Corrosion Behavior of MgTiZn and Mg4TiZn Alloys After Ball Milling and Subsequent Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. X-Ray Diffraction
2.2.2. Scanning Electron Microscopy
2.2.3. Scanning Transmission Electron Microscopy (STEM)
2.2.4. Nanoindentation
2.2.5. X-Ray Photoelectron Spectroscopy
2.3. Corrosion
3. Results and Discussion
3.1. Microstructural Characterization Using SEM, XRD, and S/TEM
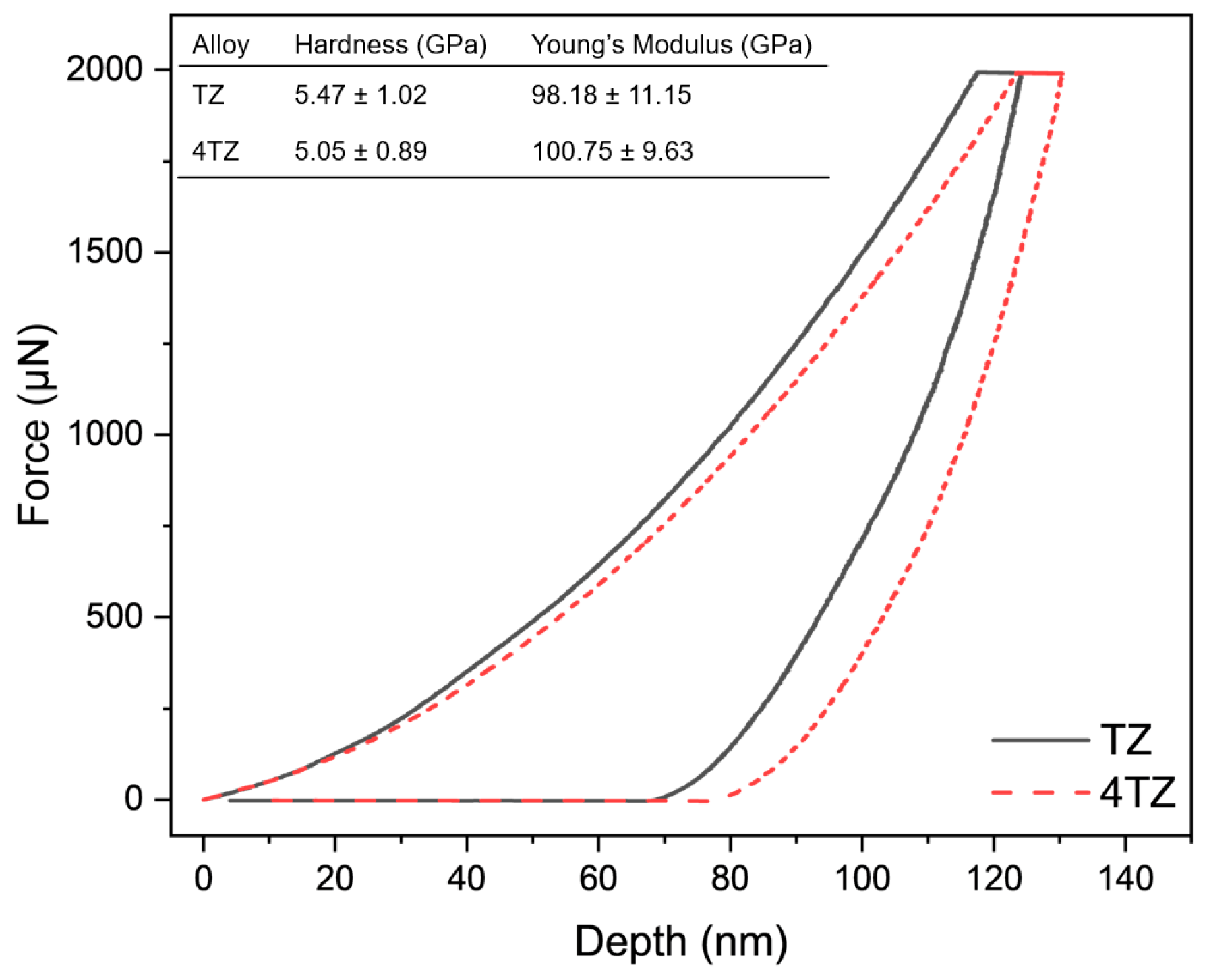
3.2. Hardness
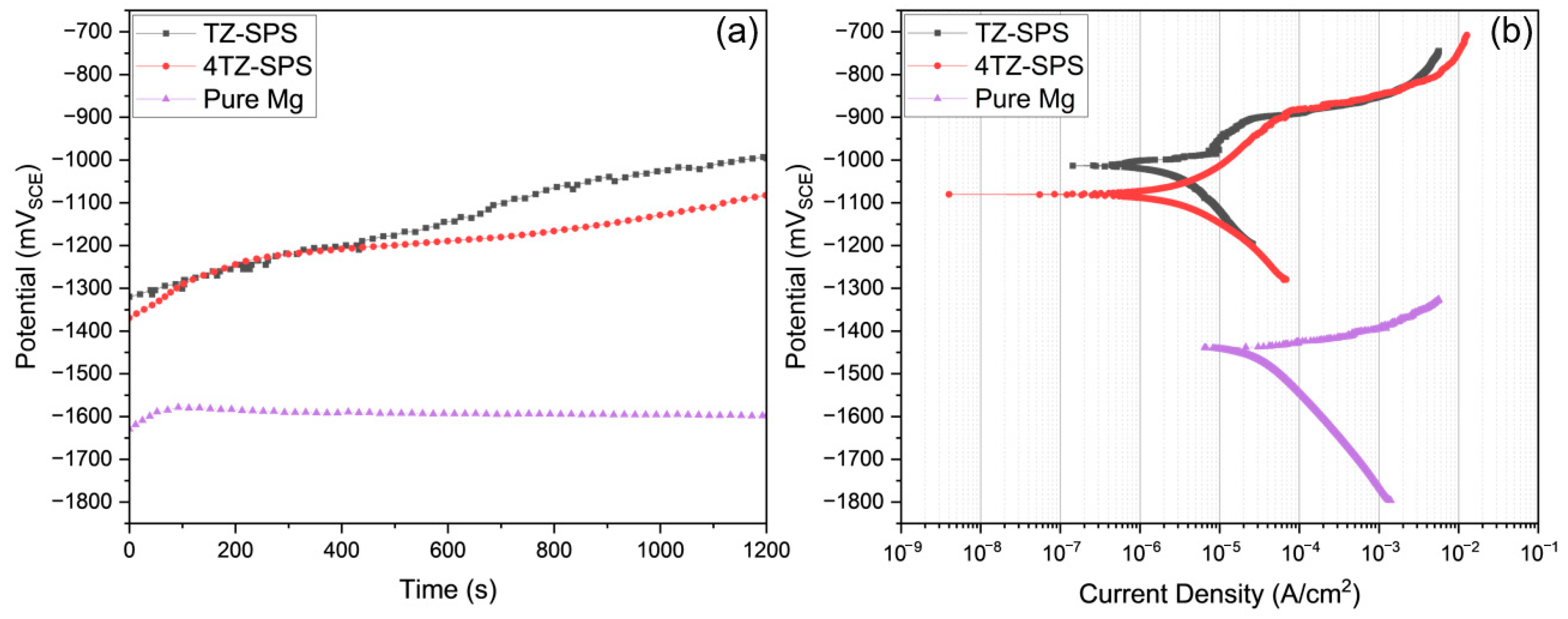
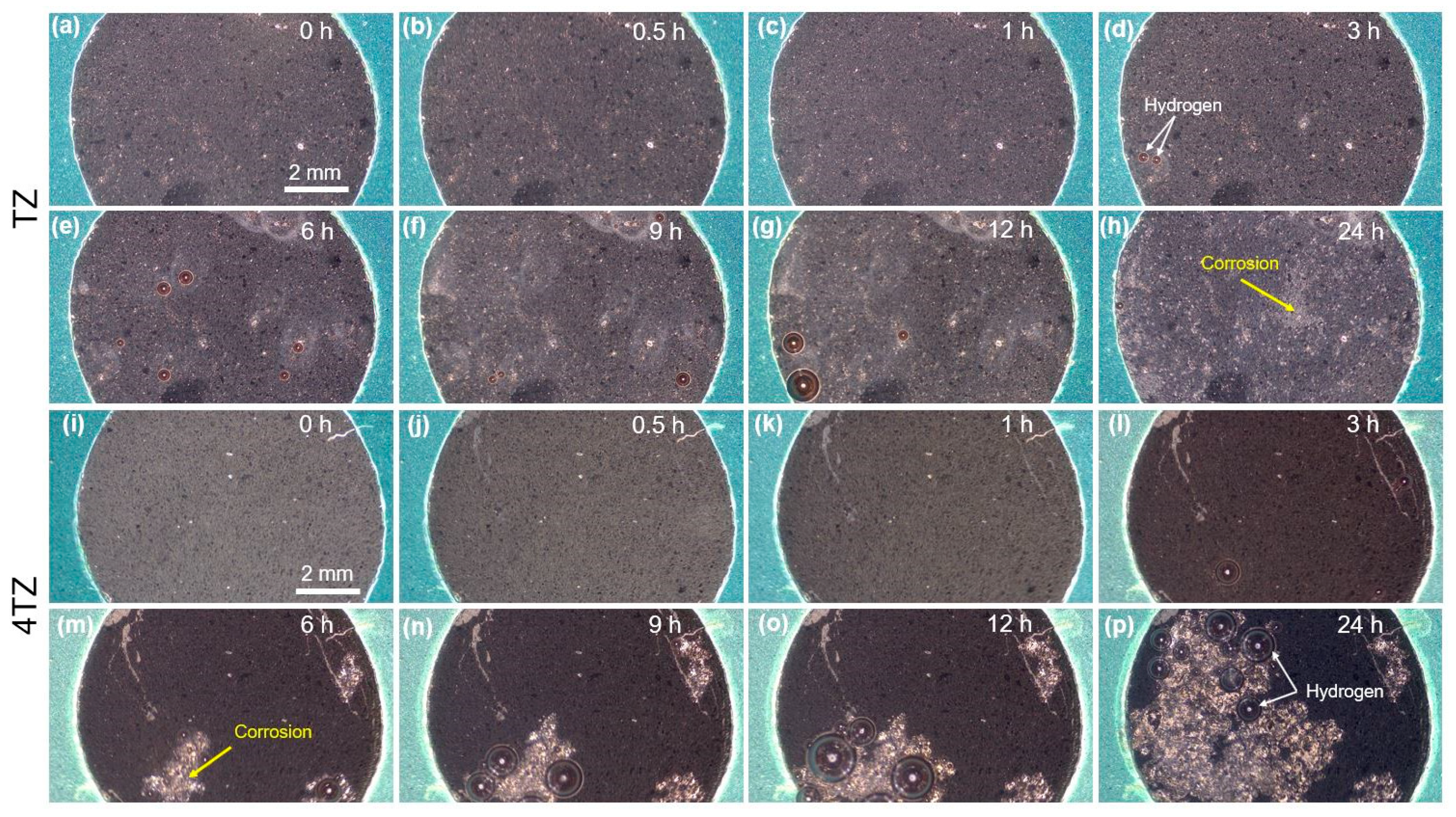
3.3. Corrosion Behavior
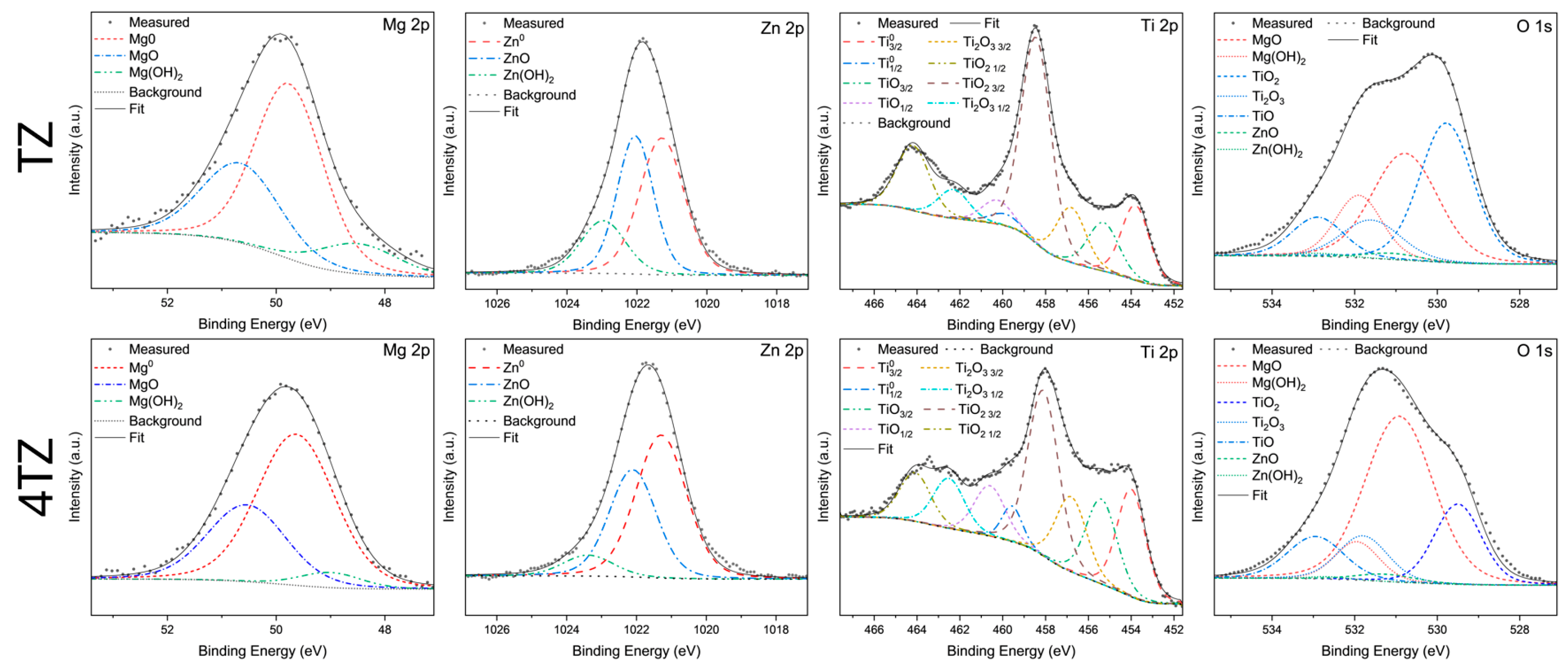
3.4. Surface Characterization Using X-Ray Photoelectron Spectroscopy
3.5. General Discussion
4. Conclusions
- MgTiZn and Mg4TiZn alloys were produced via ball milling and spark plasma sintering. XRD, SEM, and STEM analyses revealed the presence of multiple phases.
- Both TZ and 4TZ exhibited high hardness and stiffness, which were much higher than those of any commercial Mg alloy.
- Both TZ and 4TZ exhibited high corrosion resistance (indicated by the high corrosion potential and low corrosion current density) and passivation, which were attributed to the formation of a Ti rich surface film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCC | Body-centered cubic |
| BCT | Body-centered tetragonal |
| EDXS | Energy-dispersive X-ray spectroscopy |
| FCC | Face-centered cubic |
| FIB | Focused ion beam |
| HAADF | High-angle annular dark-field |
| HEBM | High-energy ball milling |
| HCP | Hexagonal close-packed |
| OCP | Open circuit potential |
| PDP | Potentiodynamic polarization |
| RPM | Revolutions per minute |
| SAED | Selected area electron diffraction |
| SCE | Saturated calomel electrode |
| SEM | Scanning electron microscopy |
| SPS | Spark plasma sintering |
| STEM | Scanning transmission electron microscopy |
| TEM | Transmission electron microscopy |
| TZ | Equal molar MgTiZn alloy |
| 4TZ | Mg4TiZn alloy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| MPEA | Multi-principle element alloy |
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Final Rule: Multi-Pollutant Emissions Standards for Model Years 2027 and Later Light-Duty and Medium-Duty Vehicles; U.S. Environmental Protection Agency: Washington, DC, USA, 2024.
- AbdelGawad, M.; Usman, C.A.; Shunmugasamy, V.C.; Karaman, I.; Mansoor, B. Corrosion behavior of Mg-Zn-Zr-RE alloys under physiological environment—Impact on mechanical integrity and biocompatibility. J. Magnes. Alloys 2022, 10, 1542–1572. [Google Scholar] [CrossRef]
- Birbilis, N.; Easton, M.A.; Sudholz, A.D.; Zhu, S.M.; Gibson, M.A. On the corrosion of binary magnesium-rare earth alloys. Corros. Sci. 2009, 51, 683–689. [Google Scholar] [CrossRef]
- Koo, Y.; Jang, Y.; Yun, Y. A study of long-term static load on degradation and mechanical integrity of Mg alloys-based biodegradable metals. Mater. Sci. Eng. B 2017, 219, 45–54. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gajević, S.; Sharma, L.K.; Pradhan, R.; Miladinović, S.; Ašonja, A.; Stojanović, B. Magnesium-Titanium Alloys: A Promising Solution for Biodegradable Biomedical Implants. Materials 2024, 17, 5157. [Google Scholar] [CrossRef]
- Hoffmann, I.; Cheng, Y.-T.; Puleo, D.A.; Song, G.; Waldo, R.A. Mg-Ti: A Possible Biodegradable, Biocompatible, Mechanically Matched Material for Temporary Implants. MRS Online Proc. Libr. 2011, 1301, 111–115. [Google Scholar] [CrossRef]
- Wang, K.; Tong, X.; Lin, J.; Wei, A.; Li, Y.; Dargusch, M.; Wen, C. Binary Zn–Ti alloys for orthopedic applications: Corrosion and degradation behaviors, friction and wear performance, and cytotoxicity. J. Mater. Sci. Technol. 2021, 74, 216–229. [Google Scholar] [CrossRef]
- Qi, M.H.; Xu, J.L.; Lai, T.; Huang, J.; Ma, Y.C.; Luo, J.M.; Zheng, Y.F. Novel bioactive Ti-Zn alloys with high strength and low modulus for biomedical applications. J. Alloys Compd. 2023, 931, 167555. [Google Scholar] [CrossRef]
- Șutic, A.-T.; Chelariu, R.; Cimpoeșu, R.; Roman, A.-M.; Istrate, B.; Goanță, V.; Benchea, M.; Moscu, M.; Alexandru, A.; Cimpoeşu, N.; et al. Corrosion Behavior and Mechanical Properties of Zn–Ti Alloys as Biodegradable Materials. Metals 2024, 14, 764. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.-H.; Shan, D.; Yim, C.D.; You, B.S. The role of second phases in the corrosion behavior of Mg–5Zn alloy. Corros. Sci. 2012, 60, 238–245. [Google Scholar] [CrossRef]
- Beura, V.K.; Garg, P.; Joshi, V.V.; Solanki, K.N. Numerical Investigation of Micro-Galvanic Corrosion in Mg Alloys: Role of the Cathodic Intermetallic Phase Size and Spatial Distributions. In Magnesium Technology 2020; Jordon, J.B., Miller, V., Joshi, V.V., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 217–223. [Google Scholar]
- Sahu, M.R.; Yamamoto, A. An overview of the recent developments in biodegradable Mg-Zn alloy. J. Magnes. Alloys 2025, 13, 486–509. [Google Scholar] [CrossRef]
- Song, G.L.; Unocic, K.A.; Meyer, H.; Cakmak, E.; Brady, M.P.; Gannon, P.E.; Himmer, P.; Andrews, Q. The corrosion and passivity of sputtered Mg–Ti alloys. Corros. Sci. 2016, 104, 36–46. [Google Scholar] [CrossRef]
- Mukhtar, S.; Kamran, M.; Tayyeb, A.; Hussain, F.; Ishtiaq, M.; Riaz, F.; Asghar, W. Composition design and performance analysis of binary and ternary Mg-Zn-Ti alloys for biomedical implants. J. Biol. Phys. 2025, 51, 9. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Li, Z.; Qiu, Y.; Marcus, P.; Scully, J.R.; Gharbi, O.; Luo, H.; Gupta, R.K.; Zeng, Z.R.; Fraser, H.L.; et al. Current Progress in Aqueous Corrosion of Multi-Principal Element Alloys. Metall. Mater. Trans. A 2024, 55, 2571–2588. [Google Scholar] [CrossRef]
- Jeje, S.O.; Mpofu, P.; Malatji, N.; Kanyane, L.R.; Shongwe, M.B. Multi-Principal Element Alloy Coatings: A Review of Deposition Techniques, Applications, and Future Prospects. Met. Mater. Int. 2025. Available online: https://link.springer.com/article/10.1007/s12540-025-01943-6#citeas (accessed on 26 May 2025). [CrossRef]
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. ASM Handbook Volume 3: Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 2016; ISBN 978-1-62708-070-5. [Google Scholar]
- Liang, L.; Huang, Q.; Wu, H.; Ouyang, Z.; Liu, T.; He, H.; Xiao, J.; Lei, G.; Zhou, K. Stimulation of in vitro and in vivo osteogenesis by Ti-Mg alloys with the sustained-release function of magnesium ions. Colloids Surf. B Biointerfaces 2021, 197, 111360. [Google Scholar] [CrossRef]
- Ralls, A.M.; Daroonparvar, M.; Menezes, P.L. Spark Plasma Sintering of Mg-based Alloys: Microstructure, Mechanical Properties, Corrosion Behavior, and Tribological Performance. J. Magnes. Alloys 2024, 12, 405–442. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Sübütay, H.; Şavklıyıldız, İ. The relationship between structural evolution and high energy ball milling duration in tin reinforced Mg alloys. Mater. Today Commun. 2023, 35, 105868. [Google Scholar] [CrossRef]
- Huang, S.J.; Muneeb, A.; Abbas, A.; Sankar, R. The effect of Mg content and milling time on the solid solubility and microstructure of Ti–Mg alloys processed by mechanical milling. J. Mater. Res. Technol. 2021, 11, 1424–1433. [Google Scholar] [CrossRef]
- Esteves, L.; Witharamage, C.S.; Christudasjustus, J.; Walunj, G.; O’Brien, S.P.; Ryu, S.; Borkar, T.; Akans, R.E.; Gupta, R.K. Corrosion behavior of AA5083 produced by high-energy ball milling. J. Alloys Compd. 2021, 857, 158268. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L.; Huot, J.; Schulz, R. Effect of ball milling on the corrosion resistance of magnesium in aqueous media. Electrochim. Acta 2004, 49, 2461–2470. [Google Scholar] [CrossRef]
- Christudasjustus, J.; Witharamage, C.S.; Walunj, G.; Borkar, T.; Gupta, R.K. The influence of spark plasma sintering temperatures on the microstructure, hardness, and elastic modulus of the nanocrystalline Al-xV alloys produced by high-energy ball milling. J. Mater. Sci. Technol. 2022, 122, 68–76. [Google Scholar] [CrossRef]
- Khan, M.U.F.; Patil, A.; Christudasjustus, J.; Borkar, T.; Gupta, R.K. Spark plasma sintering of a high-energy ball milled Mg-10 wt% Al alloy. J. Magnes. Alloys 2020, 8, 319–328. [Google Scholar] [CrossRef]
- Somasundaram, M.; Uttamchand, N.K.; Annamalai, A.R.; Jen, C.-P. Insights on Spark Plasma Sintering of Magnesium Composites: A Review. Nanomaterials 2022, 12, 2178. [Google Scholar] [CrossRef]
- Digole, S.; Karki, S.; Mugale, M.; Choudhari, A.; Gupta, R.K.; Borkar, T. Spark Plasma Sintering of Pure Titanium: Microstructure and Mechanical Characteristics. Materials 2024, 17, 3469. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Tong, S.; Zhang, Y.; Yuan, Y.; Chen, X.; Pan, F. Effect of impurity elements on the corrosion behavior of Mg-Al alloys: A first principles study. J. Mater. Res. Technol. 2025, 36, 8632–8642. [Google Scholar] [CrossRef]
- Bryan, Z.L.; Alieninov, P.; Berglund, I.S.; Manuel, M.V. A diffusion mobility database for magnesium alloy development. Calphad 2015, 48, 123–130. [Google Scholar] [CrossRef]
- Murray, J.L. The Mg−Ti (Magnesium-Titanium) system. Bull. Alloy Phase Diagr. 1986, 7, 245–248. [Google Scholar] [CrossRef]
- Huang, S.-J.; Mose, M.P. High-energy ball milling-induced crystallographic structure changes of AZ61-Mg alloy for improved hydrogen storage. J. Energy Storage 2023, 68, 107773. [Google Scholar] [CrossRef]
- Bhuyan, R.K.; Kisan, B.; Parida, S.K.; Patra, S.; Kumar, S. Synthesis of Nano-Composites Mg2TiO4 Powders via Mechanical Alloying Method and Characterization. In Magnesium Alloys Structure and Properties; Tański, T., Jarka, P., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Zhou, J.; Han, Y.; Yu, H.; Zhang, P.; Su, Q.; Li, H.; Ning, K.; Cheng, K.; Zhao, D.; Wang, J.; et al. Nanocrystalline AZ91 alloy: Preparation, exceptional thermal stability and enhanced hardness. Vacuum 2024, 224, 113088. [Google Scholar] [CrossRef]
- Kumar, T.S.; Thankachan, T.; Shalini, S.; Čep, R.; Kalita, K. Microstructure, hardness and wear behavior of ZrC particle reinforced AZ31 surface composites synthesized via friction stir processing. Sci. Rep. 2023, 13, 20089. [Google Scholar] [CrossRef]
- AZO Materials. Magnesium AZ31B-F Alloy (UNS M11311). Available online: https://www.azom.com/article.aspx?ArticleID=9239 (accessed on 13 June 2025).
- AZO Materials. Magnesium WE43-T6 Alloy (UNS M16600). Available online: https://www.azom.com/article.aspx?ArticleID=8539 (accessed on 13 June 2025).
- MatWeb. Magnesium Elektron Elektron® 43 Magnesium Wrought Alloy. Available online: https://www.matweb.com/search/datasheet.aspx?matguid=a3a15f442b4c4d41a8e6aaf13372bac1&ckck=1 (accessed on 13 June 2025).
- Xia, Z.; Miao, L.; Huang, R.; Yan, C.; Xin, Y.; Feng, B.; Xu, J.; Huang, G.; Zhao, L. Mechanical properties and corrosion behavior of a ZK60 magnesium alloy containing profuse twins and precipitates. J. Mater. Res. Technol. 2024, 29, 1767–1778. [Google Scholar] [CrossRef]
- Alaa Adnan, F.; Jamal Abdulkader, N.; Salab Hamza, M. Corrosion Behavior of Zinc Alloy Based Metal Matrix Composites Reinforced with nano BN. J. Phys. Conf. Ser. 2019, 1279, 012031. [Google Scholar] [CrossRef]
- Acharya, M.G.; Shetty, A.N. Performance of anionic dimeric surfactant on AZ31 Magnesium alloy in neutral medium unveiled through experimental and theoretical investigation. Surf. Sci. Technol. 2024, 2, 16. [Google Scholar] [CrossRef]
- Feng, B.; Liu, G.; Yang, P.; Huang, S.; Qi, D.; Chen, P.; Wang, C.; Du, J.; Zhang, S.; Liu, J. Different role of second phase in the micro-galvanic corrosion of WE43 Mg alloy in NaCl and Na2SO4 solution. J. Magnes. Alloys 2022, 10, 1598–1608. [Google Scholar] [CrossRef]
- Wang, W.; Mraied, H.; Diyatmika, W.; Chu, J.P.; Li, L.; Cai, W. Effects of nanoscale chemical heterogeneity on the wear, corrosion, and tribocorrosion resistance of Zr-based thin film metallic glasses. Surf. Coat. Technol. 2020, 402, 126324. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, M.; Geng, Y.; Zhang, S.; Xin, T.; Chen, G.; Zhou, Y.; Zhou, X.; Wu, R.; Shi, Q. Microstructural evolution and its influence on mechanical and corrosion behaviors in a high-Al/Zn containing duplex Mg-Li alloy after friction stir processing. J. Mater. Sci. Technol. 2024, 184, 245–255. [Google Scholar] [CrossRef]
- Nogueira, R.A.; Pinto, L.; Angelo, A.; Claro, A.P.R.A.; Grandini, C.R. Interstitial oxygen’s influence on the corrosion behavior of ti-9mo alloys. Mater. Res. 2013, 16, 1405–1410. [Google Scholar] [CrossRef]
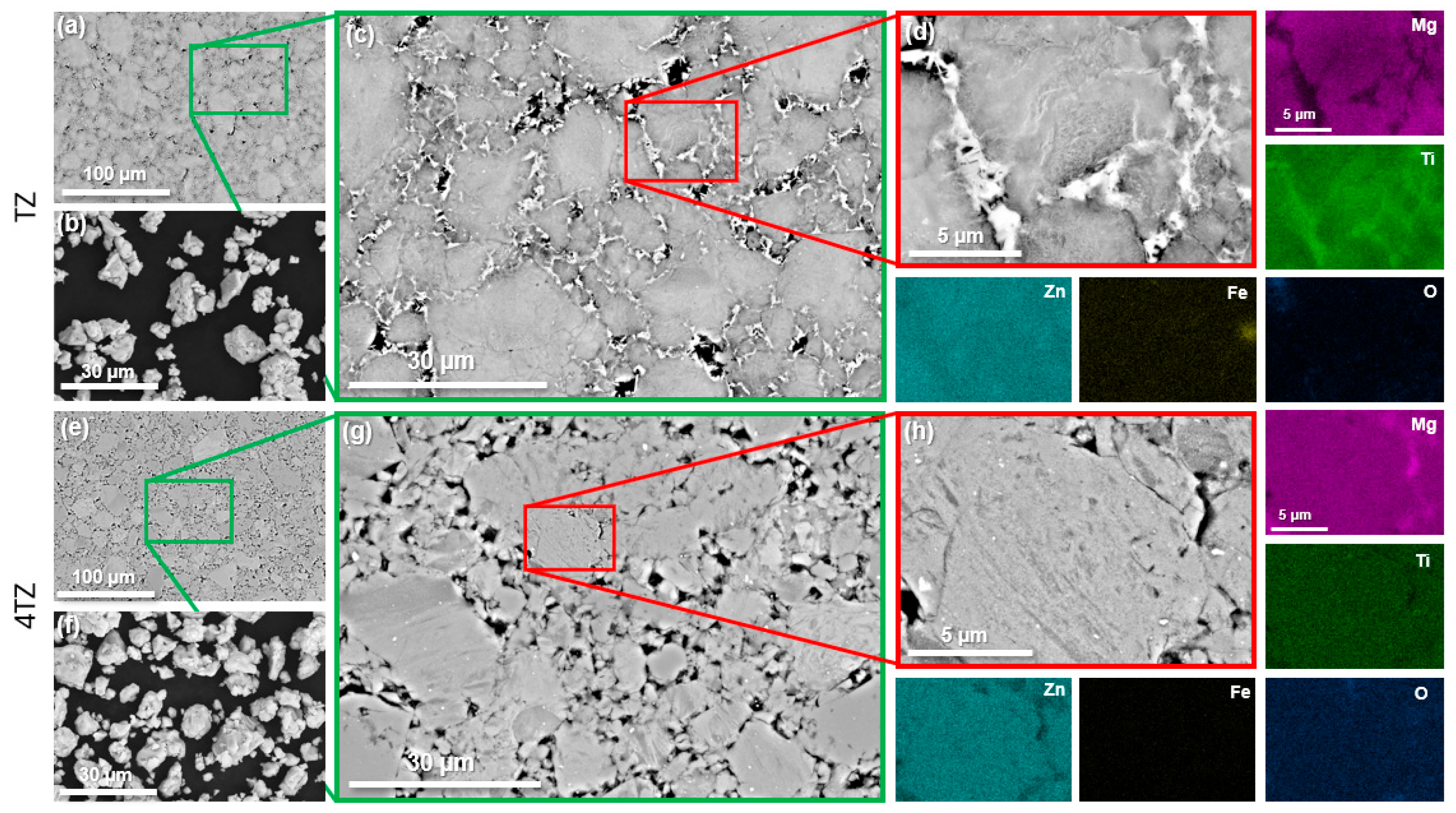
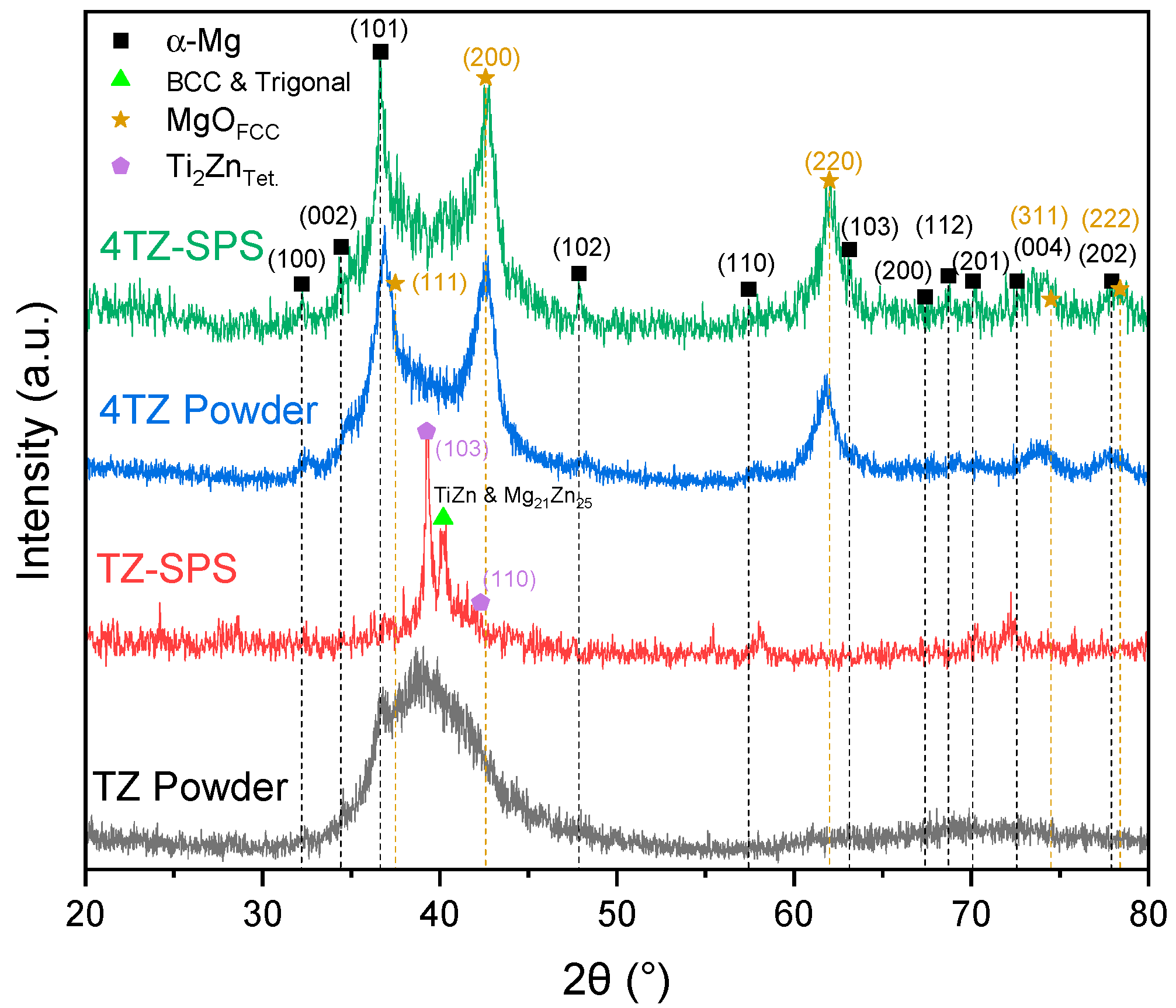
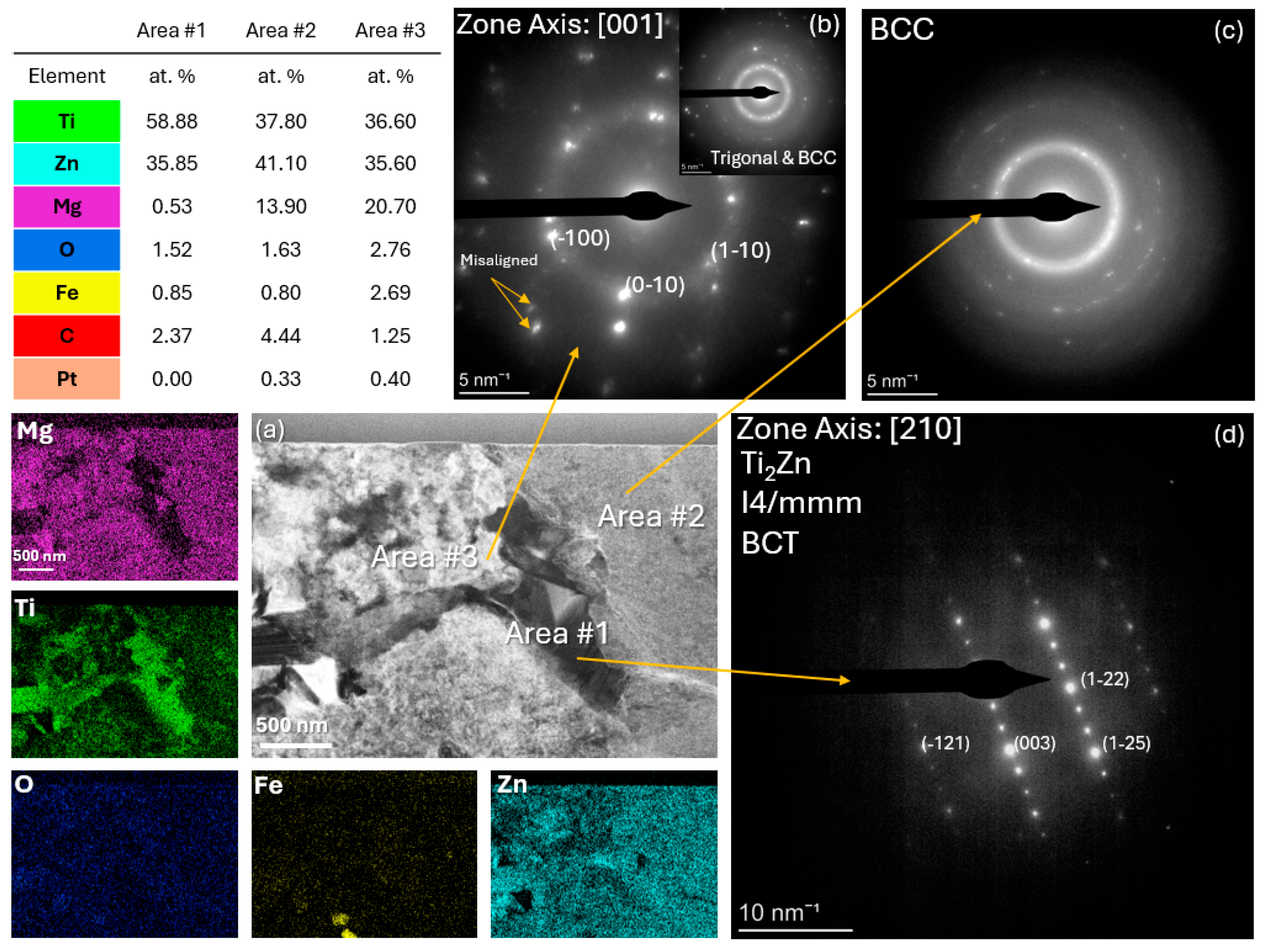


| Alloy | Mg wt.% | Ti wt.% | Zn wt.% |
|---|---|---|---|
| MgTiZn “TZ” | 17.7 | 34.8 | 47.5 |
| Mg4TiZn “4TZ” | 46.2 | 22.7 | 31.1 |
| Alloy | Hardness (GPa) | Young’s Modulus (GPa) | Refs. |
|---|---|---|---|
| TZ | 5.47 ± 1.02 | 98.18 ± 11.15 | This work |
| 4TZ | 5.05 ± 0.89 | 100.75 ± 9.63 | This work |
| Zn-(0.05–0.3)Ti | 0.41–0.58 | - | [11] |
| Zn-(0.1–1)Ti | 0.33–0.69 | - | [13] |
| Ti-(5–30)Zn | 1.5–3.8 | 4.3–26.8 | [12] |
| ZK60 (Mg-5.5Zn) | 0.69 | - | [44] |
| AZ31 | 0.59 | 44.8 | [40,41] |
| WE43C | 0.93 | 44.0 | [42,43] |
| Alloy | Ecorr (mVSCE) | icorr (µA/cm2) | Solution | Refs. |
|---|---|---|---|---|
| TZ | −972 ± 78 | 3.65 ± 0.65 | 0.1 M NaCl | This work |
| 4TZ | −1078 ± 2 | 4.58 ± 1.64 | 0.1 M NaCl | This work |
| Zn-0.05Ti | −1036 ± 78 | 19.7 ± 0.4 | HBSS | [11] |
| Zn-0.1Ti | −1038 ± 94 | 21.6 ± 0.6 | HBSS | [11] |
| Zn-0.2Ti | −1045 ± 72 | 22.8 ± 0.7 | HBSS | [11] |
| Zn-0.3Ti | −1049 ± 82 | 27.4 ± 0.3 | HBSS | [11] |
| Zn-0.10Ti | −1005.6 | 33.4 | 0.9% NaCl saline | [13] |
| Zn-0.25Ti | −1035.3 | 16.2 | 0.9% NaCl saline | [13] |
| Zn-1.00Ti | −1048.4 | 15.6 | 0.9% NaCl saline | [13] |
| Ti-5Zn | −187 | 0.69 | SBF | [12] |
| Ti-10Zn | −202 | 0.97 | SBF | [12] |
| Ti-20Zn | −178 | 0.74 | SBF | [12] |
| Ti-30Zn | −245 | 3.63 | SBF | [12] |
| ZA-8 | −1050 | 127.54 | 0.6 M NaCl | [45] |
| ZK60 (Mg-5.5Zn) | −1500 | 50.4 | 3 wt.% NaCl | [44] |
| AZ31 (Mg-3Al-1Zn) | −1483 | 154.03 | 0.1 M NaCl | [46] |
| WE43C | −1720 ± 20 | 65.7 ± 2 | 0.6 M NaCl | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmer, A.; Agrawal, R.; Mugale, M.; Borkar, T.; Gupta, R. Corrosion Behavior of MgTiZn and Mg4TiZn Alloys After Ball Milling and Subsequent Spark Plasma Sintering. Materials 2025, 18, 3279. https://doi.org/10.3390/ma18143279
Helmer A, Agrawal R, Mugale M, Borkar T, Gupta R. Corrosion Behavior of MgTiZn and Mg4TiZn Alloys After Ball Milling and Subsequent Spark Plasma Sintering. Materials. 2025; 18(14):3279. https://doi.org/10.3390/ma18143279
Chicago/Turabian StyleHelmer, Alexander, Rahul Agrawal, Manoj Mugale, Tushar Borkar, and Rajeev Gupta. 2025. "Corrosion Behavior of MgTiZn and Mg4TiZn Alloys After Ball Milling and Subsequent Spark Plasma Sintering" Materials 18, no. 14: 3279. https://doi.org/10.3390/ma18143279
APA StyleHelmer, A., Agrawal, R., Mugale, M., Borkar, T., & Gupta, R. (2025). Corrosion Behavior of MgTiZn and Mg4TiZn Alloys After Ball Milling and Subsequent Spark Plasma Sintering. Materials, 18(14), 3279. https://doi.org/10.3390/ma18143279









