrossRef]
- Furushima, R.; Tanaka, S.; Kato, Z.; Uematsu, K. Orientation distribution-Lotgering factor relationship in a polycrystalline material–As an example of bismuth titanate prepared by a magnetic field. J. Ceram. Soc. 2010, 118, 921–926. [Google Scholar] [CrossRef]
- Valle, L.C.M.; Santana, A.I.C.; Rezende, M.C.; Dille, J.; Mattos, O.R.; de Almeida, L.H. The influence of heat treatments on the corrosion behaviour of nickel-based alloy 718. J. Alloys Compd. 2019, 809, 151781. [Google Scholar] [CrossRef]


| Specimen | Ecorr (mVsce) | Icorr (µA/cm2) | Epit (mVsce) |
|---|---|---|---|
| GRX-810-AB | 187 | 1.80 | 505 |
| GRX-810-HIP | −406 | 11 | 437 |
| Specimen | Rs (Ωcm2) | Qf (Ω−1/cm2) | Rf (Ωcm2) | n | Х2 |
|---|---|---|---|---|---|
| GRX-810 AB | 19.16 | 2.5 × 10−5 | 4.8 × 1011 | 0.94 | 6.14 × 10−4 |
| GRX-810 HIP | 16.13 | 1.11 × 10−4 | 1.4 × 104 | 0.73 | 7.9 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoniyi, P.; Alfred, S.; Looby, K.; Bamiduro, O.; Amiri, M.; Owolabi, G. Corrosion Behavior of Additively Manufactured GRX-810 Alloy in 3.5 wt.% NaCl. Materials 2025, 18, 3252. https://doi.org/10.3390/ma18143252
Omoniyi P, Alfred S, Looby K, Bamiduro O, Amiri M, Owolabi G. Corrosion Behavior of Additively Manufactured GRX-810 Alloy in 3.5 wt.% NaCl. Materials. 2025; 18(14):3252. https://doi.org/10.3390/ma18143252
Chicago/Turabian StyleOmoniyi, Peter, Samuel Alfred, Kenneth Looby, Olu Bamiduro, Mehdi Amiri, and Gbadebo Owolabi. 2025. "Corrosion Behavior of Additively Manufactured GRX-810 Alloy in 3.5 wt.% NaCl" Materials 18, no. 14: 3252. https://doi.org/10.3390/ma18143252
APA StyleOmoniyi, P., Alfred, S., Looby, K., Bamiduro, O., Amiri, M., & Owolabi, G. (2025). Corrosion Behavior of Additively Manufactured GRX-810 Alloy in 3.5 wt.% NaCl. Materials, 18(14), 3252. https://doi.org/10.3390/ma18143252









